Warfarin: The End or the End of One Size Fits All Therapy?
Abstract
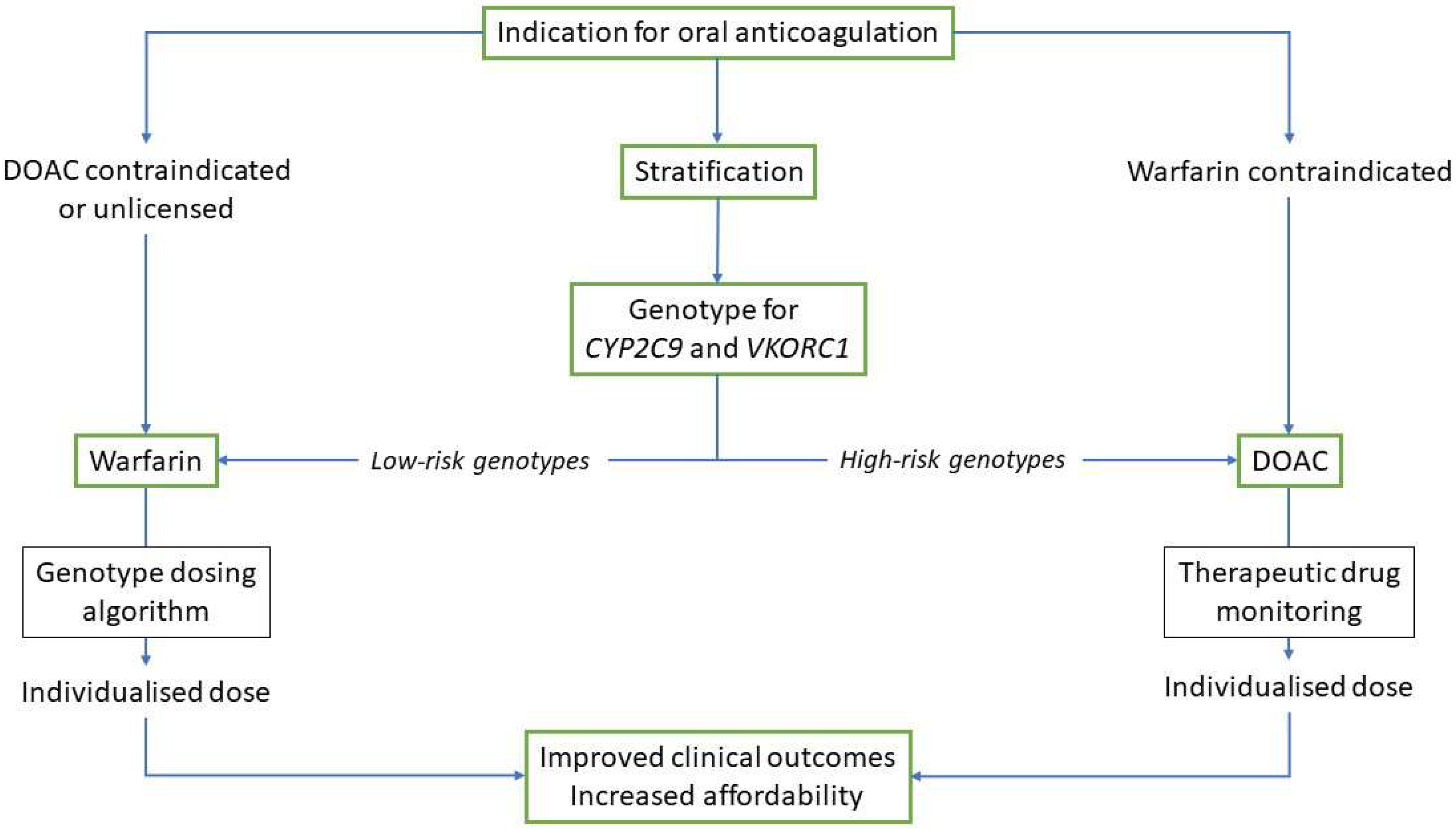
1. Introduction
2. Warfarin Pharmacology and Pharmacogenetics
3. Direct Oral Anticoagulants
- (a)
- Patients with renal impairment: all the drug labels for DOACs have criteria, which either recommend a dose reduction or absolutely contraindicate the use of the DOAC [44]. While it may be relatively easy to avoid the use of DOACs in patients with severe forms of renal impairment, a group that may be at particular risk are patients with incipient renal impairment, where there may be asymptomatic and slow decline in renal function with age or an acute decline in an elderly patient because of a concomitant urinary tract infection. This is compounded by the fact that monitoring of renal function in patients on DOACs is poorly performed.
