The Role of Intravascular Imaging in Coronary Chronic Total Occlusion PCI: Enhancing Procedural Success Through Real-Time Visualization
Abstract
1. Introduction
2. Intravascular Ultrasound
2.1. IVUS Applications in the Antegrade Approach
- Proximal cap puncture
- Antegrade wiring
- Antegrade dissection and re-entry
2.2. IVUS Applications in the Retrograde Approach
- Retrograde wiring
- Reverse controlled antegrade retrograde tracking
3. IVUS for Lesion Preparation and Stenting in CTO PCI
3.1. Clinical Data
3.2. Critical Evidence Analysis and Controversies in IVUS-Guided CTO Interventions
3.3. Future Implications
4. Optical Coherence Tomography (OCT)
4.1. Practical Utility
4.2. Clinical Data
4.3. OCT Versus IVUS: Clinical Controversies and Evidence Gaps
4.4. Future Implications
5. Coronary Computed Tomography Angiography (CCTA)
5.1. Introduction
5.2. CCTA for Periprocedural Guidance in CTO PCI
5.3. CCTA Co-Registration: Clinical Evidence
5.4. CCTA Co-Registration: Implementation Challenges and Evidence Limitations
6. Future Perspectives
7. Critical Limitations and Implementation Barriers
8. Personalized Medicine Perspectives and Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Fefer, P.; Knudtson, M.L.; Cheema, A.N.; Galbraith, P.D.; Osherov, A.B.; Yalonetsky, S.; Gannot, S.; Samuel, M.; Weisbrod, M.; Bierstone, D.; et al. Current perspectives on coronary chronic total occlusions: The Canadian multicenter chronic total occlusions registry. J. Am. Coll. Cardiol. 2012, 4, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, S.P.; Stuijfzand, W.J.; Opolski, M.P.; van Rossum, A.C.; Nap, A.; Knaapen, P. Percutaneous Coronary Intervention of Chronic Total Occlusions: When and How to Treat. Cardiovasc. Revascularization Med. 2019, 20, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Werner, G.S.; Martin-Yuste, V.; Hildick-Smith, D.; Boudou, N.; Sianos, G.; Gelev, V.; Rumoroso, J.R.; Erglis, A.; Christiansen, E.H.; Escaned, J.; et al. A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur. Heart J. 2018, 39, 2484–2493. [Google Scholar] [CrossRef] [PubMed]
- Werner, G.S.; Hildick-Smith, D.; Yuste, V.M.; Boudou, N.; Sianos, G.; Gelev, V.; Rumoroso, J.R.; Erglis, A.; Christiansen, E.H.; Escaned, J.; et al. Three-year outcomes of A Randomized Multicentre Trial Comparing Revascularization and Optimal Medical Therapy for Chronic Total Coronary Occlusions (EuroCTO). Euro Interv. 2023, 19, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.M.; Kang, S.J.; Yoon, S.H.; Park, H.W.; Kang, S.M.; Lee, J.Y.; Lee, S.W.; Kim, Y.H.; Lee, C.W.; Park, S.W.; et al. Meta-analysis of outcomes after intravascular ultrasound-guided versus angiography-guided drug-eluting stent implantation in 26,503 patients enrolled in three randomized trials and 14 observational studies. Am. J. Cardiol. 