Polymer Therapeutics: Biomarkers and New Approaches for Personalized Cancer Treatment
Abstract
1. Introduction: Polymer Therapeutics and the Requirement for Biomarkers
2. Biomarkers: Current Understanding, Problems and PT-Based Considerations
3. Hurdle 1: The Bloodstream
3.1. Complement Activation
3.2. Anti-Polymer Antibodies
3.3. Protein Corona Formation/Opsonization
3.4. Blood Clotting
4. Hurdle 2: Targeting the Tumor and Tumor Uptake
4.1. Passive Targeting by the EPR Effect
4.2. Active Targeting
4.3. Tumor Microenvironment (TME)
4.4. Tumor Uptake
5. Hurdle 3: Intracellular Release of Active Agent
5.1. Enzymatic Triggers
5.2. pH/Redox Triggers
6. Hurdle 4: Further Concepts
7. Hurdle 5: Moving towards the Finishing Line: Improved Detection, Better Models, New Biomarkers and New PT-Based Therapies
8. Conclusions: Last of the Hurdles but Only the Beginning of the Race
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Duncan, R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003, 2, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Gaspar, R. Nanomedicine(s) under the microscope. Mol. Pharm. 2011, 8, 2101–2141. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R. Polymer therapeutics: Top 10 selling pharmaceuticals—What next? J. Control. Release 2014, 190, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R. Polymer conjugates as anticancer nanomedicines. Nat. Rev. Cancer 2006, 6, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Vicent, M.J.; Duncan, R. Polymer conjugates: Nanosized medicines for treating cancer. Trends Biotechnol. 2006, 24, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Vicent, M.J.; Dieudonne, L.; Carbajo, R.J.; Pineda-Lucena, A. Polymer conjugates as therapeutics: Future trends, challenges and opportunities. Expert Opin. Drug Deliv. 2008, 5, 593–614. [Google Scholar] [CrossRef] [PubMed]
- Greco, F.; Vicent, M.J. Combination therapy: Opportunities and challenges for polymer-drug conjugates as anticancer nanomedicines. Adv. Drug Deliv. Rev. 2009, 61, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R. Polymer therapeutics at a crossroads? Finding the path for improved translation in the twenty-first century. J. Drug Target. 2017, 25, 759–780. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.K. Phase II and phase III failures: 2013–2015. Nat. Rev. Drug Discov. 2016, 15, 817–818. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.J.; Wiley, D.T.; Zuckerman, J.E.; Webster, P.; Chao, J.; Lin, J.; Yen, Y.; Davis, M.E. CRLX101 nanoparticles localize in human tumors and not in adjacent, nonneoplastic tissue after intravenous dosing. Proc. Natl. Acad. Sci. USA 2016, 113, 3850–3854. [Google Scholar] [CrossRef] [PubMed]
- Langer, C.J.; O’Byrne, K.J.; Socinski, M.A.; Mikhailov, S.M.; Lesniewski-Kmak, K.; Smakal, M.; Ciuleanu, T.E.; Orlov, S.V.; Dediu, M.; Heigener, D.; et al. Phase III trial comparing paclitaxel poliglumex (CT-2103, PPX) in combination with carboplatin versus standard paclitaxel and carboplatin in the treatment of PS 2 patients with chemotherapy-naive advanced non-small cell lung cancer. J. Thorac. Oncol. 2008, 3, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.A.; Awada, A.; O’Shaughnessy, J.; Rugo, H.S.; Twelves, C.; Im, S.-A.; Zhao, C.; Hoch, U.; Hannah, A.L.; Cortes, J. Phase III trial of etirinotecan pegol (EP) versus treatment of physician’s choice (TPC) in patients (pts) with advanced breast cancer (aBC) whose disease has progressed following anthracycline (A), taxane (T) and capecitabine (C): The beacon study. J. Clin. Oncol. 2015, 33, 1001. [Google Scholar] [CrossRef]
- Allievi, C.; Strepponi, I.; Bastrup, U.; Piazzoni, L.; Tavazzi, S.; Pisano, R.; Pezzoni, G.; Fornasier, M.; Bernareggi, A.; Singer, J.W. Biodistribution of paclitaxel poliglumex (PPX) in lung: Analysis of gender-related alterations in a preclinical model. J. Clin. Oncol. 2006, 24, 17003. [Google Scholar] [CrossRef]
- Melancon, M.P.; Wang, W.; Wang, Y.; Shao, R.; Ji, X.; Gelovani, J.G.; Li, C. A novel method for imaging in vivo degradation of poly(l-glutamic acid), a biodegradable drug carrier. Pharm. Res. 2007, 24, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Ryan, C.; Alumkal, J.; Ryan, C.W.; Sun, J.; Eilers, K.M. A phase II study of paclitaxel poliglumex in combination with transdermal estradiol for the treatment of metastatic castration-resistant prostate cancer after docetaxel chemotherapy. Anticancer Drugs 2010, 21, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Natfji, A.A.; Osborn, H.M.I.; Greco, F. Feasibility of polymer-drug conjugates for non-cancer applications. Curr. Opin. Colloid Interface Sci. 2017, 31, 51–56. [Google Scholar] [CrossRef]
- Requejo-Aguilar, R.; Alastrue-Agudo, A.; Cases-Villar, M.; Lopez-Mocholi, E.; England, R.; Vicent, M.J.; Moreno-Manzano, V. Combined polymer-curcumin conjugate and ependymal progenitor/stem cell treatment enhances spinal cord injury functional recovery. Biomaterials 2017, 113, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Conejos-Sánchez, I.; Cardoso, I.; Oteo-Vives, M.; Romero-Sanz, E.; Paul, A.; Sauri, A.R.; Morcillo, M.A.; Saraiva, M.J.; Vicent, M.J. Polymer-doxycycline conjugates as fibril disrupters: An approach towards the treatment of a rare amyloidotic disease. J. Control. Release 2015, 198, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Conejos–Sánchez, I.; Cardoso, I.; Saraiva, M.J.; Vicent, M.J. Targeting a rare amyloidotic disease through rationally designed polymer conjugates. J. Control. Release 2014, 178, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Roncador, A.; Oppici, E.; Talelli, M.; Pariente, A.N.; Donini, M.; Dusi, S.; Voltattorni, C.B.; Vicent, M.J.; Cellini, B. Use of polymer conjugates for the intraperoxisomal delivery of engineered human alanine:glyoxylate aminotransferase as a protein therapy for primary hyperoxaluria type I. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.D.; Webster, L.; Sostek, M.; Lappalainen, J.; Barker, P.N.; Tack, J. Naloxegol for opioid-induced constipation in patients with noncancer pain. N. Engl. J. Med. 2014, 370, 2387–2396. [Google Scholar] [CrossRef] [PubMed]
