Feasibility of a Comprehensive Home Monitoring Program for Sarcoidosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Study Procedures
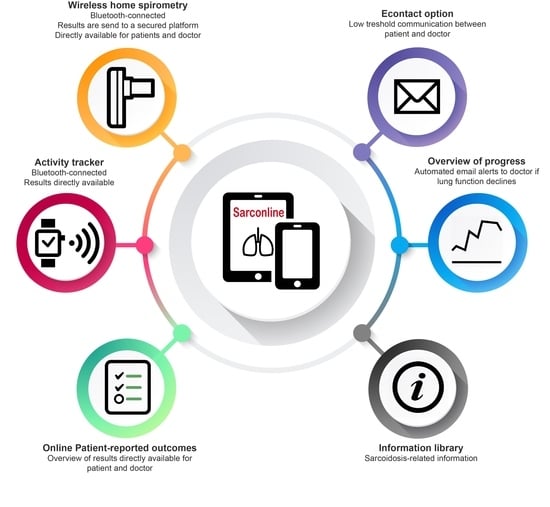
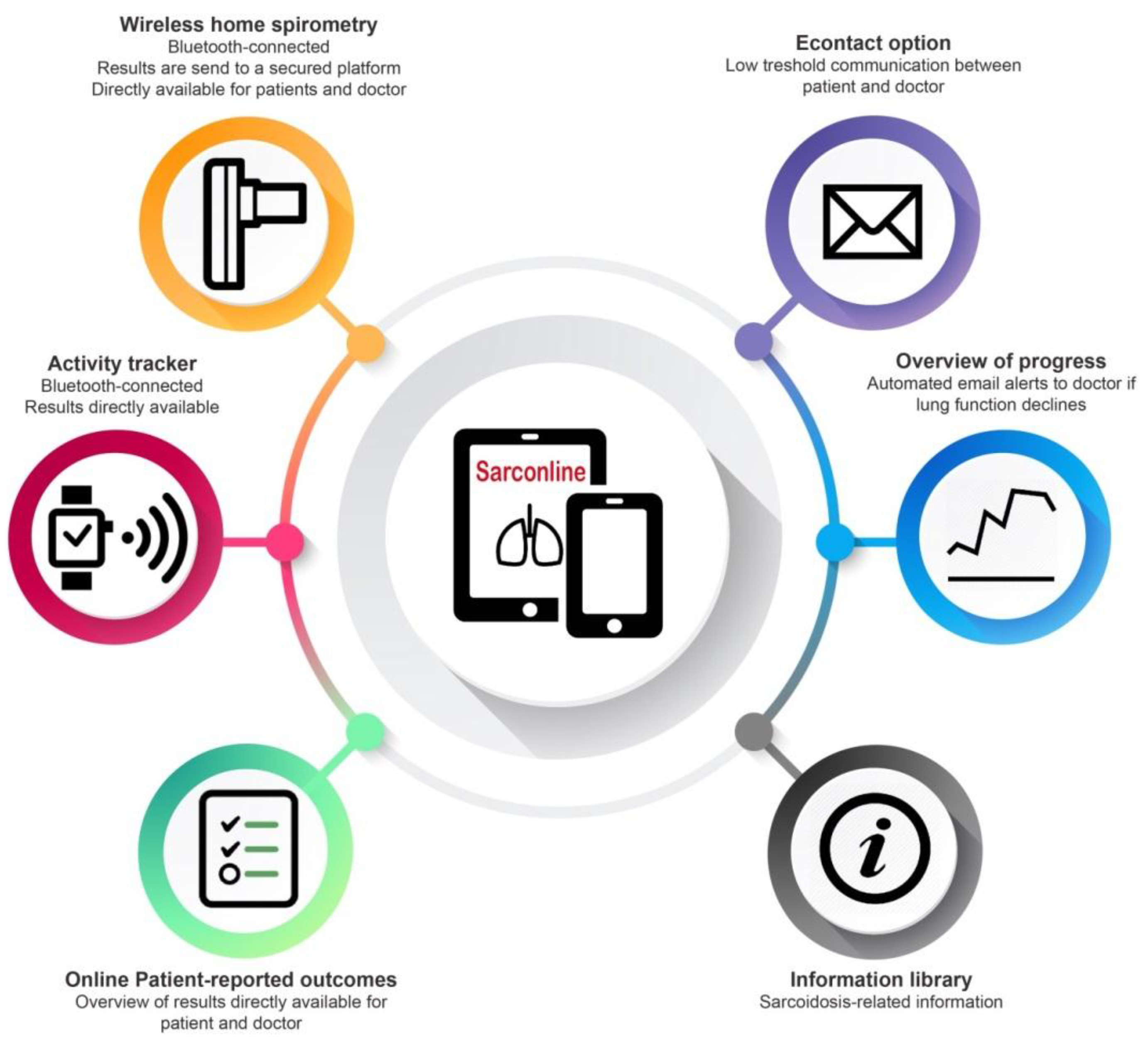
2.2.1. Home Monitoring Program
2.2.2. Home Spirometry
2.2.3. Activity Tracking
2.2.4. Patient-Reported Outcome Measures (PROMs)
2.2.5. Phone Interview
2.3. Statistical Analysis
3. Results
3.1. Home-Based Assessments
3.2. Patient Experiences
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Costabel, U.; Hunninghake, G.W. Ats/ers/wasog statement on sarcoidosis. Sarcoidosis statement committee. American thoracic society. European respiratory society. World association for sarcoidosis and other granulomatous disorders. Eur. Respir. J. 1999, 14, 735–737. [Google Scholar] [CrossRef]
- Goracci, A.; Fagiolini, A.; Martinucci, M.; Calossi, S.; Rossi, S.; Santomauro, T.; Mazzi, A.; Penza, F.; Fossi, A.; Bargagli, E.; et al. Quality of life, anxiety and depression in sarcoidosis. Gen. Hosp. Psychiatry 2008, 30, 441–445. [Google Scholar] [CrossRef]
- Judson, M.A. Quality of life in sarcoidosis. Semin. Respir. Crit. Care Med. 2017, 38, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Drent, M.; Strookappe, B.; Hoitsma, E.; De Vries, J. Consequences of sarcoidosis. Clin. Chest Med. 2015, 36, 727–737. [Google Scholar] [CrossRef]
- Paramothayan, N.S.; Lasserson, T.J.; Jones, P.W. Corticosteroids for pulmonary sarcoidosis. Cochrane Database Syst. Rev. 2005, CD001114. [Google Scholar] [CrossRef]
- Landi, C.; Carleo, A.; Cillis, G.; Rottoli, P. Sarcoidosis: Proteomics and new perspectives for improving personalized medicine. Expert Rev. Proteom. 2018, 15, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Spagnolo, P.; Oldham, J.M.; Jones, M.G.; Lee, J.S. Personalized medicine in interstitial lung diseases. Curr. Opin. Pulm. Med. 2017, 23, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Ziegelstein, R.C. Personomics. JAMA Intern. Med. 2015, 175, 888–889. [Google Scholar] [CrossRef] [PubMed]
- Moor, C.C.; Heukels, P.; Kool, M.; Wijsenbeek, M.S. Integrating patient perspectives into personalized medicine in idiopathic pulmonary fibrosis. Front. Med. (Lausanne) 2017, 4, 226. [Google Scholar] [CrossRef] [PubMed]
- Wicks, P.; Stamford, J.; Grootenhuis, M.A.; Haverman, L.; Ahmed, S. Innovations in e-health. Qual. Life Res. 2014, 23, 195–203. [Google Scholar] [CrossRef]