2014, 4, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Yock, P.J.; Yock, P.G.; Fitzgerald, P.J.; Yock, P.G. Intravascular Ultrasound: State of the Art and Future Directions. Am. J. Cardiol. 1998, 81, 27E–32E. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Yock, P. Intravascular Ultrasound: Novel Pathophysiological Insights and Current Clinical Applications. Circulation 2001, 103, 604–616. [Google Scholar] [CrossRef]
- Chieffo, A.; Latib, A.; Caussin, C.; Presbitero, P.; Galli, S.; Menozzi, A.; Varbella, F.; Mauri, F.; Valgimigli, M.; Arampatzis, C.; et al. A prospective, randomized trial of intravascular-ultrasound guided compared to angiography guided stent implantation in complex coronary lesions: The AVIO trial. Am. Heart J. 2013, 165, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Okamura, A.; Iwakura, K.; Iwamoto, M.; Nagai, H.; Sumiyoshi, A.; Tanaka, K.; Tanaka, T.; Inoue, K.; Koyama, Y.; Fujii, K. Tip Detection Method Using the New IVUS Facilitates the 3-Dimensional Wiring Technique for CTO Intervention. JACC Cardiovasc. Interv. 2020, 13, 74–82. [Google Scholar]
- McLeod, C.; Moran, C.M.; McBride, K.A.; Pye, S.D. Evaluation of Intravascular Ultrasound Catheter-Based Transducers Using the Resolution Integral. Ultrasound Med. Biol. 2018, 44, 2802–2812. [Google Scholar]
- Sung, J.H.; Chang, J.H.; Chang, J.H.; Pantea, C. Mechanically Rotating Intravascular Ultrasound (IVUS) Transducer: A Review. Sensors 2021, 21, 3907. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Wu, H.; Kim, S.; Dai, X.; Jiang, X. Recent Advances in Transducers for Intravascular Ultrasound (IVUS) Imaging. Sensors 2021, 21, 3540. [Google Scholar] [CrossRef] [PubMed]
- Gersh, B.J.; Gersh, B.J. Trends in Outcomes After Percutaneous Coronary Intervention for Chronic Total Occlusions: A 25-Year Experience From the Mayo Clinic. Yearb. Cardiol. 2008, 2008, 252–253. [Google Scholar] [CrossRef]
- Dong, S.; Smorgick, Y.; Nahir, M.; Lotan, C.; Mosseri, M.; Nassar, H.; Gotsman, M.S.; Hasin, Y. Predictors for successful angioplasty of chronic totally occluded coronary arteries. J. Interv. Cardiol. 2005, 18, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dingli, P.; Ryan, N.; Gonzalo, N.; Dingli, P. Intravascular ultrasound guidance of percutaneous coronary intervention in ostial chronic total occlusions: A description of the technique and procedural results. Int. J. Cardiovasc. Imaging 2017, 33, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Miyazaki, S.; Morii, I.; Daikoku, S.; Goto, Y.; Nonogi, H. Percutaneous transluminal coronary angioplasty of chronic total occlusions. Determinants of primary success and long-term clinical outcome. Catheter. Cardiovasc. Interv. 2000, 49, 258–264. [Google Scholar] [PubMed]