- Srinivasarao, M.; Galliford, C.V.; Low, P.S. Principles in the design of ligand-targeted cancer therapeutics and imaging agents. Nat. Rev. Drug Discov. 2015, 14, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Eliasof, S.; Lazarus, D.; Peters, C.G.; Case, R.I.; Cole, R.O.; Hwang, J.; Schluep, T.; Chao, J.; Lin, J.; Yen, Y.; et al. Correlating preclinical animal studies and human clinical trials of a multifunctional, polymeric nanoparticle. Proc. Natl. Acad. Sci. USA 2013, 110, 15127–15132. [Google Scholar] [CrossRef] [PubMed]
- Dawidczyk, C.M.; Kim, C.; Park, J.H.; Russell, L.M.; Lee, K.H.; Pomper, M.G.; Searson, P.C. State-of-the-art in design rules for drug delivery platforms: Lessons learned from FDA-approved nanomedicines. J. Control. Release 2014, 187, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Zagorodko, O.; Arroyo-Crespo, J.J.; Nebot, V.J.; Vicent, M.J. Polypeptide-based conjugates as therapeutics: Opportunities and challenges. Macromol. Biosci. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Koziolova, E.; Chytil, P.; Tsukigawa, K.; Fang, J.; Haratake, M.; Ulbrich, K.; Etrych, T.; Maeda, H. Pronounced cellular uptake of pirarubicin versus that of other anthracyclines: Comparison of HPMA copolymer conjugates of pirarubicin and doxorubicin. Mol. Pharm. 2016, 13, 4106–4115. [Google Scholar] [CrossRef] [PubMed]
- Říhová, B. Biocompatibility of biomaterials: Hemocompatibility, immunocompatiblity and biocompatibility of solid polymeric materials and soluble targetable polymeric carriers. Adv. Drug Deliv. Rev. 1996, 21, 157–176. [Google Scholar] [CrossRef]
- Šimečková, J.; Ríhová, B.; Plocová, D.; Kopecek, J. The activity of complement in the presence of N-(2-hydroxypropyl)methacrylamide copolymers. J. Bioact. Compat. Polym. 1986, 1, 20–31. [Google Scholar] [CrossRef]
- Plank, C.; Mechtler, K.; Szoka, F.C., Jr.; Wagner, E. Activation of the complement system by synthetic DNA complexes: A potential barrier for intravenous gene delivery. Hum. Gene Ther. 1996, 7, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Hamad, I.; Hunter, A.C.; Szebeni, J.; Moghimi, S.M. Poly(ethylene glycol)s generate complement activation products in human serum through increased alternative pathway turnover and a MASP-2-dependent process. Mol. Immunol. 2008, 46, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Hunter, A.C.; Dadswell, C.M.; Savay, S.; Alving, C.R.; Szebeni, J. Causative factors behind poloxamer 188 (Pluronic F68, FlocorTM)-induced complement activation in human sera. A protective role against poloxamer-mediated complement activation by elevated serum lipoprotein levels. Biochim. Biophys. Acta 2004, 1689, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Seymour, L.W.; Ferry, D.R.; Anderson, D.; Hesslewood, S.; Julyan, P.J.; Poyner, R.; Doran, J.; Young, A.M.; Burtles, S.; Kerr, D.J.; et al. Hepatic drug targeting: Phase I evaluation of polymer-bound doxorubicin. J. Clin. Oncol. 2002, 20, 1668–1676. [Google Scholar] [CrossRef] [PubMed]
- Sausville, E.A.; Garbo, L.E.; Weiss, G.J.; Shkolny, D.; Yurkovetskiy, A.V.; Bethune, C.; Ramanathan, R.K.; Fram, R.J. Phase I study of XMT-1001 given IV every 3 weeks to patients with advanced solid tumors. J. Clin. Oncol. 2010, 28, e13121. [Google Scholar] [CrossRef]
- Szebeni, J. Complement activation-related pseudoallergy: A new class of drug-induced acute immune toxicity. Toxicology 2005, 216, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, G.; Griffin, J.I.; Brenneman, B.; Banda, N.K.; Holers, V.M.; Backos, D.S.; Wu, L.; Moghimi, S.M.; Simberg, D. Complement proteins bind to nanoparticle protein corona and undergo dynamic exchange in vivo. Nat. Nanotechnol. 2017, 12, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, J.; Alving, C.R.; Rosivall, L.; Bunger, R.; Baranyi, L.; Bedocs, P.; Toth, M.; Barenholz, Y. Animal models of complement-mediated hypersensitivity reactions to liposomes and other lipid-based nanoparticles. J. Liposome Res. 2007, 17, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Jackman, J.A.; Meszaros, T.; Fulop, T.; Urbanics, R.; Szebeni, J.; Cho, N.J. Comparison of complement activation-related pseudoallergy in miniature and domestic pigs: Foundation of a validatable immune toxicity model. Nanomedicine 2016, 12, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Chauvierre, C.; Leclerc, L.; Labarre, D.; Appel, M.; Marden, M.C.; Couvreur, P.; Vauthier, C. Enhancing the tolerance of poly(isobutylcyanoacrylate) nanoparticles with a modular surface design. Int. J. Pharm. 2007, 338, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Benasutti, H.; Wang, G.; Vu, V.P.; Scheinman, R.; Groman, E.; Saba, L.; Simberg, D. Variability of complement response toward preclinical and clinical nanocarriers in the general population. Bioconjug. Chem. 2017, 28, 2747–2755. [Google Scholar] [CrossRef] [PubMed]
- Ganson, N.J.; Povsic, T.J.; Sullenger, B.A.; Alexander, J.H.; Zelenkofske, S.L.; Sailstad, J.M.; Rusconi, C.P.; Hershfield, M.S. Pre-existing anti-polyethylene glycol antibody linked to first-exposure allergic reactions to pegnivacogin, a pegylated RNA aptamer. J. Allergy Clin. Immunol. 2016, 137, 1610–1613. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Lai, S.K. Anti-peg immunity: Emergence, characteristics, and unaddressed questions. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 655–677. [Google Scholar] [CrossRef] [PubMed]
- Lubich, C.; Allacher, P.; de la Rosa, M.; Bauer, A.; Prenninger, T.; Horling, F.M.; Siekmann, J.; Oldenburg, J.; Scheiflinger, F.; Reipert, B.M. The mystery of antibodies against polyethylene glycol (PEG)—What do we know? Pharm. Res. 2016, 33, 2239–2249. [Google Scholar] [CrossRef] [PubMed]
- Garay, R.P.; El-Gewely, R.; Armstrong, J.K.; Garratty, G.; Richette, P. Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Expert Opin. Drug Deliv. 2012, 9, 1319–1323. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jacobs, T.M.; McCallen, J.D.; Moore, D.T.; Huckaby, J.T.; Edelstein, J.N.; Lai, S.K. Analysis of pre-existing IGG and IGM antibodies against polyethylene glycol (PEG) in the general population. Anal. Chem. 2016, 88, 11804–11812. [Google Scholar] [CrossRef] [PubMed]
- Kierstead, P.H.; Okochi, H.; Venditto, V.J.; Chuong, T.C.; Kivimae, S.; Frechet, J.M.J.; Szoka, F.C. The effect of polymer backbone chemistry on the induction of the accelerated blood clearance in polymer modified liposomes. J. Control. Release 2015, 213, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Monopoli, M.P.; Aberg, C.; Salvati, A.; Dawson, K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012, 7, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Lynch, I.; Ejtehadi, M.R.; Monopoli, M.P.; Bombelli, F.B.; Laurent, S. Protein-nanoparticle interactions: Opportunities and challenges. Chem. Rev. 2011, 111, 5610–5637. [Google Scholar] [CrossRef] [PubMed]
