Long-Term Outcomes in Aortic Stenosis: Mortality Analysis in a Selected Patient Group
Abstract
1. Introduction
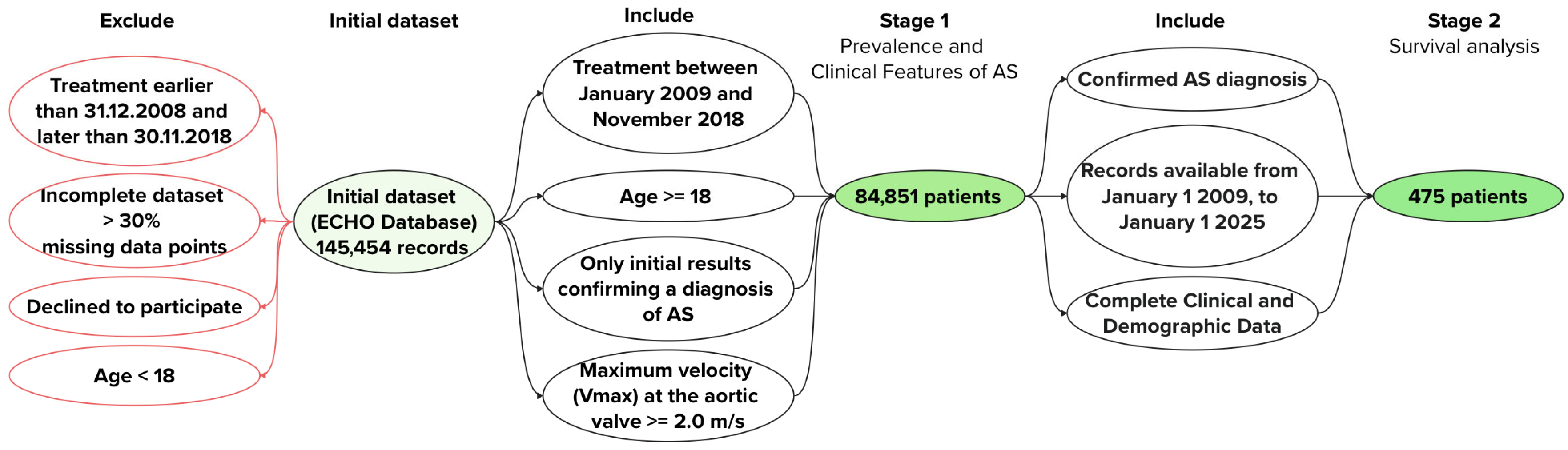
2. Materials and Methods
- Study Design
- Stage 1
- Inclusion Criteria
- Patients treated between 1 January 2009, and 30 November 2018, who underwent transthoracic echocardiography (TTE) at the Federal State Budgetary Institution “V.A. Almazov National Medical Research Centre”;
- Patients with a maximum velocity (Vmax) across the aortic valve ≥ 2.0 m/s;
- Age ≥ 18 years at the time of inclusion;
- Patient consent for participation in the study.
- Exclusion Criteria
- Patients under 18 years of age at the time of inclusion;
- Patients with insufficient data for further analysis.
- Propensity Score Matching
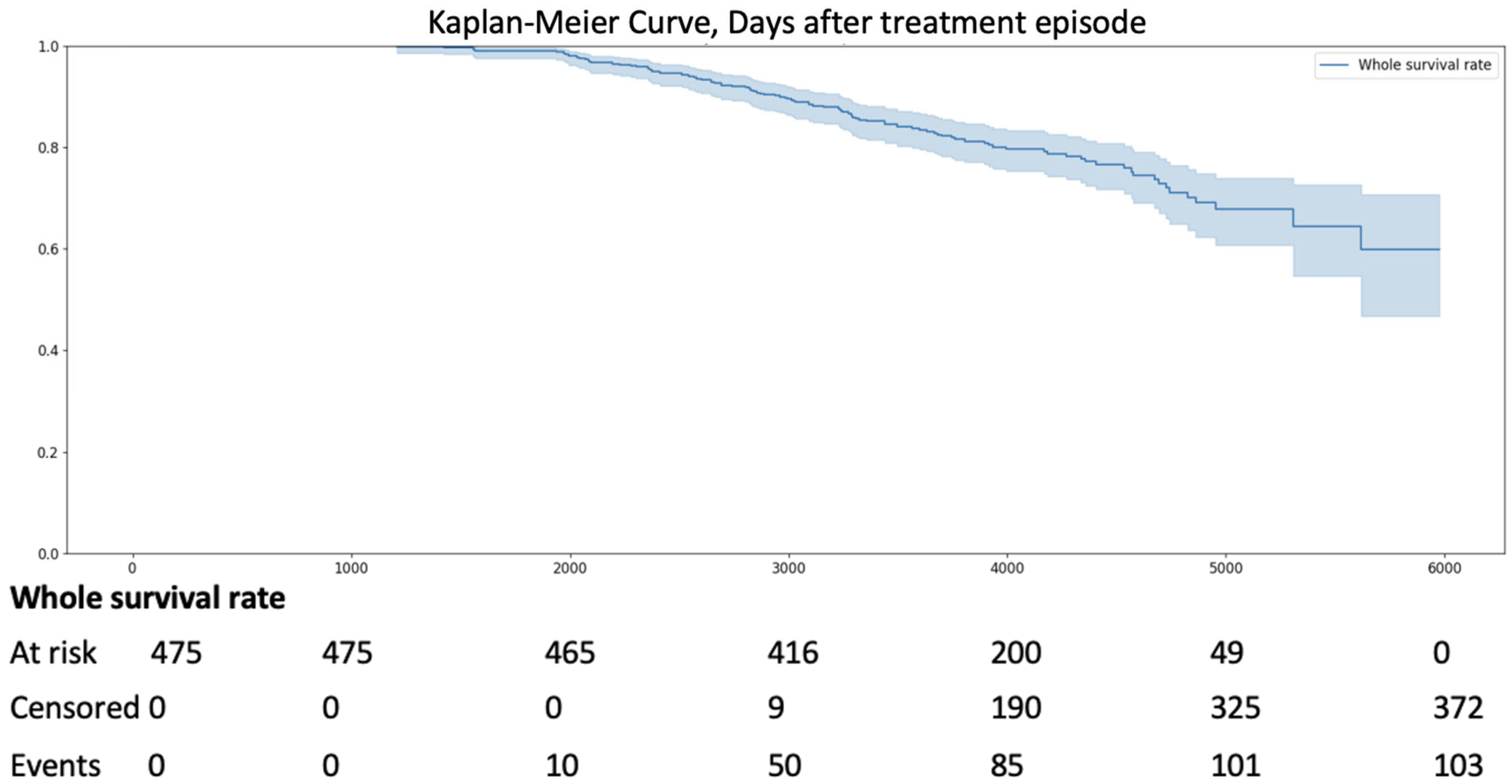
- Stage 2
- Confirmed AS diagnosis. Patients were required to have a confirmed diagnosis of AS based on TTE findings (Vmax ≥ 2.0 m/s) at the time of inclusion in 2009, ensuring a homogeneous cohort with verified AS for survival analysis.
