Study on the Performance and Corrosion Failure Process of Porous Titanium-Based Coated Electrodes
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Experimental Methods
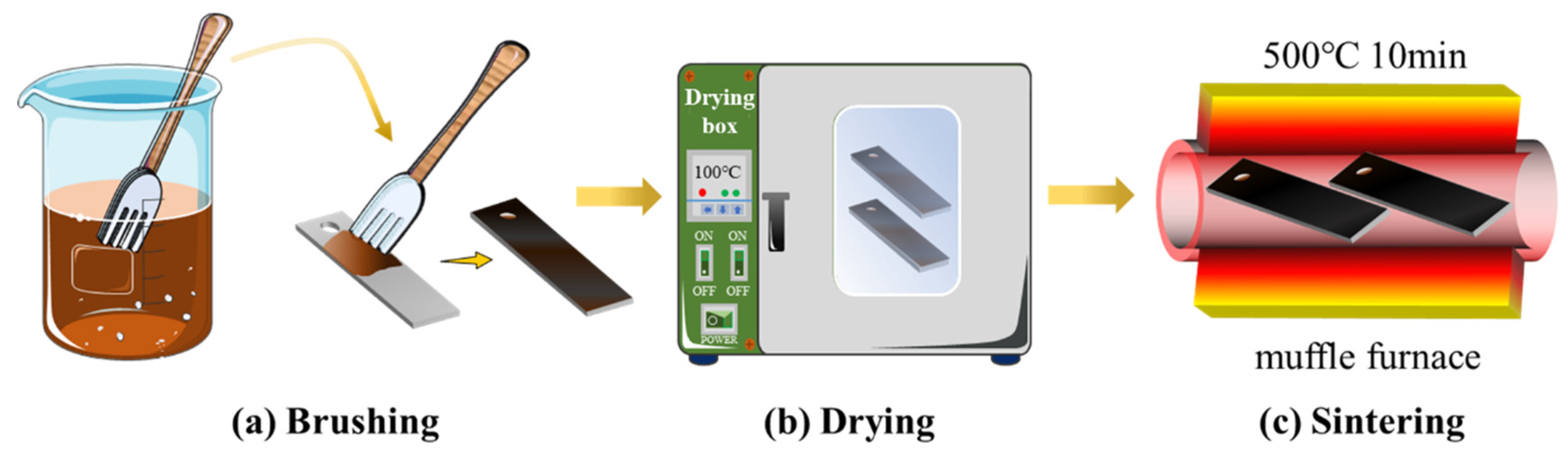
