Single-Center Cross-Sectional Analysis of Patients with RA, SpA, and PsA: Data from the Prescription Database
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
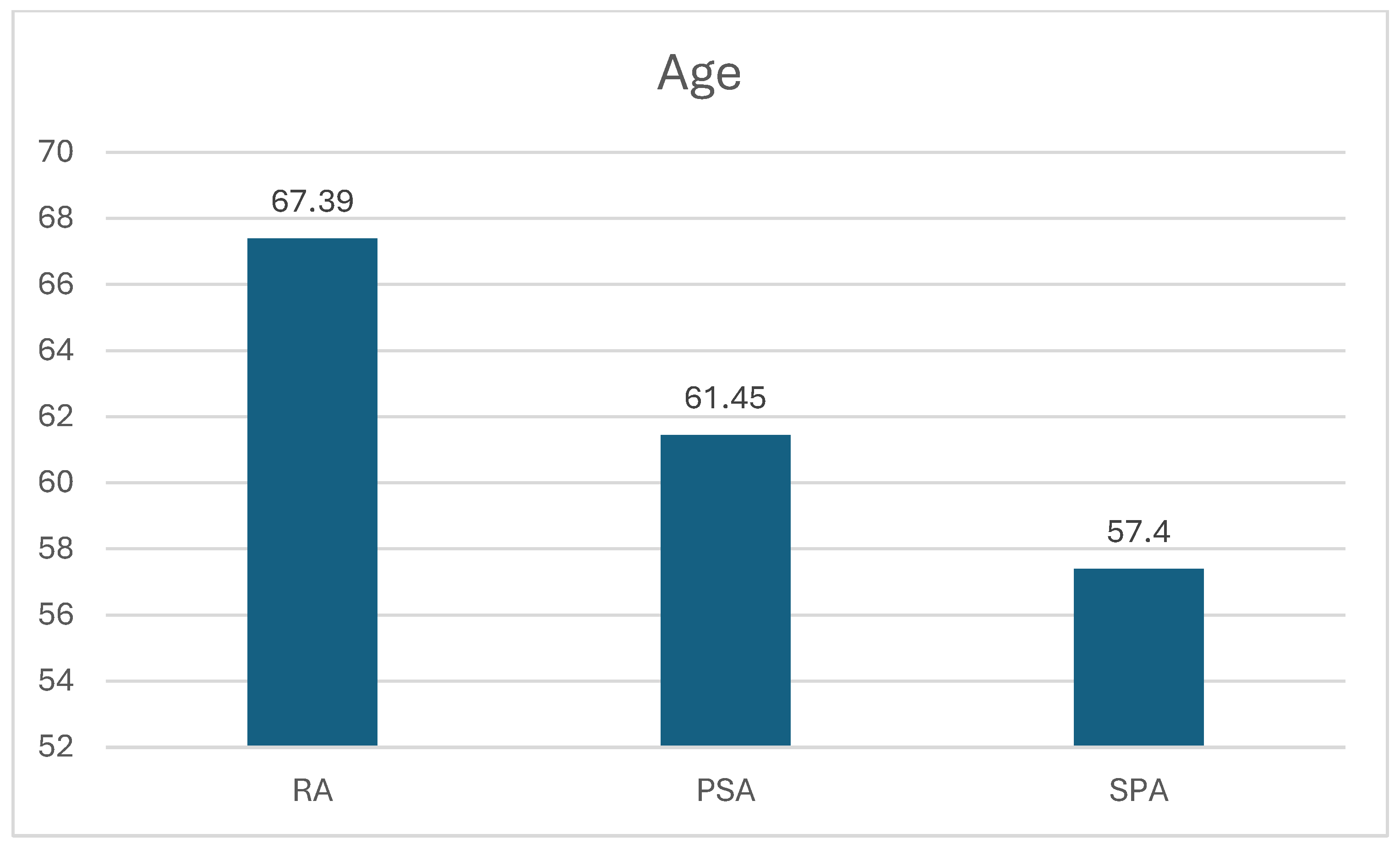
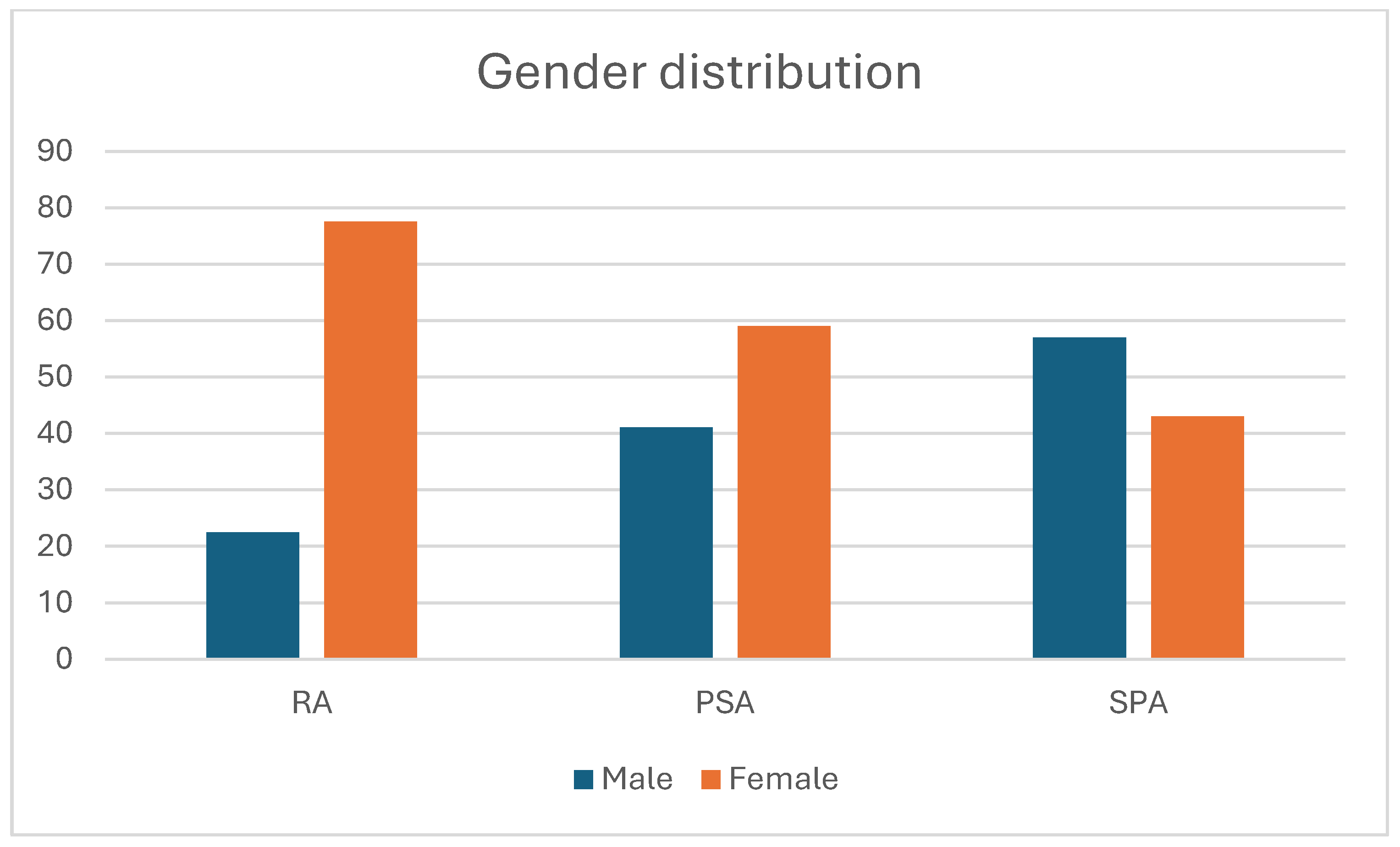
3.1. Rheumatoid Arthritis Patients
3.2. Spondyloarthritis Patients
3.3. Psoriatic Arthritis Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smolen, J.S.; Landewé, R.B.M.; Bergstra, S.A.; Kerschbaumer, A.; Sepriano, A.; Aletaha, D.; Caporali, R.; Edwards, C.J.; Hyrich, K.L.; Pope, J.E.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann. Rheum. Dis. 2023, 82, 3–18. [Google Scholar] [CrossRef]
- Ramiro, S.; Nikiphorou, E.; Sepriano, A.; Ortolan, A.; Webers, C.; Baraliakos, X.; Landewé, R.B.M.; van den Bosch, F.E.; Boteva, B.; Bremander, A.B.I.; et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann. Rheum. Dis. 2023, 82, 19–34. [Google Scholar] [CrossRef]
- Gossec, L.; Kerschbaumer, A.; Ferreira, R.J.O.; Aletaha, D.; Baraliakos, X.; Bertheussen, H.; Boehncke, W.H.; Esbensen, B.A.; McInnes, I.B.; McGonagle, D.; et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2023 update. Ann. Rheum. Dis. 2024, 83, 706–719. [Google Scholar] [CrossRef]
- Cantini, F.; Niccoli, L.; Nannini, C.; Cassarà, E.; Kaloudi, O.; Favalli, E.G.; Becciolini, A.; Benucci, M.; Li-Gobbi, F.; Guiducci, S.; et al. Second-line biologic therapy optimization in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. Semin. Arthritis Rheum. 2017, 47, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Cantini, F.; Goletti, D.; Benucci, M.; Foti, R.; Damiani, A.; Niccoli, L. Tailored first-line biologic and targeted synthetic disease modifying anti-rheumatic drugs therapy in patients with rheumatoid arthritis: 2021 updated ITABIO statements. Expert. Opin. Drug Saf. 2022, 21, 613–623. [Google Scholar] [CrossRef]
- Manfredi, A.; Fornaro, M.; Bazzani, C.; Perniola, S.; Cauli, A.; Rai, A.; Favalli, E.G.; Rossini, M.; Foti, R.; Conti, F.; et al. Retention rate of biologic and targeted synthetic anti-rheumatic drugs in elderly rheumatoid arthritis patients: Data from GISEA registry. Front. Med. 2024, 11, 1349533. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.A.; Suissa, S.; Skovron, M.L.; Frisell, T.; Askling, J.; Michaud, K.; Pedro, S.; Meissner, Y.; Boers, M.; Hoffman, V.; et al. Infection outcomes in patients with rheumatoid arthritis treated with abatacept and other disease-modifying antirheumatic drugs: Results from a 10-year international post-marketing study. Semin. Arthritis Rheum. 2024, 64, 152313. [Google Scholar] [CrossRef] [PubMed]
- Lend, K.; Koopman, F.A.; Lampa, J.; Jansen, G.; Hetland, M.L.; Uhlig, T.; Nordström, D.; Nurmohamed, M.; Gudbjornsson, B.; Rudin, A.; et al. Methotrexate Safety and Efficacy in Combination Therapies in Patients with Early Rheumatoid Arthritis: A Post Hoc Analysis of a Randomized Controlled Trial. Arthritis Rheumatol. 2024, 76, 363–376. [Google Scholar] [CrossRef]
- Lend, K.; Lampa, J.; Padyukov, L.; Hetland, M.L.; Heiberg, M.S.; Nordström, D.C.; Nurmohamed, M.T.; Rudin, A.; Östergaard, M.; Haavardsholm, E.A.; et al. Association of rheumatoid factor, anti-citrullinated protein antibodies and shared epitope with clinical response to initial treatment in patients with early rheumatoid arthritis: Data from a randomised controlled trial. Ann. Rheum. Dis. 2024, 83, 1657–1665. [Google Scholar] [CrossRef]
- Yamada, Z.; Muraoka, S.; Kawazoe, M.; Hirose, W.; Kono, H.; Yasuda, S.; Sugihara, T.; Nanki, T. Long-term effects of abatacept on atherosclerosis and arthritis in older vs. younger patients with rheumatoid arthritis: 3-year results of a prospective, multicenter, observational study. Arthritis Res. Ther. 2024, 26, 87. [Google Scholar] [CrossRef]
- Lee, K.A.; Kim, B.Y.; Kim, S.S.; Cheon, Y.H.; Lee, S.I.; Kim, S.H.; Jung, J.H.; Kim, G.T.; Hur, J.W.; Lee, M.S.; et al. Effect of abatacept versus conventional synthetic disease modifying anti-rheumatic drugs on rheumatoid arthritis-associated interstitial lung disease Korean. J. Intern. Med. 2024, 39, 855–864. [Google Scholar]
- Parisi, S.; Ditto, M.C.; Ghellere, F.; Panaro, S.; Piccione, F.; Borrelli, R.; Fusaro, E. Update on tocilizumab in rheumatoid arthritis: A narrative review. Front. Immunol. 2025, 16, 1470488. [Google Scholar] [CrossRef]