- Availability of long-term follow-up data. Patients were included if they had continuous medical records available from 1 January 2009, to 1 January 2025, allowing for a 16-year observation period to assess all-cause mortality using the Kaplan–Meier method.
- Complete clinical and demographic data. Patients were selected only if they had comprehensive baseline data (age, gender, valve morphology [BAV vs. TAV], comorbidities, and TTE parameters).
- I13.X
- E10.X
- E11.X
- I25.X
- J44.0—Chronic obstructive pulmonary disease with (acute) lower respiratory infection
- J44.1—Chronic obstructive pulmonary disease with (acute) exacerbation
- J44.9—Chronic obstructive pulmonary disease, unspecified
- J45.X
- E66.X
- E78.X
- I50.X
- Therapy
- Statistical Methods
- Pre-analysis Data Screening
- Missing-data Handling
- Missingness ranged from 0% to 15.2% per variable (median = 4.3%).
- Little’s test for missing completely at random was significant (χ2 = 103.4, df = 78, p = 0.018), indicating that the data are not missing completely at random (MCAR).
- Complete-case analysis was used when missingness ≤ 5%
- For variables with 5–20% missingness, we applied multiple imputation by fully conditional specification:
- ○
- predictive mean matching (k = 5) for continuous, logistic/multinomial for binary or nominal factors,
- ○
- all model covariates and the outcome included,
- ○
- convergence inspected via trace plots of means/SDs.
- Any variable with >20% missingness (none in this study) would have been excluded a priori.
3. Results
4. Discussion
5. Clinical Implications
6. Limitations
7. Conclusions
8. Highlights
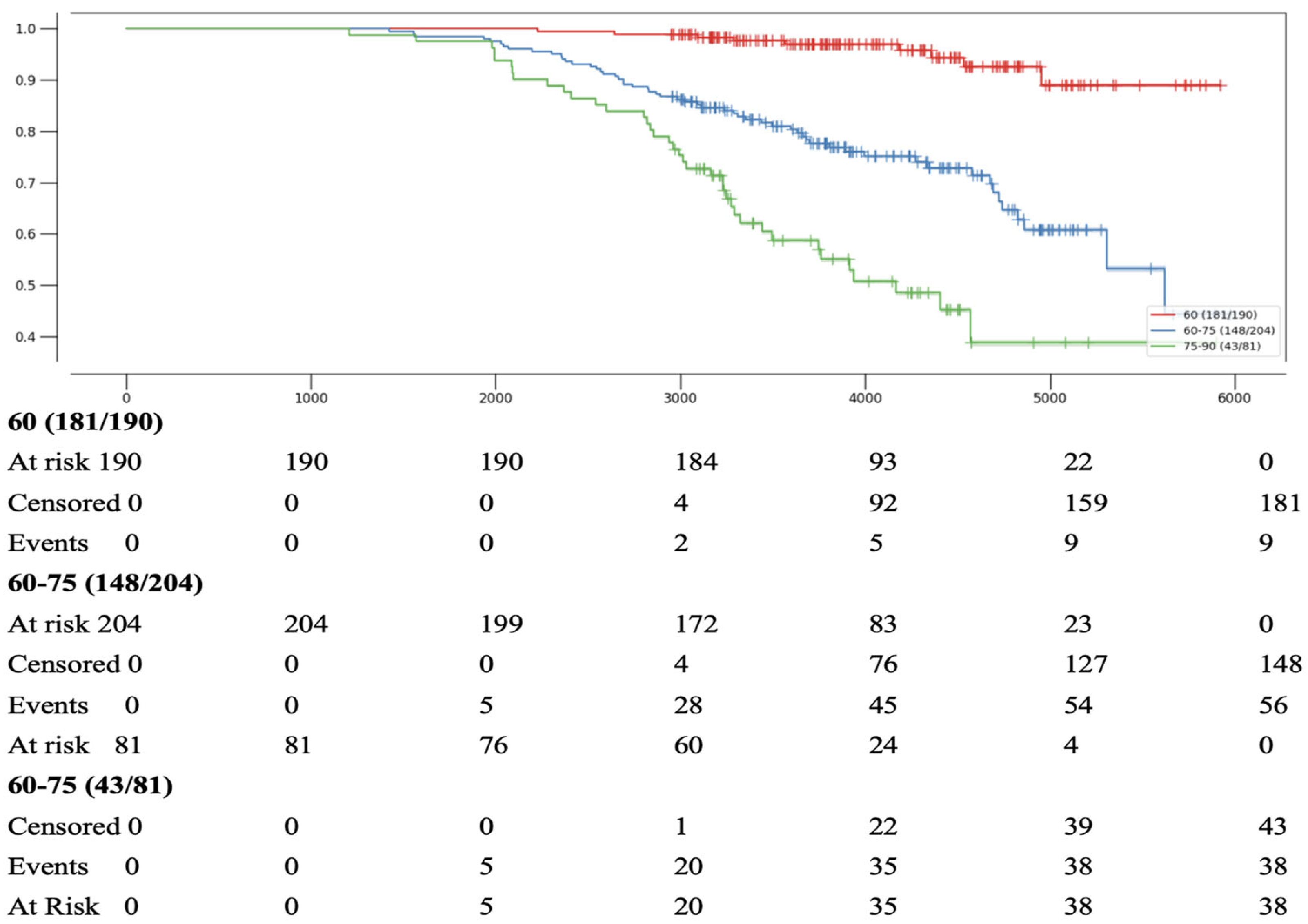
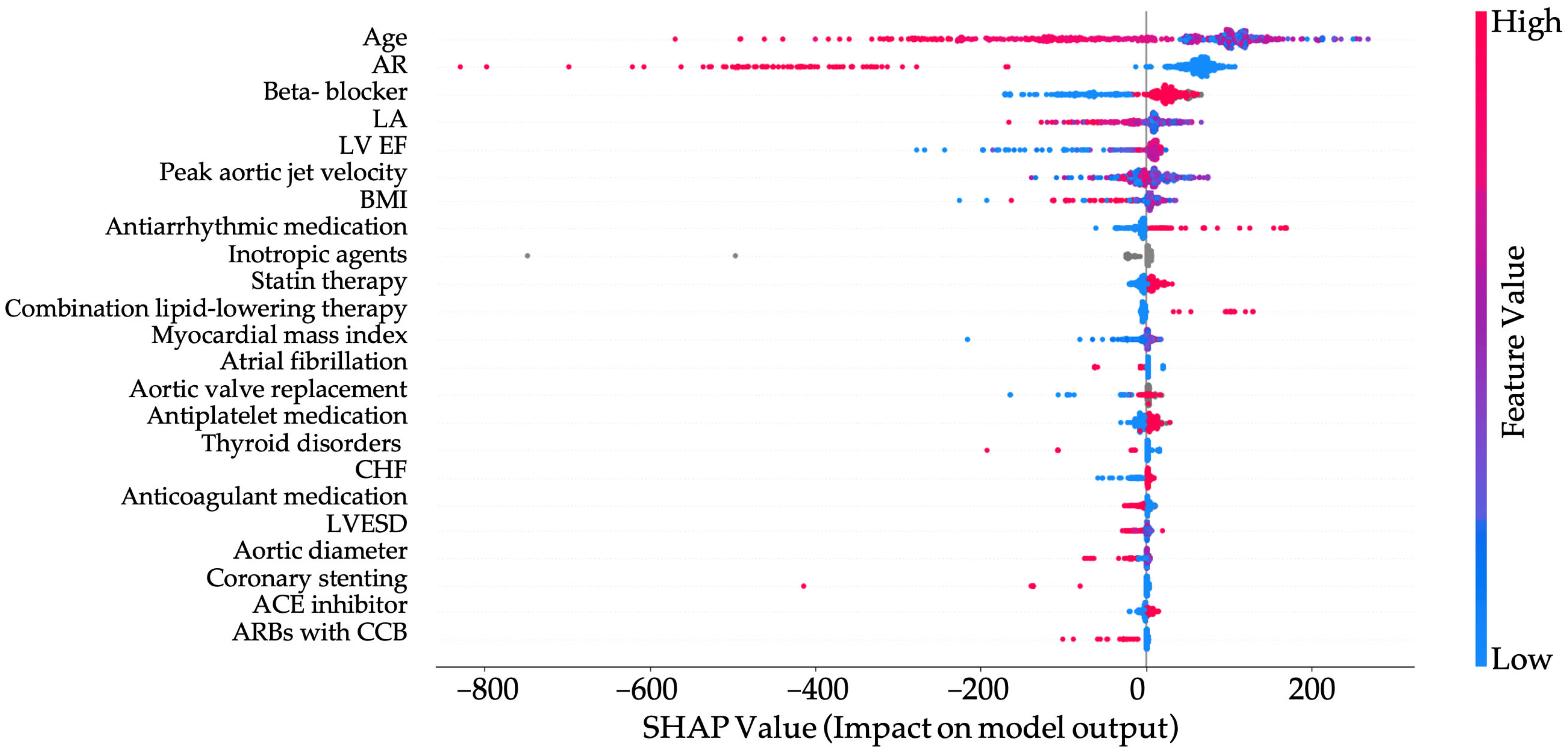
- Survival in patients with aortic stenosis was significantly reduced in those aged >68.5 years and in the presence of concomitant aortic regurgitation (AR).
- Beta-blocker therapy was associated with improved survival, demonstrating a protective effect in patients with varying AS severity.
