Triprotic Ammonium Oleate Ionic Liquid Crystal Lubricant for Copper-Copper Friction and Wear Reduction
Abstract
1. Introduction
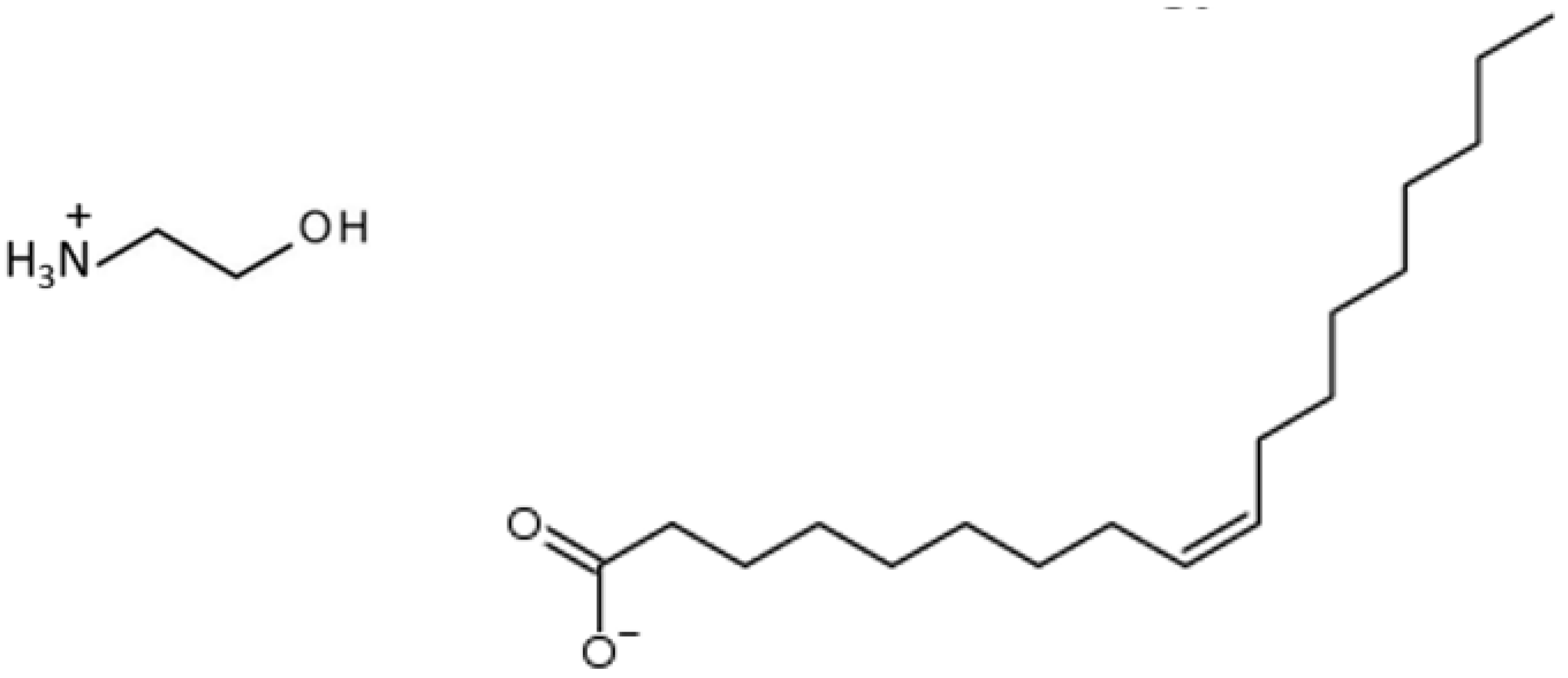
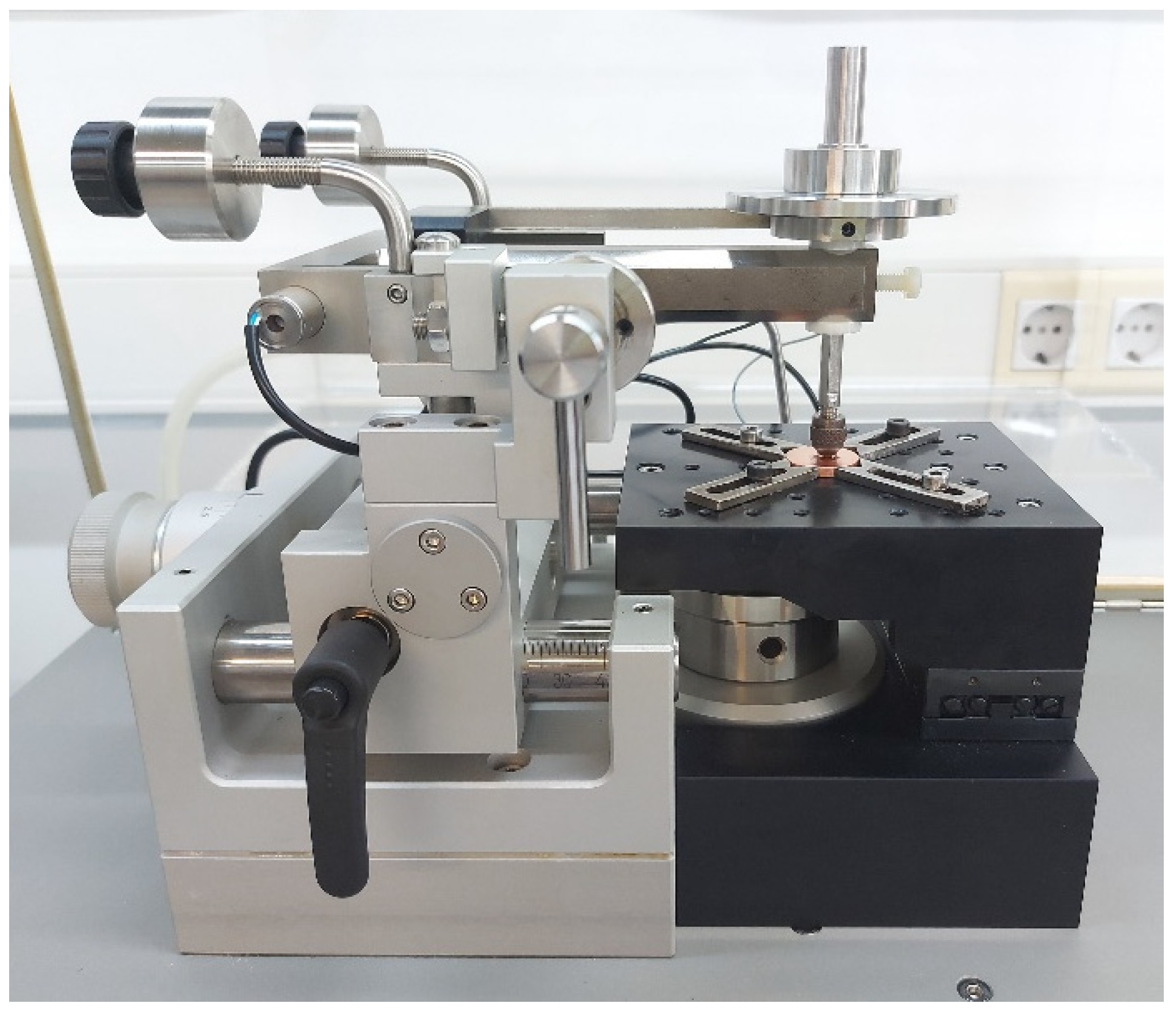
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cai, M.; Yu, Q.; Liu, W.; Zhou, F. Ionic liquid lubricants: When chemistry meets tribology. Chem. Soc. Rev. 2020, 49, 7753–7818. [Google Scholar] [CrossRef] [PubMed]
- Greaves, T.L.; Drummond, C.J. Protic ionic liquids: Evolving structure–property relationships and expanding applications. Chem. Rev. 2015, 115, 11379–11448. [Google Scholar] [CrossRef] [PubMed]
- Vega, M.R.O.; Baldin, E.K.; Pereira, D.; Martins, M.C.S.; Pranke, P.; Horn, F.; Pinheiro, I.; Vieira, A.; Espiña, B.; Mattedi, S.; et al. Toxicity of oleate-based amino protic ionic liquids towards Escherichia coli, Danio rerio embryos and human skin cells. J. Hazard. Mater. 2022, 422, 126896. [Google Scholar] [CrossRef] [PubMed]
- Dang, R.K.; Dhami, S.S.; Goyal, D.; Chauhan, A. Effect of TiO2 and CuO Based Nanolubricants on the Static Thermal Performance of Circular Journal Bearings. Tribol. Ind. 2021, 43, 420–433. [Google Scholar] [CrossRef]
