Metabolic Signature of FLT3-Mutated AML: Clinical and Therapeutic Implications
Abstract
1. Introduction
2. Clinical and Molecular Features of FLT3-Mutated AML
3. Metabolic Dependencies in FLT3-Mutated AML
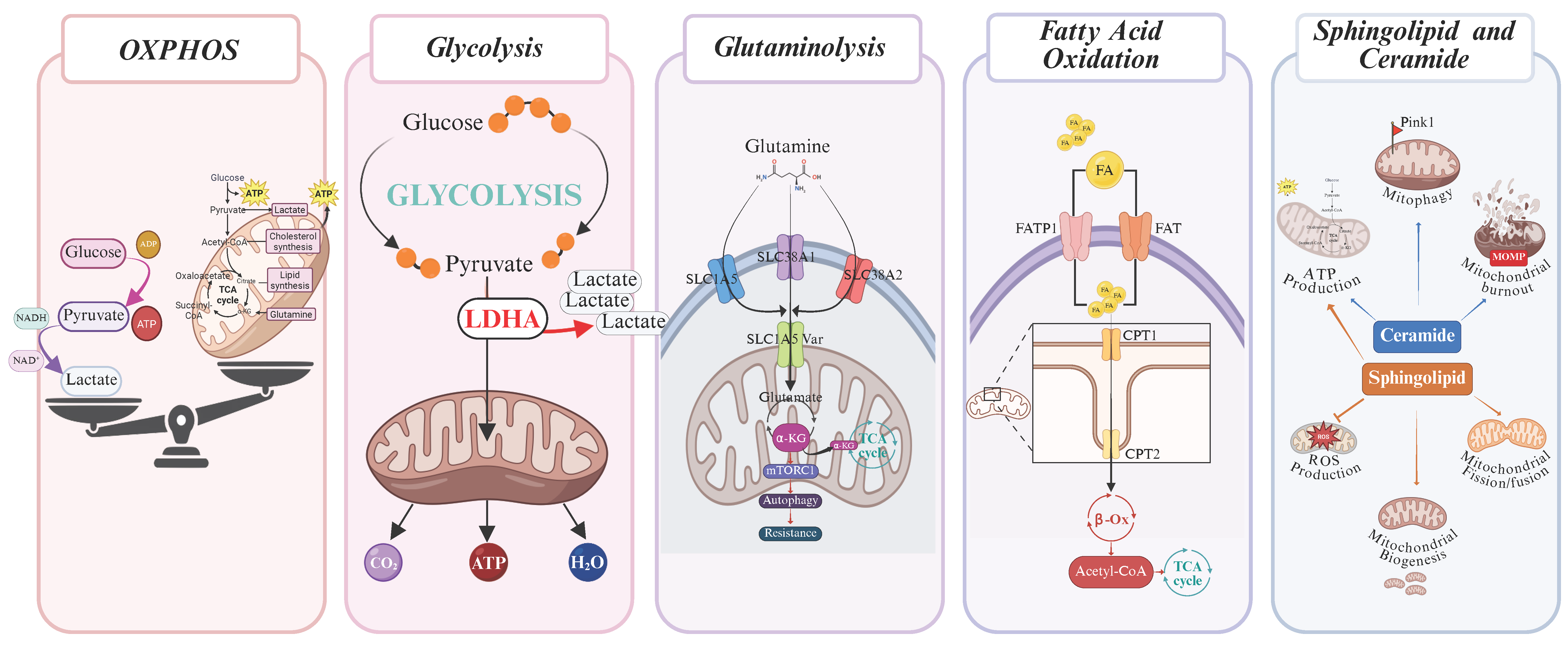
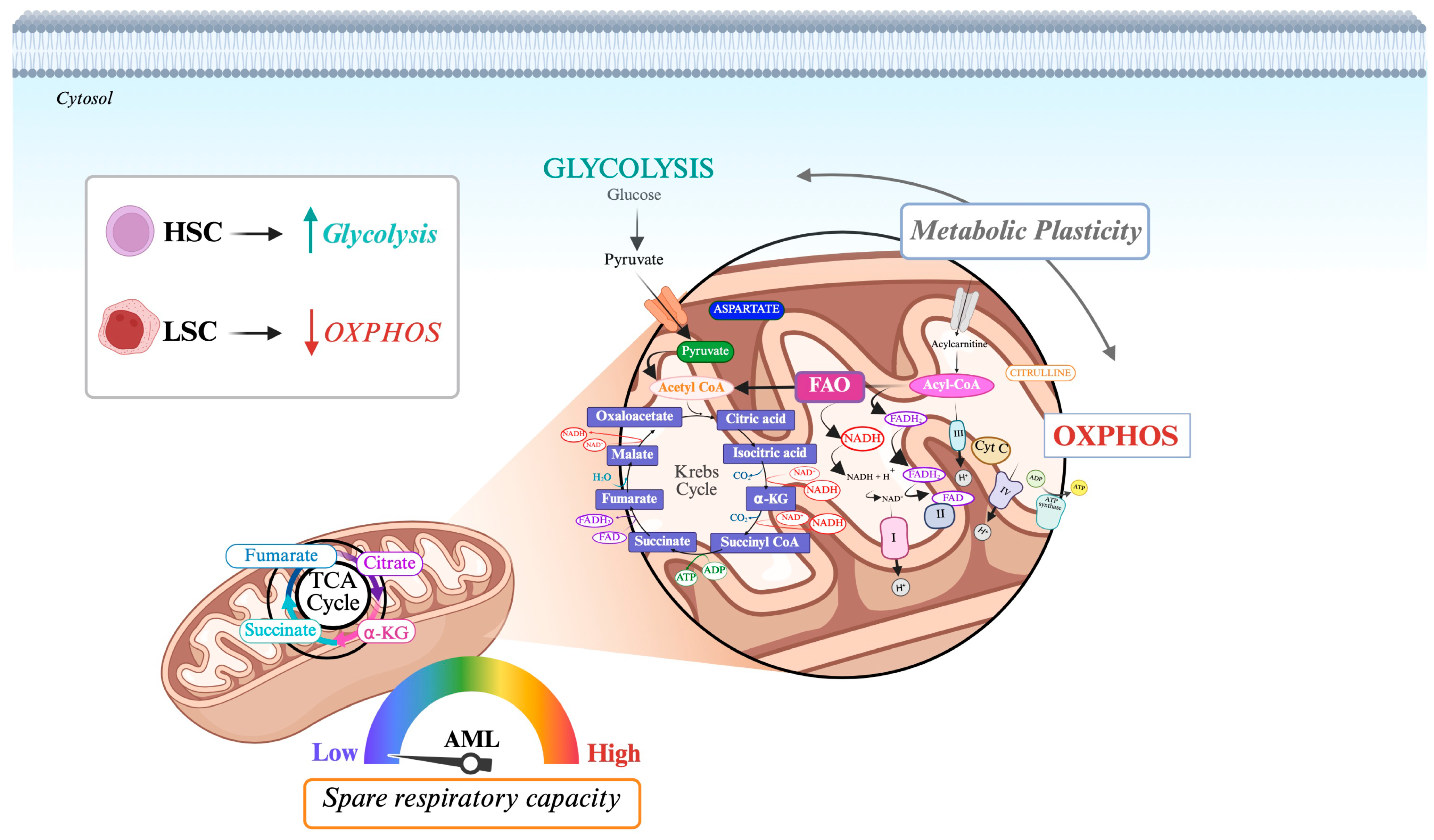
3.1. Oxidative Phosphorylation (OXPHOS)
3.2. Aerobic Glycolysis
3.3. Glutaminolysis
3.4. Fatty Acid Oxidation (FAO)
3.5. Sphingolipid Metabolism and Ceramide
3.6. Anabolic Reprogramming in FLT3-ITD AML
3.7. ROS Dynamics in FLT3-Mutated AML
3.8. Metabolic Targeting: Balancing Antileukemic Efficacy and Normal Cell Function
4. Metabolic Vulnerabilities of FLT3-Mutated AML
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Döhner, H.; Weisdorf, D.J.; Bloomfield, C.D. Acute Myeloid Leukemia. N. Engl. J. Med. 2015, 373, 1136–1152. [Google Scholar] [CrossRef]
- Löwenberg, B.; Ossenkoppele, G.J.; Putten, W.V.; Schouten, H.C.; Graux, C.; Ferrant, A.; Sonneveld, P.; Maertens, J.; Jongen-Lavrencic, M.; von Lilienfeld-Toal, M.; et al. High-Dose Daunorubicin in Older Patients with Acute Myeloid Leukemia. N. Engl. J. Med. 2009, 361, 1235–1248. [Google Scholar] [CrossRef]
- Park, H.J.; Gregory, M.A. Acute Myeloid Leukemia in Elderly Patients: New Targets, New Therapies. Aging Cancer 2023, 4, 51–73. [Google Scholar] [CrossRef]
- Dombret, H.; Gardin, C. An Update of Current Treatments for Adult Acute Myeloid Leukemia. Blood 2016, 127, 53–61. [Google Scholar] [CrossRef]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N.; et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef]
- Shimony, S.; Stahl, M.; Stone, R.M. Acute Myeloid Leukemia: 2025 Update on Diagnosis, Risk-Stratification, and Management. Am. J. Hematol. 2025, 100, 860–891. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Lu, M.; Jung, K.H.; Park, J.H.; Yu, L.; Onuchic, J.N.; Kaipparettu, B.A.; Levine, H. Elucidating Cancer Metabolic Plasticity by Coupling Gene Regulation with Metabolic Pathways. Proc. Natl. Acad. Sci. USA 2019, 116, 3909–3918. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Warburg, O.; Wind, F.; Negelein, E. The Metabolism of Tumors in the Body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, S.; Wang, J.; Guo, X.; Song, Y.; Fu, K.; Gao, Z.; Liu, D.; He, W.; Yang, L.-L. Energy Metabolism in Health and Diseases. Signal Transduct. Target. Ther. 2025, 10, 69. [Google Scholar] [CrossRef]
- Yoshida, G.J. Metabolic Reprogramming: The Emerging Concept and Associated Therapeutic Strategies. J. Exp. Clin. Cancer Res. 2015, 34, 111. [Google Scholar] [CrossRef] [PubMed]
- Takacova, M.; Kajanova, I.; Kolarcikova, M.; Lapinova, J.; Zatovicova, M.; Pastorekova, S. Understanding Metabolic Alterations and Heterogeneity in Cancer Progression through Validated Immunodetection of Key Molecular Com-ponents: A Case of Carbonic Anhydrase IX. Cancer Metastasis Rev. 2021, 40, 1035–1053. [Google Scholar] [CrossRef]
- Banella, C.; Catalano, G.; Travaglini, S.; Pelosi, E.; Ottone, T.; Zaza, A.; Guerrera, G.; Angelini, D.F.; Niscola, P.; Divona, M.; et al. Ascorbate Plus Buformin in AML: A Metabolic Targeted Treatment. Cancers 2022, 14, 2565. [Google Scholar] [CrossRef]
- Catalano, G.; Zaza, A.; Banella, C.; Pelosi, E.; Castelli, G.; Marinis, E.D.; Smigliani, A.; Travaglini, S.; Ottone, T.; Divona, M.; et al. MCL1 Regulates AML Cells Metabolism via Direct Interaction with HK2. Metabolic Signature at Onset Predicts Overall Survival in AMLs’ Patients. Leukemia 2023, 37, 1600–1610. [Google Scholar] [CrossRef]
- Moloney, J.N.; Stanicka, J.; Cotter, T.G. Subcellular Localization of the FLT3-ITD Oncogene Plays a Significant Role in the Production of NOX- and P22phox-Derived Reactive Oxygen Species in Acute Myeloid Leukemia. Leuk. Res. 2017, 52, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, G.; Mengucci, C.; Padella, A.; Fonzi, E.; Picone, G.; Delpino, C.; Nanni, J.; Tommaso, R.D.; Franchini, E.; Papayannidis, C.; et al. Integrated Genomic-Metabolic Classification of Acute Myeloid Leukemia Defines a Sub-group with NPM1 and Cohesin/DNA Damage Mutations. Leukemia 2021, 35, 2813–2826. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.J.; Metallo, C.M. Metabolic Consequences of Oncogenic IDH Mutations. Pharmacol. Ther. 2015, 152, 54–62. [Google Scholar] [CrossRef]
- Nardozza, A.M.; Guarnera, L.; Travaglini, S.; Ottone, T.; Divona, M.; Bellis, E.D.; Savi, A.; Banella, C.; Noguera, N.I.; Di Fusco, D.; et al. Characterization of a Novel IDH2-R159H Mutation in Acute Myeloid Leukaemia: Effects on Cell Metabolism and Differentiation. Br. J. Haematol. 2024, 204, 719–723. [Google Scholar] [CrossRef]
- Banella, C.; Catalano, G.; Travaglini, S.; Divona, M.; Masciarelli, S.; Guerrera, G.; Fazi, F.; Coco, F.L.; Voso, M.T.; Noguera, N. PML/RARa Interferes with NRF2 Transcriptional Activity Increasing the Sensitivity to Ascorbate of Acute Promyelocytic Leukemia Cells. Cancers 2019, 12, 95. [Google Scholar] [CrossRef]
- Travaglini, S.; Silvestrini, G.; Attardi, E.; Fanciulli, M.; Scalera, S.; Antonelli, S.; Maurillo, L.; Palmieri, R.; Divona, M.; Ciuffreda, L.; et al. Evolution of Transcriptomic Profiles in Relapsed Inv(16) Acute Myeloid Leukemia. Leuk. Res. 2024, 145, 107568. [Google Scholar] [CrossRef]
- Bolkun, L.; Pienkowski, T.; Sieminska, J.; Godzien, J.; Pietrowska, K.; Kłoczko, J.; Wierzbowska, A.; Moniuszko, M.; Ratajczak, M.; Kretowski, A.; et al. Metabolomic Profile of Acute Myeloid Leukaemia Parallels of Prognosis and Response to Therapy. Sci. Rep. 2023, 23, 21809. [Google Scholar] [CrossRef]
- Sung, J.-Y.; Yun, W.; Kim, H.-Y.; Kim, H.-J.; Choi, J.R.; Kim, S.-H.; Jung, C.W.; Lee, S.-T. Metabolic Subtype Reveals Potential Therapeutic Vulnerability in Acute Promyelocytic Leukaemia. Clin. Transl. Med. 2022, 12, e964. [Google Scholar] [CrossRef]
- Balasundaram, N.; Ganesan, S.; Chendamarai, E.; Palani, H.K.; Venkatraman, A.; Alex, A.A.; David, S.; Kumar, S.P.; Radhakrishnan, N.R.; Yasar, M.; et al. Metabolic Adaptation Drives Arsenic Trioxide Resistance in Acute Promyelocytic Leukemia. Blood Adv. 2022, 6, 652–663. [Google Scholar] [CrossRef]
- Ferret, Y.; Boissel, N.; Helevaut, N.; Madic, J.; Nibourel, O.; Marceau-Renaut, A.; Bucci, M.; Geffroy, S.; Celli-Lebras, K.; Castaigne, S.; et al. Clinical Relevance of IDH1/2 Mutant Allele Burden during Follow-up in Acute Myeloid Leukemia. A Study by the French ALFA Group. Haematologica 2018, 103, 822–829. [Google Scholar] [CrossRef]
- Chen, W.-L.; Wang, J.-H.; Zhao, A.-H.; Xu, X.; Wang, Y.-H.; Chen, T.-L.; Li, J.-M.; Mi, J.-Q.; Zhu, Y.-M.; Liu, Y.-F.; et al. A Distinct Glucose Metabolism Signature of Acute Myeloid Leukemia with Prognostic Value. Blood 2014, 124, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Larrue, C.; Saland, E.; Vergez, F.; Serhan, N.; Delabesse, E.; Mas, V.M.-D.; Hospital, M.-A.; Tamburini, J.; Manenti, S.; Sarry, J.E.; et al. Antileukemic Activity of 2-Deoxy-d-Glucose through Inhibition of N-Linked Glycosylation in Acute Myeloid Leukemia with FLT3-ITD or c-KIT Mutations. Mol. Cancer Ther. 2015, 14, 2364–2373. [Google Scholar] [CrossRef] [PubMed]