- Puma, J.A.; Sketch, M.H.; Tcheng, J.E.; Harrington, R.A.; Phillips, H.R.; Stack, R.S.; Califf, R.M. Percutaneous Revascularization of Chronic Coronary Occlusions: An Overview. J. Am. Coil Cardiol. 1995, 26, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, M.; Ogata, N.; Araki, H.; Ashida, K.; Isomura, N.; Mikoshiba, Y.; Obara, C. Intravascular ultrasound guided wiring for chronic total occlusions. Indian Heart J. 2006, 58, 15–20. [Google Scholar] [PubMed]
- Ito, T.; Ito, S.; Suzuki, T.; Ito, T. Novel technique using intravascular ultrasound-guided guidewire cross in coronary intervention for uncrossable chronic total occlusions. Circ. J. 2004, 68, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Park, H.S.; Jang, G.-L.; Lee, D.-Y.; Lee, H.; Lee, J.H.; Kang, H.J.; Yang, D.H.; Cho, Y.; Chae, S.-C.; et al. Intravascular ultrasound guided recanalization of stumpless chronic total occlusion. Int. J. Cardiol. 2011, 148, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.; Katoh, O.; Tuschikane, E.; Oida, A.; Suzuki, T.; Takase, S. A novel modification of the retrograde approach for the recanalization of chronic total occlusion of the coronary arteries intravascular ultrasound-guided reverse controlled antegrade and retrograde tracking. JACC Cardiovasc. Interv. 2010, 3, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.; Terashima, M.; Suzuki, T. Value of intravascular ultrasound in the management of coronary chronic total occlusions. Catheter. Cardiovasc. Interv. 2009, 4, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Furuichi, S.; Airoldi, F.; Colombo, A. Intravascular ultrasound-guided wiring for chronic total occlusion. Catheter. Cardiovasc. Interv. 2007, 4, 856–859. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-T.; Lee, W.-H.; Kuo, H.-F.; Chen, M.Z.; Hsu, P.-C.; Chu, C.-S.; Su, H.-M.; Lin, T.-H.; Yen, H.-W.; Chiu, C.-A. Ping-Pong Guide Catheters to Facilitate Real-Time Intravascular Ultrasound-Guided Recanalization of Stumpless Chronic Total Occlusion. JACC Case Rep. 2019, 1, 792–795. [Google Scholar] [CrossRef] [PubMed]
- Bavishi, C.; Sardar, P.; Chatterjee, S.; Khan, A.R.; Shah, A.; Ather, S.; Lemos, P.A.; Moreno, P.; Stone, G.W. Intravascular ultrasound–guided vs angiography-guided drug-eluting stent implantation in complex coronary lesions: Meta-analysis of randomized trials. Am. Hear. J. 2017, 185, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Okamura, A.; Nagai, H.; Iwakura, K. Tip detection-antegrade dissection and reentry using intravascular ultrasound in chronic total occlusion intervention: First human case report. Eur. Heart J. Case Rep. 2022, 6, ytac233. [Google Scholar] [CrossRef] [PubMed]
