Abstract
Alkyltetralins similar to naphthenic base oil were successfully synthesized by Friedel–Crafts alkylation of tetralin with short-chain α-olefins (C3/C4), where the applied chemical feedstock could be obtained from coal chemical industry with ease. The Et3NHCl-AlCl3 ionic liquid (IL) as catalyst exhibited excellent catalytic performance for alkylation of tetralin with n-butene or propylene. The reaction conditions were investigated in detail to achieve the high selectivity toward multi-alkyltetralins, and the selectivity for multi-alkyltetralins could reach as high as 90% with the complete conversion of tetralin. For comparison, three alkyltetralins with various side-chain alkyl groups and number of side-chains on tetralin were isolated, which behaved as lubricating base oils for investigation. The synthetic oils exhibited an excellent low temperature fluidity, and good potential for compatibility with additives. Di-propyltetralins (DPT) and di-butyltetralins (DBT) displayed the lower friction and wear values than mono-butyltetralins (MBT), which had comparable tribological properties to the commercial naphthenic base oil 4006 (N4006).
1. Introduction
Naphthenic base oils are widely applied for industrial purposes, such as compressor oil, insulating oil and metal work fluid, because they offer excellent low-temperature performance, good emulsified ability and excellent solubility. The demand for naphthenic base oils extracted from naphthenic crude oil has increased rapidly in recent years. However, naphthenic crude oil is a scarce petroleum resource in the world, only accounting for 2.2% of total crude oil reserves [1,2]. Alkylnaphthalene is a unique class of synthetic fluids with similar structure to naphthenic base oils. Alkylnaphthalene as lubricant exhibit outstanding thermo-oxidative and hydrolytic stability, low volatility, and suitable solubility with additives [3]. However, the toxicity and carcinogenicity of alkylnaphthalene have attracted considerable attention, and limited its application in some fields [4,5].
Now naphthalene as by-product in the coal-tar industry is excessive surplus, and tetralin could be easily obtained from the semi-hydrogenation of naphthalene [6]. Compared with naphthalene, tetralin contains one aromatic ring in its molecular structure, therefore it is easier to control the resulting products with desirable number of side-chain alkyl groups. Meanwhile, the molecular structure of alkyltetralin is more similar to that of the typical naphthenic oil, with a lower toxic than the alkylnaphthalene. Hence, tetralin is expected to an ideal alkylating material for replacing the naphthalene with olefins to produce high-quality lubricating base oils.
The alkylation of tetralin with olefins is considered to be a typical Friedel-Crafts reaction. To date, some specific catalysts have been reported in Friedel-Crafts alkylation reaction, including Lewis acids like BF3, AlCl3, ZnCl2, TiCl4 or SnCl4, and strong Brønsted-acid such as HF, H2SO4 or superacids (HF·SbF5 and HSO3F·SbF5) [7,8]. However, these acids inevitably pose several problems concerning toxicity, potential hazards in handing, difficulties in separation, catalyst recovery, corrosion and waste disposal. Therefore, developing less harmful, more efficient and environmentally friendly catalysts have been of keen interest in recent years. Ionic liquids (ILs) have been extensively used as catalysts in organic synthesis processes due to their unique characteristics, including negligible volatility, low melting point, high thermal stability, mild reaction condition and adjustable acidic nature [9,10]. It is well known that acidic chloroaluminates ILs have potential for acting as efficient catalysts in Friedel-Crafts reaction [11]. Qiao et al. [12] reported the alkylation of benzene with 1-dodecene in the [EMIM] [AlCl4] ionic liquid (IL), the high conversion and selectivity for mono-alkylbenzene were obtained. R. I. Aminov et al. [13] demonstrated that [Et3NH] + [Al2Cl7]—could act as efficient catalyst of benzene alkylation with cycloolefins to form cycloalkyl derivatives in 58–98% yield. Zhao et al. [14] found that BuPyBr-AlCl3 exhibited excellent catalytic performance for alkylation of α-methylnaphthalene with long-chain olefins, and the conversion of olefins was higher than 90%. Yang et al. [15] found that a high yield of alkylated naphthalene (>99%) could be obtained within 60 s at 30 °C with Me3NHCl-AlCl3 IL in a microreaction system. In our group, we have successfully achieved the alkylated polyaromatic hydrocarbons (naphthalene, phenanthrene) with middle-long chain α-olefins catalyzed by ILs [16,17]. It was found that the resulting properties as lubricating base oils depended on the α-olefin, the numbers of the side-chain alkyl on the aromatic ring, as well as the structure of aromatics.
With the rapid development of methanol-to-olefins (MTO) process in China, the production capacity of butene as by-product for producing C2/C3 olefins has been continuously expanded, and the high value-added utilization of butene is urgently required [18]. Herein, based on the special structure of tetralin derived from the coal chemical industrial products, n-butene was selected for alkylating agents to produce the alkyltetralin base oils. The alkylation of tetralin with α-olefins catalyzed by various ILs was studied in detail, and high conversion of tetralin and selectivity for multi-alkyltetralins were obtained. The synthetic alkyltetralins as lubricating base oils were studied for comparison, di-propyltetralins (DPT) and di-butyltetralins (DBT) exhibited comparable tribological properties to the commercial naphthenic base oil 4006 (N4006).