- The combination of angiotensin II receptor blockers and calcium channel blockers was linked to poorer survival outcomes and should be used with caution in patients with AS.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HP | hypertension |
| AV | aortic valve |
| AR | aortic regurgitation |
| AS | aortic stenosis |
| BAV | bicuspid aortic valve |
| HLP | hyperlipidemia |
| DBP | diastolic blood pressure |
| CAD | coronary artery disease |
| BMI | body mass index |
| LV | left ventricle |
| LA | left atrium |
| LVESD | left ventricular end systolic dimension |
| SBP | systolic blood pressure |
| DM | diabetes mellitus |
| TAV | tricuspid aortic valve |
| TTE | transthoracic echocardiography |
| EF | ejection fraction |
| COPD | chronic obstructive pulmonary disease |
| CHF | chronic heart failure |
References
- Chatterjee, A.; Kazui, T.; Acharya, D. Growing prevalence of aortic stenosis—Question of age or better recognition? Int. J. Cardiol. 2023, 388, 131155. [Google Scholar] [CrossRef]
- Whelton, S.P.; Jha, K.; Dardari, Z.; Razavi, A.C.; Boakye, E.; Dzaye, O.; Verghese, D.; Shah, S.; Budoff, M.J.; Matsushita, K.; et al. Prevalence of Aortic Valve Calcium and the Long-Term Risk of Incident Severe Aortic Stenosis. JACC Cardiovasc. Imaging 2023, 17, 31–42. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., III; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, e25–e197. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Osnabrugge, R.L.; Mylotte, D.; Head, S.J.; Van Mieghem, N.M.; Nkomo, V.T.; LeReun, C.M.; Bogers, A.J.; Piazza, N.; Kappetein, A.P. Aortic Stenosis in the Elderly. JACC 2013, 62, 1002–1012. [Google Scholar] [CrossRef]
- Peters, A.S.; Duggan, J.P.; Trachiotis, G.D.; Antevil, J.L. Epidemiology of Valvular Heart Disease. Surg. Clin. N. Am. 2022, 102, 517–528. [Google Scholar] [CrossRef]
- Moncla, L.H.M.; Briend, M.; Bossé, Y.; Mathieu, P. Calcific aortic valve disease: Mechanisms, prevention and treatment. Nat. Rev. Cardiol. 2023, 20, 546–559. [Google Scholar] [CrossRef]
- Timmis, A.; Vardas, P.; Townsend, N.; Torbica, A.; Katus, H.; De Smedt, D.; Gale, C.P.; Maggioni, A.P.; Petersen, S.E.; Huculeci, R.; et al. European Society of Cardiology: Cardiovascular disease statistics 2021. Eur. Heart J. 2022, 43, 716–799. [Google Scholar] [CrossRef]
- Michelena, H.I.; Prakash, S.K.; Della Corte, A.; Bissell, M.M.; Anavekar, N.; Mathieu, P.; Bossé, Y.; Limongelli, G.; Bossone, E.; Benson, D.; et al. Bicuspid Aortic Valve. Circulation 2014, 129, 2691–2704. [Google Scholar] [CrossRef]
- Tribouilloy, C.; Bohbot, Y.; Rusinaru, D.; Belkhir, K.; Diouf, M.; Altes, A.; Delpierre, Q.; Serbout, S.; Kubala, M.; Levy, F.; et al. Excess Mortality and Undertreatment of Women with Severe Aortic Stenosis. J. Am. Heart Assoc. 2021, 10, e018816. [Google Scholar] [CrossRef]
- Hahn, R.T.; Clavel, M.A.; Mascherbauer, J.; Mick, S.L.; Asgar, A.W.; Douglas, P.S. Sex-Related Factors in Valvular Heart Disease. J. Am. Coll. Cardiol. 2022, 79, 1506–1518. [Google Scholar] [CrossRef]
- Côté, N.; Clavel, M.A. Sex Differences in the Pathophysiology, Diagnosis, and Management of Aortic Stenosis. Cardiol. Clin. 2020, 38, 129–138. [Google Scholar] [CrossRef]
- Linde, L.; Carter-Storch, R.; Christensen, N.L.; Øvrehus, K.A.; Diederichsen, A.C.P.; Laursen, K.; Jensen, P.S.; Rasmussen, L.M.; Møller, J.E.; Dahl, J.S. Sex differences in aortic valve calcification in severe aortic valve stenosis: Association between computer tomography assessed calcification and valvular calcium concentrations. Eur. Heart J.-Cardiovasc. Imaging 2020, 22, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Guzzetti, E.; Poulin, A.; Annabi, M.-S.; Zhang, B.; Kalavrouziotis, D.; Couture, C.; Dagenais, F.; Pibarot, P.; Clavel, M.-A. Transvalvular Flow, Sex, and Survival After Valve Replacement Surgery in Patients with Severe Aortic Stenosis. J. Am. Coll. Cardiol. 2020, 75, 1897–1909. [Google Scholar] [CrossRef] [PubMed]