- (b)
- Patients on interacting drugs: Although DOACs are less likely to be involved in drug–drug interactions than warfarin, they are not immune from them. For patients on certain medications—for example, itraconazole—the use of apixaban is not recommended. A recent database study from Taiwan showed that concomitant use of drugs, such as amiodarone, fluconazole, rifampicin, and phenytoin, increased the risk of major bleeding when compared with the use of DOACs alone [45]. There is no simple biomarker that can be used to individualise dosing with DOACs, unlike warfarin, where INR monitoring provides the opportunity to change dosage to maintain the INR within a therapeutic range.
- (c)
- Use in children: DOACs are currently not licensed for use in children, but there are paediatric investigation plans in place [46]. Thus, for the time being, warfarin (or other vitamin K antagonists) remain the only alternatives. There have been numerous studies in children investigating the effects of genetic polymorphisms on warfarin dosing [47,48,49,50], but no algorithm has been tested in clinical trials.
- (d)
- Mechanical heart valves: DOACs are currently contraindicated in patients with mechanical heart valves. In the RE-ALIGN trial, after enrolment of 252 patients, an increased risk of bleeding and thrombosis was seen in patients on dabigatran, compared with warfarin, which resulted in premature discontinuation of the trial [51].
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Sotiriou, A.; Patel, H.C.; Tyebally, S.; Raza, S.; Qudah, T.; Malik, K.; Patel, K.; Bhattacharyya, S.; Chow, A.; Hayward, C. Is this the beginning of the end for warfarin? EP Eur. 2017, 19, i28. [Google Scholar] [CrossRef]
- Wadelius, M.; Pirmohamed, M. Pharmacogenetics of warfarin: Current status and future challenges. Pharmacogenomics J. 2007, 7, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Rieder, M.J.; Reiner, A.P.; Gage, B.F.; Nickerson, D.A.; Eby, C.S.; McLeod, H.L.; Blough, D.K.; Thummel, K.E.; Veenstra, D.L.; Rettie, A.E. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N. Engl. J. Med. 2005, 352, 2285–2293. [Google Scholar] [CrossRef] [PubMed]
- Rettie, A.E.; Wienkers, L.C.; Gonzalez, F.J.; Trager, W.F.; Korzekwa, K.R. Impaired (S)-warfarin metabolism catalysed by the R144C allelic variant of CYP2C9. Pharmacogenetics 1994, 4, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Limdi, N.A.; Wadelius, M.; Cavallari, L.; Eriksson, N.; Crawford, D.C.; Lee, M.T.; Chen, C.H.; Motsinger-Reif, A.; Sagreiya, H.; Liu, N.; et al. Warfarin pharmacogenetics: A single VKORC1 polymorphism is predictive of dose across 3 racial groups. Blood 2010, 115, 3827–3834. [Google Scholar] [CrossRef] [PubMed]
- Cavallari, L.H.; Perera, M.A. The future of warfarin pharmacogenetics in under-represented minority groups. Future Cardiol. 2012, 8, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, M.I.; Hart, R.; Pearce, L.A. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database Syst. Rev. 2007, CD006186. [Google Scholar] [CrossRef] [PubMed]