2. Materials and Methods
2.1. Materials
Tetralin (C10H12, 98.5%), iron (Ⅲ) chloride (FeCl3, 99%) and zinc chloride (ZnCl2, GR) were purchased from Macklin. Anhydrous aluminum chloride (AlCl3, 99%), triethylamine hydrochloride (Et3NHCl, 99%), diethylamine hydrochloride (Et2NH2Cl, 98%), ethylamine hydrochloride (EtNH3Cl, 98%), trimethylamine hydrochloride (Me3NHCl, 99%), dimethylamine hydrochloride (Me2NH2Cl, 99%), methylamine hydrochloride (MeNH3Cl, 99%) and n-decane (C10H22, 98%) were purchased from Aladdin. n-Butene (C4H8, 99.2%) was obtained from Henan Xingdao Gas Technology Co., Ltd. (Luoyang, China) Propylene (C3H6, 99.9%) was obtained from Beijing Nanfei Gas Technology Co., Ltd. (Beijing, China). All the reagents were used as received without further purification. The naphthenic base oil (N4006) was purchased from PetroChina Co., Ltd. (Beijing, China), and typical molecular structures were shown in Figure S1.
2.2. Synthesis
The typical synthesis of Et3NHCl-AlCl3 IL was described as follows, the other ILs also could be prepared by this procedure. The oil was heated to 60 °C, then 3 mmol Et3NHCl, 30 mmol n-decane and 6 mmol AlCl3 were added in batches. The mixture was stirred for 1 h to obtain the homogeneous Et3NHCl-AlCl3 IL. The whole process was kept under a dry argon atmosphere protection. The Et3NHCl-AlCl3 used in this work had a molar of 2.0 of AlCl3 to Et3NHCl. The XAlCl3 is defined as the molar ratio of AlCl3/AlCl3 + Et3NHCl.
The alkylation of tetralin was carried out in a three-necked round-bottomed flask equipped with a magnetic agitator and a reflux condenser. A total of 30 mmol of tetralin and n-decane, and 3 mmol of Et3NHCl-AlCl3 were placed into a three-necked flask at 20–100 °C with 500 rpm. Then, n-butene or propylene gas was bubbled into the reaction system through a gas inlet tube for 0–60 min. The flow rate of n-butene or propylene was controlled by a calibrated glass rotameter. Another gas tube was inserted to the reaction system which connected the upper end of the condenser and the other side arm of the flask to ensure that the unreacted olefins could return to the mixture and react fully. After the end of ventilation, the system was stirred for an additional 30 min. The liquid–liquid phase was formed after the reaction was terminated. The upper layer contained the alkylated products, unreacted reactants and solvent. After cooling, it was separated from the lower IL and then washed with water for analysis.
Finally, the alkylated products were distilled in vacuum to remove solvent and reactants. DPT, MBT and DBT were fractions obtained by vacuum distillation at 140–160 °C, 130–150 °C and 150–170 °C, respectively, and their lubricating properties were further researched.
2.3. Analysis Conditions
Fourier-transform infrared spectroscopy (FTIR) characterization of ILs was performed by mixing acetonitrile and ILs at the volume ratio 1:8, then a thin liquid film of the mixture was injected into the KBr windows. The FTIR spectra were recorded on IRAffinity-1 from Shimadzu (Kyoto, Japan).
Quantitative analyses of all samples were carried out with Shimadzu GC-2010 Pro equipped with a DB-5HT (15 m × 0.25 mm × 0.10 μm). The column injector temperature and FID detector temperatures were 330 °C. The initial column temperature was maintained at 50 °C for 2 min and raised to 200 °C at 15 °C/min which maintained for 2 min. Then, ramped to 280 °C at 5 °C/min and held for 3 min. Qualitative analyses were determined using gas chromatography–mass spectrometry (GC/MS) equipped with a Rtx-5MS column (30 m × 0.25 mm × 0.25 μm). The final column temperature was 300 °C (16 min holding), and the other conditions were the same as for gas chromatography (GC).
The conversion of tetralin was quantified by external standard method, and the standard curve of tetralin is shown in Figure S2. Since the standard of products was difficult to obtain, the selectivity of the products was calculated by area normalization method.
2.4. Physicochemical Properties Test
The usual series of lubricant properties tests were performed: density, aniline point, kinematic viscosity, pour point, flash point and oxidation onset temperature. The density at 20 °C was determined using a density meter (DMA 1001, Anton Paar, Graz, Austria) according to the ASTM D4052 method. The mixed aniline point was measured by mixing 5 mL sample, 5 mL n-heptane and 10 mL aniline using an aniline point tester (SYD-262, Shanghai Changji, Shanghai, China) according to the GB/T 262 method. The kinematic viscosities at 40 °C and 100 °C were measured according to the ASTM D445 method (BSY-108, Dalian Beigang, Dalian, China). The pour point was measured using a multifunctional low temperature tester (SYD-510F1, Shanghai Changji) according to the GB/T 3535 method. The flash point was measured according to the ASTM D92 method (CLA 5, Anton Paar). The oxidation onset temperature was obtained using a differential scanning calorimetry (DSC 204HP, NETZSCH, Selb, Germany) according to the SN/T 3950 method. Samples were heated at a rate of 10 °C/min to 350 °C under ambient pressure and oxygen flow rate of 100 mL/min.