- Dang, R.K.; Goyal, D.; Chauhan, A.; Dhami, S.S. Numerical and Experimental Studies on Performance Enhancement of Journal Bearings Using Nanoparticles Based Lubricants. Arch. Comp. Methods Eng. 2021, 28, 3887–3915. [Google Scholar] [CrossRef]
- Khan, A.; Gusain, R.; Sahai, M.; Khatri, O.P. Fatty acids-derived protic ionic liquids as lubricant additive to synthetic lube base oil for enhancement of tribological properties. J. Mol. Liq. 2019, 293, 111444. [Google Scholar] [CrossRef]
- Gusain, R.; Khatri, O.P. Fatty acid ionic liquids as environmentally friendly lubricants for low friction and wear. RSC Adv. 2016, 6, 3462. [Google Scholar] [CrossRef]
- Zhou, Y.; Qu, J. Ionic liquids as lubricant additives: A review. ACS Appl. Mater. Interfaces 2017, 9, 3209–3222. [Google Scholar] [CrossRef]
- Nyrdek, T.; Stapels, M.; Kunz, W. Newly synthesized ionic liquids and additives to existing lubricant oils. Proc. Int. Mech. Eng. Part J J. Eng. Tribol. 2022, 236, 1409–1419. [Google Scholar]
- Aviles, M.D.; Pamies, R.; Sanes, J.; Carrion, F.J.; Bermudez, M.D. Fatty Acid-Derived Ionic Liquid Lubricant. Protic Ionic Liquid Crystals as Protic Ionic Liquid Additives. Coatings 2019, 9, 710. [Google Scholar] [CrossRef]
- Vega, M.R.O.; Parise, K.; Ramos, L.B.; Boff, U.; Mattedi, S.; Schaeffer, L.; Malfatti, C.F. Protic ionic liquids used as metal-forming green lubricants for aluminum: Effect of anion-chain lengh. Mater. Res. 2017, 20, 675–687. [Google Scholar] [CrossRef]
- Vega, M.R.O.; Ercolani, J.; Mattedi, S.; Aguzzoli, C.; Ferreira, C.A.; Rocha, A.S.; Malfatti, C.F. Oleate-based protic ionic liquids as lubricants for aluminum 1100. Ind. Eng. Chem. Res. 2018, 57, 12386–12396. [Google Scholar] [CrossRef]
- Vega, M.R.O.; Mattedi, S.; Schroeder, R.M.; Malfatti, C.F. 2-hydroxyethylammonium oleate protic ionic liquid as corrosion inhibitor for aluminum in neutral medium. Mater. Corros. 2020, 72, 543–556. [Google Scholar] [CrossRef]
- Kreivaitis, R.; Gumbyte, M.; Kupčinskas, A.; Kazancev, K.; Makarevičienė, V. Investigating the tribological properties of PILs derived from different ammonium cations and long chain carboxylic acid anion. Tribol. Int. 2020, 141, 105905. [Google Scholar] [CrossRef]