- Velardo, C.; Shah, S.A.; Gibson, O.; Clifford, G.; Heneghan, C.; Rutter, H.; Farmer, A.; Tarassenko, L.; Team, E.C. Digital health system for personalised copd long-term management. BMC Med. Inform. Decis. Mak. 2017, 17, 19. [Google Scholar] [CrossRef]
- Steven Kohn, M. Editorial commentary: Wearable devices and personalized healthcare. Trends Cardiovasc. Med. 2018, 28, 151–152. [Google Scholar] [CrossRef]
- Moor, C.C.; van Manen, M.J.G.; van Hagen, P.M.; Miedema, J.R.; van den Toorn, L.M.; Gur-Demirel, Y.; Berendse, A.P.C.; van Laar, J.A.M.; Wijsenbeek, M.S. Needs, perceptions and education in sarcoidosis: A live interactive survey of patients and partners. Lung 2018, 196, 569–575. [Google Scholar] [CrossRef]
- Broos, C.E.; Wapenaar, M.; Looman, C.W.N.; In 't Veen, J.; van den Toorn, L.M.; Overbeek, M.J.; Grootenboers, M.; Heller, R.; Mostard, R.L.; Poell, L.H.C.; et al. Daily home spirometry to detect early steroid treatment effects in newly treated pulmonary sarcoidosis. Eur. Respir. J. 2018, 51, 1702089. [Google Scholar] [CrossRef]
- Moor, C.C.; Wapenaar, M.; Miedema, J.R.; Geelhoed, J.J.M.; Chandoesing, P.P.; Wijsenbeek, M.S. A home monitoring program including real-time wireless home spirometry in idiopathic pulmonary fibrosis: A pilot study on experiences and barriers. Respir. Res. 2018, 19, 105. [Google Scholar] [CrossRef]
- Simpson, A.J.; Honkoop, P.J.; Kennington, E.; Snoeck-Stroband, J.B.; Smith, I.; East, J.; Coleman, C.; Caress, A.; Chung, K.F.; Sont, J.K.; et al. Perspectives of patients and healthcare professionals on mhealth for asthma self-management. Eur. Respir. J. 2017, 49, 1601966. [Google Scholar] [CrossRef]
- Duncan, M.; Murawski, B.; Short, C.E.; Rebar, A.L.; Schoeppe, S.; Alley, S.; Vandelanotte, C.; Kirwan, M. Activity trackers implement different behavior change techniques for activity, sleep, and sedentary behaviors. Interact. J. Med. Res. 2017, 6, e13. [Google Scholar] [CrossRef]
- Patel, A.S.; Siegert, R.J.; Creamer, D.; Larkin, G.; Maher, T.M.; Renzoni, E.A.; Wells, A.U.; Higginson, I.J.; Birring, S.S. The development and validation of the king's sarcoidosis questionnaire for the assessment of health status. Thorax 2013, 68, 57–65. [Google Scholar] [CrossRef]