- Irtyuga, O.; Babakekhyan, M.; Kostareva, A.; Uspensky, V.; Gordeev, M.; Faggian, G.; Malashicheva, A.; Metsker, O.; Shlyakhto, E.; Kopanitsa, G. Analysis of Prevalence and Clinical Features of Aortic Stenosis in Patients with and without Bicuspid Aortic Valve Using Machine Learning Methods. J. Pers. Med. 2023, 13, 1588. [Google Scholar] [CrossRef] [PubMed]
- Irtyuga, O.; Kopanitsa, G.; Kostareva, A.; Metsker, O.; Uspensky, V.; Mikhail, G.; Faggian, G.; Sefieva, G.; Derevitskii, I.; Malashicheva, A.; et al. Application of Machine Learning Methods to Analyze Occurrence and Clinical Features of Ascending Aortic Dilatation in Patients with and without Bicuspid Aortic Valve. J. Pers. Med. 2022, 12, 794. [Google Scholar] [CrossRef]
- Rich, J.T.; Neely, J.G.; Paniello, R.C.; Voelker, C.C.J.; Nussenbaum, B.; Wang, E.W. A practical guide to understanding Kaplan-Meier curves. Otolaryngol.–Head Neck Surg. 2010, 143, 331–336. [Google Scholar] [CrossRef]
- Iribarren, A.C.; AlBadri, A.; Wei, J.; Nelson, M.D.; Li, D.; Makkar, R.; Merz, C.N.B. Sex differences in aortic stenosis: Identification of knowledge gaps for sex-specific personalized medicine. Am. Heart J. Plus: Cardiol. Res. Pract. 2022, 21. [Google Scholar] [CrossRef]
- Bernal, E.; Ariza-Solé, A.; Bayés-Genís, A.; Formiga, F.; Díez-Villanueva, P.; Romaguera, R.; González-Saldívar, H.; Martínez-Sellés, M. Management of Nonagenarian Patients with Severe Aortic Stenosis: The Role of Comorbidity. Heart Lung Circ. 2018, 27, 219–226. [Google Scholar] [CrossRef]
- Dismorr, M.; Granbom-Koski, M.; Ellfors, E.; Rück, A.; Settergren, M.; Sartipy, U.; Glaser, N. Sex differences and long-term clinical outcomes after transcatheter aortic valve replacement: A SWEDEHEART study. Am. Heart J. 2024, 277, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sellés, M.; Díez-Villanueva, P.; Sánchez-Sendin, D.; Hevia, A.C.; Doblas, J.J.G.; de la Villa, B.G.; Cornide, L.; Tello, A.A.; Ogando, R.A.; Vera, T.R.; et al. Comorbidity and intervention in octogenarians with severe symptomatic aortic stenosis. Int. J. Cardiol. 2015, 189, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Díez-Villanueva, P.; Salamanca, J.; Rojas, A.; Alfonso, F. Importance of frailty and comorbidity in elderly patients with severe aortic stenosis. J. Geriatr. Cardiol. 2017, 14, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Sharma, R.P.; Cubeddu, R.J.; Aaron, L.; Abdelfattah, O.M.; Koulogiannis, K.P.; Marcoff, L.; Naguib, M.; Kapadia, S.R.; Makkar, R.R.; et al. The Mortality Burden of Untreated Aortic Stenosis. J. Am. Coll. Cardiol. 2023, 82, 2101–2109. [Google Scholar] [CrossRef]
- Mengi, S.; Januzzi, J.L.; Cavalcante, J.L.; Avvedimento, M.; Galhardo, A.; Bernier, M.; Rodés-Cabau, J. Aortic Stenosis, Heart Failure, and Aortic Valve Replacement. JAMA Cardiol. 2024, 9, 1159–1168. [Google Scholar] [CrossRef]
- Sengeløv, M.; Cheng, S.; Biering-Sørensen, T.; Matsushita, K.; Konety, S.; Solomon, S.D.; Folsom, A.R.; Shah, A.M. Ideal Cardiovascular Health and the Prevalence and Severity of Aortic Stenosis in Elderly Patients. J. Am. Heart Assoc. 2018, 7, e007234. [Google Scholar] [CrossRef]
- Saeed, S.; Scalise, F.; Chambers, J.B.; Mancia, G. Hypertension in aortic stenosis: A focused review and recommendations for clinical practice. J. Hypertens. 2020, 38, 1211–1219. [Google Scholar] [CrossRef]
- Rassa, A.; Zahr, F. Hypertension and Aortic Stenosis: A Review. Curr. Hypertens. Rev. 2018, 14, 6–14. [Google Scholar] [CrossRef]
- Yamamoto, K.; Takeji, Y.; Taniguchi, T.; Morimoto, T.; Shirai, S.; Tabata, H.; Ohno, N.; Murai, R.; Osakada, K.; Murata, K.; et al. Safety of Calcium Channel Blockers in Patients with Severe Aortic Stenosis and Hypertension. Circ. J. 2024. [Google Scholar] [CrossRef]
