Delayed vs. Concomitant Urethrectomy for Non-Metastatic Urothelial Carcinoma of the Urinary Bladder Undergoing Radical Cystectomy: Perioperative and Survival Outcomes from a Single Tertiary Centre in the United Kingdom
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort Characteristics According to Urethrectomy Timing
2.2. Surgical Procedure and Pathological Analysis
2.3. Statistical Analysis
3. Results
3.1. Study Cohort Characteristics According to Urethrectomy Time
3.2. Multivariable Logistic Modelling for Perioperative Outcomes
3.3. Survival Analysis According to Urethrectomy Time
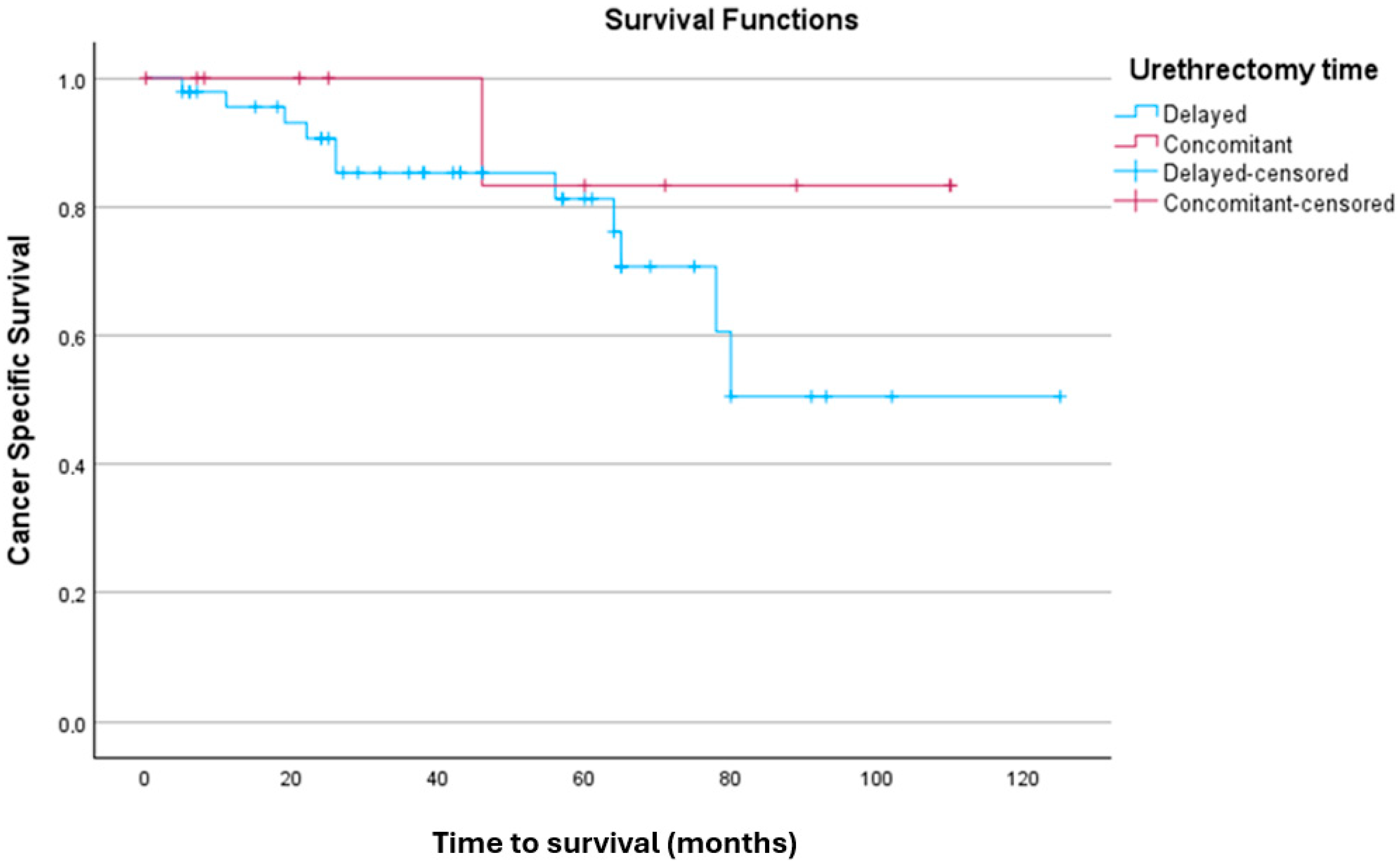
3.3.1. Kaplan–Meier Analysis for CSS
3.3.2. Kaplan–Meier Analysis for OS
3.3.3. Multivariable Cox Regression Models for PFS, CSS, and OS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alfred Witjes, J.; Max Bruins, H.; Carrión, A.; Cathomas, R.; Compérat, E.; Efstathiou, J.A.; Fietkau, R.; Gakis, G.; Lorch, A.; Martini, A.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2023 Guidelines. Eur. Urol. 2024, 85, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Basile, G.; de Angelis, M.; Leni, R.; Re, C.; Longoni, M.; Mari, A.; Soria, F.; Pradere, B.; Del Giudice, F.; Laukhtina, E.; et al. Implications for diagnosis and treatment strategies in non-muscle invasive bladder cancer with variant histology: A systematic review. Minerva Urol. Nephrol. 2023, 75, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Zhang, J.; Chen, S.; Wang, G.; Du, Y.; Tao, Z.; Xu, C.; Tang, Q.; Yang, Y.; Zhou, L.; et al. Long-term impact of synchronous and metachronous bladder cancer on prognosis after radical nephroureterectomy for upper urinary tract urothelial carcinoma: Results from a large population-based cohort in China. Int. J. Surg. 2025. advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Gakis, G.; Black, P.C.; Bochner, B.H.; Boorjian, S.A.; Stenzl, A.; Thalmann, G.N.; Kassouf, W. Systematic Review on the Fate of the Remnant Urothelium after Radical Cystectomy. Eur. Urol. 2017, 71, 545–557. [Google Scholar] [CrossRef]
- Chan, Y.; Fisher, P.; Tilki, D.; Evans, C.P. Urethral recurrence after cystectomy: Current preventative measures, diagnosis and management. BJU Int. 2016, 117, 563–569. [Google Scholar] [CrossRef]
- Laukhtina, E.; Mori, K.; DAndrea, D.; Moschini, M.; Abufaraj, M.; Soria, F.; Mari, A.; Krajewski, W.; Albisinni, S.; Teoh, J.Y.-C.; et al. Incidence, risk factors and outcomes of urethral recurrence after radical cystectomy for bladder cancer: A systematic review and meta-analysis. Urol. Oncol. 2021, 39, 806–815. [Google Scholar] [CrossRef]
- Lopez-Beltran, A.; Cookson, M.S.; Guercio, B.J.; Cheng, L. Advances in diagnosis and treatment of bladder cancer. BMJ 2024, 384, e076743. [Google Scholar] [CrossRef]