- Maiolo, D.; Del Pino, P.; Metrangolo, P.; Parak, W.J.; Baldelli, B.F. Nanomedicine delivery: Does protein corona route to the target or off road? Nanomedicine 2015, 10, 3231–3247. [Google Scholar] [CrossRef] [PubMed]
- Walkey, C.D.; Chan, W.C. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem. Soc. Rev. 2012, 41, 2780–2799. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef] [PubMed]
- Dell’Orco, D.; Lundqvist, M.; Oslakovic, C.; Cedervall, T.; Linse, S. Modeling the time evolution of the nanoparticle-protein corona in a body fluid. PLoS ONE 2010, 5, e10949. [Google Scholar] [CrossRef] [PubMed]
- Casals, E.; Pfaller, T.; Duschl, A.; Oostingh, G.J.; Puntes, V. Time evolution of the nanoparticle protein corona. ACS Nano 2010, 4, 3623–3632. [Google Scholar] [CrossRef] [PubMed]
- Hadjidemetriou, M.; Al-Ahmady, Z.; Kostarelos, K. Time-evolution of in vivo protein corona onto blood-circulating pegylated liposomal doxorubicin (DOXIL) nanoparticles. Nanoscale 2016, 8, 6948–6957. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, M.J.; Laurent, S.; Aghaie, A.; Rezaee, F.; Mahmoudi, M. Personalized protein coronas: A “key” factor at the nanobiointerface. Biomater. Sci. 2014, 2, 1210–1221. [Google Scholar] [CrossRef]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Nedelkov, D.; Kiernan, U.A.; Niederkofler, E.E.; Tubbs, K.A.; Nelson, R.W. Investigating diversity in human plasma proteins. Proc. Natl. Acad. Sci. USA 2005, 102, 10852–10857. [Google Scholar] [CrossRef] [PubMed]
- Meister, S.; Zlatev, I.; Stab, J.; Docter, D.; Baches, S.; Stauber, R.H.; Deutsch, M.; Schmidt, R.; Ropele, S.; Windisch, M.; et al. Nanoparticulate flurbiprofen reduces amyloid-β42 generation in an in vitro blood–brain barrier model. Alzheimers Res. Ther. 2013, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Papi, M.; Caputo, D.; Palmieri, V.; Coppola, R.; Palchetti, S.; Bugli, F.; Martini, C.; Digiacomo, L.; Pozzi, D.; Caracciolo, G. Clinically approved pegylated nanoparticles are covered by a protein corona that boosts the uptake by cancer cells. Nanoscale 2017, 9, 10327–10334. [Google Scholar] [CrossRef] [PubMed]
- Hamad-Schifferli, K. Exploiting the novel properties of protein coronas: Emerging applications in nanomedicine. Nanomedicine 2015, 10, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nano 2013, 8, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Salvati, A.; Pitek, A.S.; Monopoli, M.P.; Prapainop, K.; Bombelli, F.B.; Hristov, D.R.; Kelly, P.M.; Aberg, C.; Mahon, E.; Dawson, K.A. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat. Nano 2013, 8, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Mirshafiee, V.; Mahmoudi, M.; Lou, K.; Cheng, J.; Kraft, M.L. Protein corona significantly reduces active targeting yield. Chem. Commun. 2013, 49, 2557–2559. [Google Scholar] [CrossRef] [PubMed]
- Walkey, C.D.; Olsen, J.B.; Song, F.; Liu, R.; Guo, H.; Olsen, D.W.; Cohen, Y.; Emili, A.; Chan, W.C. Protein corona fingerprinting predicts the cellular interaction of gold and silver nanoparticles. ACS Nano 2014, 8, 2439–2455. [Google Scholar] [CrossRef] [PubMed]
- Bigdeli, A.; Palchetti, S.; Pozzi, D.; Hormozi-Nezhad, M.R.; Baldelli Bombelli, F.; Caracciolo, G.; Mahmoudi, M. Exploring cellular interactions of liposomes using protein corona fingerprints and physicochemical properties. ACS Nano 2016, 10, 3723–3737. [Google Scholar] [CrossRef] [PubMed]
- Sakulkhu, U.; Maurizi, L.; Mahmoudi, M.; Motazacker, M.; Vries, M.; Gramoun, A.; Ollivier Beuzelin, M.G.; Vallee, J.P.; Rezaee, F.; Hofmann, H. Ex situ evaluation of the composition of protein corona of intravenously injected superparamagnetic nanoparticles in rats. Nanoscale 2014, 6, 11439–11450. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Cui, J.; Sun, H.; Nguyen, T.H.; Alcantara, S.; De Rose, R.; Kent, S.J.; Porter, C.J.H.; Caruso, F. Templated polymer replica nanoparticles to facilitate assessment of material-dependent pharmacokinetics and biodistribution. ACS Appl. Mater. Interfaces 2017, 9, 33683–33694. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; De Rose, R.; Alt, K.; Alcantara, S.; Paterson, B.M.; Liang, K.; Hu, M.; Richardson, J.J.; Yan, Y.; Jeffery, C.M.; et al. Engineering poly(ethylene glycol) particles for improved biodistribution. ACS Nano 2015, 9, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Hadjidemetriou, M.; Al-Ahmady, Z.; Mazza, M.; Collins, R.F.; Dawson, K.; Kostarelos, K. In vivo biomolecule corona around blood-circulating, clinically used and antibody-targeted lipid bilayer nanoscale vesicles. ACS Nano 2015, 9, 8142–8156. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.; Shmeeda, H.; Barenholz, Y. Pharmacokinetics of pegylated liposomal doxorubicin. Clin. Pharmacokinet. 2003, 42, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.L.; Harada, T.; Christian, D.A.; Pantano, D.A.; Tsai, R.K.; Discher, D.E. Minimal “self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science 2013, 339, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-M.J.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Sun, W.; Qian, C.; Wang, C.; Bomba, H.N.; Gu, Z. Anticancer platelet-mimicking nanovehicles. Adv. Mater. 2015, 27, 7043–7050. [Google Scholar] [CrossRef] [PubMed]
- Parodi, A.; Quattrocchi, N.; van de Ven, A.L.; Chiappini, C.; Evangelopoulos, M.; Martinez, J.O.; Brown, B.S.; Khaled, S.Z.; Yazdi, I.K.; Enzo, M.V.; et al. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat. Nano 2013, 8, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Grenier, P.; Mahmoudi, M.; Lima, E.M.; Appel, E.A.; Dormont, F.; Lim, J.M.; Karnik, R.; Langer, R.; Farokhzad, O.C. Mechanistic understanding of in vivo protein corona formation on polymeric nanoparticles and impact on pharmacokinetics. Nat. Commun. 2017, 8, 777. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, J.; Nizzero, S.; Liu, H.; Li, F.; Zhang, G.; Li, Z.; Shen, H.; Blanco, E.; Ferrari, M. A chloroquine-induced macrophage-preconditioning strategy for improved nanodelivery. Sci. Rep. 2017, 7, 13738. [Google Scholar] [CrossRef] [PubMed]