- Kreivaitis, R.; Gumbyte, M.; Kupčinskas, A.; Kazancev, K.; Makarevičienė, V.; Ta, K.N.; Horng, J.H. Investigation of tribological properties of two protic ionic liquids as additives in water for steel–steel and alumina–steel contacts. Wear 2020, 456–457, 203390. [Google Scholar] [CrossRef]
- Guo, H.; Pang, J.; Adukure, A.R.; Iglesias, P. Influence of hydrogen bonding and iconicity of protic ionic liquids on lubricating steel-steel and steel-aluminum contacts: Potential ecofriendly lubricants and additives. Tribol. Lett. 2020, 68, 114. [Google Scholar] [CrossRef]
- Pandey, P.; Anthony, E.; Somers, A.E.; Hait, S.K.; Forsyth, M.; Ramakumar, S.S.V. Short Chain imidazolium ionic liquids: Synthesis and oil miscibility in various base oil by use of surfactant as high performance friction and antiwear lubricant additive. Tribol. Lett. 2021, 69, 95. [Google Scholar] [CrossRef]
- Ramajo, B.; Blanco, D.; Rivera, N.; Viesca, J.L.; González, R.; Hernández-Battez, A. Long-term thermal stability of fatty acid anion-based ionic liquids. J. Mol. Liq. 2021, 328, 115492. [Google Scholar] [CrossRef]