- Valenti, R.; Vergara, R.; Migliorini, A.; Parodi, G.; Carrabba, N.; Cerisano, G.; Dovellini, E.V.; Antoniucci, D. Predictors of reocclusion after successful drug-eluting stent-supported percutaneous coronary intervention of chronic total occlusion. J. Am. Coll. Cardiol. 2013, 61, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, Y.; Liu, Y.; Cao, F.; Chen, Y. Long-term clinical outcomes of successful revascularization with drug-eluting stents for chronic total occlusions: A systematic review and meta-analysis. Catheter. Cardiovasc. Interv. 2017, 89, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Sianos, G.; Werner, G.S.; Galassi, A.R.; Papafaklis, M.I.; Escaned, J.; Hildick-Smith, D.; Christiansen, E.H.; Gershlick, A.; Carlino, M.; Karlas, A.; et al. Recanalisation of chronic total coronary occlusions: 2012 consensus document from the EuroCTO club. EuroIntervention 2012, 8, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-S.; Song, Y.-J.; Kang, W.; Jin, H.-Y.; Seo, J.-S.; Yang, T.-H.; Kim, D.-K.; Cho, K.-I.; Kim, B.-H.; Park, Y.H.; et al. Intravascular ultrasound-guided implantation of drug-eluting stents to improve outcome: A meta-analysis. JACC Cardiovasc. Interv. 2014, 7, 233–243. [Google Scholar]
- Shin, D.-H.; Hong, S.-J.; Mintz, G.; Kim, J.-S.; Kim, B.-K.; Ko, Y.-G.; Choi, D.; Jang, Y.; Hong, M.-K. Effects of Intravascular Ultrasound-Guided Versus Angiography-Guided New-Generation Drug-Eluting Stent Implantation: Meta-Analysis With Individual Patient-Level Data From 2345 Randomized Patients. JACC Cardiovasc. Interv. 2016, 9, 2232–2239. [Google Scholar]
- Wang, F.; Gao, X.F.; Wang, Z.M.; Wang, F. Intravascular ultrasound guidance reduces cardiac death and coronary revascularization in patients undergoing drug-eluting stent implantation: Results from a meta-analysis of 9 randomized trials and 4724 patients. Int. J. Cardiovasc. Imaging 2019, 35, 239–247. [Google Scholar]
- Fujii, K.; Mintz, G.S.; Kobayashi, Y.; Carlier, S.G.; Takebayashi, H.; Yasuda, T.; Moussa, I.; Dangas, G.; Mehran, R.; Lansky, A.J.; et al. Contribution of stent underexpansion to recurrence after sirolimus-eluting stent implantation for in-stent restenosis. Circulation 2004, 109, 1085–1088. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Hong, M.K.; Mintz, G.S.; Lee, C.W. Intravascular ultrasound predictors of angiographic restenosis after sirolimus-eluting stent implantation. Eur. Heart J. 2006, 27, 1305–1310. [Google Scholar]