- Jacque, N.; Ronchetti, A.M.; Larrue, C.; Meunier, G.; Birsen, R.; Willems, L.; Saland, E.; Decroocq, J.; Maciel, T.T.; Lambert, M.; et al. Targeting Glutaminolysis Has Antileukemic Activity in Acute Myeloid Leukemia and Synergizes with BCL-2 Inhibition. Blood 2015, 126, 1346–1356. [Google Scholar] [CrossRef]
- Liyanage, S.U.; Hurren, R.; Voisin, V.; Bridon, G.; Wang, X.; Xu, C.; MacLean, N.; Siriwardena, T.P.; Gronda, M.; Yehudai, D.; et al. Leveraging Increased Cytoplasmic Nucleoside Kinase Activity to Target MtDNA and Oxidative Phosphorylation in AML. Blood 2017, 129, 2657–2666. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, M.R.; Mirabilii, S.; Allegretti, M.; Licchetta, R.; Calarco, A.; Torrisi, M.R.; Foà, R.; Nicolai, R.; Peluso, G.; Tafuri, A. Targeting the Leukemia Cell Metabolism by the CPT1a Inhibition: Functional Preclinical Effects in Leukemias. Blood 2015, 126, 1925–1929. [Google Scholar] [CrossRef]
- Cai, T.; Lorenzi, P.L.; Rakheja, D.; Pontikos, M.A.; Lodi, A.; Han, L.; Zhang, Q.; Ma, H.; Rahmani, M.; Bhagat, T.D.; et al. Gls Inhibitor CB-839 Modulates Cellular Metabolism in AML and Potently Suppresses AML Cell Growth When Combined with 5-Azacitidine. Blood 2016, 128, 4064. [Google Scholar] [CrossRef]
- Kiyoi, H.; Naoe, T. Biology, Clinical Relevance, and Molecularly Targeted Therapy in Acute Leukemia with FLT3 Mutation. Int. J. Hematol. 2006, 83, 301–308. [Google Scholar] [CrossRef]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and Management of AML in Adults: 2022 Recommendations from an International Expert Panel on Behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- Bruno, S.; Bandini, L.; Patuelli, A.; Robustelli, V.; Venturi, C.; Mancini, M.; Forte, D.; Santis, S.D.; Monaldi, C.; Grassi, A.; et al. Case Report: A Novel Activating FLT3 Mutation in Acute Myeloid Leukemia. Front. Oncol. 2021, 11, 728613. [Google Scholar] [CrossRef]
- Travaglini, S.; Gurnari, C.; Antonelli, S.; Marchesi, F.; Angelis, G.D.; Ottone, T.; Divona, M.; Cristiano, A.; Hajrullaj, H.; Mengarelli, A.; et al. Functional Characterization and Response to FLT3 Inhibitors in Acute Myeloid Leukaemia with a Non-Canonical FLT3 Mutation: A Proof of Concept. Br. J. Haematol. 2023, 203, 327–330. [Google Scholar] [CrossRef]
- Kennedy, V.E.; Smith, C.C. FLT3 Targeting in the Modern Era: From Clonal Selection to Combination Therapies. Int. J. Hematol. 2023, 120, 528–540. [Google Scholar] [CrossRef]
- Ruglioni, M.; Crucitta, S.; Luculli, G.I.; Tancredi, G.; Del Giudice, M.L.; Mechelli, S.; Galimberti, S.; Danesi, R.; Del Re, M. Understanding Mechanisms of Resistance to FLT3 Inhibitors in Adult FLT3-Mutated Acute Myeloid Leukemia to Guide Treatment Strategy. Crit. Rev. Oncol. Hematol. 2024, 201, 104424. [Google Scholar] [CrossRef]
- Travaglini, S.; Gurnari, C.; Ottone, T.; Voso, M.T. Advances in the Pathogenesis of FLT3 -Mutated Acute Myeloid Leukemia and Targeted Treatments. Curr. Opin. Oncol. 2024, 36, 569–576. [Google Scholar] [CrossRef]
- Maryanovich, M.; Zaltsman, Y.; Ruggiero, A.; Goldman, A.; Shachnai, L.; Zaidman, S.L.; Porat, Z.; Golan, K.; Lapidot, T.; Gross, A. An MTCH2 Pathway Repressing Mitochondria Metabolism Regulates Haematopoietic Stem Cell Fate. Nat. Commun. 2015, 6, 7901. [Google Scholar] [CrossRef] [PubMed]
- Folmes, C.D.L.; Dzeja, P.P.; Nelson, T.J.; Terzic, A. Metabolic Plasticity in Stem Cell Homeostasis and Differentiation. Cell Stem Cell 2012, 11, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Suda, T. Metabolic Requirements for the Maintenance of Self-Renewing Stem Cells. Nat. Rev. Mol. Cell Biol. 2014, 15, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Amarachintha, S.; Wilson, A.F.; Pang, Q. SCO2 Mediates Oxidative Stress-Induced Glycolysis to Oxidative Phosphorylation Switch in Hematopoietic Stem Cells. Stem Cells 2016, 34, 960–971. [Google Scholar] [CrossRef]
- Ito, K.; Bonora, M.; Ito, K. Metabolism as Master of Hematopoietic Stem Cell Fate. Int. J. Hematol. 2019, 109, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Maiso, P.; Huynh, D.; Moschetta, M.; Sacco, A.; Aljawai, Y.; Mishima, Y.; Asara, J.M.; Roccaro, A.M.; Kimmelman, A.C.; Ghobrial, I.M. Metabolic Signature Identifies Novel Targets for Drug Resistance in Multiple Myeloma. Cancer Res. 2015, 75, 2071–2082. [Google Scholar] [CrossRef] [PubMed]
- Herst, P.M.; Howman, R.A.; Neeson, P.J.; Berridge, M.V.; Ritchie, D.S. The Level of Glycolytic Metabolism in Acute Myeloid Leukemia Blasts at Diagnosis Is Prognostic for Clinical Outcome. J. Leukoc. Biol. 2010, 89, 51–55. [Google Scholar] [CrossRef]
- Kominsky, D.J.; Klawitter, J.; Brown, J.L.; Boros, L.G.; Melo, J.V.; Eckhardt, S.G.; Serkova, N.J. Abnormalities in Glucose Uptake and Metabolism in Imatinib-Resistant Human BCR-ABL–Positive Cells. Clin. Cancer Res. 2009, 15, 3442–3450. [Google Scholar] [CrossRef] [PubMed]
- Lagadinou, E.D.; Sach, A.; Callahan, K.; Rossi, R.M.; Neering, S.J.; Minhajuddin, M.; Ashton, J.M.; Pei, S.; Grose, V.; O’Dwyer, K.M.; et al. BCL-2 Inhibition Targets Oxidative Phosphorylation and Selectively Eradicates Quiescent Human Leukemia Stem Cells. Cell Stem Cell 2013, 12, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Yi, Q.; Xu, B.; Li, S.; Wang, T.; Liu, F.; Zhu, B.; Hoffmann, P.R.; Ji, G.; Lei, P.; et al. ORP4L Is Essential for T-Cell Acute Lymphoblastic Leukemia Cell Survival. Nat. Commun. 2016, 7, 12702. [Google Scholar] [CrossRef]
- Boag, J.M.; Beesley, A.H.; Firth, M.J.; Freitas, J.R.; Ford, J.; Hoffmann, K.; Cummings, A.J.; Klerk, N.H.D.; Kees, U.R. Altered Glucose Metabolism in Childhood Pre-B Acute Lymphoblastic Leukaemia. Leukemia 2006, 20, 1731–1737. [Google Scholar] [CrossRef]
- Spinelli, J.B.; Haigis, M.C. The Multifaceted Contributions of Mitochondria to Cellular Metabolism. Nat. Cell Biol. 2018, 20, 745–754. [Google Scholar] [CrossRef]
- Tjahjono, E.; Daneman, M.R.; Meika, B.; Revtovich, A.V.; Kirienko, N.V. Mitochondrial Abnormalities as a Target of Intervention in Acute Myeloid Leukemia. Front. Oncol. 2024, 14, 1532857. [Google Scholar] [CrossRef]
- Morganti, C.; Bonora, M.; Ito, K. Metabolism and HSC Fate: What NADPH Is Made For. Trends Cell Biol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Schönberger, K.; Cabezas-Wallscheid, N. Bidirectional Interplay between Metabolism and Epigenetics in Hematopoietic Stem Cells and Leukemia. EMBO J. 2023, 42, e112348. [Google Scholar] [CrossRef]
- Tavor, S.; Petit, I.; Porozov, S.; Avigdor, A.; Dar, A.; Leider-Trejo, L.; Shemtov, N.; Deutsch, V.; Naparstek, E.; Nagler, A.; et al. CXCR4 Regulates Migration and Development of Human Acute Myelogenous Leukemia Stem Cells in Transplanted NOD/SCID Mice. Cancer Res. 2004, 64, 2817–2824. [Google Scholar] [CrossRef] [PubMed]