- Pechar, M.; Pola, R.; Janouskova, O.; Sieglova, I.; Kral, V.; Fabry, M.; Tomalova, B.; Kovar, M. Polymer cancerostatics targeted with an antibody fragment bound via a coiled coil motif: In vivo therapeutic efficacy against murine BCL1 leukemia. Macromol. Biosci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Tomalova, B.; Sirova, M.; Rossmann, P.; Pola, R.; Strohalm, J.; Chytil, P.; Cerny, V.; Tomala, J.; Kabesova, M.; Rihova, B.; et al. The structure-dependent toxicity, pharmacokinetics and anti-tumour activity of HPMA copolymer conjugates in the treatment of solid tumours and leukaemia. J. Control. Release 2016, 223, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Tsukigawa, K.; Fang, J. A retrospective 30 years after discovery of the enhanced permeability and retention effect of solid tumors: Next-generation chemotherapeutics and photodynamic therapy—Problems, solutions, and prospects. Microcirculation 2016, 23, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Vascular permeability in cancer and infection as related to macromolecular drug delivery, with emphasis on the epr effect for tumor-selective drug targeting. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2012, 88, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Abd Ellah, N.H.; Abouelmagd, S.A. Surface functionalization of polymeric nanoparticles for tumor drug delivery: Approaches and challenges. Expert Opin. Drug Deliv. 2017, 14, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Thurber, G.M.; Weissleder, R. A systems approach for tumor pharmacokinetics. PLoS ONE 2011, 6, e24696. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Nichols, J.W.; Toh, K.; Nomoto, T.; Cabral, H.; Miura, Y.; Christie, R.J.; Yamada, N.; Ogura, T.; Kano, M.R.; et al. Vascular bursts enhance permeability of tumour blood vessels and improve nanoparticle delivery. Nat. Nanotechnol. 2016, 11, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Vasey, P.A.; Kaye, S.B.; Morrison, R.; Twelves, C.; Wilson, P.; Duncan, R.; Thomson, A.H.; Murray, L.S.; Hilditch, T.E.; Murray, T.; et al. Phase I clinical and pharmacokinetic study of PK1 [N-(2-hydroxypropyl)methacrylamide copolymer doxorubicin]: First member of a new class of chemotherapeutic agents-drug-polymer conjugates. Cancer research campaign phase I/II committee. Clin. Cancer Res. 1999, 5, 83–94. [Google Scholar] [PubMed]
- Ramanathan, R.K.; Korn, R.L.; Sachdev, J.C.; Fetterly, G.J.; Marceau, K.; Marsh, V.; Neil, J.M.; Newbold, R.G.; Raghunand, N.; Prey, J.; et al. Abstract CT224: Pilot study in patients with advanced solid tumors to evaluate feasibility of ferumoxytol (FMX) as tumor imaging agent prior to MM-398, a nanoliposomal irinotecan (nal-IRI). Cancer Res. 2014, 74, CT224. [Google Scholar] [CrossRef]
- Koukourakis, M.I.; Koukouraki, S.; Giatromanolaki, A.; Archimandritis, S.C.; Skarlatos, J.; Beroukas, K.; Bizakis, J.G.; Retalis, G.; Karkavitsas, N.; Helidonis, E.S. Liposomal doxorubicin and conventionally fractionated radiotherapy in the treatment of locally advanced non-small-cell lung cancer and head and neck cancer. J. Clin. Oncol. 1999, 17, 3512–3521. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, O.; Medina, L.A.; Estrada-Lobato, E.; Ramirez-Tirado, L.A.; Mendoza-Garcia, V.O.; de la Garza-Salazar, J. High liposomal doxorubicin tumour tissue distribution, as determined by radiopharmaceutical labelling with (99m)Tc-LD, is associated with the response and survival of patients with unresectable pleural mesothelioma treated with a combination of liposomal doxorubicin and cisplatin. Cancer Chemother. Pharmacol. 2014, 74, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Karathanasis, E.; Suryanarayanan, S.; Balusu, S.R.; McNeeley, K.; Sechopoulos, I.; Karellas, A.; Annapragada, A.V.; Bellamkonda, R.V. Imaging nanoprobe for prediction of outcome of nanoparticle chemotherapy by using mammography. Radiology 2009, 250, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Shields, A.F.; Siegel, B.A.; Miller, K.D.; Krop, I.; Ma, C.X.; LoRusso, P.M.; Munster, P.N.; Campbell, K.; Gaddy, D.F.; et al. 64Cu-MM-302 positron emission tomography quantifies variability of enhanced permeability and retention of nanoparticles in relation to treatment response in patients with metastatic breast cancer. Clin. Cancer Res. 2017, 23, 4190–4202. [Google Scholar] [CrossRef] [PubMed]
- Cabral, H.; Matsumoto, Y.; Mizuno, K.; Chen, Q.; Murakami, M.; Kimura, M.; Terada, Y.; Kano, M.R.; Miyazono, K.; Uesaka, M.; et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011, 6, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Rogers, N.J.; Jeffery, H.C.; Claire, S.; Lewis, D.J.; Zikeli, G.; Hodges, N.J.; Egginton, S.; Nash, G.B.; Pikramenou, Z. Tailoring iridium luminescence and gold nanoparticle size for imaging of microvascular blood flow. Nanomedicine 2017, 12, 2725–2740. [Google Scholar] [CrossRef] [PubMed]
- Sirova, M.; Horkova, V.; Etrych, T.; Chytil, P.; Rihova, B.; Studenovsky, M. Polymer donors of nitric oxide improve the treatment of experimental solid tumours with nanosized polymer therapeutics. J. Drug Target. 2017, 25, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Fang, J.; Maeda, H. Enhanced delivery of macromolecular antitumor drugs to tumors by nitroglycerin application. Cancer Sci. 2009, 100, 2426–2430. [Google Scholar] [CrossRef] [PubMed]
- Nagamitsu, A.; Greish, K.; Maeda, H. Elevating blood pressure as a strategy to increase tumor-targeted delivery of macromolecular drug smancs: Cases of advanced solid tumors. Jpn. J. Clin. Oncol. 2009, 39, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.P.; Martin, J.D.; Liu, H.; Lacorre, D.A.; Jain, S.R.; Kozin, S.V.; Stylianopoulos, T.; Mousa, A.S.; Han, X.; Adstamongkonkul, P.; et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat. Commun. 2013, 4, 2516. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Qin, H.; Nakamura, H.; Tsukigawa, K.; Shin, T.; Maeda, H. Carbon monoxide, generated by heme oxygenase-1, mediates the enhanced permeability and retention effect in solid tumors. Cancer Sci. 2012, 103, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Fang, J.; Liao, L.; Nakamura, H.; Maeda, H. Styrene-maleic acid copolymer-encapsulated CORM2, a water-soluble carbon monoxide (CO) donor with a constant CO-releasing property, exhibits therapeutic potential for inflammatory bowel disease. J. Control. Release 2014, 187, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H. Solid tumor physiology and hypoxia-induced chemo/radio-resistance: Novel strategy for cancer therapy: Nitric oxide donor as a therapeutic enhancer. Nitric Oxide 2008, 19, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H.; Yamaya, M.; Nakayama, K.; Sasaki, T.; Ebihara, S.; Kanda, A.; Asada, M.; Inoue, D.; Suzuki, T.; Okazaki, T.; et al. Randomized phase II trial comparing nitroglycerin plus vinorelbine and cisplatin with vinorelbine and cisplatin alone in previously untreated stage IIIB/IV non-small-cell lung cancer. J. Clin. Oncol. 