- Laukhtina, E.; Boehm, A.; Peyronnet, B.; Bravi, C.A.; Batista Da Costa, J.; Soria, F.; D’aNdrea, D.; Rajwa, P.; Quhal, F.; Yanagisawa, T.; et al. Urethrectomy at the time of radical cystectomy for non-metastatic urothelial carcinoma of the bladder: A collaborative multicenter study. World J. Urol. 2022, 40, 1689–1696. [Google Scholar] [CrossRef]
- Miki, J.; Fukuokaya, W.; Taoka, R.; Saito, R.; Matsui, Y.; Hatakeyama, S.; Kawahara, T.; Matsuda, A.; Kawai, T.; Kato, M.; et al. Oncological outcomes of prophylactic urethrectomy at the time of radical cystectomy for bladder cancer: A nationwide multi-institutional study. Int. J. Urol. 2024, 31, 1009–1016. [Google Scholar] [CrossRef]
- Hakozaki, K.; Kikuchi, E.; Ogihara, K.; Shigeta, K.; Abe, T.; Miyazaki, Y.; Kaneko, G.; Maeda, T.; Yoshimine, S.; Kanai, K.; et al. Significance of prophylactic urethrectomy at the time of radical cystectomy for bladder cancer. Jpn J. Clin. Oncol. 2021, 51, 287–295. [Google Scholar] [CrossRef]
- Chang, S.S.; Bochner, B.H.; Chou, R.; Dreicer, R.; Kamat, A.M.; Lerner, S.P.; Lotan, Y.; Meeks, J.J.; Michalski, J.M.; Morgan, T.M.; et al. Treatment of Non-Metastatic Muscle-Invasive Bladder Cancer: AUA/ASCO/ASTRO/SUO Guideline. J. Urol. 2017, 198, 552–559. [Google Scholar] [CrossRef]
- Lee, D.H.; Song, W. Risk Factors for Urethral Recurrence in Men After Radical Cystectomy with Orthotopic Urinary Diversion for Urothelial Carcinoma: A Retrospective Cohort Study. Cancer Manag. Res. 2020, 12, 6739–6746. [Google Scholar] [CrossRef]
- Laukhtina, E.; Moschini, M.; Soria, F.; Andrea, D.D.; Teoh, J.Y.-C.; Mori, K.; Albisinni, S.; Mari, A.; Krajewski, W.; Cimadamore, A.; et al. Follow-up of the Urethra and Management of Urethral Recurrence After Radical Cystectomy: A Systematic Review and Proposal of Management Algorithm by the European Association of Urology-Young Academic Urologists: Urothelial Carcinoma Working Group. Eur. Urol. Focus 2022, 8, 1635–1642. [Google Scholar] [CrossRef]
- Sherwood, J.B.; Sagalowsky, A.I. The diagnosis and treatment of urethral recurrence after radical cystectomy. Urol. Oncol. 2006, 24, 356–361. [Google Scholar] [CrossRef]
- Ahlering, T.E.; Lieskovsky, G.; Skinner, D.G. Indications for urethrectomy in men undergoing single stage radical cystectomy for bladder cancer. J. Urol. 1984, 131, 657–659. [Google Scholar] [CrossRef] [PubMed]
- Nelles, J.L.; Konety, B.R.; Saigal, C.; Pace, J.; Lai, J. Urologic Diseases in America Project. Urethrectomy following cystectomy for bladder cancer in men: Practice patterns and impact on survival. J. Urol. 2008, 180, 1933–1936, discussion 1936–1937. [Google Scholar] [CrossRef]