- Saeed, S.; Mancia, G.; Rajani, R.; Parkin, D.; Chambers, J.B. Antihypertensive treatment with calcium channel blockers in patients with moderate or severe aortic stenosis: Relationship with all-cause mortality. Int. J. Cardiol. 2020, 298, 122–125. [Google Scholar] [CrossRef]
- Bang, C.N.; Greve, A.M.; Rossebø, A.B.; Ray, S.; Egstrup, K.; Boman, K.; Nienaber, C.; Okin, P.M.; Devereux, R.B.; Wachtell, K. Antihypertensive Treatment with β-Blockade in Patients with Asymptomatic Aortic Stenosis and Association with Cardiovascular Events. J. Am. Heart Assoc. 2017, 6, e006709. [Google Scholar] [CrossRef]
- Chan, K.L.; Teo, K.; Dumesnil, J.G.; Ni, A.; Tam, J.; ASTRONOMER Investigators. Effect of Lipid Lowering with Rosuvastatin on Progression of Aortic Stenosis: Results of the ASTRONOMER Trial. Circulation 2010, 121, 306–314. [Google Scholar] [CrossRef]
- Rossebø, A.B.; Pedersen, T.R.; Boman, K.; Brudi, P.; Chambers, J.B.; Egstrup, K.; Gerdts, E.; Gohlke-Bärwolf, C.; Holme, I.; Kesäniemi, Y.A.; et al. Intensive Lipid Lowering with Simvastatin and Ezetimibe in Aortic Stenosis. N. Engl. J. Med. 2008, 359, 1343–1356. [Google Scholar] [CrossRef]
- Hirashima, Y.; Nakano, T.; Torisu, K.; Aihara, S.; Wakisaka, M.; Kitazono, T. SGLT2 inhibition mitigates transition from acute kidney injury to chronic kidney disease by suppressing ferroptosis. Sci. Rep. 2024, 14, 20386. [Google Scholar] [CrossRef]
- Kerut, E.K.; Kerut, C.M.; Giles, T.D. SGLT2 inhibitors and valvular heart disease: Potential impact on outcomes in patients with aortic stenosis. Echocardiography 2024, 41, 175–182. [Google Scholar] [CrossRef]
- Paolisso, P.; Belmonte, M.; Gallinoro, E.; Scarsini, R.; Bergamaschi, L.; Portolan, L.; Armillotta, M.; Esposito, G.; Moscarella, E.; Benfari, G.; et al. SGLT2-inhibitors in diabetic patients with severe aortic stenosis and cardiac damage undergoing transcatheter aortic valve implantation (TAVI). Cardiovasc. Diabetol. 2024, 23, 420. [Google Scholar] [CrossRef]
- Kaltoft, M.; Langsted, A.; Nordestgaard, B.G. Obesity as a Causal Risk Factor for Aortic Valve Stenosis. J. Am. Coll. Cardiol. 2020, 75, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz, D.; Yoshida, Y.; Jin, Z.; Nakanishi, K.; Mannina, C.; Elkind, M.S.; Rundek, T.; Homma, S.; Sacco, R.L.; Di Tullio, M.R. Factors associated with the progression of aortic valve calcification in older adults. Int. J. Cardiol. 2023, 381, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Kontogeorgos, S.; Rosengren, A.; Sandström, T.Z.; Fu, M.; Lindgren, M.; Basic, C.; Svanvik, M.; Djekic, D.; Thunström, E. Association Between Body Mass Index and Risk of Aortic Stenosis in Women in the Swedish Medical Birth Registry. J. Am. Heart Assoc. 2024, 13, e034891. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Dina, C.; Small, A.M.; Shaffer, C.M.; Levinson, R.T.; Helgadóttir, A.; Capoulade, R.; Munter, H.M.; Martinsson, A.; Cairns, B.J.; et al. Dyslipidemia, inflammation, calcification, and adiposity in aortic stenosis: A genome-wide study. Eur. Heart J. 2023, 44, 1927–1939. [Google Scholar] [CrossRef]
- Kerut, E.K.; Kerut, C.M.; Giles, T.D. Aortic root and ascending aorta dimensions in adults with aortic stenosis: A retrospective echocardiographic study. Echocardiography 2023, 40, 691–698. [Google Scholar] [CrossRef]
- Chaker, Z.; Badhwar, V.; Alqahtani, F.; Aljohani, S.; Zack, C.J.; Holmes, D.R.; Rihal, C.S.; Alkhouli, M. Sex Differences in the Utilization and Outcomes of Surgical Aortic Valve Replacement for Severe Aortic Stenosis. J. Am. Heart Assoc. 2017, 6, e006370. [Google Scholar] [CrossRef]
- Iung, B.; Delgado, V.; Rosenhek, R.; Price, S.; Prendergast, B.; Wendler, O.; De Bonis, M.; Tribouilloy, C.; Evangelista, A.; Bogachev-Prokophiev, A.; et al. Contemporary Presentation and Management of Valvular Heart Disease: The EURObservational Research Programme Valvular Heart Disease II Survey. Circulation 2019, 140, 1156–1169. [Google Scholar] [CrossRef]