2.5. Tribological Tests
The friction and wear properties of the alkyltetralins were evaluated on an Optimol SRV-V oscillating reciprocating friction-and-wear system. All tests were conducted at 25 °C under the load of 100 N and stroke length of 1 mm for 30 min duration. The wear scars developed on the disc samples were characterized using non-contact optical 3D surface profilometry (Zygo, Zegage).
3. Results and Discussions
3.1. Alkylation of Tetralin
The alkylation of tetralin with n-butene or propylene goes through a typical Friedel-Crafts reaction. The products were characterized by GC/MS and GC. As shown in Figure 1, tetralin is substituted with up to three butyl or four propyl groups and the alkyltetralins are the mixture of many isomers. The multi-butyltetralins (di- and tri-butyltetralins) and the multi-propyltetralins (di-, tri-, and tetra-propyltetralins) are our desired products since increasing the number of alkyl groups can improve the tribological properties of the base oil. In the present paper, in order to synthesize high selectivity of desired products multi-alkyltetralins, the effects of ILs and reaction conditions on the alkylation were investigated.
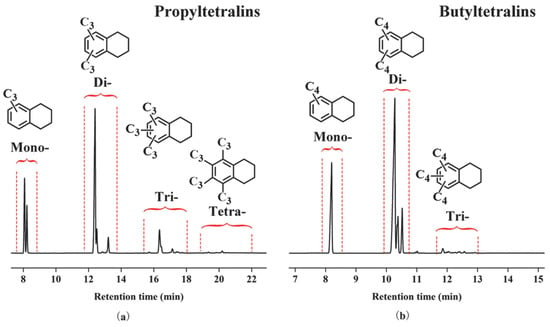
Figure 1.
Gas chromatography (GC) spectra of products produced using tetralin with (a) propylene and (b) n-butene.
The alkylation of tetralin with n-butene was investigated in detail in the following section, and the alkylation of propylene are shown in Figures S3–S7. In the alkylation of tetralin with propylene, the effect of molar fraction of AlCl3, catalyst dosage and reaction time were observed in the same trend as that of tetralin with n-butene.
3.2. Effects of ILs for Alkylation
3.2.1. IL Catalyst Types
Due to the nature of the “designability” of IL, it is possible to tune the catalytic performance by a rational combination of cations and anions. The effects of cations and anions composing ILs on the alkylation of tetralin with n-butene are listed in Table 1. In general, the reaction rate of alkylation affected by the concentration and stability of carbocation. ILs with a certain strong Lewis acidity greatly facilitate the formation of enough carbocations for the alkylation reaction. From the product distribution summarized in Table 1, we observed that Et3NHCl-MCly (M = Zn, Fe) hardly catalyze the reaction. AlCl3-based ILs exhibit high tetralin conversion and better multi-butyltetralins selectivity under identical reaction conditions. Among the AlCl3-based ILs, EtNH3Cl-AlCl3 and Et2NH2Cl-AlCl3 have poor fluidity at low temperature [19]. The Et3NHCl-AlCl3 IL exhibits the highest selectivity of multi-butyltrtralins, indicating the catalyst shows the best catalytic performance than other AlCl3-based ILs.

Table 1.
Results of alkylation of tetralin with n-butene by different ionic liquids (ILs).
In order to clarify the relationship between the acidity of ILs and catalytic performance, the acidity of ILs with different compositions was determined by FTIR spectroscopy using acetonitrile as probe and the results are shown in Figure 2. Acetonitrile as a weaker Lewis base, can coordinate with Lewis acid sites and form a CN-Lewis complex which has a characteristic absorption peak in the 2200–2400 cm−1 region. As Lewis acid strength increases, the C≡N stretching region blue shifts to higher wavenumber and a new band appear at high frequency [20]. Pure acetonitrile showed two infrared spectroscopy (IR) bands at 2252 and 2292 cm−1, associating with stretching vibratios of C≡N. When acetonitrile was mixed with Et3NHCl-MCly, it can be seen from Figure 2a that the appearance of a new band at higher wavenumber occurring at 2309 cm−1 for Et3NHCl-FeCl3, 2313 cm−1 for Et3NHCl-ZnCl2, 2336 cm−1 for Et3NHCl-AlCl3, illustrating that the higher Lewis acidity of Et3NHCl-AlCl3 than the other two ILs. Fe2Cl7−, Zn2Cl5− and Al2Cl7− are the main anionic species of ILs, respectively. The complexing ability of Al2Cl7− with Cl− is stronger than that of Fe2Cl7− and Zn2Cl5−, which is the main reason for the acidity of ILs [21,22].