- Möhle, R.; Bautz, F.; Rafii, S.; Moore, M.A.; Brugger, W.; Kanz, L. The Chemokine Receptor CXCR-4 Is Expressed on CD34+ Hematopoietic Progenitors and Leukemic Cells and Mediates Transendothelial Migration Induced by Stromal Cell-Derived Factor-1. Blood 1998, 91, 4523–4530. [Google Scholar] [CrossRef]
- Jin, L.; Hope, K.J.; Zhai, Q.; Smadja-Joffe, F.; Dick, J.E. Targeting of CD44 Eradicates Human Acute Myeloid Leukemic Stem Cells. Nat. Med. 2006, 12, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Chipuk, J.E.; Bouchier-Hayes, L.; Green, D.R. Mitochondrial Outer Membrane Permeabilization during Apoptosis: The Innocent Bystander Scenario. Cell Death Differ. 2006, 13, 1396–1402. [Google Scholar] [CrossRef]
- Sriskanthadevan, S.; Jeyaraju, D.V.; Chung, T.E.; Prabha, S.; Xu, W.; Skrtic, M.; Jhas, B.; Hurren, R.; Gronda, M.; Wang, X.; et al. AML Cells Have Low Spare Reserve Capacity in Their Respiratory Chain That Renders Them Susceptible to Oxidative Metabolic Stress. Blood 2015, 125, 2120–2130. [Google Scholar] [CrossRef]
- Zhang, P.; Brinton, L.T.; Gharghabi, M.; Sher, S.; Williams, K.; Cannon, M.; Walker, J.S.; Canfield, D.; Beaver, L.; Cempre, C.B.; et al. Targeting OXPHOS de Novo Purine Synthesis as the Nexus of FLT3 Inhibitor-Mediated Synergistic Antileukemic Actions. Sci. Adv. 2022, 8, eabp9005. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Ganapathy-Kanniappan, S.; Kunjithapatham, R.; Geschwind, J.-F.H. Statins Impair Glucose Uptake in Tumor Cells. Cancer Biol. Ther. 2013, 14, 92–94. [Google Scholar] [CrossRef]
- Huang, I.N.; Melvin, J.N. Effects of Ratio Reinforcement Schedules on Choice Behavior. J. Gen. Psychol. 1990, 117, 99–106. [Google Scholar] [CrossRef]
- Tang, Z.; Yuan, S.; Hu, Y.; Zhang, H.; Wu, W.; Zeng, Z.; Yang, J.; Yun, J.; Xu, R.; Huang, P. Over-Expression of GAPDH in Human Colorectal Carcinoma as a Preferred Target of 3-Bromopyruvate Propyl Ester. J. Bioenerg. Biomembr. 2012, 44, 117–125. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Y.; Shingu, T.; Feng, L.; Chen, Z.; Ogasawara, M.; Keating, M.J.; Kondo, S.; Huang, P. Metabolic Alterations in Highly Tumorigenic Glioblastoma Cells: Preference for Hypoxia and High Dependency on Glycolysis. J. Biol. Chem. 2011, 286, 32843–32853. [Google Scholar] [CrossRef] [PubMed]
- Geschwind, J.-F.; Georgiades, C.S.; Ko, Y.H.; Pedersen, P.L. Recently Elucidated Energy Catabolism Pathways Provide Opportunities for Novel Treatments in Hepatocellular Carcinoma. Expert. Rev. Anticancer. Ther. 2004, 4, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does It Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Li, M.; Xu, X.; Xuan, L.; Huang, G.; Liu, Q. Resistance to Chemotherapy Is Associated with Altered Glucose Metabolism in Acute Myeloid Leukemia. Oncol. Lett. 2016, 12, 334–342. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Israelsen, W.J.; Lee, D.; Yu, V.W.C.; Jeanson, N.T.; Clish, C.B.; Cantley, L.C.; Vander Heiden, M.G.; Scadden, D.T. Cell-State-Specific Metabolic Dependency in Hematopoiesis and Leukemogenesis. Cell 2014, 158, 1309–1323. [Google Scholar] [CrossRef]
- Ju, H.-Q.; Zhan, G.; Huang, A.; Sun, Y.; Wen, S.; Yang, J.; Lu, W.; Xu, R.; Li, J.; Li, Y.; et al. ITD Mutation in FLT3 Tyrosine Kinase Promotes Warburg Effect and Renders Therapeutic Sensitivity to Glycolytic Inhibition. Leukemia 2017, 31, 2143–2150. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Bounds, P.L.; Dang, C. V Otto Warburg’s Contributions to Current Concepts of Cancer Metabolism. Nat. Rev. Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. We Need to Talk about the Warburg Effect. Nat. Metab. 2020, 2, 127–129. [Google Scholar] [CrossRef]
- Erdem, A.; Marin, S.; Pereira-Martins, D.A.; Cortés, R.; Cunningham, A.; Pruis, M.G.; Boer, B.D.; Heuvel, F.A.J.V.D.; Geugien, M.; Wierenga, A.T.J.; et al. The Glycolytic Gatekeeper PDK1 Defines Different Metabolic States between Genetically Distinct Subtypes of Human Acute Myeloid Leukemia. Nat. Commun. 2022, 13, 1105. [Google Scholar] [CrossRef]
- Peng, C.-J.; Fan, Z.; Luo, J.-S.; Wang, L.-N.; Li, Y.; Liang, C.; Zhang, X.-L.; Luo, X.-Q.; Huang, L.-B.; Tang, Y.-L. The Potential Transcriptomic and Metabolomic Mechanisms of ATO and ATRA in Treatment of FLT3-ITD Acute Myeloid Leukemia. Technol. Cancer Res. Treat. 2024, 23, 15330338231223080. [Google Scholar] [CrossRef]
- Huang, A.; Ju, H.-Q.; Liu, K.; Zhan, G.; Liu, D.; Wen, S.; Garcia-Manero, G.; Huang, P.; Hu, Y. Metabolic Alterations and Drug Sensitivity of Tyrosine Kinase Inhibitor Resistant Leukemia Cells with a FLT3/ITD Mutation. Cancer Lett. 2016, 377, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Soltani, M.; Zhao, Y.; Xia, Z.; Ganjalikhani Hakemi, M.; Bazhin, A.V. The Importance of Cellular Metabolic Pathways in Pathogenesis and Selective Treatments of Hematological Malignancies. Front. Oncol. 2021, 11, 767026. [Google Scholar] [CrossRef]
- Vettore, L.; Westbrook, R.L.; Tennant, D.A. New Aspects of Amino Acid Metabolism in Cancer. Br. J. Cancer 2020, 122, 150–156. [Google Scholar] [CrossRef]
- Cormerais, Y.; Massard, P.A.; Vucetic, M.; Giuliano, S.; Tambutté, E.; Durivault, J.; Vial, V.; Endou, H.; Wempe, M.F.; Parks, S.K.; et al. The Glutamine Transporter ASCT2 (SLC1A5) Promotes Tumor Growth Independently of the Amino Acid Transporter LAT1 (SLC7A5). J. Biol. Chem. 2018, 293, 2877–2887. [Google Scholar] [CrossRef]
- Willems, L.; Jacque, N.; Jacquel, A.; Neveux, N.; Trovati Maciel, T.; Lambert, M.; Schmitt, A.; Poulain, L.; Green, A.S.; Uzunov, M.; et al. Inhibiting Glutamine Uptake Represents an Attractive New Strategy for Treating Acute Myeloid Leukemia. Blood 2013, 122, 3521–3532. [Google Scholar] [CrossRef]
- Nicklin, P.; Bergman, P.; Zhang, B.; Triantafellow, E.; Wang, H.; Nyfeler, B.; Yang, H.; Hild, M.; Kung, C.; Wilson, C.; et al. Bidirectional Transport of Amino Acids Regulates MTOR and Autophagy. Cell 2009, 136, 521–534. [Google Scholar] [CrossRef]
- Khamari, R.; Degand, C.; Fovez, Q.; Trinh, A.; Chomy, A.; Laine, W.; Dekiouk, S.; Ghesquiere, B.; Quesnel, B.; Marchetti, P.; et al. Key Role of Glutamine Metabolism in Persistence of Leukemic Cells upon Exposition to FLT3 Tyrosine Kinase Inhibitors. Exp. Hematol. 2024, 137, 104253. [Google Scholar] [CrossRef] [PubMed]
- Alshamleh, I.; Kurrle, N.; Makowka, P.; Bhayadia, R.; Kumar, R.; Süsser, S.; Seibert, M.; Ludig, D.; Wolf, S.; Koschade, S.E.; et al. PDP1 Is a Key Metabolic Gatekeeper and Modulator of Drug Resistance in FLT3-ITD-Positive Acute Myeloid Leukemia. Leukemia 2023, 37, 2367–2382. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.S.; Frankfurt, O.; Orford, K.W.; Bennett, M.; Flinn, I.W.; Maris, M.; Konopleva, M. Phase 1 Study of CB-839, a First-in-Class, Orally Administered Small Molecule Inhibitor of Glutaminase in Patients with Relapsed/Refractory Leukemia. Blood 2015, 126, 2566. [Google Scholar] [CrossRef]
- Firmanty, P.; Chomczyk, M.; Dash, S.; Konopleva, M.; Baran, N. Feasibility and Safety of Targeting Mitochondria Function and Metabolism in Acute Myeloid Leukemia. Curr. Pharmacol. Rep. 2024, 10, 388–404. [Google Scholar] [CrossRef]