| Parameter | Men with AS (n = 4998) | Men Without AS (n = 35,690) | p-Value |
|---|---|---|---|
| Age, years, Me [Q1; Q3] | 64 [55; 74] | 57 [45; 65] | <0.0001 |
| Aortic diameter at sinuses of Valsalva, mm, Me [Q1; Q3] | 37 [34; 39] | 36 [33; 39] | <0.0001 |
| Ascending aorta diameter, mm, Me [Q1; Q3] | 37 [34; 41] | 34 [31; 37] | <0.0001 |
| BMI, kg/m2, Me [Q1; Q3] | 27 [24; 30] | 27 [25; 31] | 0.23 |
| Peak aortic jet velocity, m/s, Me [Q1; Q3] | 2.74 [2.3; 3.7] | 1.6 [1.3; 1.13] | <0.0001 |
| Peak aortic valve gradient, mmHg, Me [Q1; Q3] | 30 [20; 53] | 6 [5; 8] | <0.0001 |
| LV EF, %, Me [Q1; Q3] | 60 [51; 65] | 58 [48; 64] | <0.0001 |
| Office SBP, mmHg, Me [Q1; Q3] | 140 [127; 150] | 130 [120; 140] | <0.0001 |
| Office DBP, mmHg, Me [Q1; Q3] | 80 [80; 88] | 80 [80; 88] | 0.31 |
| AR, n (%) | 901 (18.03) | 1405 (3.94) | <0.0001 |
| HP, n (%) | 3150 (63.03) | 28,920 (81.03) | <0.0001 |
| DM, n (%) | 485 (9.7) | 3218 (9.02) | 0.11 |
| CAD, n (%) | 1963 (39.3) | 14,250 (39.9) | 0.38 |
| COPD, n (%) | 521 (10.2) | 3707 (10.4) | 0.93 |
| Asthma, n (%) | 110 (2.2) | 741 (2.08) | 0.56 |
| Obesity, n (%) | 417 (8.3) | 4581 (12.8) | <0.0001 |
| HLP, n (%) | 1313 (26.3) | 3685 (10.3) | <0.0001 |
| CHF, n (%) | 2671 (53.4) | 14,908 (41.8) | <0.0001 |
| Parameter | Women with AS (n = 6322) | Women Without AS (n = 37,841) | p-Value |
|---|---|---|---|
| Age, years, Me [Q1; Q3] | 70 [61; 76] | 58 [41; 68] | <0.0001 |
| Aortic diameter at sinuses of Valsalva, mm, Me [Q1; Q3] | 32 [30; 35] | 32 [30; 34] | <0.0001 |
| Ascending aorta diameter, mm, Me [Q1; Q3] | 34 [32; 37] | 31 [28; 34] | <0.0001 |
| BMI, kg/m2, Me [Q1; Q3] | 29 [25; 32] | 27 [23; 31] | <0.0001 |
| Peak aortic jet velocity, m/s, Me [Q1; Q3] | 2.8 [2.3; 3.9] | 1.35 [1.2; 1.5] | <0.0001 |
| Peak aortic valve gradient, mmHg, Me [Q1; Q3] | 32 [20; 60] | 7 [5; 9] | <0.0001 |
| LV EF, %, Me [Q1; Q3] | 62 [58; 65] | 63 [58; 65] | 0.37 |
| Office SBP, mmHg, Me [Q1; Q3] | 140 [125; 150] | 130 [120; 140] | <0.0001 |
| Office DBP, mmHg, Me [Q1; Q3] | 80 [80; 90] | 80 [75; 86] | <0.0001 |
| AR, n (%) | 880 (13.9) | 1274 (3.4) | <0.0001 |
| HP, n (%) | 3794 (60.01) | 26,996 (71.3) | <0.0001 |
| DM, n (%) | 848 (13.4) | 3875 (10.2) | <0.0001 |
| CAD, n (%) | 2254 (35.7) | 9973 (26.4) | <0.0001 |
| COPD, n (%) | 445 (7.04) | 2145 (5.7) | <0.0001 |
| Asthma, n (%) | 218 (3.5) | 1138 (3.01) | 0.06 |
| Obesity, n (%) | 694 (10.9) | 3863 (10.2) | 0.06 |
| HLP, n (%) | 1763 (27.9) | 8901 (23.5) | <0.0001 |
| CHF, n (%) | 3445 (54.5) | 14,170 (37.5) | <0.0001 |
| Parameter | Men with BAV, AS (n = 536) | Men with BAV, no AS (n = 447) | p-Value | Men with TAV, AS (n = 4423) | Men with TAV, no AS (n = 35,280) | p-Value |
|---|---|---|---|---|---|---|
| Age, years, Me [Q1; Q3] | 50 [34; 60] | 29 [21; 46] | <0.0001 | 66 [57; 74] | 57 [46; 65] | <0.0001 |
| Aortic diameter at sinuses of Valsalva, mm, Me [Q1; Q3] | 37 [34; 41] | 37 [33; 41] | <0.05 | 36 [34; 39] | 36 [33; 39] | <0.0001 |
| Ascending aorta diameter, mm, Me [Q1; Q3] | 39 [35; 44] | 24.8 [21.8; 27.8] | <0.0001 | 37 [34; 40] | 34 [31; 37] | <0.0001 |
| BMI, kg/m2, Me [Q1; Q3] | 26.3 [23.9; 30] | 24.8 [21.8; 27.8] | <0.0001 | 27.3 [24.5; 30.3] | 27.4 [24.5; 30.6] | 0.48 |
| Peak aortic jet velocity, m/s, Me [Q1; Q3] | 2.7 [2.3; 3.5] | 1.6 [1.4; 1.8] | <0.0001 | 2.75 [2.3; 3.7] | 1.25 [1.1; 1.4] | <0.0001 |
| Peak aortic valve gradient, mmHg, Me [Q1; Q3] | 29 [20; 50] | 10 [7; 12] | <0.0001 | 30 [20; 53] | 6 [5; 8] | <0.0001 |
| LV EF, %, Me [Q1; Q3] | 63.8 [57.4; 69] | 64.2 [59.5; 69.7] | 0.04 | 62.4 [53.4; 68] | 60.9 [51.5; 67] | <0.0001 |
| Office SBP, mmHg, Me [Q1; Q3] | 135 [124; 142] | 130 [120; 140] | 0.12 | 140 [129; 150] | 130 [120; 140] | <0.0001 |
| Office DBP, mmHg, Me [Q1; Q3] | 80 [75; 85] | 80 [80; 85] | 0.86 | 80 [80; 88] | 80 [80; 87] | 0.46 |