- Jones, G.; Sebba, A.; Gu, J.; Lowenstein, M.B.; Calvo, A.; Gomez-Reino, J.J.; Siri, D.A.; Tomšič, M.; Alecock, E.; Woodworth, T.G.; et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: The AMBITION study. Ann. Rheum. Dis. 2010, 69, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Fornaro, M.; Caporali, R.; Biggioggero, M.; Bugatti, S.; De Stefano, L.; Cauli, A.; Congia, M.; Conti, F.; Chimenti, M.S.; Bazzani, C.; et al. Effectiveness and safety of filgotinib in rheumatoid arthritis patients: Data from the GISEA registry. Clin. Exp. Rheumatol. 2024, 42, 1043–1050. [Google Scholar] [CrossRef]
- van Gendt, J.; Emaus, R.; Visschedijk, M.C.; Touw, D.J.; Bouwknegt, D.G.; de Leeuw, K.; Prins, J.R.; Malik, M.P.; Mian, P. Pharmacokinetics of Monoclonal Antibodies Throughout Pregnancy: A Systematic Literature Review. Clin. Pharmacokinet. 2024, 63, 589–622. [Google Scholar] [CrossRef] [PubMed]
- Avci, A.B.; Feist, E.; Burmester, G.R. Rheumatoid factors revisited in the age of biologic therapy. Rheumatology 2025, 64 (Suppl. S2), ii15–ii24. [Google Scholar] [CrossRef]
- Ytterberg, S.R.; Bhatt, D.L.; Mikuls, T.R.; Koch, G.G.; Fleischmann, R.; Rivas, J.L.; Germino, R.; Menon, S.; Sun, Y.; Wang, C.; et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N. Engl. J. Med. 2022, 386, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Adorni, M.P.; Papotti, B.; Borghi, M.O.; Raschi, E.; Zimetti, F.; Bernini, F.; Meroni, P.L.; Ronda, N. Effect of the JAK/STAT Inhibitor Tofacitinib on Macrophage Cholesterol Metabolism. Int. J. Mol. Sci. 2023, 24, 12571. [Google Scholar] [CrossRef]
- Li, N.; Gou, Z.P.; Du, S.Q.; Zhu, X.H.; Lin, H.; Liang, X.F.; Wang, Y.S.; Feng, P. Effect of JAK inhibitors on high- and low-density lipoprotein in patients with rheumatoid arthritis: A systematic review and network meta-analysis. Clin. Rheumatol. 2022, 41, 677–688. [Google Scholar] [CrossRef]
- Benucci, M.; Li Gobbi, F.; Damiani, A.; Russo, E.; Guiducci, S.; Manfredi, M.; Lari, B.; Grossi, V.; Infantino, M. Real-Life Comparison of Four JAK Inhibitors in Rheumatoid Arthritis (ELECTRA-i Study). J. Clin. Med. 2024, 13, 1821. [Google Scholar] [CrossRef]