- Linkins, L.A.; Choi, P.T.; Douketis, J.D. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: A meta-analysis. Ann. Int. Med. 2003, 139, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Pirmohamed, M.; James, S.; Meakin, S.; Green, C.; Scott, A.K.; Walley, T.J.; Farrar, K.; Park, B.K.; Breckenridge, A.M. Adverse drug reactions as cause of admission to hospital: Prospective analysis of 18,820 patients. BMJ 2004, 329, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, S.; Jorgensen, A.; Zhang, E.J.; Hanson, A.; Gillman, M.S.; Bumpstead, S.; Toh, C.H.; Williamson, P.; Daly, A.K.; Kamali, F.; et al. A multi-factorial analysis of response to warfarin in a UK prospective cohort. Genome Med. 2016, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Wadelius, M.; Chen, L.Y.; Lindh, J.D.; Eriksson, N.; Ghori, M.J.; Bumpstead, S.; Holm, L.; McGinnis, R.; Rane, A.; Deloukas, P. The largest prospective warfarin-treated cohort supports genetic forecasting. Blood 2009, 113, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, M.D.; Awad, T.; Johnson, J.A.; Gage, B.F.; Falkowski, M.; Gardina, P.; Hubbard, J.; Turpaz, Y.; Langaee, T.Y.; Eby, C.; et al. CYP4F2 genetic variant alters required warfarin dose. Blood 2008, 111, 4106–4112. [Google Scholar] [CrossRef] [PubMed]
- Edson, K.Z.; Prasad, B.; Unadkat, J.D.; Suhara, Y.; Okano, T.; Guengerich, F.P.; Rettie, A.E. Cytochrome P450-dependent catabolism of vitamin K: ω-hydroxylation catalyzed by human CYP4F2 and CYP4F11. Biochemistry 2013, 52, 8276–8285. [Google Scholar] [CrossRef] [PubMed]
- Danese, E.; Montagnana, M.; Johnson, J.A.; Rettie, A.E.; Zambon, C.F.; Lubitz, S.A.; Suarez-Kurtz, G.; Cavallari, L.H.; Zhao, L.; Huang, M.; et al. Impact of the CYP4F2 p.V433m polymorphism on coumarin dose requirement: Systematic review and meta-analysis. Clin. Pharmacol. Ther. 2012, 92, 746–756. [Google Scholar] [CrossRef] [PubMed]
- International Warfarin Pharmacogenetics Consortium; Klein, T.E.; Altman, R.B.; Eriksson, N.; Gage, B.F.; Kimmel, S.E.; Lee, M.T.; Limdi, N.A.; Page, D.; Roden, D.M.; et al. Estimation of the warfarin dose with clinical and pharmacogenetic data. N. Engl. J. Med. 2009, 360, 753–764. [Google Scholar] [PubMed]
- Pirmohamed, M.; Kamali, F.; Daly, A.K.; Wadelius, M. Oral anticoagulation: A critique of recent advances and controversies. Trends Pharmacol. Sci. 2015, 36, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, S.E.; French, B.; Anderson, J.L.; Gage, B.F.; Johnson, J.A.; Rosenberg, Y.D.; Geller, N.L.; Kasner, S.E.; Eby, C.S.; Joo, J.; et al. Rationale and design of the clarification of optimal anticoagulation through genetics trial. Am. Heart J. 2013, 166, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Van Schie, R.M.; Wadelius, M.I.; Kamali, F.; Daly, A.K.; Manolopoulos, V.G.; de Boer, A.; Barallon, R.; Verhoef, T.I.; Kirchheiner, J.; Haschke-Becher, E.; et al. Genotype-guided dosing of coumarin derivatives: The European pharmacogenetics of anticoagulant therapy (EU-PACT) trial design. Pharmacogenomics 2009, 10, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Pirmohamed, M.; Burnside, G.; Eriksson, N.; Jorgensen, A.L.; Toh, C.H.; Nicholson, T.; Kesteven, P.; Christersson, C.; Wahlstrom, B.; Stafberg, C.; et al. A randomized trial of genotype-guided dosing of warfarin. N. Engl. J. Med. 2013, 369, 2294–2303. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, S.E.; French, B.; Kasner, S.E.; Johnson, J.A.; Anderson, J.L.; Gage, B.F.; Rosenberg, Y.D.; Eby, C.S.; Madigan, R.A.; McBane, R.B.; et al. A pharmacogenetic versus a clinical algorithm for warfarin dosing. N. Engl. J. Med. 2013, 369, 2283–2293. [Google Scholar] [CrossRef] [PubMed]