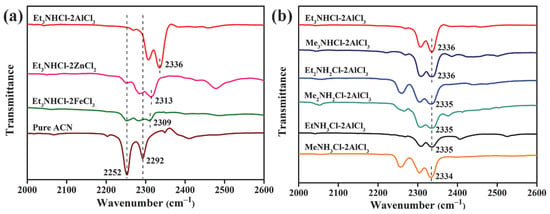
Figure 2.
Fourier-transform infrared spectroscopy (FTIR) spectra of ILs with different (a) anions and (b) cations using acetonitrile as probe.
As shown in Figure 2b, for AlCl3-based ILs with different cations, new bands at approximately 2336 cm−1 were obvserved. The FTIR spectra illustrate that the Lewis acidic strength of all AlCl3-based ILs is stronger than that of Et3NHCl-MCly (M = Zn, Fe), and Et3NHCl-AlCl3 and Me3NHCl-AlCl3 show slightly higher acidity than other AlCl3-based ILs. These results are consistent with their catalytic performance. It is noted that anions have a decisive influence on the Lewis acidity of ILs, whille organic cations have slight effects.
3.2.2. Acidity of IL
Adjusting the acidity of the IL is accessible by changing the molar fraction of AlCl3 in IL, which affects the catalytic activity in alkylation reactions. The effect of molar fraction of AlCl3 (XAlCl3 = 0.4–0.75) on the alkylation of tetralin was investigated. As shown in Figure 3, no alkylation product was occurred when XAlCl3 ≤ 0.5, it was attributed to that chloroaluminate melts are designated as basic and neutral when XAlCl3 < 0.5 and XAlCl3 = 0.5, respectively [10]. Further increasing XAlCl3, the acidity of the IL increased so that the alkylation of tetralin could be carried out well. When XAlCl3 was increased to 0.6, tetralin was almost completely converted. The increase of XAlCl3 from 0.6 to 0.67 led to an increase of the content of the multi-butyltetralins from 65.5% to 93.7%. As XAlCl3 increased to 0.75, the selectivity of multi-butyltetralins declined slightly to 88.8% and may generate other by-products due to overhigh acidity of IL. Therefore, in order to obtain high selectivity of multi-butyltetralins, the optimal molar fraction of AlCl3 was found to be at XAlCl3 = 0.67.
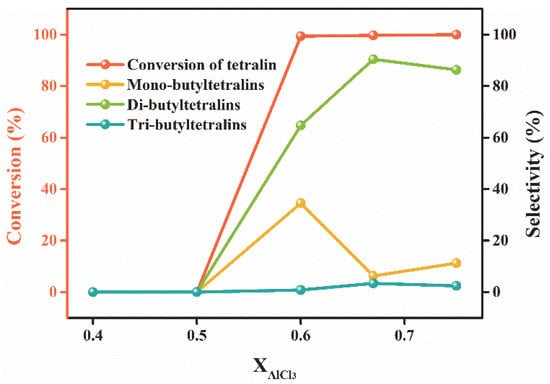
Figure 3.
Catalytic results of ILs with different molar fractions of AlCl3 on the alkylation of n-butene. Reaction conditions: T = 60 °C; IL/tetralin = 0.1; flow rate of n-butene = 20 mL/min; t = 40 min.
3.3. Effect of Reaction Conditions
3.3.1. Reaction Temperature
The reaction temperature affects the dynamics and thermodynamics of the reaction, thereby affecting the catalyst activity and product distribution. Therefore, the effect of reaction temperature on the alkylation of tetralin catalyzed by Et3NHCl-AlCl3 was investigated in detail. As shown in Figure 4, it is observed that the temperature has significant effect on the products distribution, while tetralin conversion could be maintained above 99% in the temperature range of 20 °C to 100 °C. When the reaction temperature was 60 °C, the IL exhibited excellent catalytic activity and the multi-butyltetralins selectivity reached the maximum (93.7%). As the temperature increased to above 80 °C, the degree of alkylation of tetralin decreased and the selectivity to multi-butyltetralins declined. This phenomenon illustrates that the overhigh temperature is meaningless for enhance the catalytic activity and is not beneficial to obtain multi-alkyltetralin owing to the alkylation reaction is an exothermic reaction. Hence, the optimal reaction temperature for the alkylation of tetralin with n-butene was 60 °C.
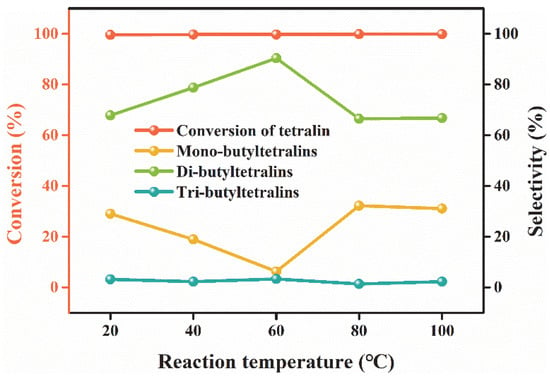
Figure 4.
Effect of reaction temperature on the alkylation of n-butene. Reaction conditions: XAlCl3 = 0.67; IL/tetralin = 0.1; flow rate of n-butene = 20 mL/min; t = 40 min.