- Giollo, A.; Zen, M.; Larosa, M.; Astorri, D.; Salvato, M.; Calligaro, A.; Botsios, K.; Bernardi, C.; Bianchi, G.; Doria, A. Early characterization of difficult-to-treat rheumatoid arthritis by suboptimal initial management: A multicentre cohort study. Rheumatology 2023, 62, 2083–2089. [Google Scholar] [CrossRef] [PubMed]
- Paudel, M.L.; Li, R.; Naik, C.; Shadick, N.; Weinblatt, M.E.; Solomon, D.H. Prevalence and characteristics of adults with difficult-to-treat rheumatoid arthritis in a large patient registry. Rheumatology 2025, 64, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Kanda, R.; Miyazaki, Y.; Nakayamada, S.; Fukuyo, S.; Kubo, S.; Miyagawa, I.; Yamaguchi, A.; Satoh-Kanda, Y.; Ohkubo, N.; Todoroki, Y.; et al. Effective Second-Line b/tsDMARDs for Patients with Rheumatoid Arthritis Unresponsive to First-Line b/tsDMARDs from the FIRST Registry. Rheumatol. Ther. 2025, 12, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, R.; Iqbal, I. Risk: Benefit profile of etanercept in elderly patients with rheumatoid arthritis, ankylosing spondylitis or psoriatic arthritis. Drugs Aging 2007, 24, 239–254. [Google Scholar] [CrossRef]
- Förger, F.; Zbinden, A.; Villiger, P.M. Certolizumab treatment during late pregnancy in patients with rheumatic diseases: Low drug levels in cord blood but possible risk for maternal infections. A case series of 13 patients. Jt. Bone Spine 2016, 83, 341–343. [Google Scholar] [CrossRef]
- Sun, X.; Cui, Z.; Wang, Q.; Liu, L.; Ding, X.; Wang, J.; Cai, X.; Li, B.; Li, X. Formation and clinical effects of anti-drug antibodies against biologics in psoriasis treatment: An analysis of current evidence. Autoimmun. Rev. 2024, 23, 103530. [Google Scholar] [CrossRef]
- Sen, R.; Caplan, L.; Danila, M.I. Cardiovascular disease in spondyloarthritis: A narrative review of risk factors and the effect of treatments. Curr. Opin. Rheumatol. 2024, 36, 95–107. [Google Scholar] [CrossRef]
- Picchianti-Diamanti, A.; Aiello, A.; De Lorenzo, C.; Migliori, G.B.; Goletti, D. Management of tuberculosis risk, screening and preventive therapy in patients with chronic autoimmune arthritis undergoing biotechnological and targeted immunosuppressive agents. Front. Immunol. 2025, 16, 1494283. [Google Scholar] [CrossRef]