2.2.1. Pretreatment Process
2.2.2. Preparation of Metal Oxide Layer
2.3. Characterization of Electrodes
3. Results and Discussion
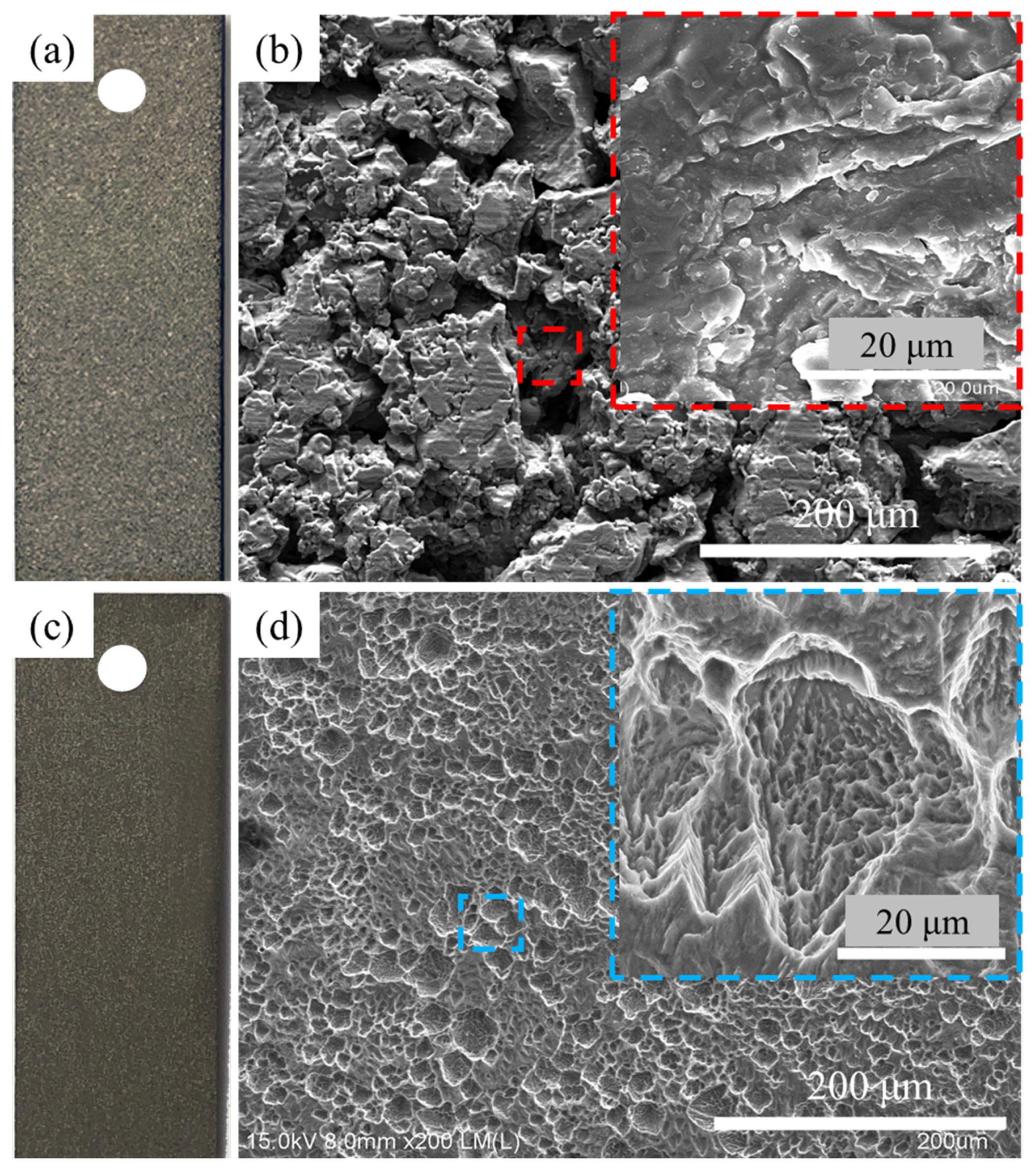