2006, 24, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Chandra, R.; Cuccarese, M.F.; Pfirschke, C.; Engblom, C.; Stapleton, S.; Adhikary, U.; Kohler, R.H.; Mohan, J.F.; Pittet, M.J.; et al. Radiation therapy primes tumors for nanotherapeutic delivery via macrophage-mediated vascular bursts. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.P.; Stylianopoulos, T.; Martin, J.D.; Popovic, Z.; Chen, O.; Kamoun, W.S.; Bawendi, M.G.; Fukumura, D.; Jain, R.K. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat. Nanotechnol. 2012, 7, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Sessa, C.; Guibal, A.; Del Conte, G.; Ruegg, C. Biomarkers of angiogenesis for the development of antiangiogenic therapies in oncology: Tools or decorations? Nat. Clin. Pract. Oncol. 2008, 5, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkova, Y.; Beztsinna, N.; Baues, M.; Klein, D.; Rix, A.; Golombek, S.K.; Al Rawashdeh, W.E.; Gremse, F.; Barz, M.; Koynov, K.; et al. Balancing passive and active targeting to different tumor compartments using riboflavin-functionalized polymeric nanocarriers. Nano Lett. 2017, 17, 4665–4674. [Google Scholar] [CrossRef] [PubMed]
- Eldar-Boock, A.; Blau, R.; Ryppa, C.; Baabur-Cohen, H.; Many, A.; Vicent, M.J.; Kratz, F.; Sanchis, J.; Satchi-Fainaro, R. Integrin-targeted nano-sized polymeric systems for paclitaxel conjugation: A comparative study. J. Drug Target. 2017, 25, 829–844. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, J. Nab-paclitaxel (Abraxane®): An albumin-bound cytotoxic exploiting natural delivery mechanisms into tumors. Breast Cancer Res. 2009, 11, S21. [Google Scholar] [CrossRef]
- Desai, N.; Trieu, V.; Damascelli, B.; Soon-Shiong, P. Sparc expression correlates with tumor response to albumin-bound paclitaxel in head and neck cancer patients. Transl. Oncol. 2009, 2, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Low, P.S.; Kularatne, S.A. Folate-targeted therapeutic and imaging agents for cancer. Curr. Opin. Chem. Biol. 2009, 13, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Bissell, M.J.; Radisky, D. Putting tumours in context. Nat. Rev. Cancer 2001, 1, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Eikenes, L.; Tari, M.; Tufto, I.; Bruland, O.S.; de Lange Davies, C. Hyaluronidase induces a transcapillary pressure gradient and improves the distribution and uptake of liposomal doxorubicin (Caelyx) in human osteosarcoma xenografts. Br. J. Cancer 2005, 93, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Cuevas, C.; Chang, A.E.; Goel, V.K.; Von Hoff, D.D.; Hingorani, S.R. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012, 21, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, S.R.; Bullock, A.J.; Seery, T.E.; Zheng, L.; Sigal, D.; Ritch, P.S.; Braiteh, F.S.; Zalupski, M.; Bahary, N.; Harris, W.P.; et al. Randomized phase II study of PEGPH20 plus nab-paclitaxel/gemcitabine (PAG) vs AG in patients (PTs) with untreated, metastatic pancreatic ductal adenocarcinoma (mPDA). J. Clin. Oncol. 2017, 35, 4008. [Google Scholar] [CrossRef]
- Miller, M.A.; Zheng, Y.-R.; Gadde, S.; Pfirschke, C.; Zope, H.; Engblom, C.; Kohler, R.H.; Iwamoto, Y.; Yang, K.S.; Askevold, B.; et al. Tumour-associated macrophages act as a slow-release reservoir of nano-therapeutic PT(IV) pro-drug. Nat. Commun. 2015, 6, 8692. [Google Scholar] [CrossRef] [PubMed]
- Daldrup-Link, H.E.; Golovko, D.; Ruffell, B.; Denardo, D.G.; Castaneda, R.; Ansari, C.; Rao, J.; Tikhomirov, G.A.; Wendland, M.F.; Corot, C.; et al. MRI of tumor-associated macrophages with clinically applicable iron oxide nanoparticles. Clin. Cancer Res. 2011, 17, 5695–5704. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Gadde, S.; Pfirschke, C.; Engblom, C.; Sprachman, M.M.; Kohler, R.H.; Yang, K.S.; Laughney, A.M.; Wojtkiewicz, G.; Kamaly, N.; et al. Predicting therapeutic nanomedicine efficacy using a companion magnetic resonance imaging nanoparticle. Sci. Transl. Med. 2015, 7, 314ra183. [Google Scholar] [CrossRef] [PubMed]
- Zimel, M.N.; Horowitz, C.B.; Rajasekhar, V.K.; Christ, A.B.; Wei, X.; Wu, J.; Wojnarowicz, P.M.; Wang, D.; Goldring, S.R.; Purdue, P.E.; et al. HPMA-copolymer nanocarrier targets tumor-associated macrophages in primary and metastatic breast cancer. Mol. Cancer Ther. 2017, 16, 2701–2710. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Xia, J.X.; Wang, J. Nanomedicine-mediated cancer stem cell therapy. Biomaterials 2016, 74, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Huang, G.; Chen, Z.; Zhang, Y. Nanomaterials in targeting cancer stem cells for cancer therapy. Front. Pharmacol. 2017, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, J.; Rhim, J.S.; Kopeček, J. HPMA copolymer-based combination therapy toxic to both prostate cancer stem/progenitor cells and differentiated cells induces durable anti-tumor effects. J. Control. Release 2013, 172, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Polymer therapeutics and the EPR effect. J. Drug Target. 2017, 25, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Mosesson, Y.; Mills, G.B.; Yarden, Y. Derailed endocytosis: An emerging feature of cancer. Nat. Rev. Cancer 2008, 8, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Lanzetti, L.; Di Fiore, P.P. Behind the scenes: Endo/exocytosis in the acquisition of metastatic traits. Cancer Res. 2017, 77, 1813–1817. [Google Scholar] [CrossRef] [PubMed]
- De Leon, J.; Susce, M.T.; Murray-Carmichael, E. The amplichip CYP450 genotyping test: Integrating a new clinical tool. Mol. Diagn. Ther. 2006, 10, 135–151. [Google Scholar] [CrossRef] [PubMed]
- Kunjachan, S.; Rychlik, B.; Storm, G.; Kiessling, F.; Lammers, T. Multidrug resistance: Physiological principles and nanomedical solutions. Adv. Drug Deliv. Rev. 2012, 65, 1852–1865. [Google Scholar] [CrossRef] [PubMed]