- Harding, J.J.; Telli, M.L.; Munster, P.N.; Le, M.H.; Molineaux, C.; Bennett, M.K.; Mittra, E.; Burris, H.A.; Clark, A.S.; Dunphy, M.; et al. Safety and Tolerability of Increasing Doses of CB-839, a First-in-Class, Orally Administered Small Molecule Inhibitor of Glutaminase, in Solid Tumors. J. Clin. Oncol. 2015, 33, 2512. [Google Scholar] [CrossRef]
- Maher, M.; Diesch, J.; Casquero, R.; Buschbeck, M. Epigenetic-Transcriptional Regulation of Fatty Acid Metabolism and Its Alterations in Leukaemia. Front. Genet. 2018, 9, 405. [Google Scholar] [CrossRef]
- Snaebjornsson, M.T.; Janaki-Raman, S.; Schulze, A. Greasing the Wheels of the Cancer Machine: The Role of Lipid Metabolism in Cancer. Cell Metab. 2020, 31, 62–76. [Google Scholar] [CrossRef]
- Balko, J.M.; Schwarz, L.J.; Luo, N.; Estrada, M.V.; Giltnane, J.M.; Dávila-González, D.; Wang, K.; Sánchez, V.; Dean, P.T.; Combs, S.E.; et al. Triple-Negative Breast Cancers with Amplification of JAK2 at the 9p24 Locus Demonstrate JAK2-Specific Dependence. Sci. Transl. Med. 2016, 8, 334ra53. [Google Scholar] [CrossRef]
- Samudio, I.; Konopleva, M. Targeting Leukemia’s “Fatty Tooth”. Blood 2015, 126, 1874–1875. [Google Scholar] [CrossRef]
- Pabst, T.; Kortz, L.; Fiedler, G.M.; Ceglarek, U.; Idle, J.R.; Beyoğlu, D. The Plasma Lipidome in Acute Myeloid Leukemia at Diagnosis in Relation to Clinical Disease Features. BBA Clin. 2017, 7, 105–114. [Google Scholar] [CrossRef]
- Mesbahi, Y.; Trahair, T.N.; Lock, R.B.; Connerty, P. Exploring the Metabolic Landscape of AML: From Haematopoietic Stem Cells to Myeloblasts and Leukaemic Stem Cells. Front. Oncol. 2022, 12, 807266. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, D.; Schroeder, T. Symmetric and Asymmetric Activation of Hematopoietic Stem Cells. Curr. Opin. Hematol. 2021, 28, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Carracedo, A.; Weiss, D.; Arai, F.; Ala, U.; Avigan, D.E.; Schafer, Z.T.; Evans, R.M.; Suda, T.; Lee, C.-H.; et al. A PML–PPAR-δ Pathway for Fatty Acid Oxidation Regulates Hematopoietic Stem Cell Maintenance. Nat. Med. 2012, 18, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Samudio, I.; Harmancey, R.; Fiegl, M.; Kantarjian, H.; Konopleva, M.; Korchin, B.; Kaluarachchi, K.; Bornmann, W.; Duvvuri, S.; Taegtmeyer, H.; et al. Pharmacologic Inhibition of Fatty Acid Oxidation Sensitizes Human Leukemia Cells to Apoptosis Induction. J. Clin. Investig. 2010, 120, 142–156. [Google Scholar] [CrossRef]
- Bonnefont, J.-P.; Bastin, J.; Behin, A.; Djouadi, F. Bezafibrate for an Inborn Mitochondrial Beta-Oxidation Defect. N. Engl. J. Med. 2009, 360, 838–840. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Li, H.; Purevsuren, J.; Yamada, K.; Furui, M.; Takahashi, T.; Mushimoto, Y.; Kobayashi, H.; Hasegawa, Y.; Taketani, T.; et al. Bezafibrate Can Be a New Treatment Option for Mitochondrial Fatty Acid Oxidation Disorders: Evaluation by in Vitro Probe Acylcarnitine Assay. Mol. Genet. Metab. 2012, 107, 87–91. [Google Scholar] [CrossRef]
- Ogretmen, B. Sphingolipid Metabolism in Cancer Signalling and Therapy. Nat. Rev. Cancer 2018, 18, 33–50. [Google Scholar] [CrossRef]
- Schömel, N.; Geisslinger, G.; Wegner, M.-S. Influence of Glycosphingolipids on Cancer Cell Energy Metabolism. Prog. Lipid Res. 2020, 79, 101050. [Google Scholar] [CrossRef]
- Canals, D.; Clarke, C.J. Compartmentalization of Sphingolipid Metabolism: Implications for Signaling and Therapy. Pharmacol. Ther. 2022, 232, 108005. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.A.; Lewis, A.C.; Zhu, W.; Toubia, J.; Pitman, M.R.; Wallington-Beddoe, C.T.; Moretti, P.A.B.; Iarossi, D.; Samaraweera, S.E.; Cummings, N.; et al. Targeting Sphingosine Kinase 1 Induces MCL1-Dependent Cell Death in Acute Myeloid Leukemia. Blood 2017, 129, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.Z.; Kaufmann, K.B.; Wang, W.; Chan-Seng-Yue, M.; Gan, O.I.; Laurenti, E.; Garcia-Prat, L.; Takayanagi, S.; Ng, S.W.K.; Xu, C.; et al. Sphingosine-1-Phosphate Receptor 3 Potentiates Inflammatory Programs in Normal and Leukemia Stem Cells to Promote Differentiation. Blood Cancer Discov. 2021, 2, 32–53. [Google Scholar] [CrossRef]
- Mendoza, A.E.-H.D.; Castello-Cros, R.; Imbuluzqueta, E.; Cirauqui, C.; Pippa, R.; Odero, M.D.; Blanco-Prieto, M.J. Lipid Nanosystems Enhance the Bioavailability and the Therapeutic Efficacy of FTY720 in Acute Myeloid Leukemia. J. Biomed. Nanotechnol. 2015, 11, 691–701. [Google Scholar] [CrossRef]
- Cristóbal, I.; Garcia-Orti, L.; Cirauqui, C.; Alonso, M.M.; Calasanz, M.J.; Odero, M.D. PP2A Impaired Activity Is a Common Event in Acute Myeloid Leukemia and Its Activation by Forskolin Has a Potent Anti-Leukemic Effect. Leukemia 2011, 25, 606–614. [Google Scholar] [CrossRef]
- Arriazu, E.; Vicente, C.; Pippa, R.; Peris, I.; Martínez-Balsalobre, E.; García-Ramírez, P.; Marcotegui, N.; Igea, A.; Alignani, D.; Rifón, J.; et al. A New Regulatory Mechanism of Protein Phosphatase 2A Activity via SET in Acute Myeloid Leukemia. Blood Cancer J. 2020, 10, 3. [Google Scholar] [CrossRef]
- Bai, A.-P.; Guo, Y. Ceramide Is a Potential Activator of Immune Responses Against Tumors. Gastroenterology 2018, 155, 579–580. [Google Scholar] [CrossRef]
- Morad, S.A.F.; Cabot, M.C. Ceramide-Orchestrated Signalling in Cancer Cells. Nat. Rev. Cancer 2013, 13, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Maceyka, M.; Harikumar, K.B.; Milstien, S.; Spiegel, S. Sphingosine-1-Phosphate Signaling and Its Role in Disease. Trends Cell Biol. 2012, 22, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, K.; Hanada, K. Structure, Functions and Regulation of CERT, a Lipid-transfer Protein for the Delivery of Ceramide at the ER—Golgi Membrane Contact Sites. FEBS Lett. 2019, 593, 2366–2377. [Google Scholar] [CrossRef]
- Dany, M.; Gencer, S.; Nganga, R.; Thomas, R.J.; Oleinik, N.; Baron, K.D.; Szulc, Z.M.; Ruvolo, P.; Kornblau, S.; Andreeff, M.; et al. Targeting FLT3-ITD Signaling Mediates Ceramide-Dependent Mitophagy and Attenuates Drug Resistance in AML. Blood 2016, 128, 1944–1958. [Google Scholar] [CrossRef]
- Lewis, A.C.; Pope, V.S.; Tea, M.N.; Li, M.; Nwosu, G.O.; Nguyen, T.M.; Wallington-Beddoe, C.T.; Moretti, P.A.B.; Anderson, D.; Creek, D.J.; et al. Ceramide-Induced Integrated Stress Response Overcomes Bcl-2 Inhibitor Resistance in Acute Myeloid Leukemia. Blood 2022, 139, 3737–3751. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.; Joyce, P.; Ross, D.M.; Bremmell, K.; Jambhrunkar, M.; Wong, S.S.; Prestidge, C.A. Combating Acute Myeloid Leukemia via Sphingosine Kinase 1 Inhibitor-Nanomedicine Combination Therapy with Cytarabine or Venetoclax. Pharmaceutics 2024, 16, 209. [Google Scholar] [CrossRef]
- Sun, X.; Li, Y.; Du, J.; Liu, F.; Wu, C.; Xiao, W.; Yu, G.; Chen, X.; Gale, R.P.; Zeng, H. Targeting Ceramide Transfer Protein Sensitizes AML to FLT3 Inhibitors via a GRP78-ATF6-CHOP Axis. Nat. Commun. 2025, 16, 1358. [Google Scholar] [CrossRef]