3.1. Surface Morphology of the Different Titanium Sheet
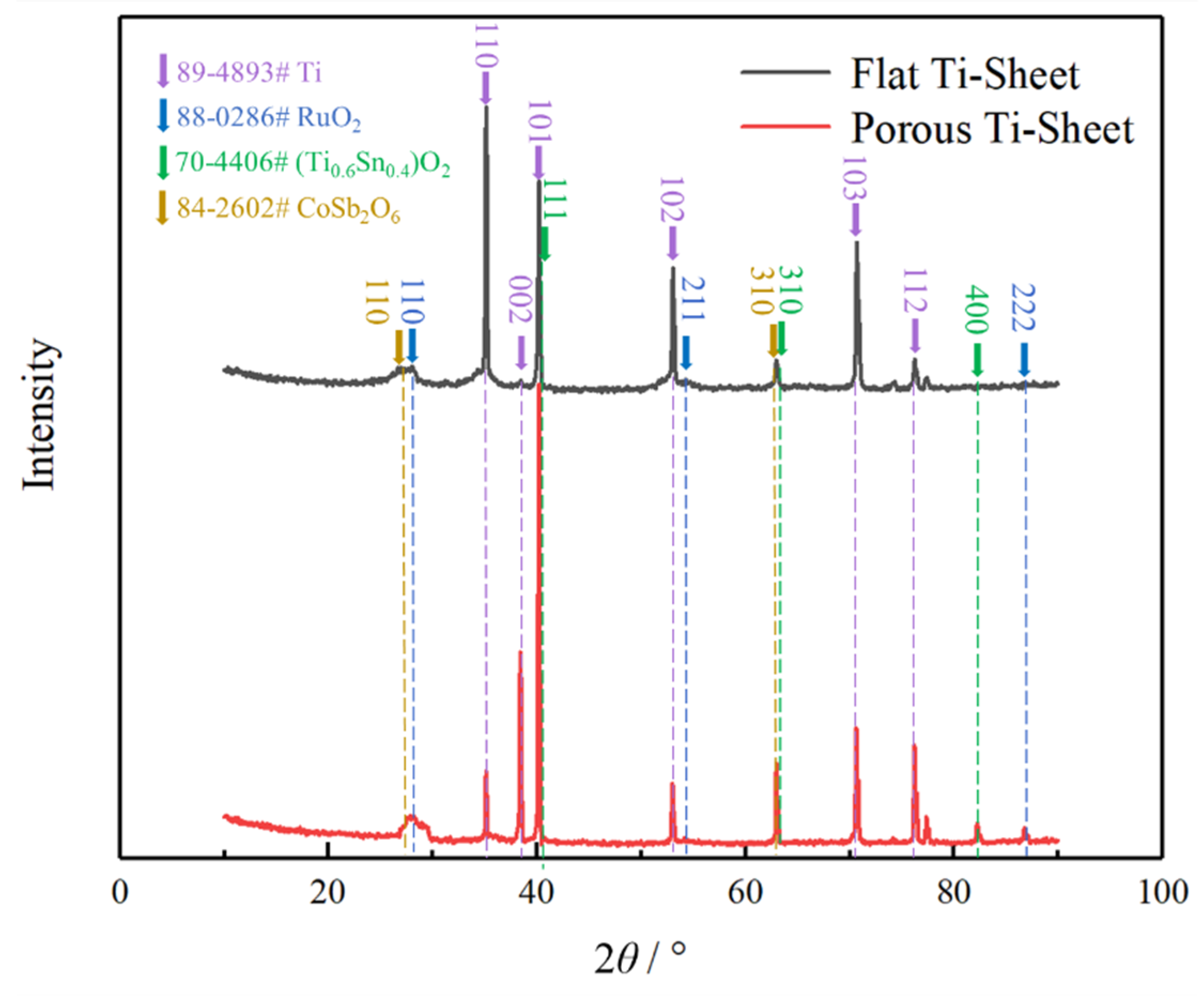
3.2. Phase Characterization of Coatings
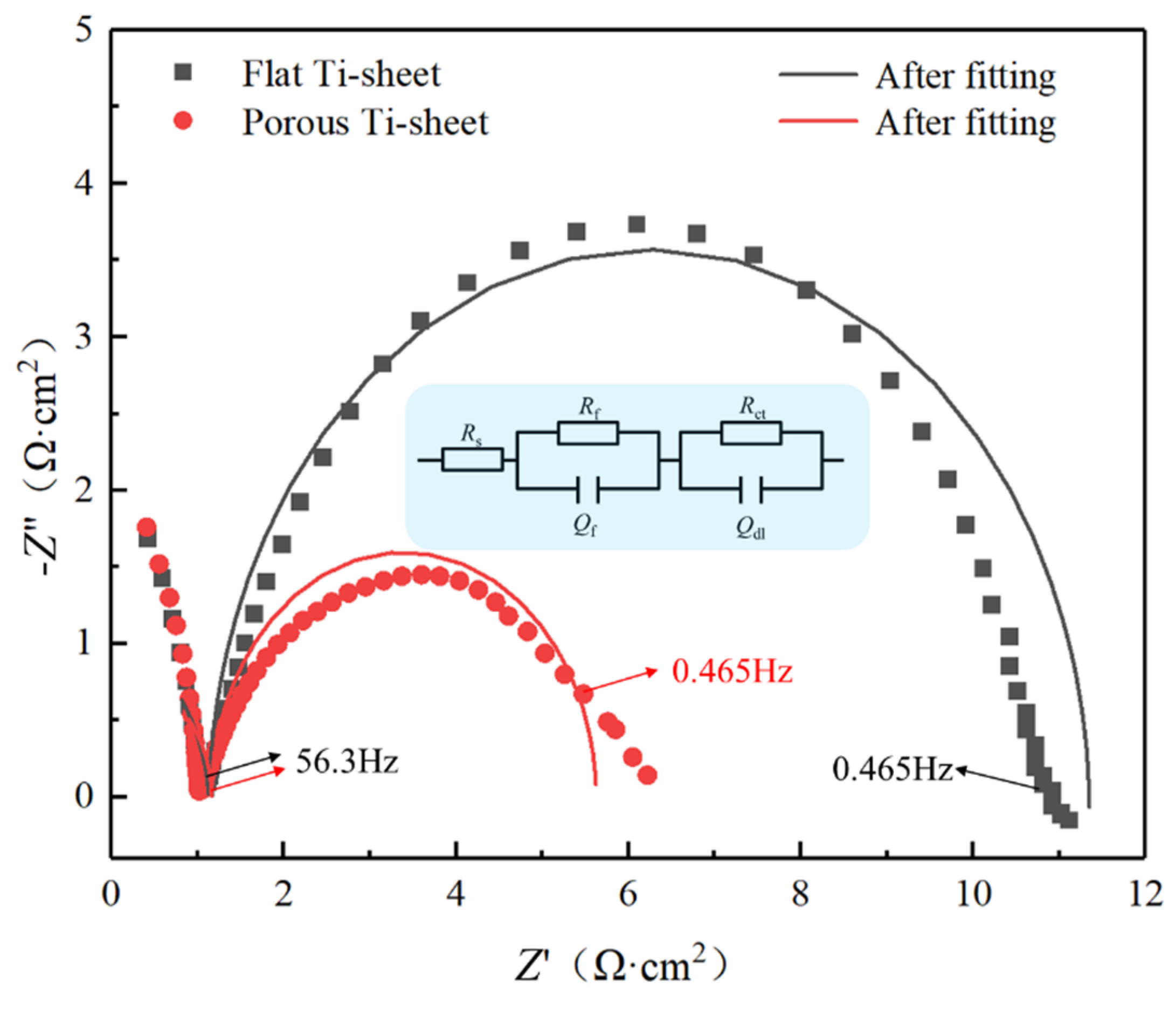
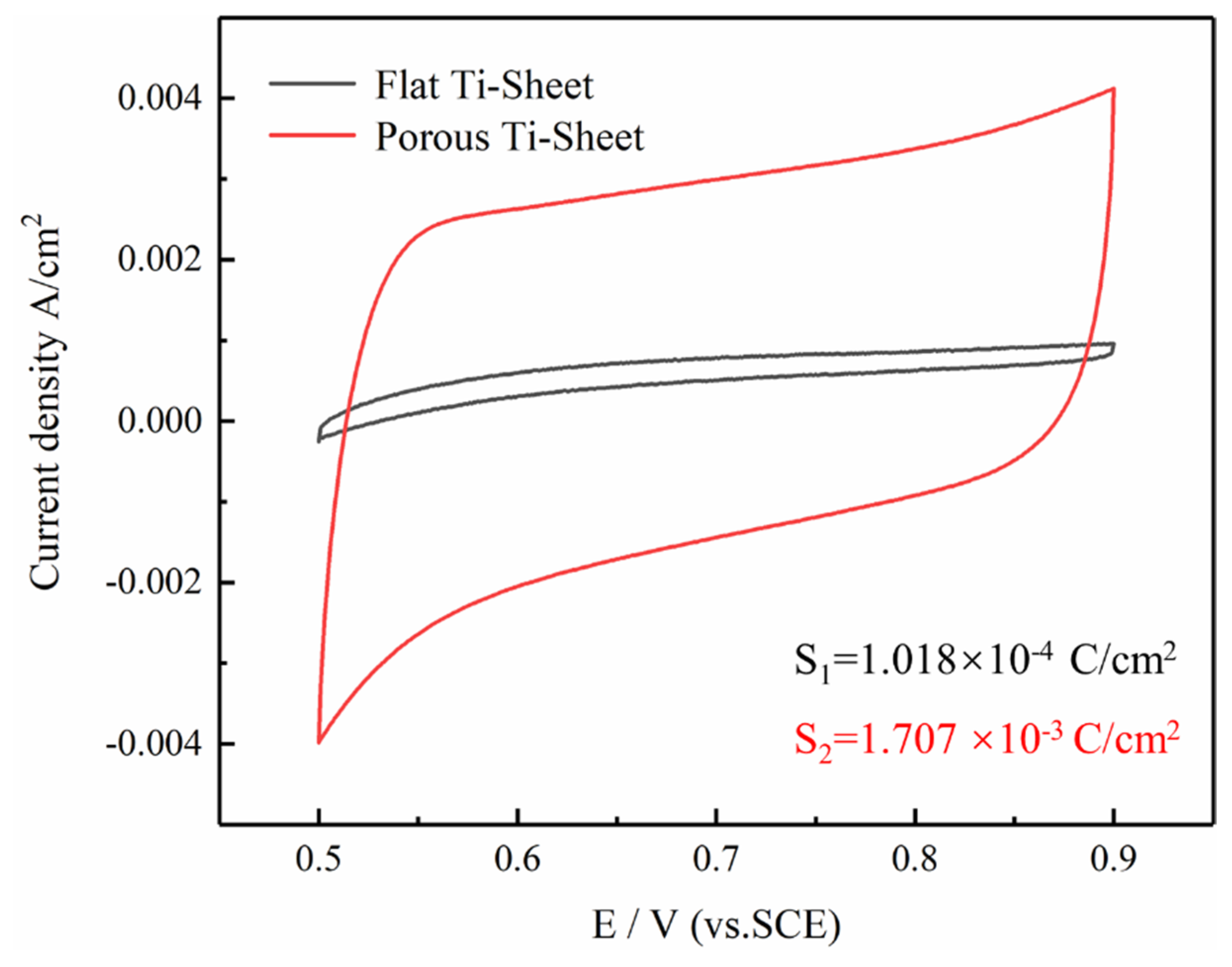