- Brooks, R. Euroqol: The current state of play. Health Policy 1996, 37, 53–72. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- De Vries, J.; Michielsen, H.; Van Heck, G.L.; Drent, M. Measuring fatigue in sarcoidosis: The fatigue assessment scale (FAS). Br. J. Health Psychol. 2004, 9, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Schenkel, F.A.; Ganesh, S.; O'Conner, J.; Sadeghi, R.; Bembi, M.; Duong, M.; Barr, M.L.; Hackmann, A.E. Pilot experience with a novel bluetooth tablet-based technology for home monitoring and education after lung transplantation. J. Heart Lung Transpl. 2018, 37, S38. [Google Scholar] [CrossRef]
- de Jong, M.; van der Meulen-de Jong, A.; Romberg-Camps, M.; Degens, J.; Becx, M.; Markus, T.; Tomlow, H.; Cilissen, M.; Ipenburg, N.; Verwey, M.; et al. Development and feasibility study of a telemedicine tool for all patients with IBD: Myibdcoach. Inflamm. Bowel Dis. 2017, 23, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Odeh, B.; Kayyali, R.; Nabhani-Gebara, S.; Philip, N.; Robinson, P.; Wallace, C.R. Evaluation of a telehealth service for COPD and HF patients: Clinical outcome and patients' perceptions. J. Telemed. Telecare 2015, 21, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Williams, V.; Price, J.; Hardinge, M.; Tarassenko, L.; Farmer, A. Using a mobile health application to support self-management in COPD: A qualitative study. Br. J. Gen. Pract. 2014, 64, e392–e400. [Google Scholar] [CrossRef]
- Russell, A.M.; Adamali, H.; Molyneaux, P.L.; Lukey, P.T.; Marshall, R.P.; Renzoni, E.A.; Wells, A.U.; Maher, T.M. Daily home spirometry: An effective tool for detecting progression in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2016, 194, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Bahmer, T.; Watz, H.; Develaska, M.; Waschki, B.; Rabe, K.F.; Magnussen, H.; Kirsten, D.; Kirsten, A.M. Physical activity and fatigue in patients with sarcoidosis. Respiration 2018, 95, 18–26. [Google Scholar] [CrossRef]
- Froidure, S.; Kyheng, M.; Grosbois, J.M.; Lhuissier, F.; Stelianides, S.; Wemeau, L.; Wallaert, B. Daily life physical activity in patients with chronic stage IV sarcoidosis: A multicenter cohort study. Health Sci. Rep. 2019, 2, e109. [Google Scholar] [CrossRef]
- Pilzak, K.; Zebrowska, A.; Sikora, M.; Hall, B.; Lakomy, O.; Kostorz, S.; Ziora, D.; Jastrzebski, D. Physical functioning and symptoms of chronic fatigue in sarcoidosis patients. Adv. Exp. Med. Biol. 2018, 1040, 13–21. [Google Scholar]
- Maher, C.; Ryan, J.; Ambrosi, C.; Edney, S. Users' experiences of wearable activity trackers: A cross-sectional study. BMC Public Health 2017, 17, 880. [Google Scholar] [CrossRef]