- van der Heijden, A.G.; Bruins, H.M.; Carrion, A.; Cathomas, R.; Compérat, E.; Dimitropoulos, K.; Efstathiou, J.A.; Fietkau, R.; Kailavasan, M.; Lorch, A.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2025 Guidelines. Eur. Urol. 2025, 87, 582–600. [Google Scholar] [CrossRef] [PubMed]
- Van Poppel, H.; Sorgeloose, T. Radical cystectomy with or without urethrectomy? Crit. Rev. Oncol. Hematol. 2003, 47, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Spiess, P.E.; Kassouf, W.; Brown, G.; Highshaw, R.; Wang, X.; Do, K.-A.; Kamat, A.M.; Czerniak, B.; Dinney, C.P.; Grossman, H.B. Immediate versus staged urethrectomy in patients at high risk of urethral recurrence: Is there a benefit to either approach? Urology 2006, 67, 466–471. [Google Scholar] [CrossRef]
- Clark, P.B. Urethral carcinoma after cystectomy: The case for routine urethrectomy. J Urol 1984, 90, 173–179. [Google Scholar]
- Santarelli, V.; Carino, D.; Corvino, R.; Salciccia, S.; De Berardinis, E.; Krajewski, W.; Nowak, Ł.; Łaszkiewicz, J.; Szydełko, T.; Nair, R.; et al. Surgical technique and perioperative outcomes of the “Sapienza” urology residency program’s trocar placement configuration during robotic-assisted radical prostatectomy (RARP): A retrospective, single-centre observational study comparing experienced attendings vs. Post-graduate Year I-III residents as bedside assistants. Cancers 2024, 17, 20. [Google Scholar] [CrossRef]
- Zennami, K.; Sumitomo, M.; Nukaya, T.; Takenaka, M.; Ichino, M.; Takahara, K.; Sasaki, H.; Kusaka, M.; Shiroki, R. Impact of Urethra-Preserving Surgery During Radical Cystectomy: An Optimal Urethral Management in the Robotic Era. Clin. Genitourin. Cancer 2024, 22, 102146. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, L.; Pambuccian, S.E.; Barkan, G.A.; Quek, M.L.; Wojcik, E.M. Urine cytology in monitoring recurrence in urothelial carcinoma after radical cystectomy and urinary diversion. Cancer Cytopathol. 2016, 124, 273–278. [Google Scholar] [CrossRef]
- Santarelli, V.; Rosati, D.; Canale, V.; Salciccia, S.; Di Lascio, G.; Bevilacqua, G.; Tufano, A.; Sciarra, A.; Cantisani, V.; Franco, G.; et al. The Current Role of Contrast-Enhanced Ultrasound (CEUS) in the Diagnosis and Staging of Bladder Cancer: A Review of the Available Literature. Life 2024, 14, 857. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.; Panebianco, V.; Narumi, Y.; Del Giudice, F.; Muglia, V.F.; Takeuchi, M.; Ghafoor, S.; Bochner, B.H.; Goh, A.C.; Hricak, H.; et al. Diagnostic Performance of Vesical Imaging Reporting and Data System for the Prediction of Muscle-invasive Bladder Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Oncol. 2020, 3, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Morizane, S.; Honda, M.; Yumioka, T.; Iwamoto, H.; Hikita, K.; Takenaka, A. Technique of en bloc resection of the membranous urethra and bladder during robot-assisted radical cystectomy in patients without simultaneous urethrectomy. Asian J. Endosc. Surg. 2022, 15, 683–687. [Google Scholar] [CrossRef]