- Swanton, C.; Marani, M.; Pardo, O.; Warne, P.H.; Kelly, G.; Sahai, E.; Elustondo, F.; Chang, J.; Temple, J.; Ahmed, A.A.; et al. Regulators of Mitotic Arrest and Ceramide Metabolism Are Determinants of Sensitivity to Paclitaxel and Other Chemotherapeutic Drugs. Cancer Cell 2007, 11, 498–512. [Google Scholar] [CrossRef]
- Chung, L.H.; Liu, D.; Liu, X.T.; Qi, Y. Ceramide Transfer Protein (CERT): An Overlooked Molecular Player in Cancer. Int. J. Mol. Sci. 2021, 22, 13184. [Google Scholar] [CrossRef]
- Sabatier, M.; Birsen, R.; Lauture, L.; Mouche, S.; Angelino, P.; Dehairs, J.; Goupille, L.; Boussaid, I.; Heiblig, M.; Boet, E.; et al. C/EBPα Confers Dependence to Fatty Acid Anabolic Pathways and Vulnerability to Lipid Oxidative Stress–Induced Ferroptosis in FLT3 -Mutant Leukemia. Cancer Discov. 2023, 13, 1720–1747. [Google Scholar] [CrossRef]
- Chang, H.-H.; Lee, L.-C.; Hsu, T.; Peng, Y.-H.; Huang, C.-H.; Yeh, T.-K.; Lu, C.-T.; Huang, Z.-T.; Hsueh, C.-C.; Kung, F.-C.; et al. Development of Potent and Selective Inhibitors of Methylenetetrahydrofolate Dehydrogenase 2 for Targeting Acute Myeloid Leukemia: SAR, Structural Insights, and Biological Characterization. J. Med. Chem. 2024, 67, 21106–21125. [Google Scholar] [CrossRef]
- Pikman, Y.; Puissant, A.; Alexe, G.; Furman, A.; Chen, L.M.; Frumm, S.M.; Ross, L.; Fenouille, N.; Bassil, C.F.; Lewis, C.A.; et al. Targeting MTHFD2 in Acute Myeloid Leukemia. J. Exp. Med. 2016, 213, 1285–1306. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, V.; Fultang, L.; Booth, S.; Simone, D.D.; Bartnik, A.; Scarpa, U.; Gneo, L.; Panetti, S.; Potluri, S.; Almowaled, M.; et al. Invariant NKT Cells Metabolically Adapt to the Acute Myeloid Leukaemia Environment. Cancer Immunol. Immunother. 2023, 72, 543–560. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Li, M.; Liu, Z.; Tiessen, J.; Li, Y.; Zhou, J.; Zhu, Y.; Mahesula, S.; Ding, Q.; Tan, L.; et al. Guanine Nucleotide Biosynthesis Blockade Impairs MLL Complex Formation and Sensitizes Leukemias to Menin Inhibition. Nat. Commun. 2025, 16, 2641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Velasco-Hernandez, T.; Mess, J.; Lalioti, M.-E.; Romero-Mulero, M.C.; Obier, N.; Karantzelis, N.; Rettkowski, J.; Schönberger, K.; Karabacz, N.; et al. GPRC5C Drives Branched-Chain Amino Acid Metabolism in Leukemogenesis. Blood Adv. 2023, 7, 7525–7538. [Google Scholar] [CrossRef]
- Baranello, M.P.; Bauer, L.; Jordan, C.T.; Benoit, D.S.W. Micelle Delivery of Parthenolide to Acute Myeloid Leukemia Cells. Cell Mol. Bioeng. 2015, 8, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.L.; Inguva, A.; Jordan, C.T. Targeting Energy Metabolism in Cancer Stem Cells: Progress and Challenges in Leukemia and Solid Tumors. Cell Stem Cell 2021, 28, 378–393. [Google Scholar] [CrossRef]
- Latini, S.; Venafra, V.; Massacci, G.; Bica, V.; Graziosi, S.; Pugliese, G.M.; Iannuccelli, M.; Frioni, F.; Minnella, G.; Marra, J.D.; et al. Unveiling the Signaling Network of FLT3-ITD AML Improves Drug Sensitivity Prediction. Elife 2024, 12, RP90532. [Google Scholar] [CrossRef]
- Chen, Y.; Zou, Z.; Găman, M.-A.; Xu, L.; Li, J. NADPH Oxidase Mediated Oxidative Stress Signaling in FLT3-ITD Acute Myeloid Leukemia. Cell Death Discov. 2023, 9, 208. [Google Scholar] [CrossRef]
- Sillar, J.R.; Germon, Z.P.; DeIuliis, G.N.; Dun, M.D. The Role of Reactive Oxygen Species in Acute Myeloid Leukaemia. Int. J. Mol. Sci. 2019, 20, 6003. [Google Scholar] [CrossRef]
- Starkov, A.A. The Role of Mitochondria in Reactive Oxygen Species Metabolism and Signaling. Ann. N. Y. Acad. Sci. 2008, 1147, 37–52. [Google Scholar] [CrossRef]
- Wise, D.R.; DeBerardinis, R.J.; Mancuso, A.; Sayed, N.; Zhang, X.-Y.; Pfeiffer, H.K.; Nissim, I.; Daikhin, E.; Yudkoff, M.; McMahon, S.B.; et al. Myc Regulates a Transcriptional Program That Stimulates Mitochondrial Glutaminolysis and Leads to Glutamine Addiction. Proc. Natl. Acad. Sci. USA 2008, 105, 18782–18787. [Google Scholar] [CrossRef]
- Pryde, K.R.; Hirst, J. Superoxide Is Produced by the Reduced Flavin in Mitochondrial Complex I. J. Biol. Chem. 2011, 286, 18056–18065. [Google Scholar] [CrossRef]
- Zhou, F.; Yin, Y.; Su, T.; Yu, L.; Yu, C.-A. Oxygen Dependent Electron Transfer in the Cytochrome Bc1 Complex. Biochim. Biophys. Acta (BBA) Bioenerg. 2012, 1817, 2103–2109. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide Dismutases: Dual Roles in Controlling ROS Damage and Regulating ROS Signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Woolley, J.F.; Naughton, R.; Stanicka, J.; Gough, D.R.; Bhatt, L.; Dickinson, B.C.; Chang, C.J.; Cotter, T.G. H2O2 Production Downstream of FLT3 Is Mediated by P22phox in the Endoplasmic Reticulum and Is Required for STAT5 Signalling. PLoS ONE 2012, 7, e34050. [Google Scholar] [CrossRef] [PubMed]
- Lagunas-Rangel, F.A.; Linnea-Niemi, J.V.; Kudłak, B.; Williams, M.J.; Jönsson, J.; Schiöth, H.B. Role of the Synergistic Interactions of Environmental Pollutants in the Development of Cancer. Geohealth 2022, 6, e2021GH000552. [Google Scholar] [CrossRef] [PubMed]
- Stanicka, J.; Russell, E.G.; Woolley, J.F.; Cotter, T.G. NADPH Oxidase-Generated Hydrogen Peroxide Induces DNA Damage in Mutant FLT3-Expressing Leukemia Cells. J. Biol. Chem. 2015, 290, 9348–9361. [Google Scholar] [CrossRef]
- Wang, X.-X.; Wei, J.-Z.; Jiao, J.; Jiang, S.-Y.; Yu, D.-H.; Li, D. Genome-Wide DNA Methylation and Gene Expression Patterns Provide Insight into Polycystic Ovary Syndrome Development. Oncotarget 2014, 5, 6603–6610. [Google Scholar] [CrossRef]
- Böhmer, A.; Barz, S.; Schwab, K.; Kolbe, U.; Gabel, A.; Kirkpatrick, J.; Ohlenschläger, O.; Görlach, M.; Böhmer, F.-D. Modulation of FLT3 Signal Transduction through Cytoplasmic Cysteine Residues Indicates the Potential for Redox Regulation. Redox Biol. 2020, 28, 101325. [Google Scholar] [CrossRef]
- Wu, M.; Li, L.; Hamaker, M.; Small, D.; Duffield, A.S. FLT3-ITD Cooperates with Rac1 to Modulate the Sensitivity of Leukemic Cells to Chemotherapeutic Agents via Regulation of DNA Repair Pathways. Haematologica 2019, 104, 2418–2428. [Google Scholar] [CrossRef]
- Cauchy, P.; James, S.R.; Zacarias-Cabeza, J.; Ptasinska, A.; Imperato, M.R.; Assi, S.A.; Piper, J.; Canestraro, M.; Hoogenkamp, M.; Raghavan, M.; et al. Chronic FLT3-ITD Signaling in Acute Myeloid Leukemia Is Connected to a Specific Chromatin Signature. Cell Rep. 2015, 12, 821–836. [Google Scholar] [CrossRef] [PubMed]
- Sallmyr, A.; Fan, J.; Datta, K.; Kim, K.-T.; Grosu, D.; Shapiro, P.; Small, D.; Rassool, F. Internal Tandem Duplication of FLT3 (FLT3/ITD) Induces Increased ROS Production, DNA Damage, and Misrepair: Implications for Poor Prognosis in AML. Blood 2008, 111, 3173–3182. [Google Scholar] [CrossRef] [PubMed]