- Kunjachan, S.; Błauż, A.; Möckel, D.; Theek, B.; Kiessling, F.; Etrych, T.; Ulbrich, K.; Bloois, L.V.; Storm, G.; Bartosz, G.; et al. Overcoming cellular multidrug resistance using classical nanomedicine formulations. Eur. J. Pharm. Sci. 2012, 45, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Gou, J.; Liang, Y.; Miao, L.; Guo, W.; Chao, Y.; He, H.; Zhang, Y.; Yang, J.; Wu, C.; Yin, T.; et al. Improved tumor tissue penetration and tumor cell uptake achieved by delayed charge reversal nanoparticles. Acta Biomater. 2017, 62, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Harrington, K.J.; Mohammadtaghi, S.; Uster, P.S.; Glass, D.; Peters, A.M.; Vile, R.G.; Stewart, J.S. Effective targeting of solid tumors in patients with locally advanced cancers by radiolabeled pegylated liposomes. Clin. Cancer Res. 2001, 7, 243–254. [Google Scholar] [PubMed]
- Whitley, M.J.; Cardona, D.M.; Lazarides, A.L.; Spasojevic, I.; Ferrer, J.M.; Cahill, J.; Lee, C.L.; Snuderl, M.; Blazer, D.G., 3rd; Hwang, E.S.; et al. A mouse-human phase 1 co-clinical trial of a protease-activated fluorescent probe for imaging cancer. Sci. Transl. Med. 2016, 8, 320ra324. [Google Scholar] [CrossRef] [PubMed]
- Commisso, C.; Davidson, S.M.; Soydaner-Azeloglu, R.G.; Parker, S.J.; Kamphorst, J.J.; Hackett, S.; Grabocka, E.; Nofal, M.; Drebin, J.A.; Thompson, C.B.; et al. Macropinocytosis of protein is an amino acid supply route in ras-transformed cells. Nature 2013, 497, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Kim, G. Nab-paclitaxel for the treatment of pancreatic cancer. Cancer Manag. Res. 2017, 9, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Singer, J.W.; Shaffer, S.; Baker, B.; Bernareggi, A.; Stromatt, S.; Nienstedt, D.; Besman, M. Paclitaxel poliglumex (XYOTAX; CT-2103): An intracellularly targeted taxane. Anticancer Drugs 2005, 16, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.M.; Sloane, B.F. Cysteine cathepsins: Multifunctional enzymes in cancer. Nat. Rev. Cancer 2006, 6, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Berquin, I.M.; Sloane, B.F. Cathepsin B expression in human tumors. Adv. Exp. Med. Biol. 1996, 389, 281–294. [Google Scholar] [PubMed]
- Hardwicke, J.T.; Hart, J.; Bell, A.; Duncan, R.; Thomas, D.W.; Moseley, R. The effect of dextrin–rhEGF on the healing of full-thickness, excisional wounds in the (db/db) diabetic mouse. J. Control. Release 2011, 152, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Goszczyński, T.M.; Filip-Psurska, B.; Kempińska, K.; Wietrzyk, J.; Boratyński, J. Hydroxyethyl starch as an effective methotrexate carrier in anticancer therapy. Pharmacol. Res. Perspect. 2014, 2, e00047. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Gan, Z. The influence of pendant hydroxyl groups on enzymatic degradation and drug delivery of amphiphilic poly[glycidol-block-(ε-caprolactone)] copolymers. Macromol. Biosci. 2009, 9, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, J.; Liu, S.; Lu, Q.; He, J.; Zhou, Z.; Hu, Y. Enzyme sensitive, surface engineered nanoparticles for enhanced delivery of camptothecin. J. Control. Release 2015, 216, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R. Development of hpma copolymer-anticancer conjugates: Clinical experience and lessons learnt. Adv. Drug Deliv. Rev. 2009, 61, 1131–1148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Pan, D.; Li, J.; Hu, J.; Bains, A.; Guys, N.; Zhu, H.; Li, X.; Luo, K.; Gong, Q.; et al. Enzyme-responsive peptide dendrimer-gemcitabine conjugate as a controlled-release drug delivery vehicle with enhanced antitumor efficacy. Acta Biomater. 2017, 55, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Sat-Klopsch, Y.N.; Burger, A.M.; Bibby, M.C.; Fiebig, H.H.; Sausville, E.A. Validation of tumour models for use in anticancer nanomedicine evaluation: The EPR effect and cathepsin B-mediated drug release rate. Cancer Chemother. Pharmacol. 2013, 72, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Loadman, P.M.; Bibby, M.C.; Double, J.A.; Al-Shakhaa, W.M.; Duncan, R. Pharmacokinetics of PK1 and doxorubicin in experimental colon tumor models with differing responses to PK1. Clin. Cancer Res. 1999, 5, 3682–3688. [Google Scholar] [PubMed]
- Shabat, D.; Roth-Konforti, M.; Bauer, C. Unprecedented sensitivity in a probe for detection and imaging of cathepsin B: Chemiluminescence microscopy cell images of natively-expressed enzyme. Angew. Chem. Int. Ed. Engl. 2017, 56, 15633–15638. [Google Scholar] [CrossRef]
- Tsukigawa, K.; Nakamura, H.; Fang, J.; Otagiri, M.; Maeda, H. Effect of different chemical bonds in pegylation of zinc protoporphyrin that affects drug release, intracellular uptake, and therapeutic effect in the tumor. Eur. J. Pharm. Biopharm. 2015, 89, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Garland, M.; Yim, J.; Bogyo, M. A bright future for precision medicine: Advances in fluorescent chemical probe design and their clinical application. Cell Chem. Biol. 2016, 23, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.C.; Hsiao, M. Protease-activated nanomaterials for targeted cancer theranostics. Nanomedicine 2017, 12, 2153–2159. [Google Scholar] [CrossRef] [PubMed]
- Mito, J.K.; Ferrer, J.M.; Brigman, B.E.; Lee, C.-L.; Dodd, R.D.; Eward, W.C.; Marshall, L.F.; Cuneo, K.C.; Carter, J.E.; Ramasunder, S.; et al. Intraoperative detection and removal of microscopic residual sarcoma using wide-field imaging. Cancer 2012, 118, 5320–5330. [Google Scholar] [CrossRef] [PubMed]
- Cuneo, K.C.; Mito, J.K.; Javid, M.P.; Ferrer, J.M.; Kim, Y.; Lee, W.D.; Bawendi, M.G.; Brigman, B.E.; Kirsch, D.G. Imaging primary mouse sarcomas after radiation therapy using cathepsin-activatable fluorescent imaging agents. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Tung, C.-H.; Mahmood, U.; Bogdanov, A., Jr. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat. Biotechnol. 1999, 17, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Bremer, C.; Tung, C.