- Popovic, M.; Tasic, M.; Grubisa, M.; Grubisa, M. Efficacy And Safety Of IVUS-Guided Percutaneous Coronary Interventions. Serbian J. Exp. Clin. Res. 2015, 16, 115–119. [Google Scholar] [CrossRef]
- Mintz, G.S.; Ali, Z.; Maehara, A.; Maehara, A. Use of intracoronary imaging to guide optimal percutaneous coronary intervention procedures and outcomes. Heart 2021, 107, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.J.; Chen, L.; Xu, T.; Xue, X.J. Intravascular ultrasound-guided drug-eluting stent implantation is associated with improved clinical outcomes in patients with unstable angina and complex coronary artery true bifurcation lesions. Int. J. Cardiovasc. Imaging 2018, 34, 1685–1696. [Google Scholar]
- Mintz, G.S.; Kang, S.J.; Mintz, G.S. Outcomes with intravascular ultrasound-guided stent implantation: A meta-analysis of randomized trials in the era of drug-eluting stents. J. Thorac. Dis. 2016, 8, E841–E843. [Google Scholar][Green Version]
- Tian, N.-L.; Gami, S.-K.; Ye, F.; Zhang, J.-J.; Liu, Z.-Z.; Lin, S.; Ge, Z.; Shan, S.-J.; You, W.; Chen, L.; et al. Angiographic and clinical comparisons of intravascular ultrasound- versus angiography-guided drug-eluting stent implantation for patients with chronic total occlusion lesions: Two-year results from a randomized AIR-CTO study. Euro Interv. 2015, 10, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Investigators, T.R.C.P.; Hong, D.; Kim, S.M. Prognostic Impact of Intravascular Imaging-Guided Percutaneous Coronary Intervention in Chronic Total Occlusion. Circulation 2023, 148, 903–905. [Google Scholar] [CrossRef]
- Kim, B.-K.; Shin, D.-H.; Hong, M.-K.; Park, H.S.; Rha, S.-W.; Mintz, G.S.; Kim, J.-S.; Kim, J.S.; Lee, S.-J.; Kim, H.-Y.; et al. Clinical Impact of Intravascular Ultrasound-Guided Chronic Total Occlusion Intervention With Zotarolimus-Eluting Versus Biolimus-Eluting Stent Implantation: Randomized Study. Circ. Cardiovasc. Interv. 2015, 8, e002592. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Choi, K.H.; Song, Y.; Bin Lee, J.Y.; Lee, S.J.; Lee, S.Y.; Kim, S.M.; Yun, K.H.; Cho, J.Y.; Kim, C.J.; et al. Intravascular Imaging-Guided or Angiography-Guided Complex PCI. N. Engl. J. Med. 2023, 388, 1668–1679. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Kim, B.K.; Cho, I.; Kim, H.Y.; Rha, S.W.; Lee, S.H.; Park, S.M.; Kim, Y.H.; Chang, H.-J.; Ahn, C.-M.; et al. Effect of Coronary CTA on Chronic Total Occlusion Percutaneous Coronary Intervention: A Randomized Trial. JACC Cardiovasc. Imaging 2021, 14, 1993–2004. [Google Scholar]
- Stone, L.L.; Ghoshhajra, B.B.; Takx, R.A.P.; Stone, L.L. Real-time fusion of coronary CT angiography with X-ray fluoroscopy during chronic total occlusion PCI. Eur. Radiol. 2017, 27, 2464–2473. [Google Scholar]