- Aviles, M.D.; Jimenez, A.E.; Saurin, N.; Carrion, F.J.; Sanes, J.; Bermudez, M.D. Tribological characterization of epoxy coatings modified with ionic liquids and graphene. Tribol. Int. 2020, 149, 105516. [Google Scholar] [CrossRef]
- Alvarez, V.H.; Mattedi, S.; Martin-Pastor, M.; Aznar, M.; Iglesias, M. Synthesis and thermophysical properties of two new protic long-chain ionic liquids with the oleate anion. Fluid Phase Equilibria 2010, 299, 42–50. [Google Scholar] [CrossRef]
- Espinosa, T.; Sanes, J.; Jimenez, A.E.; Bermudez, M.D. Protic ammonium carboxylate ionic liquid lubricants of OFHC copper. Wear 2013, 303, 495–509. [Google Scholar] [CrossRef]
- Espinosa, T.; Jiménez, M.; Sanes, J.; Jimenez, A.E.; Iglesias, M.; Bermudez, M.D. Ultralow friction with a protic ionic liquid boundary film at the water-lubricated sapphire-stainless steel interface. Tribol. Lett. 2014, 53, 1–9. [Google Scholar] [CrossRef]
- Sanes, J.; Aviles, M.D.; Saurin, N.; Espinosa, T.; Carrion, F.J.; Bermudez, M.D. Synergy between graphene and ionic liquid lubricant additives. Tribol. Int. 2017, 116, 371–382. [Google Scholar] [CrossRef]