- Behrens, F.; Sewerin, P.; de Miguel, E.; Patel, Y.; Batalov, A.; Dokoupilova, E.; Kleinmond, C.; Pournara, E.; Shekhawat, A.; Jentzsch, C.; et al. Chilles study group. Efficacy and safety of secukinumab in patients with spondyloarthritis and enthesitis at the Achilles tendon: Results from a phase 3b trial. Rheumatology 2022, 61, 2856–2866. [Google Scholar] [CrossRef]
- Ni, R.; Zheng, J.; Varghese, J.; Kumar, B. The Impact of Interleukin-17 Inhibitors on Major Adverse Cardiovascular Events in Psoriasis or Psoriatic Arthritis Patients Naive to Biologic Agents: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Cureus 2024, 16, e60980. [Google Scholar] [CrossRef]
- Mease, P.; Roussou, E.; Burmester, G.R.; Goupille, P.; Gottlieb, A.; Moriarty, S.R.; Benichou, O.; Adams, D.H.; Xu, W.; Nash, P. Safety of Ixekizumab in Patients with Psoriatic Arthritis: Results from a Pooled Analysis of Three Clinical Trials. Arthritis Care Res. 2019, 71, 367–378. [Google Scholar] [CrossRef]
- Gladman, D.D.; Orbai, A.M.; Klitz, U.; Wei, J.C.; Gallo, G.; Birt, J.; Rathmann, S.; Shrom, D.; Marzo-Ortega, H. Ixekizumab and complete resolution of enthesitis and dactylitis: Integrated analysis of two phase 3 randomized trials in psoriatic arthritis. Arthritis Res. Ther. 2019, 21, 38. [Google Scholar] [CrossRef]
- Bernardini, N.; Skroza, N.; Marchesiello, A.; Mambrin, A.; Proietti, I.; Tolino, E.; Maddalena, P.; Marraffa, F.; Rossi, G.; Volpe, S.; et al. Psoriatic, patients with a history of cancer: A real-life experience with Apremilast treatment for 104 weeks. Dermatol. Ther. 2022, 35, e15306. [Google Scholar] [CrossRef]
- Mease, P.J.; Hatemi, G.; Paris, M.; Cheng, S.; Maes, P.; Zhang, W.; Shi, R.; Flower, A.; Picard, H.; Stein Gold, L. Apremilast Long-Term Safety Up to 5 Years from 15 Pooled Randomized, Placebo-Controlled Studies of Psoriasis, Psoriatic Arthritis, and Behcet’s Syndrome. Am. J. Clin. Dermatol. 2023, 24, 809–820. [Google Scholar] [CrossRef]
- Molteni, E.; Adinolfi, A.; Bondi, V.; Delvino, P.; Sakellariou, G.; Trentanni, C.; Ughi, N.; Pozzi, M.R.; Scirè, C.A. Novel insights into the management of rheumatoid arthritis: One year in review 2024. Clin. Exp. Rheumatol. 2024, 42, 947–960. [Google Scholar] [CrossRef]