- Joniau, S.; Shabana, W.; Verlinde, B.; Van Poppel, H. Prepubic urethrectomy during radical cystoprostatectomy. Eur. Urol. 2007, 51, 915–921. [Google Scholar] [CrossRef]
- Tresh, A.S.; Del Giudice, F.; Li, S.; Basran, S.; De Berardinis, E.; Carino, D.; Santarelli, V.; Rocco, B.; Shighinolfi, M.C.; Mayr, R.; et al. The impact of preoperative venous thromboembolism on patients undergoing TURBT: Perioperative outcomes and healthcare costs from US insurance claims data. BJUI Compass 2025, 6, e481. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, L.; Pan, Y.; Chen, L. A systematic review and meta-analysis of radical cystectomy in the treatment of muscular invasive bladder cancer (MIBC). Transl. Androl. Urol. 2021, 10, 3476–3485. [Google Scholar] [CrossRef]
- Cheng, H.; Clymer, J.W.; Po-Han Chen, B.; Sadeghirad, B.; Ferko, N.C.; Cameron, C.G.; Hinoul, P. Prolonged operative duration is associated with complications: A systematic review and meta-analysis. J. Surg. Res. 2018, 229, 134–144. [Google Scholar] [CrossRef]

| Urethrectomy Time | Delayed | Concomitant | p Value |
|---|---|---|---|
| Total | 47 | 11 | |
| Gender, n (%) | |||
| Male | 45 (95.7) | 7 (63.6) | 0.002 |
| Female | 2 (4.2) | 4 (36.4) | 0.002 |
| Smoking habit, n (%) | |||
| None | 15 (31.9) | 5 (45.5) | 0.495 |
| Ex smoker | 22 (46.8) | 3 (27.3) | 0.495 |
| Current smoker | 10 (21.3) | 3 (27.3) | 0.495 |
| Family history, n (%) | |||
| No | 47 (100) | 10 (90.9) | 0.037 |
| Yes | 0 (0) | 1 (9.1) | 0.037 |
| Age, mean ± SD | 71.91 ± 7.35 | 61.18 ± 12.28 | 0.08 |
| BMI, mean ± SD | 26.52 ± 3.66 | 24.96 ± 2.37 | 0.117 |
| CCI, mean ± SD | 4.62 ± 1.84 | 5.27 ± 1.80 | 0.149 |
| Recurrent bladder cancer, n (%) | 18 (38.3) | 2 (18.2) | 0.206 |
| Previous BCG therapy, n (%) | 14 (29.8) | 2 (18.2) | 0.438 |
| Previous MMC treatment, n (%) | 8 (17) | 1 (9.1) | 0.513 |
| TURBT grade 1973, n (%) | |||
| G2 | 2 (4.3) | 1 (9.1) | 0.514 |
| G3 | 45 (95.7) | 10 (90.9) | 0.514 |
| TURBT grade 2004, n (%) | |||
| LG | 2 (4.3) | 0 (0) | 0.486 |
| HG | 45 (95.7) | 11 (100) | 0.486 |
| CIS at TURBT, n (%) | |||
| No | 16 (34) | 4 (36.4%) | 0.71 |
| Yes | 31 (66) | 7 (63.6%) | 0.71 |
| T stage at TURBT, n (%) | |||
| pTa | 7 (14.9) | 6 (54.5) | <0.0001 |
| pTis | 4 (8.5) | 4 (36.4) | <0.0001 |
| pT1 | 21 (44.7) | 1 (9.1) | <0.0001 |
| pT2 | 15 (31.9) | 0 (0) | <0.0001 |
| RC approach, n (%) | |||