3.3.2. Catalyst Dosage
The effect of the dosage of IL on the alkylation of tetralin was studied by varying molar ratio of IL to tetralin from 0.05 to 0.25. Increasing catalyst dosage will produce more Lewis acidic species and increase the probability of contact between n-butene and Lewis acidic species, thereby improving mass transfer process in the reaction system, which is conducive to the generation of more carbocations [23]. As shown in Figure 5, there existed an obviously increase in selectivity of multi-butyltetralins from 47.4% to 93.7% when catalyst dosage was increased from 0.05 to 0.1, whereas a slight change occurred with further increased. The tetralin conversion reached more than 99% as long as the dosage of IL was not less than 0.1. Considering the improvement of alkylation degree of tetralin and cost saving, the optimal catalyst dosage was 0.1.
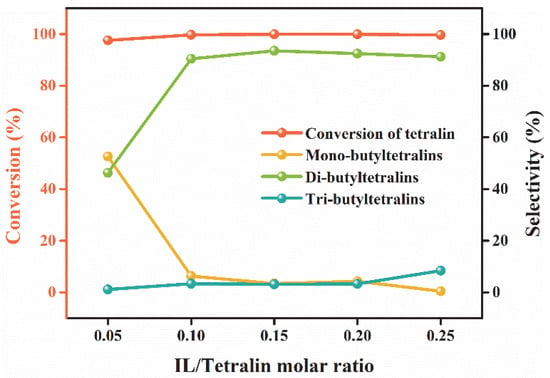
Figure 5.
Effect of catalyst dosage on the alkylation of n-butene. Reaction conditions: T = 60 °C; XAlCl3 = 0.67; flow rate of n-butene = 20 mL/min; t = 40 min.
3.3.3. Flow Rate of n-Butene
The flow rate of n-butene and stirring speed can affect the mass transfer process of reaction. Figure S8 shows the effect of stirring speed. It was observed that the selectivity of multi-butyltetralins from 78.1% to 93.7% as the stirring speed increased from 100 rpm to 500 rpm, whereas a slight change occurred with further increased. The results showed that the high stirring speed significantly promoted the mass transfer process in the reaction, which could increase the contact between the reaction phases [24].
The effect of the flow rate of n-butene on the alkylation of tetralin was examined in the range of 10 mL/min to 50 mL/min. As can be seen from Figure 6, multi-butyltetralins selectivity tended to become equilibrium when the flow rate of n-butene was higher than 20 mL/min. As the flow rate increased, the concentration of carbonium ion will be enhanced which is conducive to deep alkylation reaction. Unexpectedly, excessive n-butene may lead to olefin polymerization. Considering all of these factors, the flow rate of n-butene was fixed at 20 mL/min.
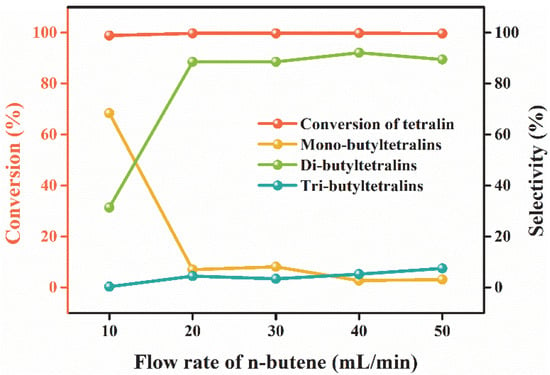
Figure 6.
Effect of the flow rate of n-butene. Reaction conditions: T = 60 °C; XAlCl3 = 0.67; IL/tetralin = 0.1; t = 40 min.
3.3.4. Reaction Time
The effect of reaction time on the alkylation of tetralin was investigated by varying reaction time from 0 to 60 min. The tetralin conversion reached almost 100% when the reaction time was longer than 20 min with n-butene, as shown in Figure 7. The selectivity of multi-butyltetralins showed a great increasing trend with the extension of time to 40 min, after which the values increased slightly. The increase in di-butyltetralins selectivity accompanied the decrease in mono-alkyltetralins selectivity. It should be noted that it is difficult to generate products with more alkyl side chains due to steric hindrance. Therefore, continuing to extend the reaction time is meaningless for the formation of multi-butyltetralins and the optimal ventilation time for n-butene was 40 min.

Figure 7.
Effect of reaction time on the alkylation of n-butene. Reaction conditions: T = 60 °C; XAlCl3 = 0.67; IL/tetralin = 0.1; flow rate of n-butene = 20 mL/min.
3.4. Catalytic Mechanism of the ILs in Alkylation
Based on the catalytic reaction results, the mechanism of the alkylation of tetralin with n-butene in the presence of AlCl3-based ILs was proposed (Scheme 1). In such reaction, n-butene can trap Lewis acidic species in the melt to form carbocations which are necessary to initiate the alkylation reaction [25]. The carbocations attack the unsaturated ring of tetralin and the carbon atom on the ring connected to the carbocations is converted to sp3 hybridization, further forming unstable σ complexes. H+ is more electrophilic than AlCl3, the H+ on the sp3 hybridized carbon on the unsaturated ring will replace AlCl3, resulting in mono-butyltetralins are generated [26]. Furthermore, mono-butyltetralins continue to react with more carbonium ions to form multi-butyltetralins. It is interesting to notice that tri-butyltetralins are formed at a slower rate and lower selectivity than mono- and di-butyltetralins. This is explained by the fact that tetralin and mono-butyltetralins possesse four and three positions for the electrophilic attack, respectively, while di-butyltetralins with only two alkylation sites exhibit greater steric hindrance.