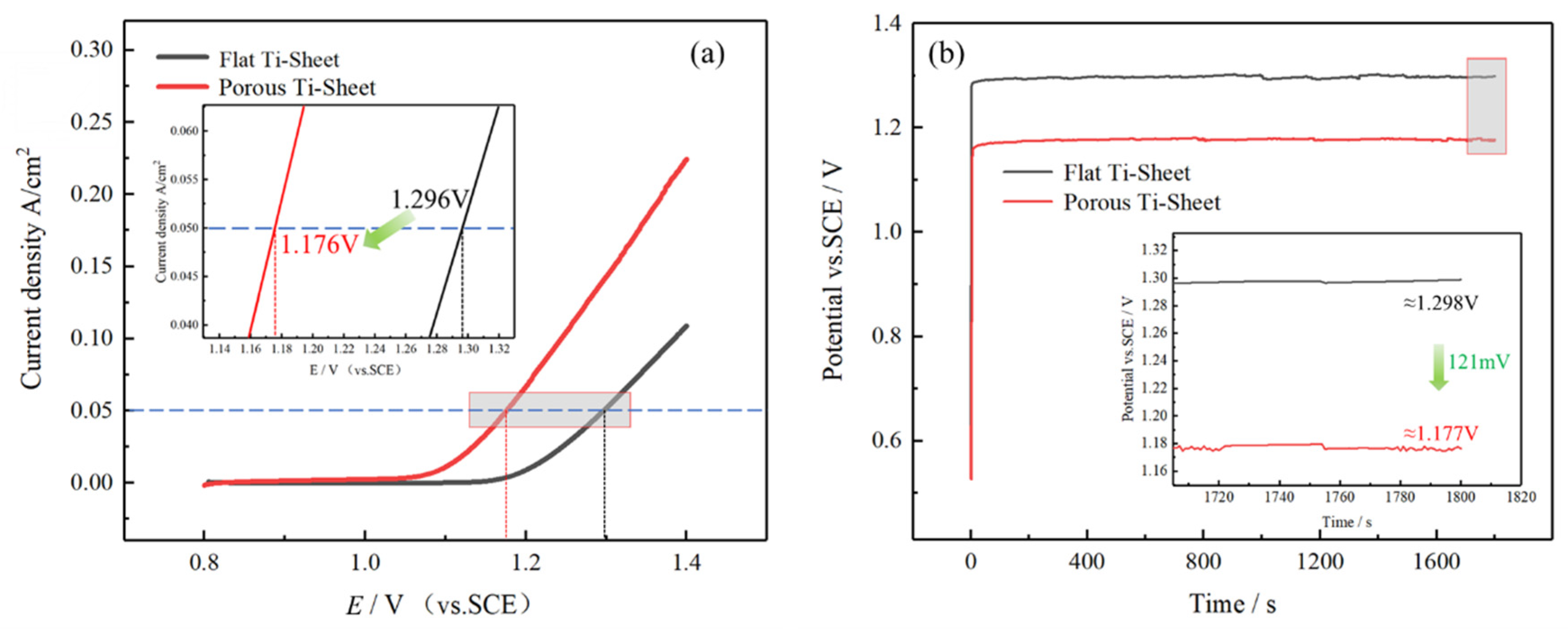
3.3. Electrochemical Catalytic Performance of Coated Electrodes
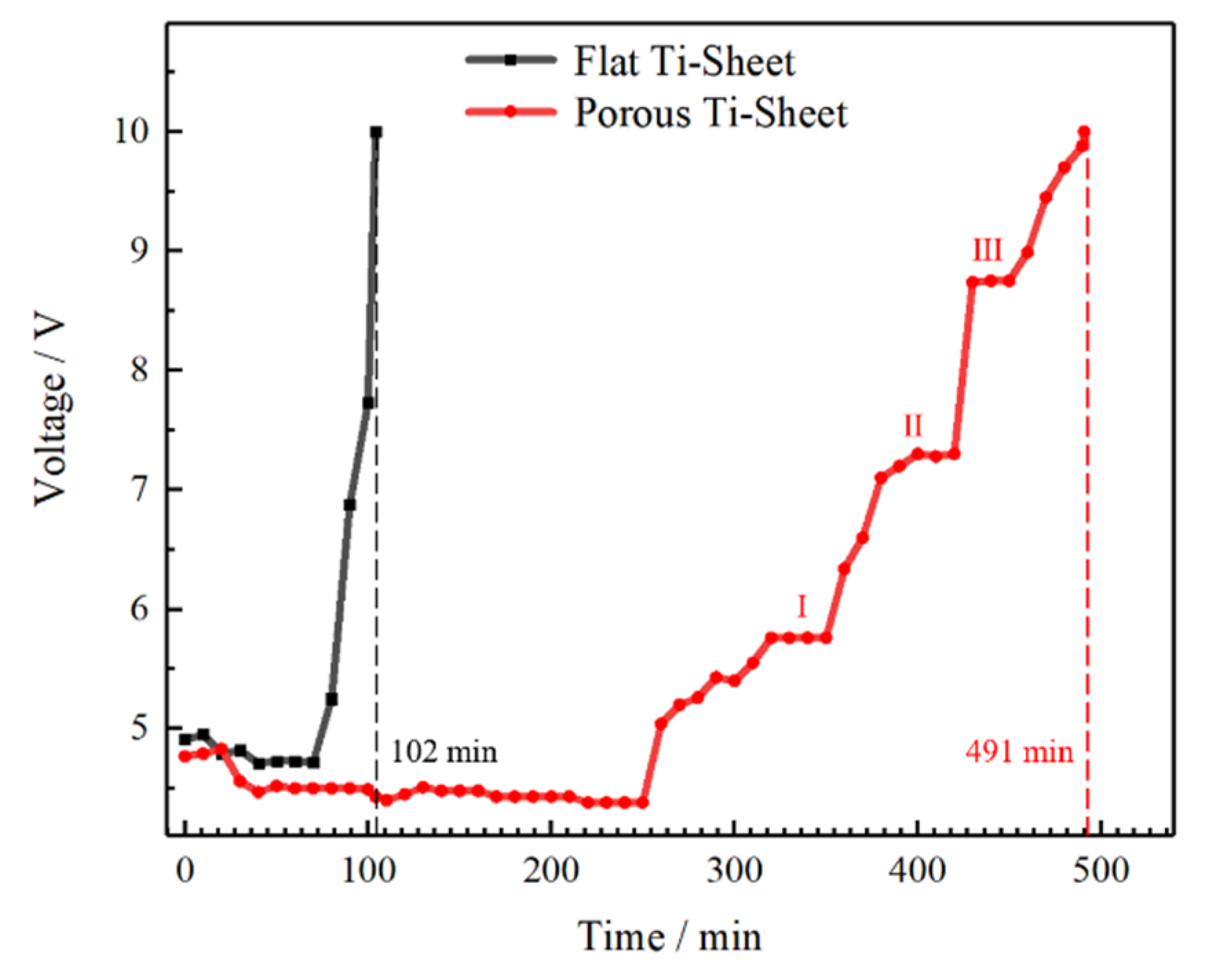
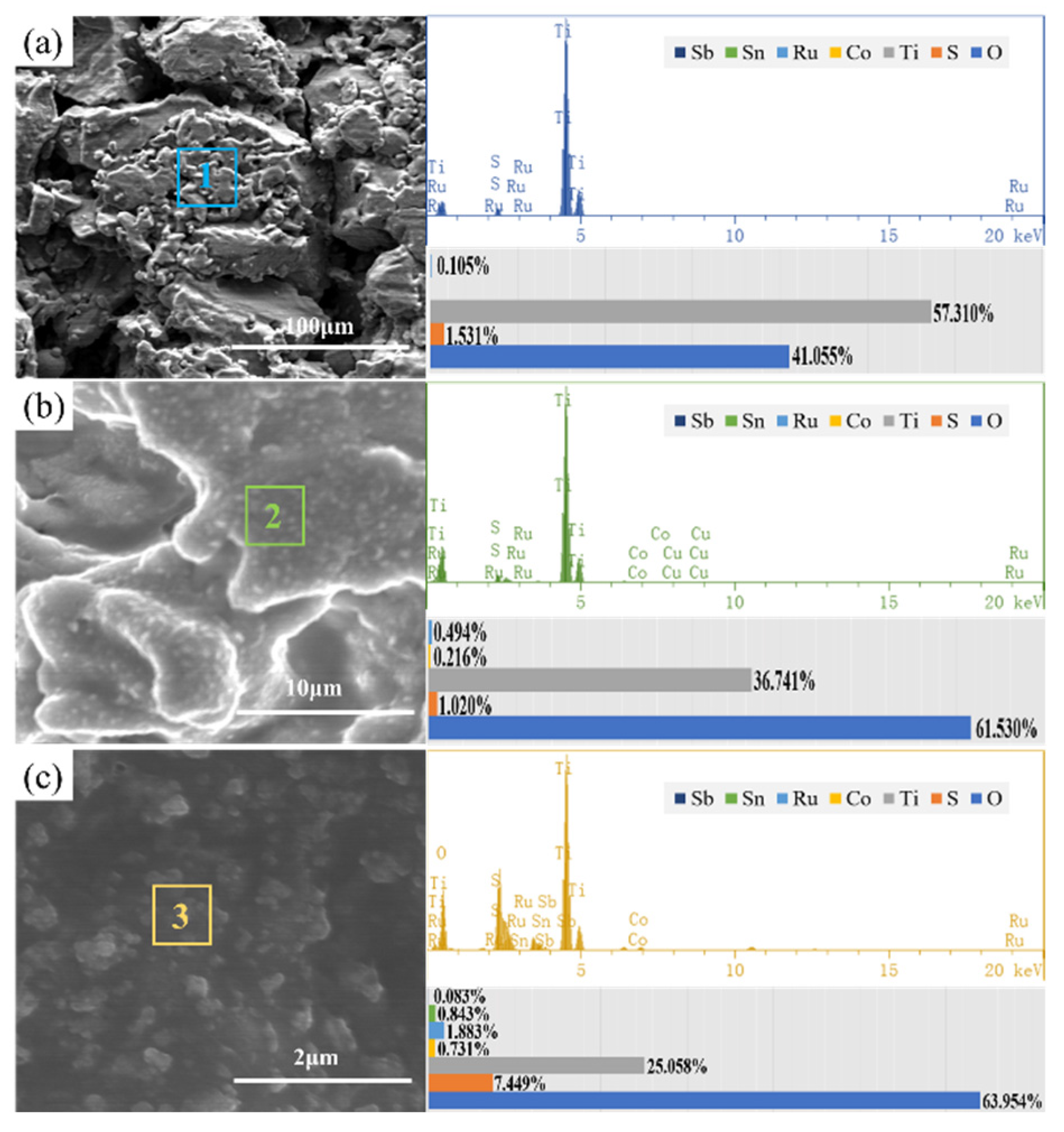