- Gallipoli, P.; Giotopoulos, G.; Tzelepis, K.; Costa, A.S.H.; Vohra, S.; Medina-Perez, P.; Basheer, F.; Marando, L.; Di Lisio, L.; Dias, J.M.L.; et al. Glutaminolysis Is a Metabolic Dependency in FLT3ITD Acute Myeloid Leukemia Unmasked by FLT3 Tyrosine Kinase Inhibition. Blood 2018, 131, 1639–1653. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.; Irwin, M.E.; Herbrich, S.M.; Cheng, T.; Patterson, L.L.; Aitken, M.J.L.; Bhalla, K.; You, M.J.; Konopleva, M.; Zweidler-McKay, P.A.; et al. Targeting the NRF2/HO-1 Antioxidant Pathway in FLT3-ITD-Positive AML Enhances Therapy Efficacy. Antioxidants 2022, 11, 717. [Google Scholar] [CrossRef]
- Rushworth, S.A.; Zaitseva, L.; Langa, S.; Bowles, K.M.; MacEwan, D.J. FLIP Regulation of HO-1 and TNF Signalling in Human Acute Myeloid Leukemia Provides a Unique Secondary Anti-Apoptotic Mechanism. Oncotarget 2010, 1, 359–366. [Google Scholar] [CrossRef][Green Version]
- Hasan, S.K.; Jayakumar, S.; Espina Barroso, E.; Jha, A.; Catalano, G.; Sandur, S.K.; Noguera, N.I. Molecular Targets of Oxidative Stress: Focus on Nuclear Factor Erythroid 2–Related Factor 2 Function in Leukemia and Other Cancers. Cells 2025, 14, 713. [Google Scholar] [CrossRef]
- Yagishita, Y.; Gatbonton-Schwager, T.N.; McCallum, M.L.; Kensler, T.W. Current Landscape of NRF2 Biomarkers in Clinical Trials. Antioxidants 2020, 9, 716. [Google Scholar] [CrossRef]
- Zhang, D.; Hou, Z.; Aldrich, K.E.; Lockwood, L.; Odom, A.L.; Liby, K.T. A Novel Nrf2 Pathway Inhibitor Sensitizes Keap1-Mutant Lung Cancer Cells to Chemotherapy. Mol. Cancer Ther. 2021, 20, 1692–1701. [Google Scholar] [CrossRef]
- Pearson, K.J.; Lewis, K.N.; Price, N.L.; Chang, J.W.; Perez, E.; Cascajo, M.V.; Tamashiro, K.L.; Poosala, S.; Csiszar, A.; Ungvari, Z.; et al. Nrf2 Mediates Cancer Protection but Not Prolongevity Induced by Caloric Restriction. Proc. Natl. Acad. Sci. USA 2008, 105, 2325–2330. [Google Scholar] [CrossRef]
- Jones, C.L.; Stevens, B.M.; D’Alessandro, A.; Culp-Hill, R.; Reisz, J.A.; Pei, S.; Gustafson, A.; Khan, N.; DeGregori, J.; Pollyea, D.A.; et al. Cysteine Depletion Targets Leukemia Stem Cells through Inhibition of Electron Transport Complex II. Blood 2019, 134, 389–394. [Google Scholar] [CrossRef]
- Ryl, T.; Kuchen, E.E.; Bell, E.; Shao, C.; Flórez, A.F.; Mönke, G.; Gogolin, S.; Friedrich, M.; Lamprecht, F.; Westermann, F.; et al. Cell-Cycle Position of Single MYC-Driven Cancer Cells Dictates Their Susceptibility to a Chemotherapeutic Drug. Cell Syst. 2017, 5, 237–250.e8. [Google Scholar] [CrossRef]
- Blagosklonny, M. V Target for Cancer Therapy: Proliferating Cells or Stem Cells. Leukemia 2006, 20, 385–391. [Google Scholar] [CrossRef]
- Wang, B.; Pei, J.; Xu, S.; Liu, J.; Yu, J. A Glutamine Tug-of-War between Cancer and Immune Cells: Recent Advances in Unraveling the Ongoing Battle. J. Exp. Clin. Cancer Res. 2024, 43, 74. [Google Scholar] [CrossRef]
- Matés, J.M.; Di Paola, F.J.; Campos-Sandoval, J.A.; Mazurek, S.; Márquez, J. Therapeutic Targeting of Glutaminolysis as an Essential Strategy to Combat Cancer. Semin. Cell Dev. Biol. 2020, 98, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-A.; Chen, C.-L.; Huang, Y.-H.; Evans, E.E.; Cheng, C.-C.; Chuang, Y.-J.; Zhang, C.; Le, A. Inhibition of Glutaminolysis in Combination with Other Therapies to Improve Cancer Treatment. Curr. Opin. Chem. Biol. 2021, 62, 64–81. [Google Scholar] [CrossRef] [PubMed]
- Akins, N.S.; Nielson, T.C.; Le, H.V. Inhibition of Glycolysis and Glutaminolysis: An Emerging Drug Discovery Approach to Combat Cancer. Curr. Top. Med. Chem. 2018, 18, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Xue, H.; Li, Z.; Huo, M.; Gao, H.; Guan, X. Exploiting the Achilles’ Heel of Cancer: Disrupting Glutamine Metabolism for Effective Cancer Treatment. Front. Pharmacol. 2024, 15, 1345522. [Google Scholar] [CrossRef]
- Stine, Z.E.; Schug, Z.T.; Salvino, J.M.; Dang, C.V. Targeting Cancer Metabolism in the Era of Precision Oncology. Nat. Rev. Drug Discov. 2022, 21, 141–162. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.; Hartmann, R.; Tsiampali, J.; Uhlmann, C.; Nickel, A.-C.; He, X.; Kamp, M.A.; Sabel, M.; Barker, R.A.; Steiger, H.-J.; et al. A Comparative Pharmaco-Metabolomic Study of Glutaminase Inhibitors in Glioma Stem-like Cells Confirms Biological Effectiveness but Reveals Differences in Target-Specificity. Cell Death Discov. 2020, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Rattigan, K.M.; Zarou, M.M.; Helgason, V. Metabolism in Stem Cell Driven Leukaemia: Parallels between Haematopoiesis and Immunity. Blood 2023, 141, 2553–2565. [Google Scholar] [CrossRef]
- Appelbaum, F.R.; Gundacker, H.; Head, D.R.; Slovak, M.L.; Willman, C.L.; Godwin, J.E.; Anderson, J.E.; Petersdorf, S.H. Age and Acute Myeloid Leukemia. Blood 2006, 107, 3481–3485. [Google Scholar] [CrossRef]
- Juliusson, G.; Antunovic, P.; Derolf, A.; Lehmann, S.; Möllgård, L.; Stockelberg, D.; Tidefelt, U.; Wahlin, A.; Höglund, M. Age and Acute Myeloid Leukemia: Real World Data on Decision to Treat and Outcomes from the Swedish Acute Leukemia Registry. Blood 2009, 113, 4179–4187. [Google Scholar] [CrossRef]
- Norsworthy, K.J.; Luo, L.; Hsu, V.; Gudi, R.; Dorff, S.E.; Przepiorka, D.; Deisseroth, A.; Shen, Y.-L.; Sheth, C.M.; Charlab, R.; et al. FDA Approval Summary: Ivosidenib for Relapsed or Refractory Acute Myeloid Leukemia with an Isocitrate Dehydrogenase-1 Mutation. Clin. Cancer Res. 2019, 25, 3205–3209. [Google Scholar] [CrossRef]
- Kim, E.S. Enasidenib: First Global Approval. Drugs 2017, 77, 1705–1711. [Google Scholar] [CrossRef]
- Bross, P.F.; Beitz, J.; Chen, G.; Chen, X.H.; Duffy, E.; Kieffer, L.; Roy, S.; Sridhara, R.; Rahman, A.; Williams, G.; et al. Approval Summary: Gemtuzumab Ozogamicin in Relapsed Acute Myeloid Leukemia. Clin. Cancer Res. 2001, 7, 1490–1496. [Google Scholar]
- Krauss, A.C.; Gao, X.; Li, L.; Manning, M.L.; Patel, P.; Fu, W.; Janoria, K.G.; Gieser, G.; Bateman, D.A.; Przepiorka, D.; et al. FDA Approval Summary: (Daunorubicin and Cytarabine) Liposome for Injection for the Treatment of Adults with High-Risk Acute Myeloid Leukemia. Clin. Cancer Res. 2019, 25, 2685–2690. [Google Scholar] [CrossRef] [PubMed]
- Addanki, S.; Kim, L.; Stevens, A. Understanding and Targeting Metabolic Vulnerabilities in Acute Myeloid Leukemia: An Updated Comprehensive Review. Cancers 2025, 17, 1355. [Google Scholar] [CrossRef] [PubMed]
- Yeşlyurt, S.G.; Koyun, D.; Toprak, S.K.; Özcan, M.; Özen, C. A Predictive Metabolomic Model for FLT3 and NPM1 Mutations in Acute Myeloid Leukemia Patients. J. Pharm. Biomed. Anal. 2025, 260, 116789. [Google Scholar] [CrossRef]
- Hvinden, I.C.; Cadoux-Hudson, T.; Schofield, C.J.; McCullagh, J.S.O. Metabolic Adaptations in Cancers Expressing Isocitrate Dehydrogenase Mutations. Cell Rep. Med. 2021, 2, 100469. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C.; Hu, W.; Feng, Z. Tumor Suppressor P53 and Metabolism. J. Mol. Cell Biol. 2019, 11, 284–292. [Google Scholar] [CrossRef]
- Dai, Y.-J.; Hu, F.; He, S.-Y.; Tian, X.-P.; Li, H.-H.; Qin, Z.-Y.; Chen, S.; Liang, Y. A Distinct Metabolic Signature in DNMT3A-Mutated Leukemia. Blood 2019, 134, 1426. [Google Scholar] [CrossRef]