- Zineh, I.; Pacanowski, M.; Woodcock, J. Pharmacogenetics and coumarin dosing—Recalibrating expectations. N. Engl. J. Med. 2013, 369, 2273–2275. [Google Scholar] [CrossRef] [PubMed]
- Furie, B. Do pharmacogenetics have a role in the dosing of vitamin K antagonists? N. Engl. J. Med. 2013, 369, 2345–2346. [Google Scholar] [CrossRef] [PubMed]
- Vosoughi, S.; Roy, D.; Aral, S. The spread of true and false news online. Science 2018, 359, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Saffian, S.; Duffull, S.; Wright, D. Warfarin dosing algorithms underpredict dose requirements in patients requiring ≥7 mg daily: A systematic review and meta-analysis. Clin. Pharmacol. Ther. 2017, 102, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Ye, F.; Xie, D.; Zhu, Y.; Zhu, J.; Tao, Y.; Yu, F. A new algorithm to predict warfarin dose from polymorphisms of CYP4F2, CYP2C9 and VKORC1 and clinical variables: Derivation in Han Chinese patients with non valvular atrial fibrillation. Thromb. Haemost. 2012, 107, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, T.; Ghosh, K.; Avery, P.; Kamali, F.; Shetty, S. Warfarin dose model for the prediction of stable maintenance dose in indian patients. Clin. Appl. Thromb./Hemost. 2018, 24, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.; Ohara, M.; Tachikawa, M.; Cavallari, L.H.; Lee, M.T.M.; Wen, M.S.; Scordo, M.G.; Nutescu, E.A.; Perera, M.A.; Miyajima, A.; et al. Population differences in s-warfarin pharmacokinetics among African Americans, Asians and Whites: Their influence on pharmacogenetic dosing algorithms. Pharmacogenomics J. 2016, 17, 494. [Google Scholar] [CrossRef] [PubMed]
- Gage, B.F.; Bass, A.R.; Lin, H.; Woller, S.C.; Stevens, S.M.; Al-Hammadi, N.; Li, J.; Rodriguez, T., Jr.; Miller, J.P.; McMillin, G.A.; et al. Effect of genotype-guided warfarin dosing on clinical events and anticoagulation control among patients undergoing hip or knee arthroplasty: The gift randomized clinical trial. JAMA 2017, 318, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Van Spall, H.G.C.; Wallentin, L.; Yusuf, S.; Eikelboom, J.W.; Nieuwlaat, R.; Yang, S.; Kabali, C.; Reilly, P.A.; Ezekowitz, M.D.; Connolly, S.J. Variation in warfarin dose adjustment practice is responsible for differences in the quality of anticoagulation control between centers and countries. An analysis of patients receiving warfarin in the randomized evaluation of long-term anticoagulation therapy (RE-LY) trial. Circulation 2012, 126, 2309–2316. [Google Scholar] [PubMed]
- Verhoef, T.I.; Redekop, W.K.; Langenskiold, S.; Kamali, F.; Wadelius, M.; Burnside, G.; Maitland-van der Zee, A.H.; Hughes, D.A.; Pirmohamed, M. Cost-effectiveness of pharmacogenetic-guided dosing of warfarin in the United Kingdom and Sweden. Pharmacogenomics J. 2016, 16, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Plumpton, C.O.; Roberts, D.; Pirmohamed, M.; Hughes, D.A. A systematic review of economic evaluations of pharmacogenetic testing for prevention of adverse drug reactions. PharmacoEconomics 2016, 34, 771–793. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.A.; Caudle, K.E.; Gong, L.; Whirl-Carrillo, M.; Stein, C.M.; Scott, S.A.; Lee, M.T.; Gage, B.F.; Kimmel, S.E.; Perera, M.A.; et al. Clinical pharmacogenetics implementation consortium (CPIC) guideline for pharmacogenetics-guided warfarin dosing: 2017 update. Clin. Pharmacol. Ther. 2017, 102, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Bank, P.C.D.; Caudle, K.E.; Swen, J.J.; Gammal, R.S.; Whirl-Carrillo, M.; Klein, T.E.; Relling, M.V.; Guchelaar, H.J. Comparison of the guidelines of the clinical pharmacogenetics implementation consortium and the dutch pharmacogenetics working group. Clin. Pharmacol. Ther. 2018, 103, 599–618. [Google Scholar] [CrossRef] [PubMed]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new Oral Anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef]
- Lopez-Lopez, J.A.; Sterne, J.A.C.; Thom, H.H.Z.; Higgins, J.P.T.; Hingorani, A.D.; Okoli, G.N.; Davies, P.A.; Bodalia, P.N.; Bryden, P.A.; Welton, N.J.; et al. Oral anticoagulants for prevention of stroke in atrial fibrillation: Systematic review, network meta-analysis, and cost effectiveness analysis. BMJ 2017, 359, j5058. [Google Scholar] [CrossRef] [PubMed]
- Barra, S.; Fynn, S. Untreated atrial fibrillation in the United Kingdom: Understanding the barriers and treatment options. J. Saudi Heart Assoc. 2015, 27, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Burn, J.; Pirmohamed, M. Direct oral anticoagulants versus warfarin: Is new always better than the old? Open Heart 2018, 5, e000712. [Google Scholar] [CrossRef] [PubMed]
- Mega, J.L.; Walker, J.R.; Ruff, C.T.; Vandell, A.G.; Nordio, F.; Deenadayalu, N.; Murphy, S.A.; Lee, J.; Mercuri, M.F.; Giugliano, R.P.; et al. Genetics and the clinical response to warfarin and edoxaban: Findings from the randomised, double-blind engage Af-Timi 48 trial. Lancet 2015, 385, 2280–2287. [Google Scholar] [CrossRef]
- Vandell, A.G.; Walker, J.; Brown, K.S.; Zhang, G.; Lin, M.; Grosso, M.A.; Mercuri, M.F. Genetics and clinical response to warfarin and edoxaban in patients with venous thromboembolism. Heart 2017, 103, 1800. [Google Scholar] [CrossRef] [PubMed]
- Gulilat, M.; Tang, A.; Gryn, S.E.; Leong-Sit, P.; Skanes, A.C.; Alfonsi, J.E.; Dresser, G.K.; Henderson, S.L.; Rose, R.V.; Lizotte, D.J.; et al. Interpatient variation in rivaroxaban and apixaban plasma concentrations in routine care. Can. J. Cardiol. 2017, 33, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Asic, A.; Marjanovic, D.; Mirat, J.; Primorac, D. Pharmacogenetics of novel oral anticoagulants: A review of identified gene variants & future perspectives. Per. Med. 2018, 15, 209–221. [Google Scholar] [PubMed]
- Tseng, A.S.; Patel, R.D.; Quist, H.E.; Kekic, A.; Maddux, J.T.; Grilli, C.B.; Shamoun, F.E. Clinical review of the pharmacogenomics of direct oral anticoagulants. Cardiovasc. Drugs Ther. 2018, 32, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Powell, J. Are new oral anticoagulant dosing recommendations optimal for all patients? JAMA 2015, 313, 1013–1014. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.; Jurk, K.; Schinzel, H. Direct oral anticoagulants in patients with chronic kidney disease: Patient selection and special considerations. Int. J. Nephrol. Renovasc. Dis. 2017, 10, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Chou, I.J.; Yeh, Y.H.; Chiou, M.J.; Wen, M.S.; Kuo, C.T.; See, L.C.; Kuo, C.F. Association between use of non-vitamin K oral anticoagulants with and without concurrent medications and risk of major bleeding in nonvalvular atrial fibrillation. JAMA 2017, 318, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Newall, F.; Branchford, B.; Male, C. Anticoagulant prophylaxis and therapy in children: Current challenges and emerging issues. J. Thromb. Haemost. 