- Manara, M.; Prevete, I.; Marchesoni, A.; D’Angelo, S.; Cauli, A.; Zanetti, A.; Ariani, A.; Bortoluzzi, A.; Parisi, S.; Scirè, C.A.; et al. The Italian Society for Rheumatology recommendations for the management of axial spondyloarthritis. Reumatismo 2021, 73, 71–88. [Google Scholar] [CrossRef]
- Marchesoni, A.; Olivieri, I.; Salvarani, C.; Pipitone, N.; D’Angelo, S.; Mathieu, A.; Cauli, A.; Punzi, L.; Ramonda, R.; Scarpa, R.; et al. Recommendations for the use of biologics and other novel drugs in the treatment of psoriatic arthritis: 2017 update from the Italian Society of Rheumatology. Clin. Exp. Rheumatol. 2017, 35, 991–1010. [Google Scholar]

| Ada | Certo | Eta | Gol | Upa | Tof | Apr | Bim | Gus | Inf | Ixe | Sec | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RA | 6.3 | 3.9 | 18 | 0.7 | 9.3 | 2.7 | 0 | 0 | 0 | 0 | 0 | 0 |
| PsA | 18 | 3.8 | 33 | 0.47 | 2.16 | 2.4 | 6.6 | 3.8 | 1.9 | 1.4 | 10.4 | 15.6 |
| SpA | 38 | 6.8 | 20.3 | 7.8 | 2.5 | 0 | 0.8 | 0.8 | 1.7 | 6.8 | 2.5 | 12 |
| p RA vs. PsA | 0.00033 | NS | 0.004 | NS | 0.001 | NS | 0.02 | NS | NS | 0.021 | 0.015 | NS |
| p RA vs. SpA | 0.00001 | NS | NS | 0.0005 | 0.03 | |||||||
| p PsA vs. SpA | 0.003 | NS | NS | 0.007 | NS |
| Drug | Age | Concomitant Therapy | Biol or Ts Failure | CV Risk Factors | Risk of Hepatotoxicity | Risk of TB and Other Infections | FR and/or Anti-CCP | ILD | Neoplasm | Diabetes |
|---|---|---|---|---|---|---|---|---|---|---|
| abatacept (11%) | 71.19 ± 10.65 | MTX 74% | 64% | 48% | 3.20% | 9.60% | 96.70% | 16% | 3.20% | 6.40% |
| LEF 19% | ||||||||||
| adalimumab (6.3%) | 61.83 ± 10.65 | MTX 55.5% | 72.20% | 33.30% | 0 | 0 | 16.60% | 0.00% | 0.00% | 11% |
| LEF 5.5% | ||||||||||
| baricitinib (2.3%) | 62.5 ± 16.11 | MTX 33% | 66% | 16% | 0 | 16% | 100% | 16% | 0% | 0% |
| certolizumab (3.9%) | 50.64 ± 16.47 | MTX 18% | 27% | 18% | 9% | 18% | 54% | 0% | 0% | 0% |
| LEF 9% | ||||||||||
| etanercept (18%) | 66.9 ± 16.23 | MTX 38.5% | 48% | 33% | 3.80% | 11.50% | 30.70% | 0.00% | 1.90% | 5.70% |
| LFE 7.7% | ||||||||||
| filgotinib (4.8%) | 70.5 ± 14.31 | MTX 7% | 100% | 21% | 0 | 0 | 93% | 0% | 0% | 0% |
| golimumab (0.7%) | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| sarilumab (11%) | 70.22 ± 13.74 | MTX 12.5% | 65.60% | 68.70% | 0.00% | 6.20% | 62.50% | 0.00% | 3% | 9.30% |