-H.; Weissleder, R. In vivo molecular target assessment of matrix metalloproteinase inhibition. Nat. Med. 2001, 7, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Sensarn, S.; Zavaleta, C.L.; Segal, E.; Rogalla, S.; Lee, W.; Gambhir, S.S.; Bogyo, M.; Contag, C.H. A clinical wide-field fluorescence endoscopic device for molecular imaging demonstrating cathepsin protease activity in colon cancer. Mol. Imaging Biol. 2016, 18, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Lazarides, A.L.; Whitley, M.J.; Strasfeld, D.B.; Cardona, D.M.; Ferrer, J.M.; Mueller, J.L.; Fu, H.L.; Bartholf DeWitt, S.; Brigman, B.E.; Ramanujam, N.; et al. A fluorescence-guided laser ablation system for removal of residual cancer in a mouse model of soft tissue sarcoma. Theranostics 2016, 6, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bhattacharyya, J.; Mastria, E.; Chilkoti, A. A quantitative study of the intracellular fate of PH-responsive doxorubicin-polypeptide nanoparticles. J. Control. Release 2017, 260, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, Y.; Han, X.; Liang, C.; Liu, J.; Song, X.; Ge, Z.; Liu, Z. Redox-sensitive nanoscale coordination polymers for drug delivery and cancer theranostics. ACS Appl. Mater. Interfaces 2017, 9, 23555–23563. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, H.; Liu, P.; Zeng, F.; Wu, S. A self-immolative prodrug nanosystem capable of releasing a drug and a NIR reporter for in vivo imaging and therapy. Biomaterials 2017, 139, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, Y.; Sa, Z.; Sun, Y.; Wang, Y.; Wang, J. A smart drug delivery system from charge-conversion polymer-drug conjugate for enhancing tumor therapy and tunable drug release. Macromol. Biosci. 2014, 14, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Escalona, G.; Sanchis, J.; Vicent, M.J. pH-responsive polyacetal-protein conjugates designed for polymer masked-unmasked protein therapy (PUMPT). Macromol. Biosci. 2018, 18. [Google Scholar] [CrossRef]
- Arminan, A.; Mendes, L.; Carrola, J.; Movellan, J.; Vicent, M.J.; Duarte, I.F. HIF-1α inhibition by diethylstilbestrol and its polyacetal conjugate in hypoxic prostate tumour cells: Insights from NMR metabolomics. J. Drug Target. 2017, 25, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Teo, J.Y.; Chin, W.; Ke, X.; Gao, S.; Liu, S.; Cheng, W.; Hedrick, J.L.; Yang, Y.Y. pH and redox dual-responsive biodegradable polymeric micelles with high drug loading for effective anticancer drug delivery. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Yen, J.; Yin, Q.; Liu, Y.; Song, Z.; Lezmi, S.; Zhang, Y.; Yang, X.; Helferich, W.G.; Cheng, J. Redox-responsive self-assembled chain-shattering polymeric therapeutics. Biomater. Sci. 2015, 3, 1061–1065. [Google Scholar] [CrossRef] [PubMed]
- Talelli, M.; Vicent, M.J. Reduction sensitive poly(l-glutamic acid) (PGA)-protein conjugates designed for polymer masked–unmasked protein therapy. Biomacromolecules 2014, 15, 4168–4177. [Google Scholar] [CrossRef] [PubMed]
- Perry, D.A.; Salvin, J.W.; Romfh, P.; Chen, P.; Krishnamurthy, K.; Thomson, L.M.; Polizzotti, B.D.; McGowan, F.X.; Vakhshoori, D.; Kheir, J.N. Responsive monitoring of mitochondrial redox states in heart muscle predicts impending cardiac arrest. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Chen, J.; Bajić, A.; Zhang, C.; Song, X.; Carroll, S.L.; Cai, Z.-L.; Tang, M.; Xue, M.; Cheng, N.; et al. Quantitative real-time imaging of glutathione. Nat. Commun. 2017, 8, 16087. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Zhao, Y.; Li, C.; Wang, M.; Xu, X.; Jin, Y. Single-cell pH imaging and detection for pH profiling and label-free rapid identification of cancer-cells. Sci. Rep. 2017, 7, 1759. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, K.; Huang, G.; Hensley, C.; Huang, X.; Ma, X.; Zhao, T.; Sumer, B.D.; DeBerardinis, R.J.; Gao, J. A nanoparticle-based strategy for the imaging of a broad range of tumours by nonlinear amplification of microenvironment signals. Nat. Mater. 2014, 13, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, W.C.; Strychor, S.; Maruca, L.; Ramalingam, S.; Zamboni, B.A.; Wu, H.; Friedland, D.M.; Edwards, R.P.; Stoller, R.G.; Belani, C.P.; et al. Pharmacokinetic study of pegylated liposomal CKD-602 (S-CKD602) in patients with advanced malignancies. Clin. Pharmacol. Ther. 2009, 86, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Sistare, F.D.; Dieterle, F.; Troth, S.; Holder, D.J.; Gerhold, D.; Andrews-Cleavenger, D.; Baer, W.; Betton, G.; Bounous, D.; Carl, K.; et al. Towards consensus practices to qualify safety biomarkers for use in early drug development. Nat. Biotechnol. 2010, 28, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.W.; Hsieh, Y.C.; Cheng, Y.A.; Chuang, K.H.; Huang, C.C.; Chuang, C.H.; Chen, I.J.; Cheng, K.W.; Lu, Y.C.; Cheng, T.C.; et al. Optimization of an anti-poly(ethylene glycol) (anti-PEG) cell-based capture system to quantify peg and pegylated molecules. Anal. Chem. 2016, 88, 12371–12379. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Gu, X.; Achanzar, W.E.; Chadwick, K.D.; Gan, J.; Brock, B.J.; Kishnani, N.S.; Humphreys, W.G.; Iyer, R.A. Quantitative analysis of polyethylene glycol (PEG) and pegylated proteins in animal tissues by LC-MS/MS coupled with in-source cid. Anal. Chem. 2014, 86, 7642–7649. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.I.; Wang, G.; Smith, W.J.; Vu, V.P.; Scheinman, R.; Stitch, D.; Moldovan, R.; Moghimi, S.M.; Simberg, D. Revealing dynamics of accumulation of systemically injected liposomes in the skin by intravital microscopy. ACS Nano 2017, 11, 11584–11593. [Google Scholar] [CrossRef] [PubMed]
- Shalek, A.K.; Benson, M. Single-cell analyses to tailor treatments. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Kanatani, S.; Tomer, R.; Sahlgren, C.; Kronqvist, P.; Kaczynska, D.; Louhivuori, L.; Kis, L.; Lindh, C.; Mitura, P.; et al. Whole-tissue biopsy phenotyping of three-dimensional tumours reveals patterns of cancer heterogeneity. Nat. Biomed. Eng. 2017, 1, 796–806. [Google Scholar] [CrossRef]
- Zenobi, R. Single-cell metabolomics: Analytical and biological perspectives. Science 2013, 342. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.; Nagy, C.; Knapp, B.; Laengle, J.; Ponweiser, E.; Groeger, M.; Starkl, P.; Bergmann, M.; Wagner, O.; Haschemi, A. Exploring metabolic configurations of single cells within complex tissue microenvironments. Cell Metab. 2017, 26, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Armiñán, A.; Palomino-Schätzlein, M.; Deladriere, C.; Arroyo-Crespo, J.J.; Vicente-Ruiz, S.; Vicent, M.J.; Pineda-Lucena, A. Metabolomics facilitates the discrimination of the specific anti-cancer effects of free- and polymer-conjugated doxorubicin in breast cancer models. Biomaterials. 2017. just accepted. [Google Scholar]