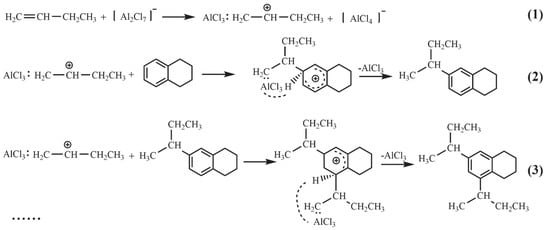
Scheme 1.
Reaction pathways of tetralin with n-butene to synthesize butyltetralins.
3.5. Multi-Alkyltetralins as Lubricating Base Oils
3.5.1. Physicochemical Properties Test
In order to illustrate the properties of single-component alkyltetralin more clearly, three synthetic oils of DPT, MBT and DBT were synthesized under different reaction conditions and vacuum distillation (the experiment details are in the Synthesis section). The compositions of synthetic oils are listed in Table 2. Physicochemical properties of the synthetic oils which can be affected greatly by structural features are shown in Table 3. It can readily be seen that the density of synthetic base oils decreases, while the flash point, pour point, viscosity, and mixed aniline point increase with the number and chain length of alkyl groups attached to tetralin. From Table 3, the results show that the density of all synthetic oils are greater than 0.9 g/cm−3. Low pour points (below −40 °C) exhibit good low-temperature fluidity, and the low mixed aniline points indicates good potential for compatibility with polar additives. In addition, the oxidation onset temperature of the synthetic oils is higher than 170 °C which exhibit good oxidation stability. It can be concluded that the synthetic oils prepared from tetralin with n-butene/propylene are expected to be used in lubrication field.

Table 2.
Composition of synthetic base oils.

Table 3.
Physicochemical properties of synthetic base oils.
3.5.2. Tribological Performance
The tribological performance of synthetic oils were tested. Commercially available N4006 was used for comparison. Figure 8 shows the evolution of the friction coefficients with time of the steel discs lubricated with four lubricants at 25 °C. From Figure 8a, an evident running-in period was noticed and the running-in period of DBT is almost shorter than that of N4006, indicating that the three alkyltetralin synthetic oils exhibit stable friction-reducing properties. As shown in Figure 8b, the average coefficient of friction (COF) values reveals that DPT and DBT give relatively low friction, while MBT gives slightly higher friction coefficient than N4006. It indicates that more attached alkyl chains further reduced the friction coefficient, even if the attached alkyl chain length was short [27].

Figure 8.
(a) The coefficient of friction (COF) variation with time, (b) Average COF for the synthetic oils and naphthenic oil 4006 (N4006).
Figure 9 shows the wear scar volumes of the steel disc lubricated by four lubricants. The results exhibit the wear volumes lubricated by DPT and DBT are similar, and lower than that of MBT and N4006. The 3D profilometry images give a clear idea of the severity of the wear which are in good agreement with their corresponding COF results shown in Figure 10. The wear scar after lubrication by N4006 is obviously larger than that lubricated by DPT and DBT. These results show that DPT and DBT possess have comparable friction-reducing and antiwear properties to N4006.
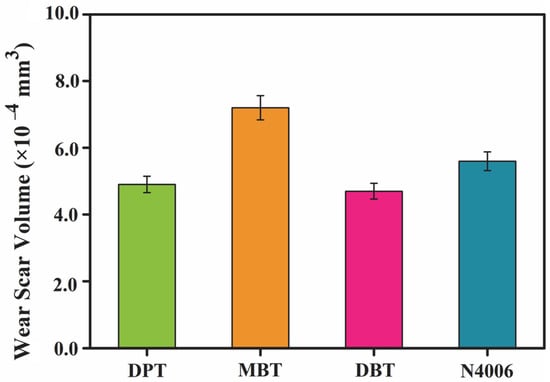
Figure 9.
Wear scar volume for the synthetic oils and N4006.
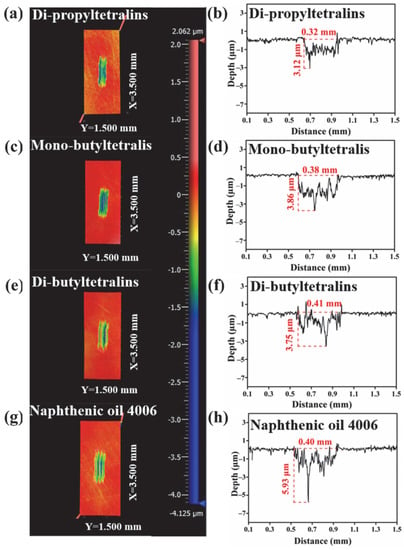
Figure 10.
(a,c,e,g) The 3D microscope images and (b,d,f,h) profiles of wear scars of the steel discs lubricated by the synthetic oils and N4006.