- Ge, X.; Li, J.; Wang, H.; Zhang, C.; Liu, Y.; Luo, J. Macroscale superlubricity under extreme pressure enabled by the combination of graphene-oxide nanosheets with ionic liquid. Carbon 2019, 151, 76–83. [Google Scholar] [CrossRef]
- Wang, C.; Sun, J.; Kong, L.; He, J. Tribological Behavior of Reduced Graphene Oxide–Al2O3 Nanofluid: Interaction among Testing Force, Rotational Speed and Nanoparticle Concentration. Materials 2022, 15, 5177. [Google Scholar] [CrossRef]


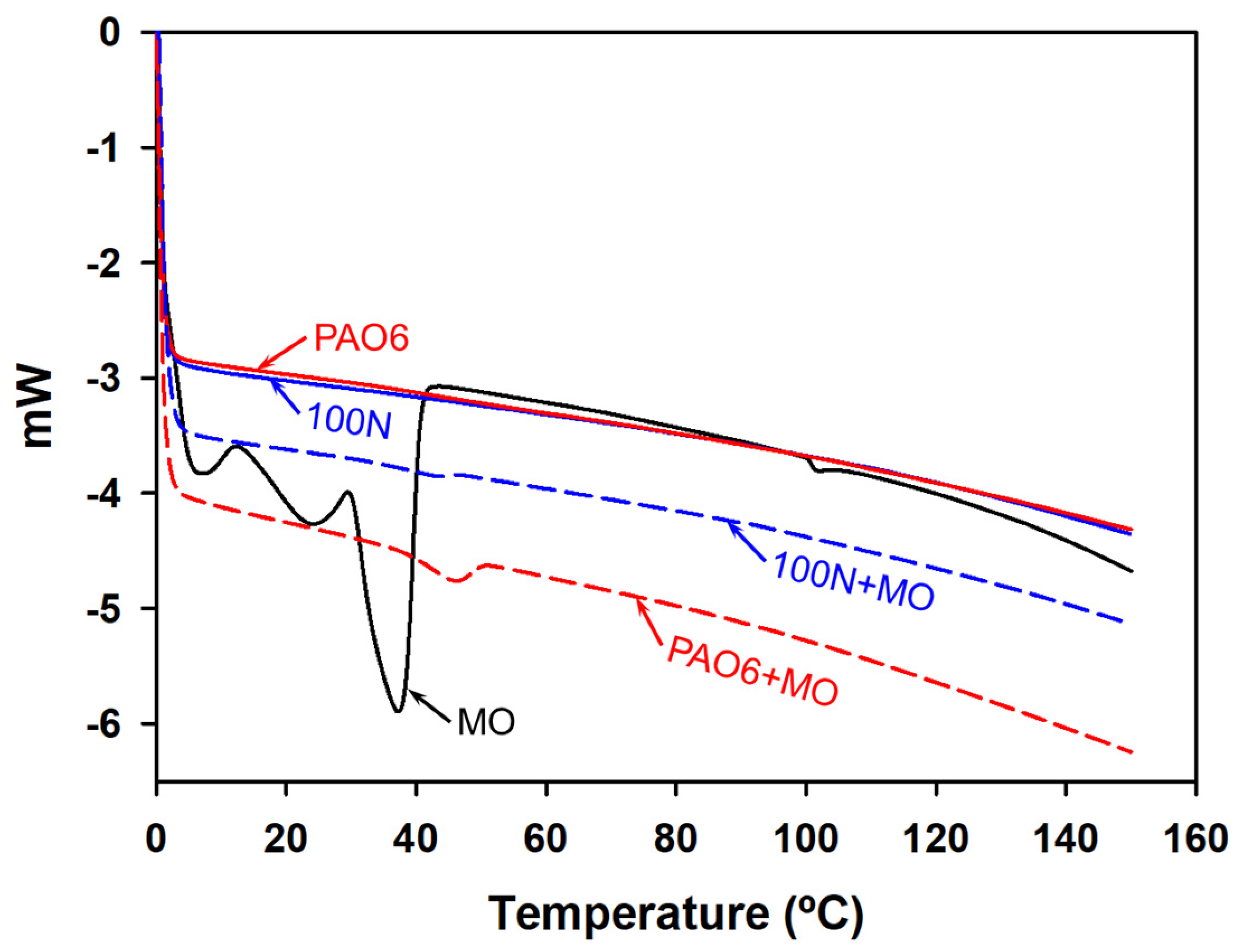
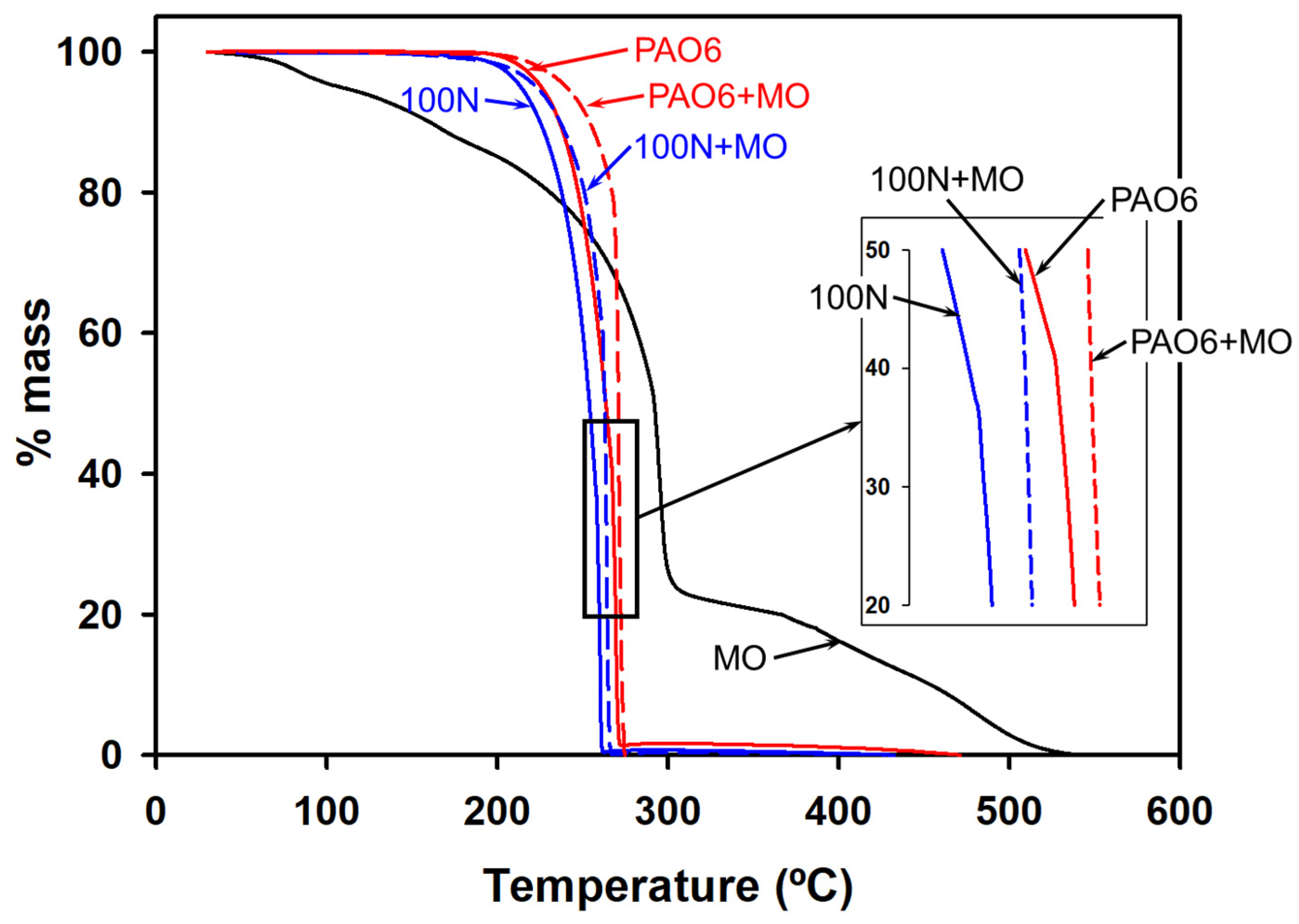
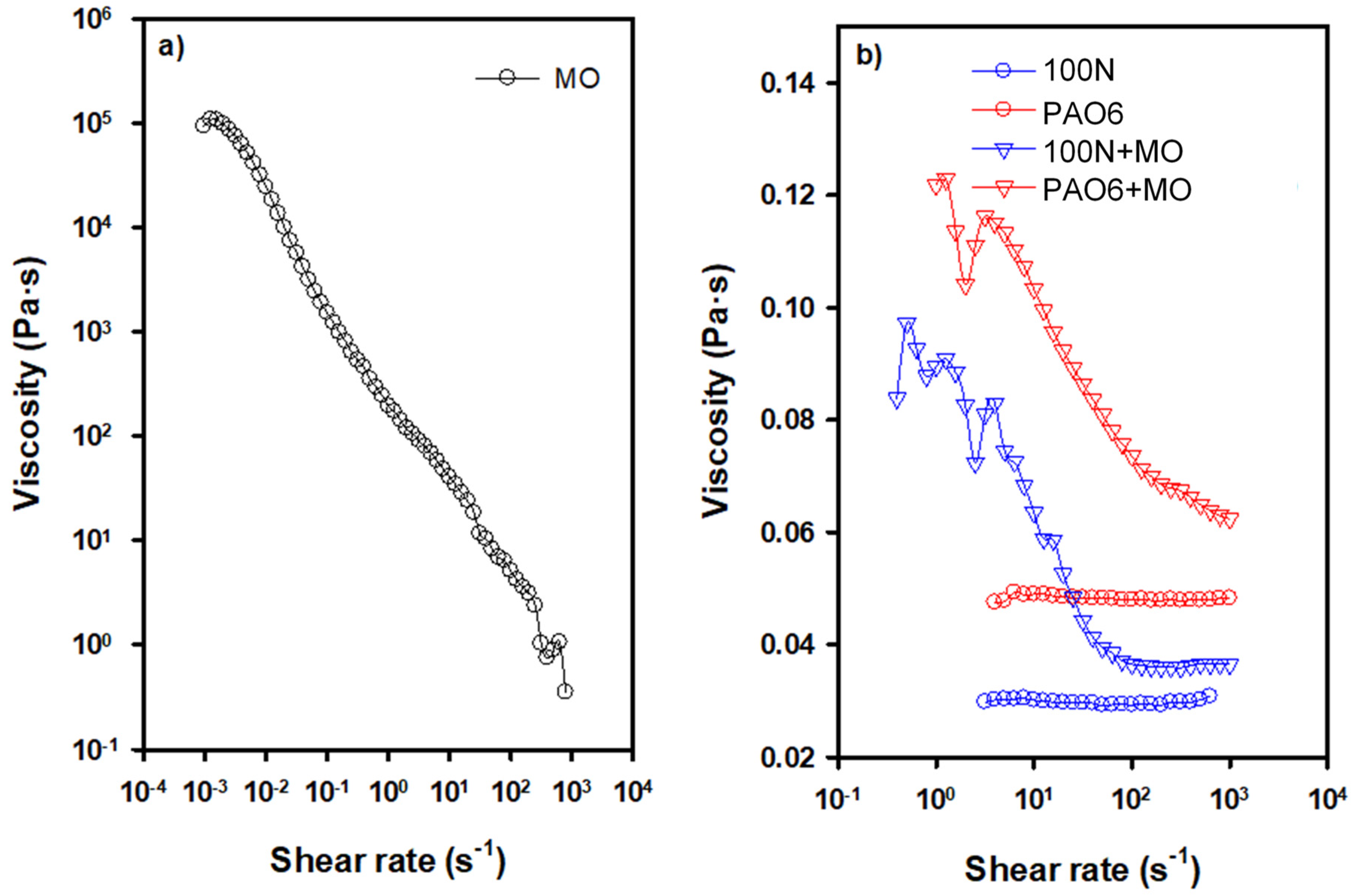
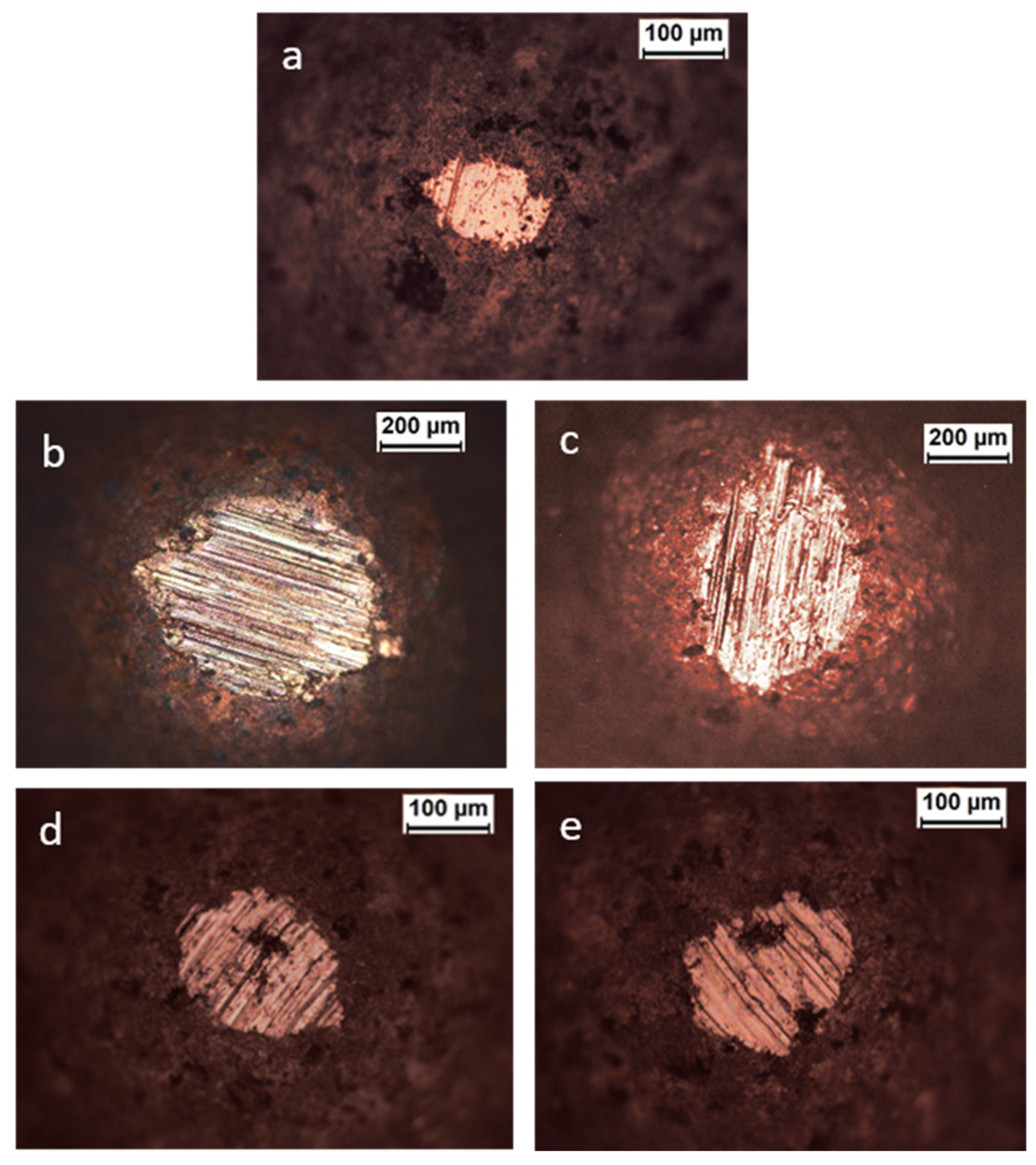
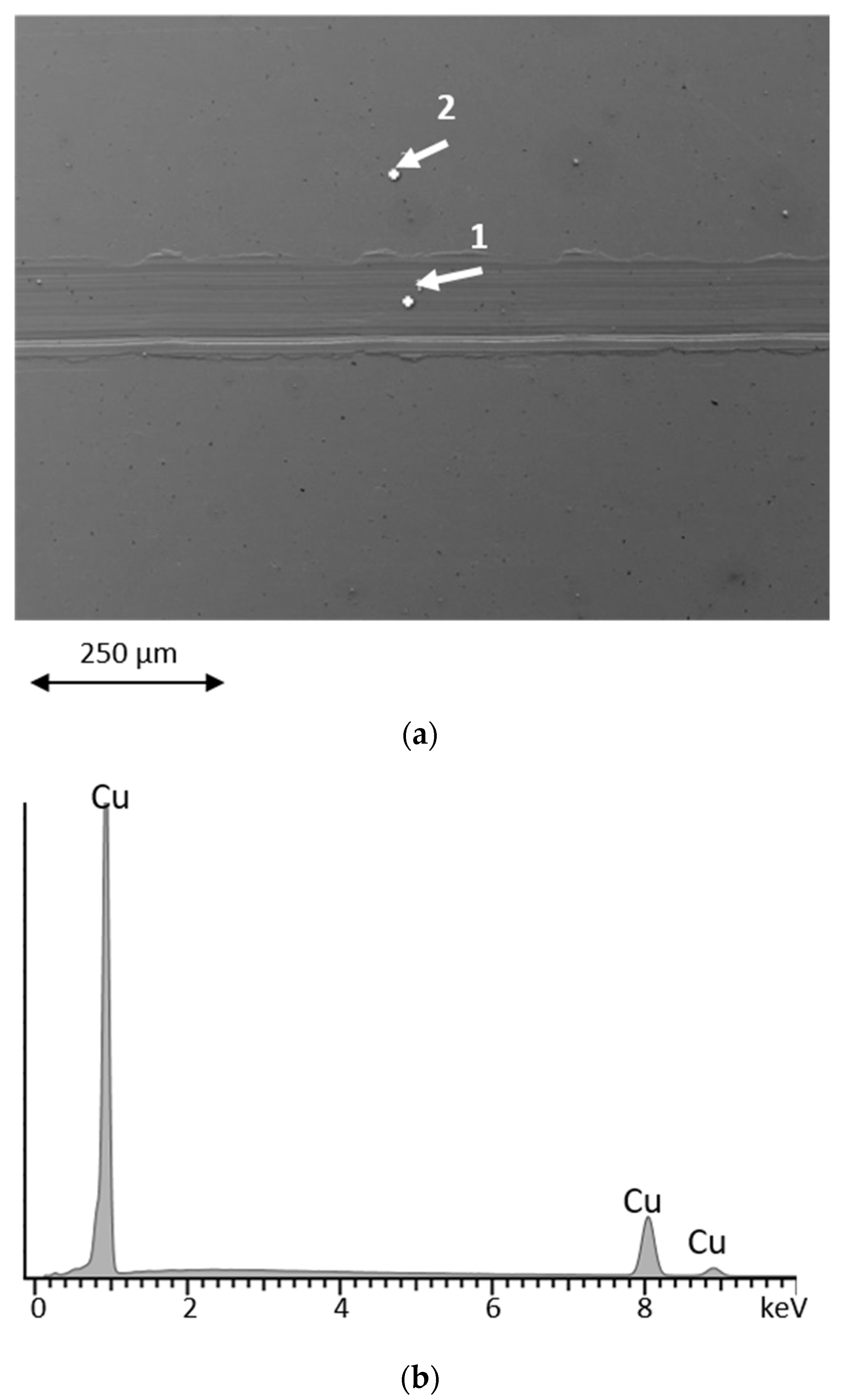
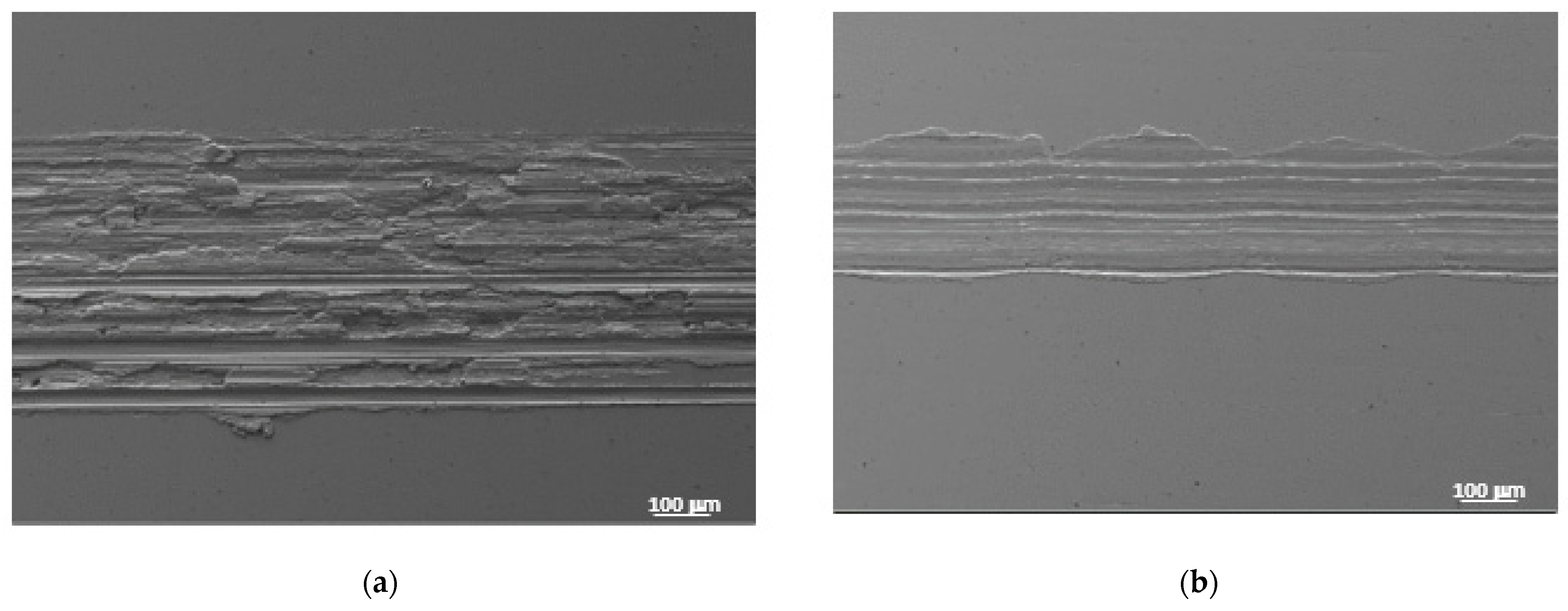

| Lubricant | T Onset (°C) | Td (−50 wt.%) * |
|---|---|---|
| MO | 62.16 | 312.3 |
| PAO6 | 258.0 | 284.8 |
| 100N | 246.9 | 270.5 |
| PAO6 + 2%MO | 288.0 | 379.7 |
| 100N + 2%MO | 274.7 | 330.7 |
| Lubricant | Initial | After 60 s |
|---|---|---|
| PAO6 |  22.5° (±1.9) |  2.2° (±0.2) |
| 100N |  20.9° (±1.4) |  1.7° (±0.1) |
| PAO6 + MO |  27.6° (±1.1) |  17.1° (±1.5) |
| 100N + MO |  26.9° (±0.9) |  8.9° (±0.9) |