| tocilizumab (30%) | 70.5 ± 13.86 | MTX 11.6% | 58.10% | 66.20% | 2.30% | 4.60% | 68.60% | 14% | 4.60% | 11.60% |
| LEF 1.1% | ||||||||||
| tofacitinib (2.7%) | 52.63 ± 12.59 | MTX 12.5% | 87.50% | 12.50% | 0 | 0 | 62.50% | 12% | 0 | 0 |
| upadacitinib (9.3%) | 64.9 ± 10.53 | MTX 3.7% | 100% | 3.70% | 0 | 0 | 92.60% | 3.70% | 3.70% | 0 |
| Drug | Age | Concomitant Therapy | Biol or Ts Failure | CV Risk Factor | Risk of Hepatotoxicity | Risk of TB and Other Infections | Extra Articular Manifestations | Enthesitis/Dactylitis | Neoplasm | Diabetes |
|---|---|---|---|---|---|---|---|---|---|---|
| adalimumab (38%) | 57.61 ± 12.88 | MTX 6.8% | 18% | 23% | 4.50% | 0.00% | 41.00% | 6.80% | 0.00% | 6.80% |
| LEF 13.6% | ||||||||||
| apremilast (0.8) | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| bimekizumab (0.8) | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| certolizumab (6.8%) | 49 ± 11.48 | MTX 12.5% | 62% | 0% | 0% | 0% | 25% | 25% | 0% | 13% |
| etanercept (20.3%) | 61.08 ± 12.24 | SSZ 4% | 29% | 17% | 4.10% | 0.00% | 0.00% | 16.60% | 0.00% | 4.10% |
| golimumab (7.8%) | 59.33 ± 11.75 | MTX 11% | 78% | 44% | 11% | 0% | 44% | 22% | 0% | 22% |
| guselkumab (1.7%) | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| infliximab (6.8%) | 53.88 ± 13 | MTX 12.5% | 37.50% | 37.50% | 0.00% | 0.00% | 37.50% | 25.00% | 0% | 12.50% |
| SSZ 12.5% | ||||||||||
| ixekizumab (2.5%) | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| secukinumab (12%) | 60.71 ± 12 | 0% | 100.00% | 50.00% | 0% | 14.30% | 0% | 71% | 0 | 7% |
| upadacitinib (2.5%) | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Drug | Age | Concomitant Therapy | Biol or Ts Failure | CV Risk Factors | Risk of Hepatotoxicity | Risk of TB and Other Infections | Extra Articular Manifestations | Enthesitis/Dactylitis | Neoplasm | Diabetes |
|---|---|---|---|---|---|---|---|---|---|---|
| adalimumab (18%) | 60.26 ± 13.29 | MTX 15.7% | 55% | 29% | 2.60% | 5.20% | 5.20% | 18.40% | 0.00% | 10.50% |
| LEF 2.6% | ||||||||||
| apremilast (6.6%) | 65.29 ± 10.11 | MTX 14% | 78% | 43% | 0% | 7% | 7% | 64% | 71% | 14% |