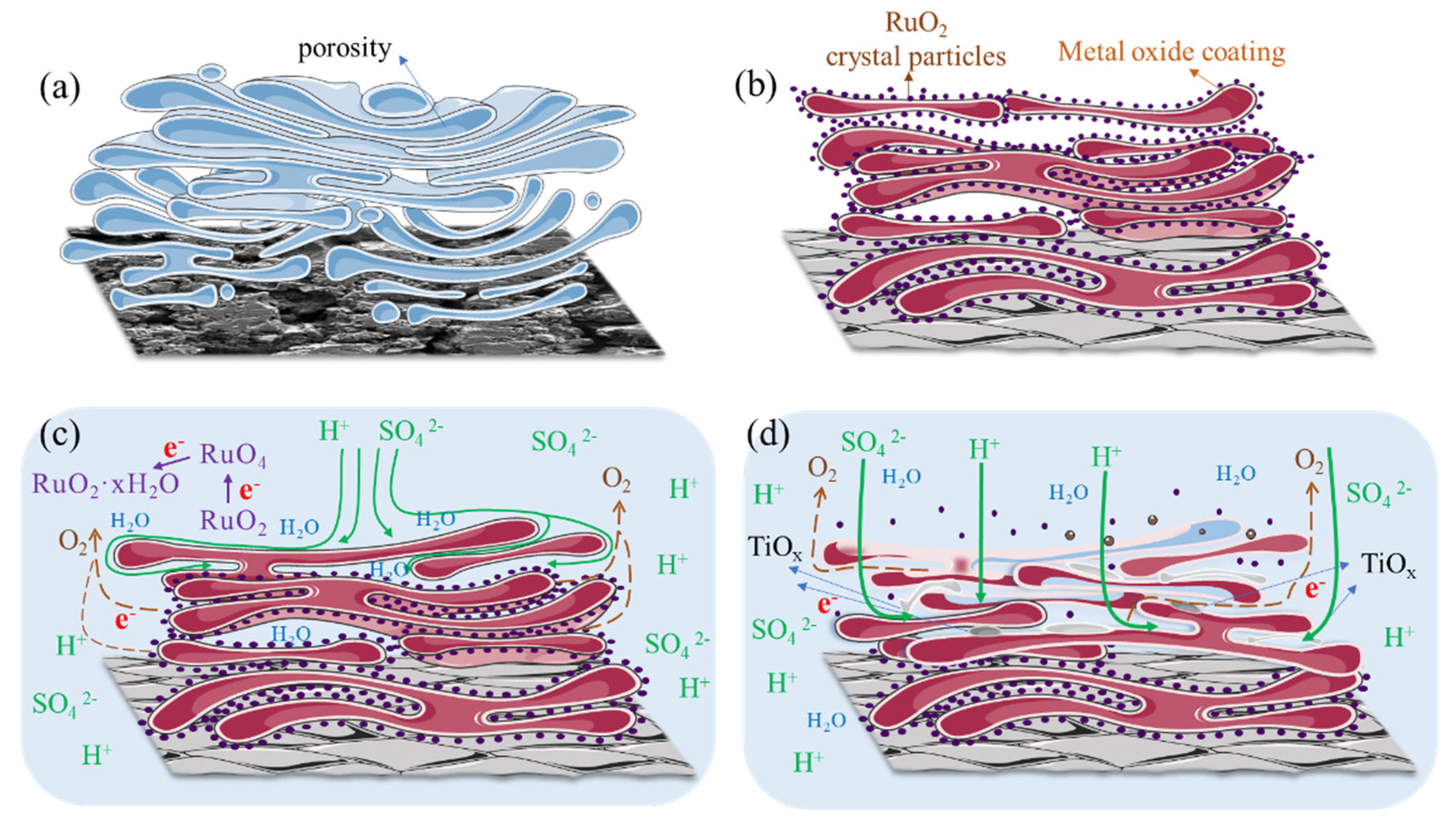
3.4. Accelerated Life Test and Corrosion Resistance of the Electrodes
4. Conclusions
- Due to its special structure, the porous titanium sheet contains as five times more active material than the flat titanium sheet. The coatings are laminated and strengthened in the form of sheets, and the bonding force between the substrate and the coating is stronger.