- Fujino, T.; Goyama, S.; Sugiura, Y.; Inoue, D.; Yamasaki, S.; Matsumoto, A.; Sato, N.; Morinaga, H.; Shikata, S.; Fukuyama, T.; et al. Mutant ASXL1 Promotes Expansion of the Phenotypic Hematopoietic Stem Cell Compartment. Blood 2019, 134, 821. [Google Scholar] [CrossRef]
- Hope, K.J.; Jin, L.; Dick, J.E. Acute Myeloid Leukemia Originates from a Hierarchy of Leukemic Stem Cell Classes That Differ in Self-Renewal Capacity. Nat. Immunol. 2004, 5, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Zhong, H. Roles of the Bone Marrow Niche in Hematopoiesis, Leukemogenesis, and Chemotherapy Resistance in Acute Myeloid Leukemia. Hematology 2018, 23, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Salvia, A.M.; Cuviello, F.; Coluzzi, S.; Nuccorini, R.; Attolico, I.; Pascale, S.P.; Bisaccia, F.; Pizzuti, M.; Ostuni, A. Expression of Some ATP-Binding Cassette Transporters in Acute Myeloid Leukemia. Hematol. Rep. 2017, 9, 7406. [Google Scholar] [CrossRef]
- Stevens, A.M.; Schafer, E.S.; Li, M.; Terrell, M.; Rashid, R.; Paek, H.; Bernhardt, M.B.; Weisnicht, A.; Smith, W.T.; Keogh, N.J.; et al. Repurposing Atovaquone as a Therapeutic against Acute Myeloid Leukemia (AML): Combination with Conventional Chemotherapy Is Feasible and Well Tolerated. Cancers 2023, 15, 1344. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.A.; Angka, L.; Rota, S.-G.; Hanlon, T.; Mitchell, A.; Hurren, R.; Wang, X.M.; Gronda, M.; Boyaci, E.; Bojko, B.; et al. Targeting Mitochondria with Avocatin B Induces Selective Leukemia Cell Death. Cancer Res. 2015, 75, 2478–2488. [Google Scholar] [CrossRef]
- Tan, S.-F.; Liu, X.; Fox, T.E.; Barth, B.M.; Sharma, A.; Turner, S.D.; Awwad, A.; Dewey, A.; Doi, K.; Spitzer, B.; et al. Acid Ceramidase Is Upregulated in AML and Represents a Novel Therapeutic Target. Oncotarget 2016, 7, 83208–83222. [Google Scholar] [CrossRef]
- Yap, T.A.; Daver, N.; Mahendra, M.; Zhang, J.; Kamiya-Matsuoka, C.; Meric-Bernstam, F.; Kantarjian, H.M.; Ravandi, F.; Collins, M.E.; Francesco, M.E.D.; et al. Complex I Inhibitor of Oxidative Phosphorylation in Advanced Solid Tumors and Acute Myeloid Leukemia: Phase I Trials. Nat. Med. 2023, 29, 115–126. [Google Scholar] [CrossRef]
- Reed, G.A.; Schiller, G.J.; Kambhampati, S.; Tallman, M.S.; Douer, D.; Minden, M.D.; Yee, K.W.; Gupta, V.; Brandwein, J.; Jitkova, Y.; et al. A Phase 1 Study of Intravenous Infusions of Tigecycline in Patients with Acute Myeloid Leukemia. Cancer Med. 2016, 5, 3031–3040. [Google Scholar] [CrossRef]
- Mussai, F.; Egan, S.; Higginbotham-Jones, J.; Perry, T.; Beggs, A.; Odintsova, E.; Loke, J.; Pratt, G.; U, K.P.; Lo, A.; et al. Arginine Dependence of Acute Myeloid Leukemia Blast Proliferation: A Novel Therapeutic Target. Blood 2015, 125, 2386–2396. [Google Scholar] [CrossRef]
- Tsai, H.-J.; Jiang, S.S.; Hung, W.-C.; Borthakur, G.; Lin, S.-F.; Pemmaraju, N.; Jabbour, E.; Bomalaski, J.S.; Chen, Y.-P.; Hsiao, H.-H.; et al. A Phase II Study of Arginine Deiminase (ADI-PEG20) in Relapsed/Refractory or Poor-Risk Acute Myeloid Leukemia Patients. Sci. Rep. 2017, 7, 11253. [Google Scholar] [CrossRef] [PubMed]
- Senapati, J.; Kantarjian, H.M.; Bazinet, A.; Reville, P.; Short, N.J.; Daver, N.; Borthakur, G.; Bataller, A.; Jabbour, E.; DiNardo, C.; et al. Lower Intensity Therapy with Cladribine/Low Dose Cytarabine/Venetoclax in Older Patients with Acute Myeloid Leukemia Compares Favorably with Intensive Chemotherapy among Patients Undergoing Allogeneic Stem Cell Transplantation. Cancer 2024, 130, 3333–3343. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Fathi, A.T.; Braun, T.P.; Ambinder, A.J.; Borthakur, G.; Redner, R.L.; Arevalo, M.; Gutierrez, S.; Limon, A.; Faller, D.V. Iadademstat and Gilteritinib for the Treatment of FLT3-Mutated Relapsed/Refractory Acute Myeloid Leukemia: The Frida Study. Blood 2023, 142, 5974. [Google Scholar] [CrossRef]
- Vidal, R.S.; Quarti, J.; Rumjanek, F.D.; Rumjanek, V.M. Metabolic Reprogramming During Multidrug Resistance in Leukemias. Front. Oncol. 2018, 8, 90. [Google Scholar] [CrossRef]
- Farge, T.; Saland, E.; Toni, F.D.; Aroua, N.; Hosseini, M.; Perry, R.; Bosc, C.; Sugita, M.; Stuani, L.; Fraisse, M.; et al. Chemotherapy-Resistant Human Acute Myeloid Leukemia Cells Are Not Enriched for Leukemic Stem Cells but Require Oxidative Metabolism. Cancer Discov. 2017, 7, 716–735. [Google Scholar] [CrossRef]
- Moschoi, R.; Imbert, V.; Nebout, M.; Chiche, J.; Mary, D.; Prebet, T.; Saland, E.; Castellano, R.; Pouyet, L.; Collette, Y.; et al. Protective Mitochondrial Transfer from Bone Marrow Stromal Cells to Acute Myeloid Leukemic Cells during Chemotherapy. Blood 2016, 128, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.A.; D’Alessandro, A.; Alvarez-Calderon, F.; Kim, J.; Nemkov, T.; Adane, B.; Rozhok, A.I.; Kumar, A.; Kumar, V.; Pollyea, D.A.; et al. ATM/G6PD-Driven Redox Metabolism Promotes FLT3 Inhibitor Resistance in Acute Myeloid Leukemia. Proc. Natl. Acad. Sci. USA 2016, 113, E6669–E6678. [Google Scholar] [CrossRef]
- Lu, X.; Han, L.; Busquets, J.; Collins, M.; Lodi, A.; Marszalek, J.R.; Konopleva, M.; Tiziani, S. The Combined Treatment with the FLT3-Inhibitor AC220 and the Complex I Inhibitor IACS-010759 Synergistically Depletes Wt- and FLT3-Mutated Acute Myeloid Leukemia Cells. Front. Oncol. 2021, 11, 686765. [Google Scholar] [CrossRef]
- Taylor, S.J.; Steidl, U. Metabolic StrugGLS after FLT3 Inhibition in AML. Blood 2018, 131, 1631–1632. [Google Scholar] [CrossRef]
- Sharma, P.; Borthakur, G. Targeting Metabolic Vulnerabilities to Overcome Resistance to Therapy in Acute Myeloid Leukemia. Cancer Drug Resist. 2023, 6, 567–589. [Google Scholar] [CrossRef] [PubMed]
- Pollyea, D.A.; Stevens, B.M.; Jones, C.L.; Winters, A.; Pei, S.; Minhajuddin, M.; D’Alessandro, A.; Culp-Hill, R.; Riemondy, K.A.; Gillen, A.E.; et al. Venetoclax with Azacitidine Disrupts Energy Metabolism and Targets Leukemia Stem Cells in Patients with Acute Myeloid Leukemia. Nat. Med. 2018, 24, 1859–1866. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, T.; Ding, X.; Chang, Y.; Liu, C.; Zhang, Y.; Hao, S.; Yin, Q.; Jiang, B. Inhibition of Mitochondrial Complex III Induces Differentiation in Acute Myeloid Leukemia. Biochem. Biophys. Res. Commun. 2021, 547, 162–168. [Google Scholar] [CrossRef]
- Alvarez-Calderon, F.; Gregory, M.A.; Pham-Danis, C.; DeRyckere, D.; Stevens, B.M.; Zaberezhnyy, V.; Hill, A.A.; Gemta, L.; Kumar, A.; Kumar, V.; et al. Tyrosine Kinase Inhibition in Leukemia Induces an Altered Metabolic State Sensitive to Mitochondrial Perturbations. Clin. Cancer Res. 2015, 21, 1360–1372. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, F.; Guan, J.; Zhou, L.; Chen, B. Action Mechanism of Metformin and Its Application in Hematological Malignancy Treatments: A Review. Biomolecules 2023, 13, 250. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, Z.; Zeng, J.; Zhu, H.; Li, J.; Cheng, X.; Jiang, T.; Zhang, L.; Zhang, C.; Chen, T.; et al. Metformin Synergistically Sensitizes FLT3-ITD-Positive Acute Myeloid Leukemia to Sorafenib by Promoting MTOR-Mediated Apoptosis and Autophagy. Leuk. Res. 2015, 39, 1421–1427. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, P.; Xiao, J.; Huang, P.; Liu, P. Modulation of Energy Metabolism to Overcome Drug Resistance in Chronic Myeloid Leukemia Cells through Induction of Autophagy. Cell Death Discov. 2022, 8, 212. [Google Scholar] [CrossRef]