- Spinelli, J.B.; Yoon, H.; Ringel, A.E.; Jeanfavre, S.; Clish, C.B.; Haigis, M.C. Metabolic recycling of ammonia via glutamate dehydrogenase supports breast cancer biomass. Science 2017, 358. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Qian, J.; Zhou, L.; Su, Y.; Zhang, R.; Dong, C.M. Biopolymer-drug conjugate nanotheranostics for multimodal imaging-guided synergistic cancer photothermal-chemotherapy. ACS Appl. Mater. Interfaces 2017, 9, 31576–31588. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Koay, E.J.; Li, T.; Wen, X.; Li, C. A hindsight reflection on the clinical studies of poly(l-glutamic acid)-paclitaxel. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Bendele, A.; Seely, J.; Richey, C.; Sennello, G.; Shopp, G. Short communication: Renal tubular vacuolation in animals treated with polyethylene-glycol-conjugated proteins. Toxicol. Sci. 1998, 42, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Turecek, P.L.; Bossard, M.J.; Schoetens, F.; Ivens, I.A. Pegylation of biopharmaceuticals: A review of chemistry and nonclinical safety information of approved drugs. J. Pharm. Sci. 2016, 105, 460–475. [Google Scholar] [CrossRef] [PubMed]
- Forest, T.; Xu, Q.; Kuruvilla, S.; Vu, H.; Vlasakova, K.; Glaab, W.E.; Hines, C.; Xun, S. Magnetic resonance and ultrastructural characterization of pegylation-associated vacuolation in nonclinical models. Toxicol. Pathol. 2017, 45, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Stone, V.; Johnston, H.; Schins, R.P. Development of in vitro systems for nanotoxicology: Methodological considerations. Crit. Rev. Toxicol. 2009, 39, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, G.; Couvreur, P.; Mura, S. Multicellular tumor spheroids: A relevant 3D model for the in vitro preclinical investigation of polymer nanomedicines. Polym. Chem. 2017, 8, 4947–4969. [Google Scholar] [CrossRef]
- Raghavan, S.; Mehta, P.; Ward, M.R.; Bregenzer, M.E.; Fleck, E.M.A.; Tan, L.; McLean, K.; Buckanovich, R.J.; Mehta, G. Personalized medicine–based approach to model patterns of chemoresistance and tumor recurrence using ovarian cancer stem cell spheroids. Clin. Cancer Res. 2017, 23, 6934–6945. [Google Scholar] [CrossRef] [PubMed]
- Rashed, M.H.; Bayraktar, E.; Helal, G.K.; Abd-Ellah, M.F.; Amero, P.; Chavez-Reyes, A.; Rodriguez-Aguayo, C. Exosomes: From garbage bins to promising therapeutic targets. Int. J. Mol. Sci. 2017, 18, 538. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.; Sahebkar, A.; Jaafari, M.R.; Goodarzi, M.; Mirzaei, H.R. Diagnostic and therapeutic potential of exosomes in cancer: The beginning of a new tale? J. Cell. Physiol. 2017, 232, 3251–3260. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Aleckovic, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; Garcia-Santos, G.; Ghajar, C.M.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through met. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.G.; Valencia, J.C.; Lai, B.; Zhang, G.; Paterson, J.K.; Rouzaud, F.; Berens, W.; Wincovitch, S.M.; Garfield, S.H.; Leapman, R.D.; et al. Melanosomal sequestration of cytotoxic drugs contributes to the intractability of malignant melanomas. Proc. Natl. Acad. Sci. USA 2006, 103, 9903–9907. [Google Scholar] [CrossRef] [PubMed]
- Safaei, R.; Larson, B.J.; Cheng, T.C.; Gibson, M.A.; Otani, S.; Naerdemann, W.; Howell, S.B. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol. Cancer Ther. 2005, 4, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Bhagwat, N.; Yee, S.S.; Ortiz, N.; Sahmoud, A.; Black, T.; Aiello, N.M.; McKenzie, L.; O’Hara, M.; Redlinger, C.; et al. Combining machine learning and nanofluidic technology to diagnose pancreatic cancer using exosomes. ACS Nano 2017, 11, 11182–11193. [Google Scholar] [CrossRef] [PubMed]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015, 523, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Milane, L.; Singh, A.; Mattheolabakis, G.; Suresh, M.; Amiji, M.M. Exosome mediated communication within the tumor microenvironment. J. Control. Release 2015, 219, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.J.; Wei, J.; Nie, G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014, 35, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of exosome-encapsulated paclitaxel to overcome mdr in cancer cells. Nanomedicine 2016, 12, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, K.B.; Gudbergsson, J.M.; Skov, M.N.; Pilgaard, L.; Moos, T.; Duroux, M. A comprehensive overview of exosomes as drug delivery vehicles—Endogenous nanocarriers for targeted cancer therapy. Biochim. Biophys. Acta (BBA) Rev. Cancer 2014, 1846, 75–87. [Google Scholar] [CrossRef] [PubMed]
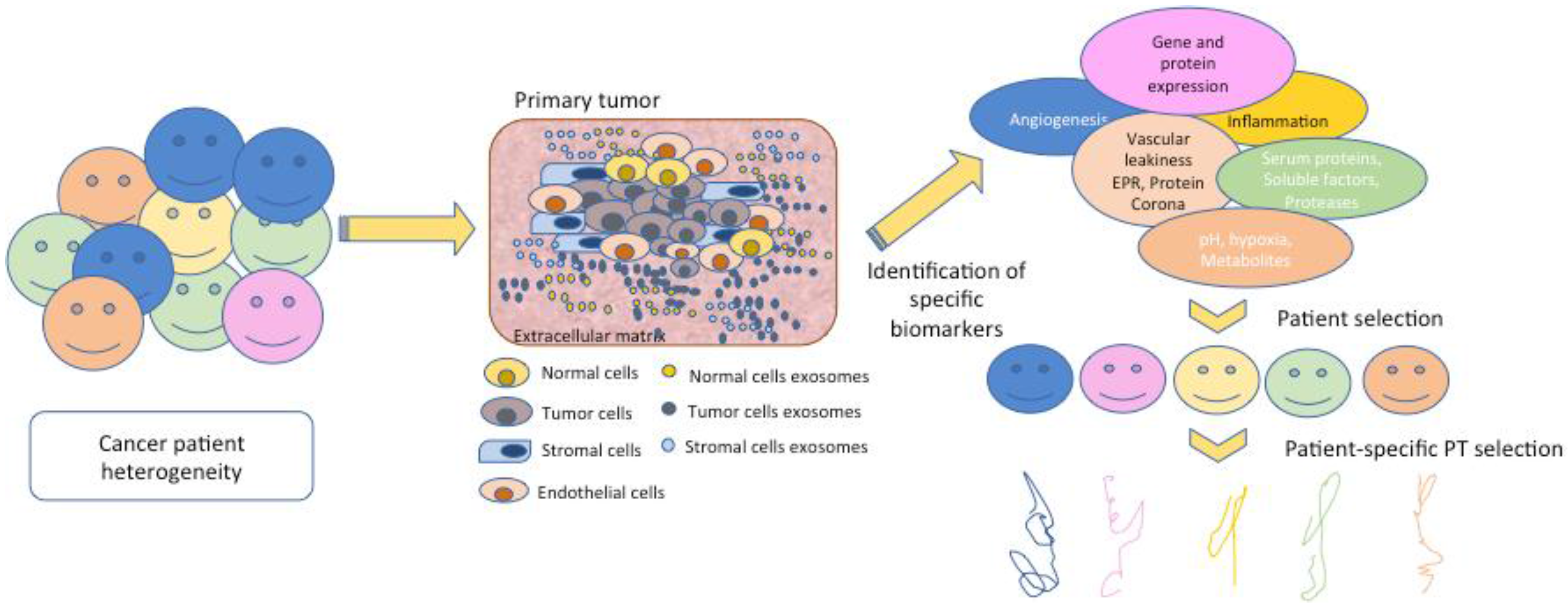
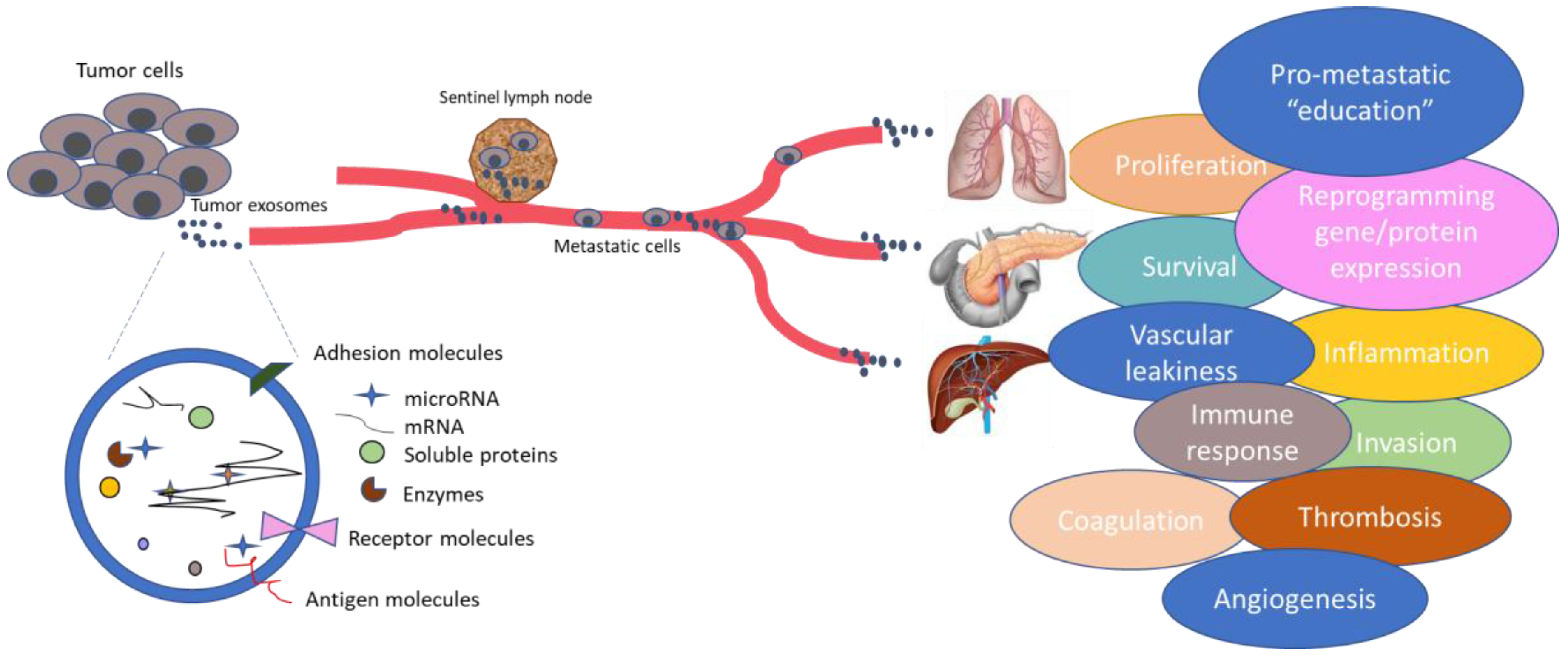
| Hurdle | Biomarker | References |
|---|---|---|
| Bloodstream | Complement Activation | [27,28,29,30,31,32,33,34] |
| Anti-polymer Antibodies | [40,41,42,43,44,45] | |
| Protein Corona/Opsonization | [49,50,51,52,53] | |
| Tumor Targeting | Passive Targeting Parameters | [81,82,83,84,85,86,87] |
| Active Targeting Parameters | [80,104,105] | |
| Tumor Microenvironmental Factors | [113,115,120] | |
| Tumor Uptake | [122,123,128] | |
| Intracellular Release | Enzyme Levels | [134,141,142] |
| pH | [26,153,156,157] | |
| Redox Status | [154,155,159,160,161] | |
| Additional Hurdles | Gene Mutations | [130] |
| Cell Metabolites | [158,175] | |
| Age | [55,56,166] | |
| Sex | [55,56] | |
| Prior PT Treatments | [166] | |
| Health/Comorbidities | [12,54] | |
| Healthy Tissue Toxicity | [167] | |
| Exosomes | [189] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atkinson, S.P.; Andreu, Z.; Vicent, M.J. Polymer Therapeutics: Biomarkers and New Approaches for Personalized Cancer Treatment. J. Pers. Med. 2018, 8, 6. https://doi.org/10.3390/jpm8010006
Atkinson SP, Andreu Z, Vicent MJ. Polymer Therapeutics: Biomarkers and New Approaches for Personalized Cancer Treatment. Journal of Personalized Medicine. 2018; 8(1):6. https://doi.org/10.3390/jpm8010006
Chicago/Turabian StyleAtkinson, Stuart P., Zoraida Andreu, and María J. Vicent. 2018. "Polymer Therapeutics: Biomarkers and New Approaches for Personalized Cancer Treatment" Journal of Personalized Medicine 8, no. 1: 6. https://doi.org/10.3390/jpm8010006
APA StyleAtkinson, S. P., Andreu, Z., & Vicent, M. J. (2018). Polymer Therapeutics: Biomarkers and New Approaches for Personalized Cancer Treatment. Journal of Personalized Medicine, 8(1), 6. https://doi.org/10.3390/jpm8010006