- The chlorine evolution potential of the coating on the porous titanium sheet is 121 mV lower than that of the flat titanium sheet, which greatly improves the electrode performance and accelerates the service life by 3.79 times.
- The corrosion process of the coating on the porous titanium sheet is divided into three stages, which is “Dissolve-Peel-Oxidize obstruct”. The final failure is caused by a combination of the abovementioned reasons.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kariman, A.; Marshall, A.T. Improving the Stability of DSA Electrodes by the Addition of TiO2 Nanoparticles. J. Electrochem. Soc. 2019, 166, E248–E251. [Google Scholar] [CrossRef]
- Chang, L.H.; Chen, B.M.; Qiao, H.H.; Huang, H.; Guo, Z.C.; He, Y.P.; Xu, R.D.; Xie, X.H. Study of the Effects of Pretreatment Processing on the Properties of Metal Oxide Coatings on Ti-Based Sheet. J. Electrochem. Soc. 2021, 168, 033501. [Google Scholar] [CrossRef]
- Nam, K.W.; Lee, E.S.; Kim, J.H.; Shin, K.H.; Kim, K.H. Effect of Etching Ti Substrate on a Catalytic Oxide Electrode. J. Electrochem. Soc. 2005, 148, B111–B115. [Google Scholar]
- Dan, J.; Chen, K. Analysis of factors affecting electrode surface morphology and electrical properties. Chloral Kali Ind. 2000, 3, 11–15. [Google Scholar]
- Yan, Z.; Zhao, Y.; Zhang, Z.; Li, G.; Li, H.; Wang, J.; Feng, Z.; Tang, M.; Yuan, X.; Zhang, R. A study on the performance of IrO2–Ta2O5 coated anodes with surface treated Ti substrates. Electrochim. Acta 2015, 157, 345–350. [Google Scholar] [CrossRef]
- Wang, S.C.; Wang, T.; Yan, W.; Chen, B.M.; Huang, H.; He, Y.P.; Guo, Z.C.; Xu, R.D. Effect of Hydrochloric Acid with Different Concentrations on the Activity and Lifetime of Titanium Based Sn-Sb-Ru Oxide Coating Electrodes. Mater. Prot. 2019, 52, 59–63. [Google Scholar]
- Wang, T.; Chen, B.M.; Zhang, Z.; Jin, L.; Zhang, X.; Guo, Z.C.; Xu, R.D. Influences of Pre-Treatment Processes on Properties of Titanium Based PbO2 Electrode. Mater. Prot. 2018, 51, 68–73. [Google Scholar]
- Xu, L.K.; Scantlebury, J.D. A study on the deactivation of an IrO2–Ta2O5 coated titanium anode. Corros. Sci. 2003, 45, 2729–2740. [Google Scholar] [CrossRef]
- Xie, X.H.; Chang, L.H.; Chen, B.M.; Li, J.M.; Huang, H.; Guo, Z.C.; He, Y.P. Effects of coating precursor states on performance of titanium-based metal oxide coating anode for Mn electrowinning. Electrochim. Acta 2022, 400, 139459. [Google Scholar] [CrossRef]
- Zinola, C.F.; Arvia, A.J. A semiempirical quantum chemistry approach to possible structures and energies of hydrogen atoms adsorbed on Pt (100) and Pt (111) clusters at a simulated Pt/aqueous electrochemical interface. Electrochim. Acta 1996, 41, 2267–2273. [Google Scholar] [CrossRef]
- Hu, J.M.; Men, H.M.; Zhang, J.Q.; Cao, C.N. Effect of preparation conditions on the properties of Ti based IrO2 + Ta2O5 anodes. Acta Metall. Sin. 2002, 38, 69–73. [Google Scholar]
- Zhang, W.; Lin, H.; Kong, H.; Lu, H.; Yang, Z.; Liu, T. Preparation and characterization of lead dioxide electrode with three-dimensional porous titanium substrate for electrochemical energy storage. Electrochim. Acta 2014, 139, 209–216. [Google Scholar] [CrossRef]
- Rolison, D.R.; Long, J.W.; Lytle, J.C.; Fischer, A.E.; Lubers, A.M. Multifunctional 3D nanoarchitectures for energy storage and conversion. Chem. Soc. Rev. 2009, 38, 226–252. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Zhao, Y.Q.; Xu, C.L. May 3D nickel foam electrode be the promising choice for supercapacitors. J. Solid State Electrochem. 2012, 16, 829–834. [Google Scholar] [CrossRef]