| bimekizumab (3.8%) | 65.88 ± 11.38 | MTX 25% | 75% | 62.50% | 0% | 0% | 0% | 75% | 0% | 0% |
| certolizumab (3.8%) | 53 ± 19.10 | 0% | 63% | 38% | 0% | 0% | 0% | 38% | 0% | 0% |
| etanercept (33%) | 62.77 ± 13.57 | MTX 8.7% | 17.30% | 32% | 4.30% | 4.30% | 0% | 10% | 1% | 10% |
| LEF 1.4% | ||||||||||
| SSZ 1.4% | ||||||||||
| filgotinib (0.47%) | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| golimumab (0.47%) | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| guselkumab (1.9%) | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| infliximab (1.4%) | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| ixekizumab (10.4%) | 67.41 ± 10.33 | MTX 9% | 100.00% | 63.60% | 0% | 4.50% | 0% | 100% | 5% | 0% |
| secukinumab (15.6%) | 56.67 ± 11.49 | MTX 6% | 75.70% | 27.20% | 3% | 6% | 0% | 97.00% | 0% | 6% |
| tofacitinib (2.4%) | 53.8 ± 10.06 | MTX 20% | 100.00% | 0% | 0% | 0% | 0% | 60% | 0% | 0% |
| upadacitinib (2.16%) | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benucci, M.; Gobbi, F.L.; Cassarà, E.A.M.; Marigliano, A.L.; Mannoni, A.; Benvenuti, E. Single-Center Cross-Sectional Analysis of Patients with RA, SpA, and PsA: Data from the Prescription Database. J. Pers. Med. 2025, 15, 366. https://doi.org/10.3390/jpm15080366
Benucci M, Gobbi FL, Cassarà EAM, Marigliano AL, Mannoni A, Benvenuti E. Single-Center Cross-Sectional Analysis of Patients with RA, SpA, and PsA: Data from the Prescription Database. Journal of Personalized Medicine. 2025; 15(8):366. https://doi.org/10.3390/jpm15080366
Chicago/Turabian StyleBenucci, Maurizio, Francesca Li Gobbi, Emanuele Antonio Maria Cassarà, Anna Lucia Marigliano, Alessandro Mannoni, and Enrico Benvenuti. 2025. "Single-Center Cross-Sectional Analysis of Patients with RA, SpA, and PsA: Data from the Prescription Database" Journal of Personalized Medicine 15, no. 8: 366. https://doi.org/10.3390/jpm15080366
APA StyleBenucci, M., Gobbi, F. L., Cassarà, E. A. M., Marigliano, A. L., Mannoni, A., & Benvenuti, E. (2025). Single-Center Cross-Sectional Analysis of Patients with RA, SpA, and PsA: Data from the Prescription Database. Journal of Personalized Medicine, 15(8), 366. https://doi.org/10.3390/jpm15080366