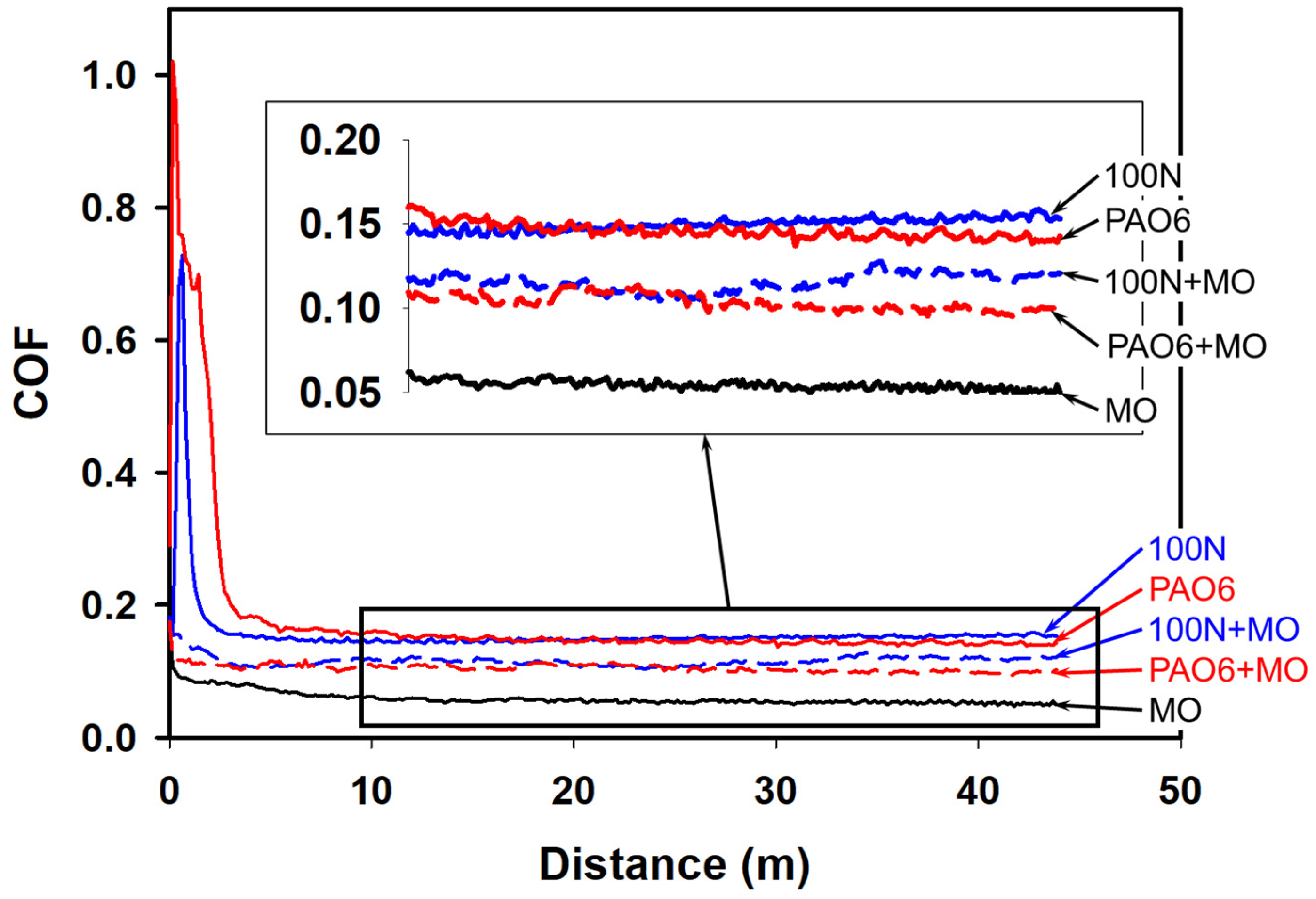
| Lubricant | Maximum COF (Distance) | Steady-State COF | Average COF |
|---|---|---|---|
| MO | 0.16 (0 m) | 0.06 | 0.06 (±0.006) |
| PAO6 | 1.00 (0.17 m) | 0.15 | 0.18 (±0.010) |
| 100N | 0.73 (0.65 m) | 0.15 | 0.16 (±0.011) |
| PAO6 + 2%MO | 0.17 (0 m) | 0.10 | 0.10 (±0.002) |
| 100N + 2%MO | 0.18 (0 m) | 0.10 | 0.11 (±0.009) |
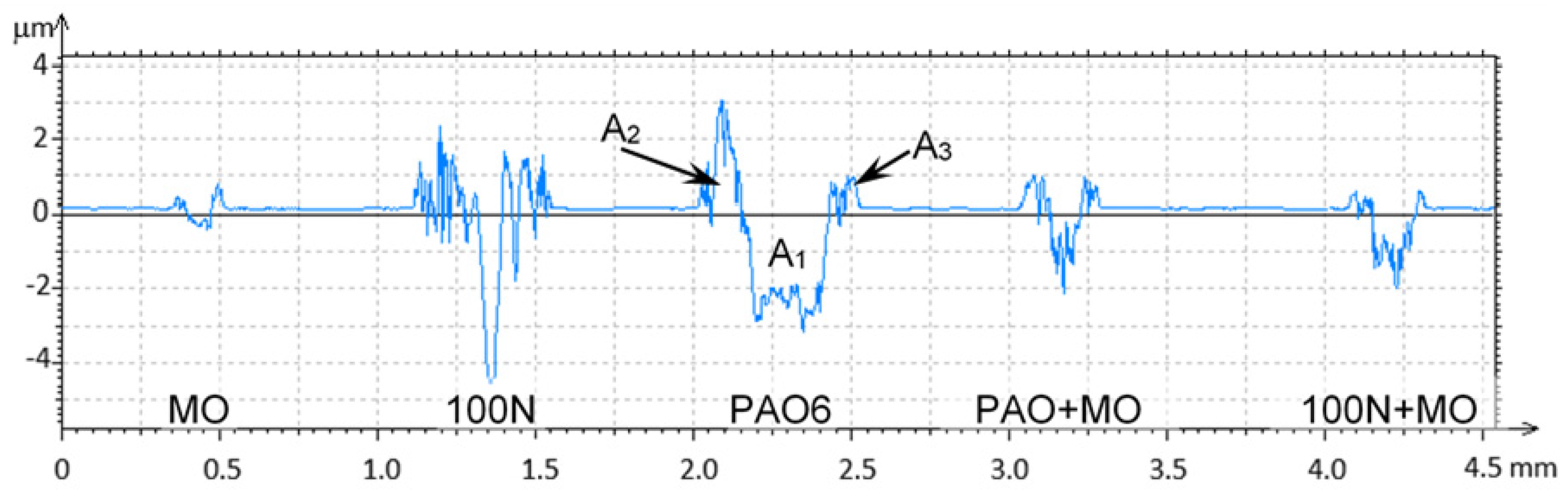
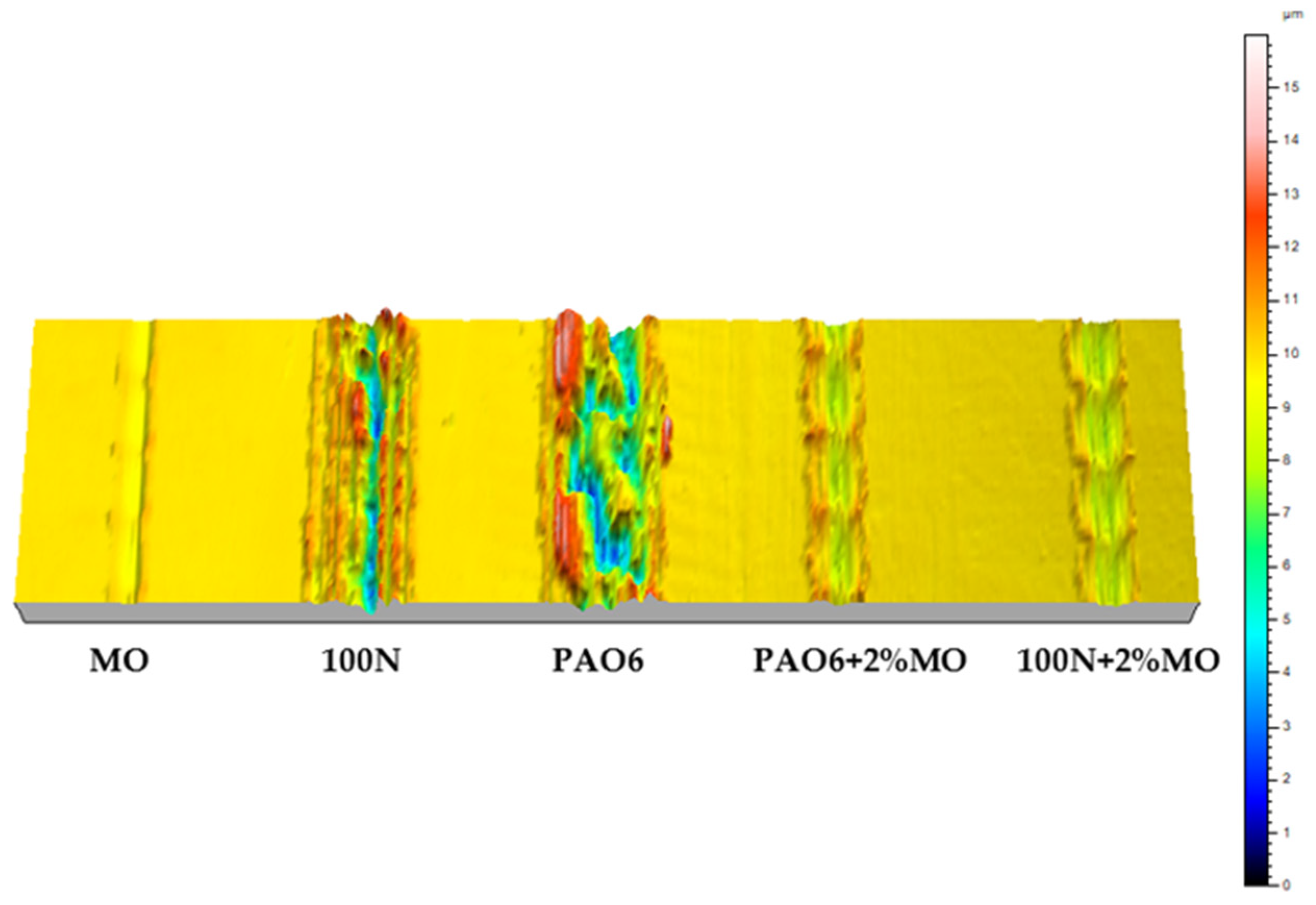
| Lubricant | Wear Rate (mm3/N·m) | Worn Area (mm2) |
|---|---|---|
| MO | 4.15 × 10−7 (±3.76 × 10−7) | 1.19 × 10−2 |
| PAO6 | 2.50 × 10−5 (±1.32 × 10−5) | 1.10 × 10−1 |
| 100N | 2.97 × 10−5 (±5.27 × 10−6) | 8.56 × 10−2 |
| PAO6 + 2%MO | 5.08 × 10−6 (±4.42 × 10−6) | 2.57 × 10−2 |
| 100N + 2%MO | 6.36 × 10−6 (±1.67 × 10−6) | 2.74 × 10−2 |
| Lubricant | Cu (wt.%) | C (wt.%) | ||
|---|---|---|---|---|
| Inside | Outside | Inside | Outside | |
| MO | 96.6 | 96.3 | 3.2 | 3.4 |
| PAO6 | 80.9 | 85.6 | 16.1 | 14.1 |
| 100N | 87.8 | 92.7 | 12.2 | 6.7 |
| PAO6 + 2%MO | 95.6 | 95.6 | 3.7 | 4.6 |