The better lubricating behavior of DBT and DPT than MBT which can be attributed to the generated-film stability of the synthetic base oils. DBT and DPT with more alkyl chain numbers and length may enhance the adsorption force to the metal surface and the cohesive energy between the lubricant molecules [28]. Compared with N4006, alkyltetralins are easier to adsorb effectively and orderly on the lubricated surface due to the π-π conjugation of polar aromatic rings, resulting in forming more stable lubricating films [29]. Hence, increasing the content of the alkyl chain in the alkyltetralin could enhance its friction-reducing and antiwear properties.
4. Conclusions
The ionic liquid composed of Et3NHCl and AlCl3 exhibited an excellent catalytic performance for the alkylation of tetralin with n-butene and propylene, respectively. The reaction conditions for the alkylation of tetralin with n-butene were optimized as follows: molar fraction for AlCl3 of 0.67, IL/tetralin molar ratio of 0.1, n-butene flow rate of 20 mL/min, reaction temperature of 60 °C, and reaction time of 40 min at 500 rpm. The n-butene and propylene combined with tetralin have a similar reaction feature, the selectivity of multi-alkyltetralins as high as 90% could be achieved at the 100% conversion of tetralin under the optimal conditions. Among the synthetic oils, it was found that the primary physicochemical properties of alkyltetralins are closely related to the number and length of alkyl chains, the mono-alkylated products showed the lower pour point and viscosity than the multi-alkylated products, however, the multi-alkylated products displayed the low friction and wear values, which had comparable tribological properties to the commercial naphthenic base oil 4006.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/lubricants10110287/s1. Figure S1: The typical molecular structures of naphthenic base oil; Figure S2: The standard curve of tetralin; Figure S3: Effect of molar fraction of AlCl3 in IL on the alkylation of propylene; Figure S4: Effect of reaction temperature on the alkylation of propylene; Figure S5: Effect of catalyst dosage on the alkylation of propylene; Figure S6: Effect of the flow rate of propylene; Figure S7: Effect of reaction time on the alkylation of propylene; Figure S8: Effect of stirring speed on the alkylation of n-butene.
Author Contributions
Conceptualization, L.L., J.D. and H.X.; methodology, J.F., C.C. and Q.T.; investigation, C.C. and Q.T.; writing—original draft preparation, J.F.; writing—review and editing, L.L., C.C. and J.F.; funding acquisition, L.L. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by the National Natural Science Foundation (U1910202, 21978194), the Key Research and Development Program of Shanxi Province (202102090301005), the Fund for Shanxi “1331 Project”, and the Shanxi Natural Science Foundation for Young Scientists (202103021223064).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yuan, H.; Li, X. The Characteristics and Utilization of Naphthenic Crude Oil. Guangzhou Chem. Ind. 2009, 37, 48–51. [Google Scholar]
- Jia, J.; Li, M.; Li, C.; Du, Q.; Yu, E. Study on Application of Naphthenic Base Oils in Metalworking Fluids. Lubr. Eng. 2016, 41, 109–114. [Google Scholar]
- Hessell, E.T.; Abramshe, R.A. Alkylated naphthalenes as high-performance synthetic fluids. J. Synth. Lubr. 2003, 20, 109–122. [Google Scholar] [CrossRef]
- Kang, H.J.; Jung, Y.; Kwon, J.H. Changes in ecotoxicity of naphthalene and alkylated naphthalenes during photodegradation in water. Chemosphere 2019, 222, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Bruyneel, B.; Kamelia, L.; Wesseling, S.; Rietjens, I.M.C.M.; Boogaard, P.J. In vitro metabolism of naphthalene and its alkylated congeners by human and rat liver microsomes via alkyl side chain or aromatic oxidation. Chem. Biol. Interact 2020, 315, 108905. [Google Scholar] [CrossRef]
- Gilbert, G.; Weil, R.C.; Hunter, R.H. Hydrorefining coal–tar naphthalene. Ind. Eng. Chem. 1961, 53, 993–996. [Google Scholar] [CrossRef]
- Rueping, M.; Nachtsheim, B.J. A review of new developments in the Friedel-Crafts alkylation-From green chemistry to asymmetric catalysis. Beilstein J. Org. Chem. 2010, 6, 6. [Google Scholar] [CrossRef]
- Yoo, K.; Burckle, E.C.; Smirniotis, P.G. Isobutane/2-Butene alkylation using large-pore zeolites: Influence of pore structure on activity and selectivity. J. Catal. 2002, 211, 6–18. [Google Scholar] [CrossRef]