| Open | 8 (17) | 1 (9.1) | 0.513 |
| Robotic | 39 (83) | 10 (90.9) | 0.513 |
| Intracorporeal UD, n (%) | 36 (76.6) | 9 (81.8) | 0.708 |
| Extended PLND template, n (%) | 19 (40.4) | 1 (9.1) | 0.049 |
| Total LNs removed, mean ± SD | 13.68 ± 6.57 | 12.36 ± 1.80 | 0.543 |
| Blood loss (ml), mean ± SD | 422.13 ± 125.74 | 430.00 ± 101.48 | 0.028 |
| Operative time (min), mean ± SD | 342.7 ± 82.7 | 379.6 ± 65.7 | 0.049 |
| Positive frozen section on ureters, n (%) | 5 (10.6) | 0 (0) | 0.258 |
| Positive frozen section on urethra, n (%) | 3 (6.4) | 2 (18.2) | 0.209 |
| Intraoperative complications, n (%) | 6 (12.8) | 2 (18.2) | 0.639 |
| Postoperative complications, n (%) | 16 (34) | 1 (9.1) | 0.09 |
| Postoperative complications, CD, n (%) | |||
| I | 5 (10.6) | 0 | |
| II | 8 (17) | 0 | 0.3 |
| III | 2 (4.3%) | 1 (9.1%) | |
| IV | 1 (2.1%) | 0 | |
| V | 0 | 0 | |
| Cumulative length of stay (d), mean ± SD | 8.7 ± 4.62 | 6.6 ± 4.21 | 0.11 |
| Readmissions, n (%) | 4 (8.5) | 4 (36.4) | 0.016 |
| Urethrectomy months, mean ± SD | 3.12 ± 2.23 | NA | NA |
| Stage at RC, n (%) | |||
| pT0 | 2 (4.3) | 0 (0) | 0.307 |
| pTa | 2 (4.3) | 0 (0) | 0.307 |
| pTis | 15 (31.9) | 6 (54.5) | 0.307 |
| pT1 | 9 (19.1) | 4 (36.4) | 0.307 |
| pT2 | 15 (31.9) | 1 (9.1) | 0.307 |
| pT3 | 4 (8.5) | 0 (0) | 0.307 |
| Concomitant CIS, n (%) | 39 (17) | 6 (45.5) | 0.042 |
| CIS on Ureters, n (%) | 13 (27.7) | 0 (0) | 0.048 |
| LVI, n (%) | 7 (14.9) | 1 (9.1) | 0.615 |
| CIS on Urethra, n (%) | 29 (61.7) | 2 (18.2) | 0.009 |
| pT0 at final urethral specimen, n (%) | 32 (68) | 8 (72.7) | 0.4 |
| Recurrence, n (%) | 17 (36.2) | 2 (18.2) | 0.252 |
| Time to recurrence (months), mean ± SD | 32.72 ± 25.76 | 81.5 ± 9.19 | 0.063 |
| Intraoperative Complications | Postoperative Complications | Readmissions | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | OR | 95% CI | p Value | OR | 95% CI | p Value | OR | 95% CI | p Value |
| Urethrectomy time (concomitant) | 3 | 0.3–32 | 0.3 | 0.16 | 0.01–1.6 | 0.13 | 17.9 | 1.2–265 | 0.036 |
| BMI | 1.22 | 0.9–2.17 | 0.2 | 0.96 | 0.8–1.1 | 0.7 | 0.73 | 0.5–1.04 | 0.08 |
| CCI | 1.8 | 1.1–3 | 0.025 | 1.1 | 0.8–1.5 | 0.6 | 0.74 | 0.4–1.4 | 0.3 |
| Gender | 0.13 | 0.03–5.15 | 0.3 | 0.4 | 0.02–4 | 0.4 | 1.03 | 0.06–17 | 0.9 |
| Smoking habit (former or active) | 1 | 0.1–10 | 0.9 | 1.05 | 0.2–4 | 0.9 | 4 | 0.5–34 | 0.2 |
| RC approach (robotic) | 3 | 0.1–100 | 0.5 | 0.3 | 0.04–1.7 | 0.17 | 0.5 | 0.01–31 | 0.7 |
| EBL | 1.003 | 1.0–1.007 | 0.03 | 1 | 1–1 | 0.9 | 0.99 | 0.98–1 | 0.1 |
| Operative Time | 1 | 0.99–1.01 | 0.5 | 1.002 | 0.99–1.01 | 0.4 | 0.99 | 0.98–1.01 | 0.6 |