2018, 16, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Biss, T.; Hamberg, A.K.; Avery, P.; Wadelius, M.; Kamali, F. Warfarin dose prediction in children using pharmacogenetics information. Br. J. Haematol. 2012, 159, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Vear, S.I.; Stein, C.M.; Ho, R.H. Warfarin pharmacogenomics in children. Pediatric Blood Cancer 2013, 60, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Hamberg, A.-K.; Wadelius, M. Pharmacogenetics-based warfarin dosing in children. Pharmacogenomics 2014, 15, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Hawcutt, D.B.; Ghani, A.A.; Sutton, L.; Jorgensen, A.; Zhang, E.; Murray, M.; Michael, H.; Peart, I.; Smyth, R.L.; Pirmohamed, M. Pharmacogenetics of warfarin in a paediatric population: Time in therapeutic range, initial and stable dosing and adverse effects. Pharmacogenomics J. 2014, 14, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Eikelboom, J.W.; Connolly, S.J.; Brueckmann, M.; Granger, C.B.; Kappetein, A.P.; Mack, M.J.; Blatchford, J.; Devenny, K.; Friedman, J.; Guiver, K.; et al. Dabigatran versus warfarin in patients with mechanical heart valves. N. Engl. J. Med. 2013, 369, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.T.; Klein, T.E. Pharmacogenetics of warfarin: Challenges and opportunities. J. Hum. Genet. 2013, 58, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Zuhlke, L.; Engel, M.E.; Karthikeyan, G.; Rangarajan, S.; Mackie, P.; Cupido, B.; Mauff, K.; Islam, S.; Joachim, A.; Daniels, R.; et al. Characteristics, complications, and gaps in evidence-based interventions in rheumatic heart disease: The global rheumatic heart disease registry (the remedy study). Eur. Heart J. 2015, 36, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Mouton, J.P.; Njuguna, C.; Kramer, N.; Stewart, A.; Mehta, U.; Blockman, M.; Fortuin-De Smidt, M.; De Waal, R.; Parrish, A.G.; Wilson, D.P.; et al. Adverse drug reactions causing admission to medical wards: A cross-sectional survey at 4 hospitals in South Africa. Medicine 2016, 95, e3437. [Google Scholar] [CrossRef] [PubMed]
- Pink, J.; Lane, S.; Pirmohamed, M.; Hughes, D.A. Dabigatran etexilate versus warfarin in management of non-valvular atrial fibrillation in UK context: Quantitative benefit-harm and economic analyses. BMJ 2011, 343, d6333. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirmohamed, M. Warfarin: The End or the End of One Size Fits All Therapy? J. Pers. Med. 2018, 8, 22. https://doi.org/10.3390/jpm8030022
Pirmohamed M. Warfarin: The End or the End of One Size Fits All Therapy? Journal of Personalized Medicine. 2018; 8(3):22. https://doi.org/10.3390/jpm8030022
Chicago/Turabian StylePirmohamed, Munir. 2018. "Warfarin: The End or the End of One Size Fits All Therapy?" Journal of Personalized Medicine 8, no. 3: 22. https://doi.org/10.3390/jpm8030022
APA StylePirmohamed, M. (2018). Warfarin: The End or the End of One Size Fits All Therapy? Journal of Personalized Medicine, 8(3), 22. https://doi.org/10.3390/jpm8030022