- Olivier-Bourbigou, H.; Magna, L.; Morvan, D. Ionic liquids and catalysis: Recent progress from knowledge to applications. Appl. Catal. A Gen. 2010, 373, 1–56. [Google Scholar] [CrossRef]
- Welton, T. Room-temperature ionic liquids. solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2083. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.; Sheng, X.; Wang, B.; Zhu, Z.; Nan, Q. The catalytic performance study of chloroaluminate ionic liquids on long-chain alkenes alkylation. Energy Fuels 2018, 32, 9763–9771. [Google Scholar] [CrossRef]
- Qiao, K.; Deng, Y. Alkylations of benzene in room temperature ionic liquids modified with HCl. J. Mol. Catal. A Chem. 2001, 171, 81–84. [Google Scholar] [CrossRef]
- Aminov, R.I.; Mazitova, A.S.; Khusnutdinov, R.I. Benzene alkylation with cycloolefins under the action of [Et3NH]+[Al2Cl7]− ionic liquid. Russ. J. Gen. Chem. 2019, 89, 2171–2177. [Google Scholar] [CrossRef]
- Zhao, Z.; Qiao, W.; Wang, G.; Li, Z.; Cheng, L. Alkylation of α-methylnaphthalene with long-chain alkenes catalyzed by butylpyridinium bromochloroaluminate ionic liquids. J. Mol. Catal. A Chem. 2005, 231, 137–143. [Google Scholar] [CrossRef]
- Yang, T.; Wang, F.; Huang, J.; Ling, S.D.; Liu, S.; Zhang, A.; Wang, Y.; Xu, J. Efficient continuous-flow synthesis of long-chain alkylated naphthalene catalyzed by ionic liquids in a microreaction system. React. Chem. Eng. 2021, 6, 1950–1960. [Google Scholar] [CrossRef]
- Li, L.; Zhao, X.; Chen, C.; Xu, H.; Liu, L.; Dong, J. Highly selective synthesis of polyalkylated naphthalenes catalyzed by ionic liquids and their tribological properties as lubricant base oil. ChemistrySelect 2019, 4, 5284–5290. [Google Scholar] [CrossRef]
- Cui, J.; Tang, Q.; Chen, C.; Xu, H.; Liu, L.; Dong, J. High-viscosity polyalkylphenanthrene oils: Synthesis and evaluation of lubricating properties. Lubr. Sci. 2022, 34, 527–536. [Google Scholar] [CrossRef]
- Shen, P.; Zhang, S. Comprehensive utilization of C4 resource as by-product of methanol to olefin process. Contemp. Chem. Ind. 2012, 41, 1333–1336. [Google Scholar]
- Zhao, Z.; Li, Z.; Wang, G.; Qiao, W.; Cheng, L. Friedel–Crafts alkylation of 2-methylnaphthalene in room temperature ionic liquids. Appl. Catal. A Gen. 2004, 262, 69–73. [Google Scholar] [CrossRef]
- Yang, Y.; Kou, Y. Determination of the Lewis acidity of ionic liquids by means of an IR spectroscopic probe. Chem. Commun. 2004, 4, 226–227. [Google Scholar] [CrossRef]
- Zhao, Z.; Qiao, W.; Wang, X.; Wang, G.; Li, Z.; Cheng, L. Effects of kinds of ionic liquid catalysts on alkylations of 1- and 2-methylnaphthalene with alkenes. Appl. Catal. A Gen. 2005, 290, 133–137. [Google Scholar] [CrossRef]
- Deng, L.; Su, Q.; Tan, X.; Wang, Y.; Dong, L.; He, H.; Li, Z.; Cheng, W. Tunable imidazolium ionic liquids as efficient catalysts for conversion of urea into cyclic carbonates. Mol. Catal. 2022, 519, 112153. [Google Scholar] [CrossRef]
- Xin, H.; Wu, Q.; Han, M.; Wang, D.; Jin, Y. Alkylation of benzene with 1-dodecene in ionic liquids [Rmim]+Al2Cl6X− (R=butyl, octyl and dodecyl; X = chlorine, bromine and iodine). Appl. Catal. A Gen. 2005, 292, 354–361. [Google Scholar] [CrossRef]
- Sronsri, C.; Sittipol, W.; Kongpop, U. Optimization of biodiesel production using magnesium pyrophosphate. Chem. Eng. Sci. 2020, 226, 115884. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Eichmann, M. Selective dimerisation of 1-butene in biphasic mode using buffered chloroaluminate ionic liquid solvents—Design and application of a continuous loop reactor. Catal. Today 2001, 66, 309–316. [Google Scholar] [CrossRef]
- Qi, G.; Jiang, F.; Sun, X.; Zhao, S. Alkylation mechanism of benzene with 1-dodecene catalyzed by Et3NHCl-AlCl3. Sci. China Chem. 2010, 53, 1102–1107. [Google Scholar] [CrossRef]
- Lu, R.; Morimoto, M.; Tani, H.; Tagawa, N.; Koganezawa, S. Tribological properties of alkyldiphenylethers in boundary lubrication. Lubricants 2019, 7, 112. [Google Scholar] [CrossRef]
- Kitakami, O.; Ichijo, M.; Daimon, H. Lubrication of surface oxidized Co–Cr thin films by phosphoric and phosphorous acid esters. J. Magn. Magn. Mater. 2001, 235, 179–182. [Google Scholar] [CrossRef]
- Hu, C.; Ai, J.; Ma, L.; Wen, P.; Fan, M.; Zhou, F.; Liu, W. Ester oils prepared from fully renewable resources and their lubricant base oil properties. ACS Omega 2021, 6, 16343–16355. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).