- Strookappe, B.; Saketkoo, L.A.; Elfferich, M.; Holland, A.; De Vries, J.; Knevel, T.; Drent, M. Physical activity and training in sarcoidosis: Review and experience-based recommendations. Expert Rev. Respir. Med. 2016, 10, 1057–1068. [Google Scholar] [CrossRef]
| Age | 53 (31–68) |
| Women | 4 (40) |
| Ethnicity | |
| Caucasian | 9 (90) |
| Surinamese Hindi | 1 (10) |
| Time since diagnosis, year | 5 (0–15) |
| Multiorgan involvement | 6 (60) |
| BMI, kg/m2 | 27 (19–35) |
| Medication | |
| Prednisone | 9 (90) |
| Methotrexate | 6 (60) |
| Other | 3 (30) |
| Pulmonary function | |
| FVC % predicted | 86 (69–105) |
| FVC (L) | 3.50 (2.53–6.47) |
| FEV1 % predicted | 81 (25–97) |
| FEV1 (L) | 2.60 (0.96–3.68) |
| FEV1/FVC (%) | 72 (27–89) |
| DLCO (%) | 74 (44–96) |
| Daily step count | 9781 (4355–17274) |
| Active, min/day | 309 (146–484) |
| Light activity, min/day | 263 (124–401) |
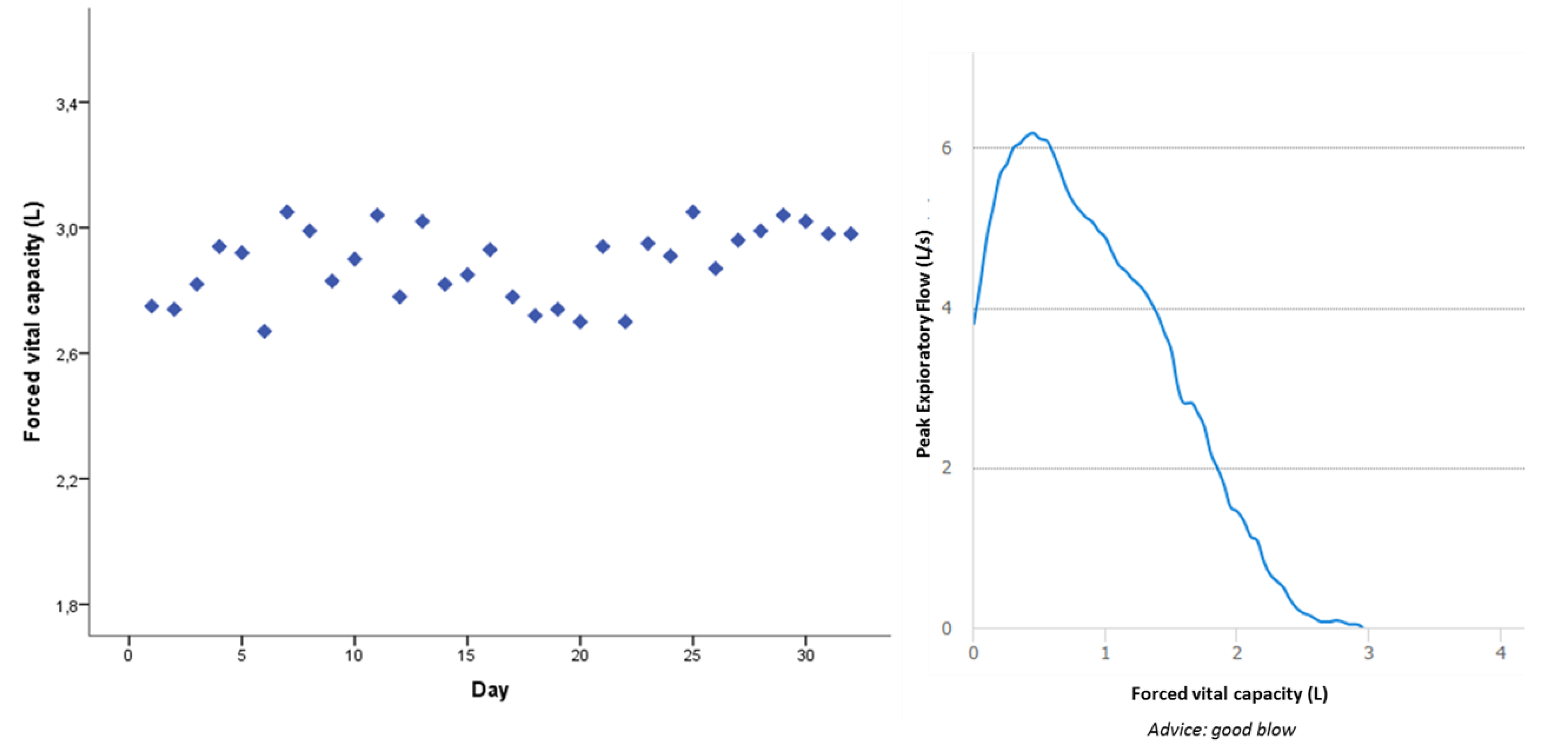
| Home-based FVC (L) | 3.3 (2.4–6.2) |
| Home-based FEV1 (L) | 2.5 (0.8–3.4) |
| EQ5D-5L index value | 0.81 (0.1–0.92) |
| EQ5D-5L VAS | 76 (14–93) |
| KSQ General health | 62 (36–77) |
| KSQ Lung | 61 (37–72) |
| HADS anxiety | 7 (2–12) |
| HADS depression | 6 (1–11) |
| FAS | 25 (17–37) |
| “It is very difficult to tell exactly how you felt four weeks ago. By completing the questionnaires, my personal goal, and symptoms on a regular basis, I get a better overview of my disease over time.” |
| “I think this app gives much more details about my health than the regular outpatient clinic visit every three months. This information could also be very helpful for my doctor and nurses.” |
| “For me, the app contains enough information and devices at the moment. Otherwise, I might become too much focused on my disease.” |
| “I use inhaled medication; this had a direct positive effect on my lung function. I appreciated it very much that I was able see this directly at home. Now, I really know why I have to use it.” |
| “I have quite some side-effects from my medication. I would appreciate if I also could monitor my side-effects in the app.” |
| “It is a reassuring idea that the healthcare team monitors you at a distance, and that they directly see it if your lung function declines.” |
| “Reminders on my email to perform my measurements would be very helpful for me; otherwise, I forget it sometimes. But I understand that other patients probably won't need those reminders.” |
| “When I did not reach my step goal at the end of the day, I went outside to walk around some more. Seeing my activity was very motivating.” |