| AR, n (%) | 123 (22.95) | 118 (26.4) | 0.20 | 778 (17.59) | 1286 (3.65) | <0.0001 |
| HP, n (%) | 316 (58.95) | 268 (59.96) | 0.5 | 2803 (63.37) | 25,532 (72.37) | <0.001 |
| DM, n (%) | 31 (5.78) | 17 (3.80) | 0.15 | 451 (10.20) | 3204 (9.08) | 0.2 |
| CAD, n (%) | 127 (23.69) | 46 (10.29) | <0.001 | 1818 (41.10) | 14,222 (40.31) | 0.31 |
| COPD, n (%) | 58 (10.82) | 23 (5.15) | <0.001 | 460 (10.40) | 3687 (10.45) | 0.92 |
| Asthma, n (%) | 22 (4.10) | 9 (2.01) | 0.06 | 88 (1.99) | 732 (2.07) | 0.71 |
| Obesity, n (%) | 61 (11.3) | 19 (4.25) | <0.0001 | 394 (8.9) | 3005 (8.52) | 0.15 |
| HLP, n (%) | 135 (25.19) | 56 (12.53) | <0.001 | 1170 (26.45) | 9061 (25.68) | 0.27 |
| CHF, n (%) | 320 (59.7) | 156 (34.90) | <0.001 | 2332 (52.72) | 14,770 (41.87) | <0.001 |
| Parameter | Women with BAV, AS (n = 365) | Women with BAV, no AS (n = 185) | p-Value | Women with TAV, AS (n = 5928) | Women with TAV, no AS (n = 40,925) | p-Value |
|---|---|---|---|---|---|---|
| Age, years, Me [Q1; Q3] | 49 [31; 61] | 31 [26; 49] | <0.0001 | 71 [62; 77] | 58 [41; 68] | <0.0001 |
| Aortic diameter at sinuses of Valsalva, mm, Me [Q1; Q3] | 32 [30; 35] | 32 [29; 36] | 0.89 | 32 [30; 35] | 32 [30; 34] | <0.0001 |
| Ascending aorta diameter, mm, Me [Q1; Q3] | 36 [32; 40] | 33 [29; 39] | <0.0001 | 34 [31; 37] | 31 [28; 34] | <0.0001 |
| BMI, kg/m2, Me [Q1; Q3] | 25.6 [22.6; 29.9] | 24.6 [21.7; 26.9] | 0.01 | 28.8 [25.2; 32.6] | 27.1 [23.5; 31.2] | <0.0001 |
| Peak aortic jet velocity, m/s, Me [Q1; Q3] | 2.9 [2.4; 3.8] | 1.62 [1.4; 1.8] | <0.001 | 2.8 [2.3; 3.9] | 1.62 [1.4; 1.8] | <0.001 |
| Peak aortic valve gradient, mmHg, Me [Q1; Q3] | 32 [22; 56] | 10 [8; 13] | <0.0001 | 31 [20; 60] | 7 [5; 9] | <0.0001 |
| LV EF, %, Me [Q1; Q3] | 66.9 [61.8; 71.4] | 66 [61; 70] | 0.15 | 65.9 [60.7; 70] | 65.7 [60.6; 70] | 0.34 |
| Office SBP, mmHg, Me [Q1; Q3] | 120 [120; 140] | 120 [110; 127.5] | 0.13 | 140 [130; 150] | 130 [120; 140] | <0.0001 |
| Office DBP, mmHg, Me [Q1; Q3] | 80 [70; 80] | 80 [70; 80] | 0.61 | 80 [80; 90] | 80 [75; 85] | <0.0001 |
| AR, n (%) | 64 (17.53) | 27 (14.59) | 0.38 | 814 (13.74) | 1284 (3.14) | <0.0001 |
| HP, n (%) | 177 (48.5) | 99 (53.5) | 0.1 | 3589 (60.5) | 26,923 (65.78) | 0.3 |
| DM, n (%) | 20 (5.48) | 9 (4.86) | 0.76 | 824 (13.90) | 3870 (9.45) | 0.1 |
| CAD, n (%) | 58 (15.89) | 18 (9.73) | 0.05 | 2183 (36.83) | 9968 (24.35) | <0.001 |
| COPD, n (%) | 15 (4.11) | 5 (2.70) | 0.4 | 426 (7.19) | 2144 (5.23) | 0.5 |
| Asthma, n (%) | 10 (2.74) | 5 (2.70) | 0.98 | 208 (3.51) | 1133 (3.01) | 0.8 |
| Obesity, n (%) | 47 (12.8) | 5 (2.70) | 0.002 | 862 (14.54) | 4026 (9.83) | <0.01 |
| HLP, n (%) | 94 (25.75) | 19 (10.27) | <0.001 | 1661 (28.02) | 8888 (21.71) | <0.01 |
| CHF, n (%) | 94 (62.7) | 179 (44.8) | 0.002 | 1451 (53.9) | 15,891 (38.8) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irtyuga, O.; Babakekhyan, M.; Metsker, O.; Starshinova, A.; Kudlay, D.; Kopanitsa, G. Long-Term Outcomes in Aortic Stenosis: Mortality Analysis in a Selected Patient Group. J. Pers. Med. 2025, 15, 410. https://doi.org/10.3390/jpm15090410
Irtyuga O, Babakekhyan M, Metsker O, Starshinova A, Kudlay D, Kopanitsa G. Long-Term Outcomes in Aortic Stenosis: Mortality Analysis in a Selected Patient Group. Journal of Personalized Medicine. 2025; 15(9):410. https://doi.org/10.3390/jpm15090410
Chicago/Turabian StyleIrtyuga, Olga, Mary Babakekhyan, Oleg Metsker, Anna Starshinova, Dmitry Kudlay, and Georgy Kopanitsa. 2025. "Long-Term Outcomes in Aortic Stenosis: Mortality Analysis in a Selected Patient Group" Journal of Personalized Medicine 15, no. 9: 410. https://doi.org/10.3390/jpm15090410
APA StyleIrtyuga, O., Babakekhyan, M., Metsker, O., Starshinova, A., Kudlay, D., & Kopanitsa, G. (2025). Long-Term Outcomes in Aortic Stenosis: Mortality Analysis in a Selected Patient Group. Journal of Personalized Medicine, 15(9), 410. https://doi.org/10.3390/jpm15090410