- Sobhakumari, A.; Orcutt, K.P.; Love-Homan, L.; Kowalski, C.E.; Parsons, A.D.; Knudson, C.M.; Simons, A.L. 2-Deoxy-d-Glucose Suppresses the In Vivo Antitumor Efficacy of Erlotinib in Head and Neck Squamous Cell Carcinoma Cells. Oncol. Res. 2016, 24, 55–64. [Google Scholar] [CrossRef]
- Bjelosevic, S.; Gruber, E.; Newbold, A.; Shembrey, C.; Devlin, J.R.; Hogg, S.J.; Kats, L.; Todorovski, I.; Fan, Z.; Abrehart, T.C.; et al. Serine Biosynthesis Is a Metabolic Vulnerability in FLT3-ITD-Driven Acute Myeloid Leukemia. Cancer Discov. 2021, 11, 1582–1599. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.; Schmidt, C.; Bruch, P.-M.; Blank, M.F.; Rohde, C.; Waclawiczek, A.; Heid, D.; Renders, S.; Göllner, S.; Vierbaum, L.; et al. Venetoclax Synergizes with Gilteritinib in FLT3 Wild-Type High-Risk Acute Myeloid Leukemia by Suppressing MCL-1. Blood 2022, 140, 2594–2610. [Google Scholar] [CrossRef]
- Milnerowicz, S.; Maszewska, J.; Skowera, P.; Stelmach, M.; Lejman, M. AML under the Scope: Current Strategies and Treatment Involving FLT3 Inhibitors and Venetoclax-Based Regimens. Int. J. Mol. Sci. 2023, 24, 15849. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.-J.; Zeng, C.-X.; Kuang, W.; Cheng, C.; Liu, H.-C.; Yan, X.-Y.; Chen, X.-P.; Zhou, G.; Cao, S. Metformin Exerts a Synergistic Effect with Venetoclax by Downregulating Mcl-1 Protein in Acute Myeloid Leukemia. J. Cancer 2021, 12, 6727–6739. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, X.; Ding, X.; Wang, Y.; Li, Z.; Zhao, R.; Cheng, H.-E.; Sun, Y. Efficacy and Safety of FLT3 Inhibitors in Monotherapy of Hematological and Solid Malignancies: A Systemic Analysis of Clinical Trials. Front. Pharmacol. 2024, 15, 1294668. [Google Scholar] [CrossRef]
- Perrone, S.; Ottone, T.; Zhdanovskaya, N.; Molica, M. How Acute Myeloid Leukemia (AML) Escapes from FMS-Related Tyrosine Kinase 3 (FLT3) Inhibitors? Still an Overrated Complication? Cancer Drug Resist. 2023, 6, 223–238. [Google Scholar] [CrossRef]
| Drug | Metabolic Target | Trial | References |
|---|---|---|---|
| 2-Deoxy-D-glucose (2-DG) | Glycolysis | Pre-clinical (in vitro and in vivo) | [68] |
| Atovaquone | OXPHOS | Pre-clinical (in vitro and in vivo) Feasibility Trial (NCT03568994) | [170] |
| Avocatin B, Etomoxir and ST-1326 | Fatty acid oxidation | Pre-clinical (in vitro and in vivo) | [57,92,171] |
| 3-bromopyruvate (3BrPA) | Glycolysis | Pre-clinical (in vitro and in vivo) | [66] |
| LCL204 | Sphingolipids | Pre-clinical (in vivo) | [172] |
| IACS-010759 | OXPHOS | Phase I (NCT02882321) | [173] |
| Telaglenastat (CB-839) | Glutaminolysis | Phase I (NCT02071927) | [174] |
| Tigecycline | OXPHOS | Phase I (NCT01332786) | [175] |
| BCT-100 | Arginine metabolism | Phase I/II (NCT03455140) | [175] |
| ADI-PEG 20 | Arginine metabolism | Phase II trial (NCT01910012) | [176] |
| Venetoclax | OXPHOS | Phase I/II/III (NCT01994837, NCT02203773, NCT02993523, NCT04801779, NCT05177731, NCT05048615, NCT03586609, NCT03625505, NCT04140487 NCT03455504) | [177,178] |
| Iadademstat | OXPHOS | NCT05546580 | [179] |
| Drug/Combination | Targeted Metabolic Pathway | Mechanism of Action | Effect | References |
|---|---|---|---|---|
| Quizartinib (1–5 nM) + CB-839 | Glutaminolysis | Inhibits glutamine metabolism; increased ROS and mitochondrial dysfunction | Synergistic lethality; overcomes resistance mechanisms (in vitro and in vivo) | [79] |
| Quizartinib (100 nM) + IACS-010759 (10 nM) | OXPHOS (Complex I inhibition) | Inhibits mitochondrial electron transport chain Complex I | Enhances sensitivity to FLT3i; impairs energy production (in vitro) | [184] |
| Venetoclax (100 nM in vitro/100 mg/kg in vivo/400 mg/die in clinical trial) + Azacitidine (1 µM in vitro/3 mg/kg in vivo/75 mg/7 days in clinical trial) | Mitochondrial metabolism, Glutathione synthesis | Impairs OXPHOS, reduces GSH levels, inhibits Bcl-2 | Targets LSCs; induces apoptosis; synergizes with FLT3i via MCL-1 suppression (in vitro, in vivo, and clinical trial) | [187] |
| Antimycin A (1 µM) | OXPHOS (Complex III inhibition) | Blocks electron flow in ETC Complex III | Reduces cell viability; promotes differentiation (in vitro) | [188] |
| Oligomycin A (0.5–10 nM in vitro/100 µg/kg in vivo) | OXPHOS (Complex V/ATP synthase inhibition) | Inhibits ATP production | Enhances sensitivity to FLT3 inhibitors (in vitro and in vivo) | [189] |
| Metformin (0.5–10 mM in vitro/200 mg/kg in vivo) | Mitochondrial metabolism/mTOR pathway | Inhibits OXPHOS and glycolysis; activates AMPK | Promotes apoptosis; synergizes with FLT3i and venetoclax (in vitro and in vivo) | [190,191] |
| Quizartinib + L-Asparaginase (2 UI/mL) | Amino acid metabolism (glutamine/asparagine depletion) | Depletes glutamine/asparagine; metabolic stress | Effective in quizartinib-resistant FLT3+ cells (in vitro) | [79] |
| Crenolanib (6 µM in vitro/15 mg/Kg/die in vivo) + MP-A08 (SPHK1 inhibitor, 6–20 µM/100 mg/kg) | Sphingolipid metabolism (ceramide pathway) | Induces ceramide accumulation; activates stress/apoptotic signaling | Promotes mitophagy and apoptosis; sensitizes to venetoclax | [110] |
| 2-Deoxy-D-glucose (2-DG, 20 mM) | Glycolysis | Inhibits hexokinase; blocks glucose utilization | Enhances cytotoxic effects of TKIs (in vitro and in vivo) | [192,193] |
| WQ-2101 (PHGDH inhibitor) | Serine synthesis | Inhibits de novo serine synthesis pathway | Enhances chemotherapy response in FLT3-mut AML (in vitro and in vivo) | [194] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banella, C.; Catalano, G.; Calvani, M.; Candi, E.; Noguera, N.I.; Travaglini, S. Metabolic Signature of FLT3-Mutated AML: Clinical and Therapeutic Implications. J. Pers. Med. 2025, 15, 431. https://doi.org/10.3390/jpm15090431
Banella C, Catalano G, Calvani M, Candi E, Noguera NI, Travaglini S. Metabolic Signature of FLT3-Mutated AML: Clinical and Therapeutic Implications. Journal of Personalized Medicine. 2025; 15(9):431. https://doi.org/10.3390/jpm15090431
Chicago/Turabian StyleBanella, Cristina, Gianfranco Catalano, Maura Calvani, Eleonora Candi, Nelida Ines Noguera, and Serena Travaglini. 2025. "Metabolic Signature of FLT3-Mutated AML: Clinical and Therapeutic Implications" Journal of Personalized Medicine 15, no. 9: 431. https://doi.org/10.3390/jpm15090431
APA StyleBanella, C., Catalano, G., Calvani, M., Candi, E., Noguera, N. I., & Travaglini, S. (2025). Metabolic Signature of FLT3-Mutated AML: Clinical and Therapeutic Implications. Journal of Personalized Medicine, 15(9), 431. https://doi.org/10.3390/jpm15090431