| “Sometimes, it was frustrating to see my step count, especially on the days that I was very tired and not feeling well. I wished I could walk more, but on some days, that was just not possible.” |
| “Everything together, the questionnaires, overview of symptoms, lung function, and activity gave a good total picture of my health.” |
| “It would be helpful to receive some more information or education about lung function. What do the different tests measure exactly and how should I interpret the results? Maybe it is an idea to make an information movie about this. For me, that is easier to understand than text.” |
| “My sarcoidosis is very stable at the moment. I think home monitoring would be more useful if your disease is getting worse, or if you start with new medication.” |
| “I have had some technical difficulties with the connection between spirometer and the app, but when I called the helpdesk, they could help me out.” |
| “If possible, I would also like to track my heart rate. If I don’t feel good, my heart rate goes up very fast. I think this would give extra information about my physical condition.” |
| Application customized to patients’ needs and wishes |
| Low threshold communication (i.e., eContact or video contact) |
| Patient education |
| Real-time availability of data for patients and healthcare providers |
| Adjustable email reminders for patients and healthcare providers |
| Integration of personal goal |
| Low burden for patients and healthcare providers |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moor, C.C.; Gür-Demirel, Y.; Wijsenbeek, M.S. Feasibility of a Comprehensive Home Monitoring Program for Sarcoidosis. J. Pers. Med. 2019, 9, 23. https://doi.org/10.3390/jpm9020023
Moor CC, Gür-Demirel Y, Wijsenbeek MS. Feasibility of a Comprehensive Home Monitoring Program for Sarcoidosis. Journal of Personalized Medicine. 2019; 9(2):23. https://doi.org/10.3390/jpm9020023
Chicago/Turabian StyleMoor, Catharina C., Yasmin Gür-Demirel, and Marlies S. Wijsenbeek. 2019. "Feasibility of a Comprehensive Home Monitoring Program for Sarcoidosis" Journal of Personalized Medicine 9, no. 2: 23. https://doi.org/10.3390/jpm9020023
APA StyleMoor, C. C., Gür-Demirel, Y., & Wijsenbeek, M. S. (2019). Feasibility of a Comprehensive Home Monitoring Program for Sarcoidosis. Journal of Personalized Medicine, 9(2), 23. https://doi.org/10.3390/jpm9020023