| Progression-Free Survival | Cancer-Specific Survival | Overall Survival | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Urethrectomy time | 4.4 | 0.45–42.37 | 0.199 | 2.95 | 0.26–33.53 | 0.382 | 0.827 | 0.16–4.075 | 0.816 |
| Positive frozen section on urethra | 2.2 | 0.27–17.85 | 0.461 | 0.63 | 0.07–5.39 | 0.672 | 1.83 | 0.28–11.73 | 0.52 |
| Urethral CIS | 1.5 | 0.26–9.73 | 0.642 | 2.15 | 0.249–22.52 | 0.551 | 1.68 | 0.16–14.45 | 0.632 |
| LVI | -* | -* | -* | -* | -* | -* | 0.72 | 0.07–7.33 | 0.782 |
| pT Stage at RC | |||||||||
| pTa | Ref | ||||||||
| pTis | 1.52 | 0.19–11.8 | 0.691 | -* | -* | -* | -* | -* | -* |
| pT1 | 3.84 | 0.43–34.9 | 0.228 | -* | -* | -* | -* | -* | -* |
| pT2 | 0.75 | 0.08–6.66 | 0.799 | -* | -* | -* | -* | -* | -* |
| pT3 | 1.11 | 0.1–12.37 | 0.930 | -* | -* | -* | -* | -* | -* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Giudice, F.; Gad, M.; Santarelli, V.; Nair, R.; Abu-Ghanem, Y.; Mensah, E.; Challacombe, B.; Kam, J.; Ibrahim, Y.; Lufti, B.; et al. Delayed vs. Concomitant Urethrectomy for Non-Metastatic Urothelial Carcinoma of the Urinary Bladder Undergoing Radical Cystectomy: Perioperative and Survival Outcomes from a Single Tertiary Centre in the United Kingdom. J. Pers. Med. 2025, 15, 375. https://doi.org/10.3390/jpm15080375
Del Giudice F, Gad M, Santarelli V, Nair R, Abu-Ghanem Y, Mensah E, Challacombe B, Kam J, Ibrahim Y, Lufti B, et al. Delayed vs. Concomitant Urethrectomy for Non-Metastatic Urothelial Carcinoma of the Urinary Bladder Undergoing Radical Cystectomy: Perioperative and Survival Outcomes from a Single Tertiary Centre in the United Kingdom. Journal of Personalized Medicine. 2025; 15(8):375. https://doi.org/10.3390/jpm15080375
Chicago/Turabian StyleDel Giudice, Francesco, Mohamed Gad, Valerio Santarelli, Rajesh Nair, Yasmin Abu-Ghanem, Elsie Mensah, Ben Challacombe, Jonathan Kam, Youssef Ibrahim, Basil Lufti, and et al. 2025. "Delayed vs. Concomitant Urethrectomy for Non-Metastatic Urothelial Carcinoma of the Urinary Bladder Undergoing Radical Cystectomy: Perioperative and Survival Outcomes from a Single Tertiary Centre in the United Kingdom" Journal of Personalized Medicine 15, no. 8: 375. https://doi.org/10.3390/jpm15080375
APA StyleDel Giudice, F., Gad, M., Santarelli, V., Nair, R., Abu-Ghanem, Y., Mensah, E., Challacombe, B., Kam, J., Ibrahim, Y., Lufti, B., Khan, A., Yeasmin, A., Chatterton, K., Amery, S., Spurna, K., Alao, R., Ali Kirmani, S. G., Crocetto, F., Barone, B., ... Khan, M. S. (2025). Delayed vs. Concomitant Urethrectomy for Non-Metastatic Urothelial Carcinoma of the Urinary Bladder Undergoing Radical Cystectomy: Perioperative and Survival Outcomes from a Single Tertiary Centre in the United Kingdom. Journal of Personalized Medicine, 15(8), 375. https://doi.org/10.3390/jpm15080375