| 100N + 2%MO | 95.1 | 96.3 | 4.4 | 3.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avilés, M.-D.; Jiménez, A.-E.; Pamies, R.; Carrión-Vilches, F.-J.; Bermúdez, M.-D. Triprotic Ammonium Oleate Ionic Liquid Crystal Lubricant for Copper-Copper Friction and Wear Reduction. Lubricants 2022, 10, 290. https://doi.org/10.3390/lubricants10110290
Avilés M-D, Jiménez A-E, Pamies R, Carrión-Vilches F-J, Bermúdez M-D. Triprotic Ammonium Oleate Ionic Liquid Crystal Lubricant for Copper-Copper Friction and Wear Reduction. Lubricants. 2022; 10(11):290. https://doi.org/10.3390/lubricants10110290
Chicago/Turabian StyleAvilés, María-Dolores, Ana-Eva Jiménez, Ramón Pamies, Francisco-José Carrión-Vilches, and María-Dolores Bermúdez. 2022. "Triprotic Ammonium Oleate Ionic Liquid Crystal Lubricant for Copper-Copper Friction and Wear Reduction" Lubricants 10, no. 11: 290. https://doi.org/10.3390/lubricants10110290
APA StyleAvilés, M.-D., Jiménez, A.-E., Pamies, R., Carrión-Vilches, F.-J., & Bermúdez, M.-D. (2022). Triprotic Ammonium Oleate Ionic Liquid Crystal Lubricant for Copper-Copper Friction and Wear Reduction. Lubricants, 10(11), 290. https://doi.org/10.3390/lubricants10110290