- Sun, J.R.; Lu, H.Y.; Lin, H.B.; Huang, W.M.; Li, H.D.; Lu JCui, T. Boron doped diamond electrodes based on porous Ti substrates. Mater. Lett. 2012, 83, 112–114. [Google Scholar] [CrossRef]
- Yang, H.T.; Liu, H.R.; Guo, Z.C.; Chen, B.M.; Zhang, Y.C.; Huang, H.; Li, X.L.; Fu, R.C.; Xu, R.D. Electrochemical behavior of rolled Pb–0.8%Ag anodes. Hydrometallurgy 2013, 140, 144–150. [Google Scholar] [CrossRef]
- Luo, S.L.; Guo, H.J.; Wang, Z.X.; Li, X.H.; Wang, J.X.; Yan, J.C. The Electrochemical Performance and Reaction Mechanism of Coated Titanium Anodes for Manganese Electrowinning. J. Electrochem. Soc. 2019, 166, E502–E511. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G. Stable Ti/RuO2-Sb2O5-SnO2 electrodes for O2 evolution. Electrochim. Acta 2005, 20, 4155–4159. [Google Scholar] [CrossRef]
- Chen, B.M.; Wang, S.C.; Liu, J.H.; Dong, C.; He, Y.P.; Yan, W.K.; Guo, Z.C.; Xu, R.D.; Yang, H.T. Corrosion resistance mechanism of a novel porous Ti/Sn-Sb-RuOx/β-PbO2 anode for zinc electrowinning. Corros. Sci. 2018, 136, 144. [Google Scholar] [CrossRef]
- Mazhari, A.H.; Jafarzadeh, K.; Mirali, S.M. An investigation of the effect of RuO2 on the deactivation and corrosion mechanism of a Ti/IrO2 + Ta2O5 coating in an OER application. J. Electrochem. Chem. 2016, 777, 66–74. [Google Scholar]
- Xu, W.; Haarberg, G.M.; Seland, F.; Sunde, S.; Ratvik Petter, A.; Susanne, H.; John, G.; Asa, A.; Erik, Z.; Akre, T. The durability of the thermally decomposed IrO2-Ta2O5 coated titanium anode in a sulfate solution. Corros. Sci. 2019, 150, 76–90. [Google Scholar] [CrossRef]
- Moradi, F.; Dehghanian, C. Addition of IrO2 to RuO2 + TiO2 coated anodes and its effect on electrochemical performance of anodes in acid media. Prog. Nat. Sci. Mater. Int. 2014, 24, 134–141. [Google Scholar] [CrossRef]
- Silva, L.M.D.; Faria, L.; Boodts, J. Electrochemical impedance spectroscopic (EIS) investigation of the deactivation mechanism, surface and electrocatalytic properties of Ti/RuO2(x)+Co3O4(1−x) electrodes. J. Electroanal. Chem. 2002, 532, 141–150. [Google Scholar] [CrossRef]


| Name | Ⅰ Alkaline Washing | Ⅱ Mixed Acid (Room Temperature) | Ⅲ Etching |
|---|---|---|---|
| Porous Ti | √ | × | × |
| Flat Ti | √ | √ | √ |
| No. | Rs (Ω·cm2) | Qf (μF·cm−2) | n1 | Rf (Ω·cm2) | Qdl (μF·cm−2) | Calculated Roughness Factor | n2 | Rct (Ω·cm2) |
|---|---|---|---|---|---|---|---|---|
| Flat | 8.77 × 10−7 | 0.763 | 1 | 2.226 | 1582 | 15.58 | 0.802 | 2.851 |
| Porous | 4.21 × 10−7 | 1.022 | 1 | 1.594 | 84,080 | 3370.95 | 0.938 | 1.755 |
| Name | Initial Potential (V) | Stable Potential (V) | Test Results | Contrast Value |
|---|---|---|---|---|
| Porous Ti | 4.77 | 4.30–4.50 | 491 min | +378.85% |
| Flat Ti | 4.91 | 4.70–4.75 | 102 min | 100% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Chang, L.; Chen, B.; Huang, H.; Guo, Z. Study on the Performance and Corrosion Failure Process of Porous Titanium-Based Coated Electrodes. Lubricants 2022, 10, 282. https://doi.org/10.3390/lubricants10110282
Li J, Chang L, Chen B, Huang H, Guo Z. Study on the Performance and Corrosion Failure Process of Porous Titanium-Based Coated Electrodes. Lubricants. 2022; 10(11):282. https://doi.org/10.3390/lubricants10110282
Chicago/Turabian StyleLi, Jiamin, Linhui Chang, Buming Chen, Hui Huang, and Zhongcheng Guo. 2022. "Study on the Performance and Corrosion Failure Process of Porous Titanium-Based Coated Electrodes" Lubricants 10, no. 11: 282. https://doi.org/10.3390/lubricants10110282
APA StyleLi, J., Chang, L., Chen, B., Huang, H., & Guo, Z. (2022). Study on the Performance and Corrosion Failure Process of Porous Titanium-Based Coated Electrodes. Lubricants, 10(11), 282. https://doi.org/10.3390/lubricants10110282