- Shah, A.; Xenogiannis, I.; Jaffer, F.A.; Shah, A.R.; Omer, M.; Megaly, M.; Vemmou, E.; Nikolakopoulos, I.; Rangan, B.; Garcia, S.; et al. Computed tomography angiography co-registration with real-time fluoroscopy in percutaneous coronary intervention for chronic total occlusions. Euro Interv. 2021, 17, e433–e435. [Google Scholar]
- Kwiecinski, J.; Oleksiak, A.; Kruk, M.; Zysk, A.; Debski, A.; Knaapen, P.; Schumacher, S.P.; Barbero, U.; Witkowski, A.; Kepka, C.; et al. Computed tomography perfusion and angiography in patients with chronic total occlusion undergoing percutaneous coronary intervention. Atherosclerosis 2023, 381, 117174. [Google Scholar] [CrossRef] [PubMed]
- Opolski, M.P.; Kwiecinski, J.; Oleksiak, A.; Kruk, M.; Debski, A.; Knaapen, P.; Schumacher, S.P.; Zysk, A.; Witkowski, A.; Kepka, C. Feasibility of computed tomography perfusion in patients with chronic total occlusion undergoing percutaneous coronary intervention. J. Cardiovasc. Comput. Tomogr. 2022, 16, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, N.; Zhang, J.; Li, M.; Lu, Z.; Wei, M.; Lu, B.; Zhang, Y. Procedural success of CTO recanalization: Comparison of the J-CTO score determined by coronary CT angiography to invasive angiography. J. Cardiovasc. Comput. Tomogr. 2015, 9, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Fujino, A.; Otsuji, S.; Hasegawa, K.; Arita, T.; Takiuchi, S.; Fujii, K.; Yabuki, M.; Ibuki, M.; Nagayama, S.; Ishibuchi, K.; et al. Accuracy of J-CTO Score Derived From Computed Tomography Versus Angiography to Predict Successful Percutaneous Coronary Intervention. JACC Cardiovasc. Imaging 2018, 11, 209–217. [Google Scholar]
- Li, J.; Wang, R.; Tesche, C.; Schoepf, U.J.; Pannell, J.T.; He, Y.; Huang, R.; Chen, Y.; Li, J.; Song, X. CT Angiography-Derived RECHARGE Score Predicts Successful Percutaneous Coronary Intervention in Patients with Chronic Total Occlusion. Korean J. Radiol. 2021, 22, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.; Yu, C.W.; Lee, H.J.; Suh, J. Coronary Computed Tomography Angiography Predicts Guidewire Crossing and Success of Percutaneous Intervention for Chronic Total Occlusion: Korean Multicenter CTO CT Registry Score as a Tool for Assessing Difficulty in Chronic Total Occlusion Percutaneous Coronary Intervention. Circ. Cardiovasc. Imaging 2017, 10, e005800. [Google Scholar]
- Opolski, M.P.; Opolski, M.P. Cardiac Computed Tomography for Planning Revascularization Procedures. J. Thorac. Imaging 2018, 33, 35–54. [Google Scholar] [CrossRef] [PubMed]
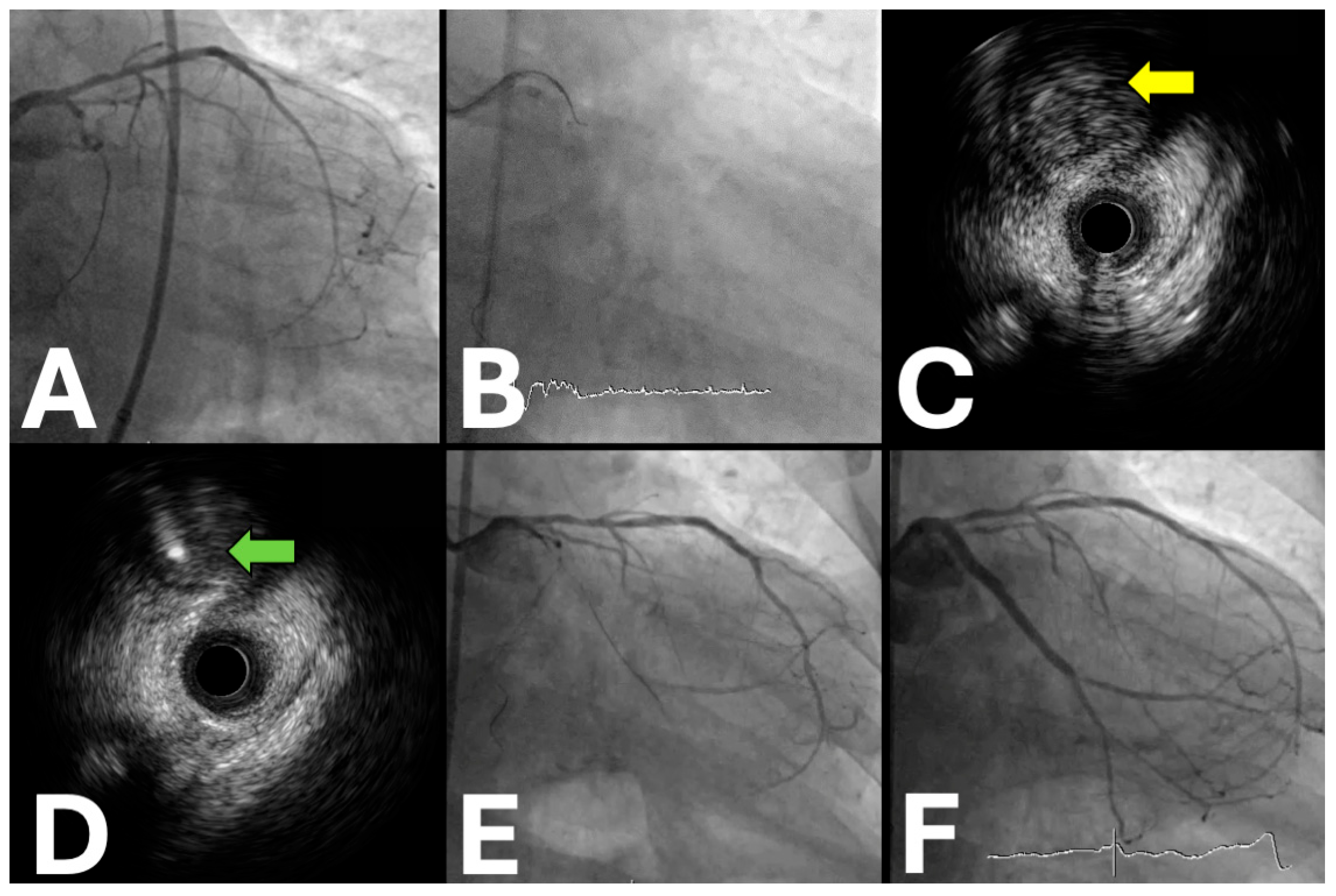
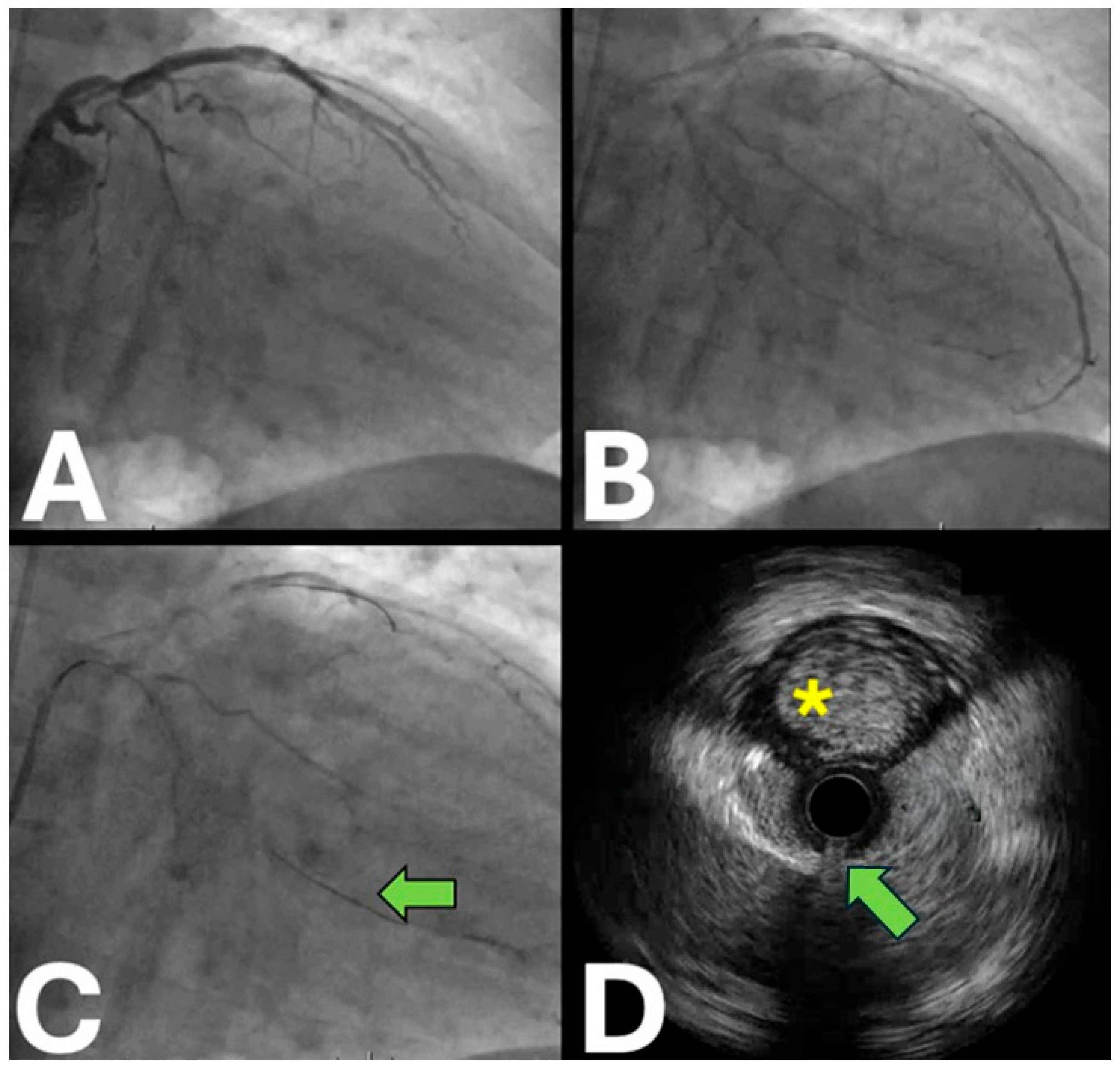
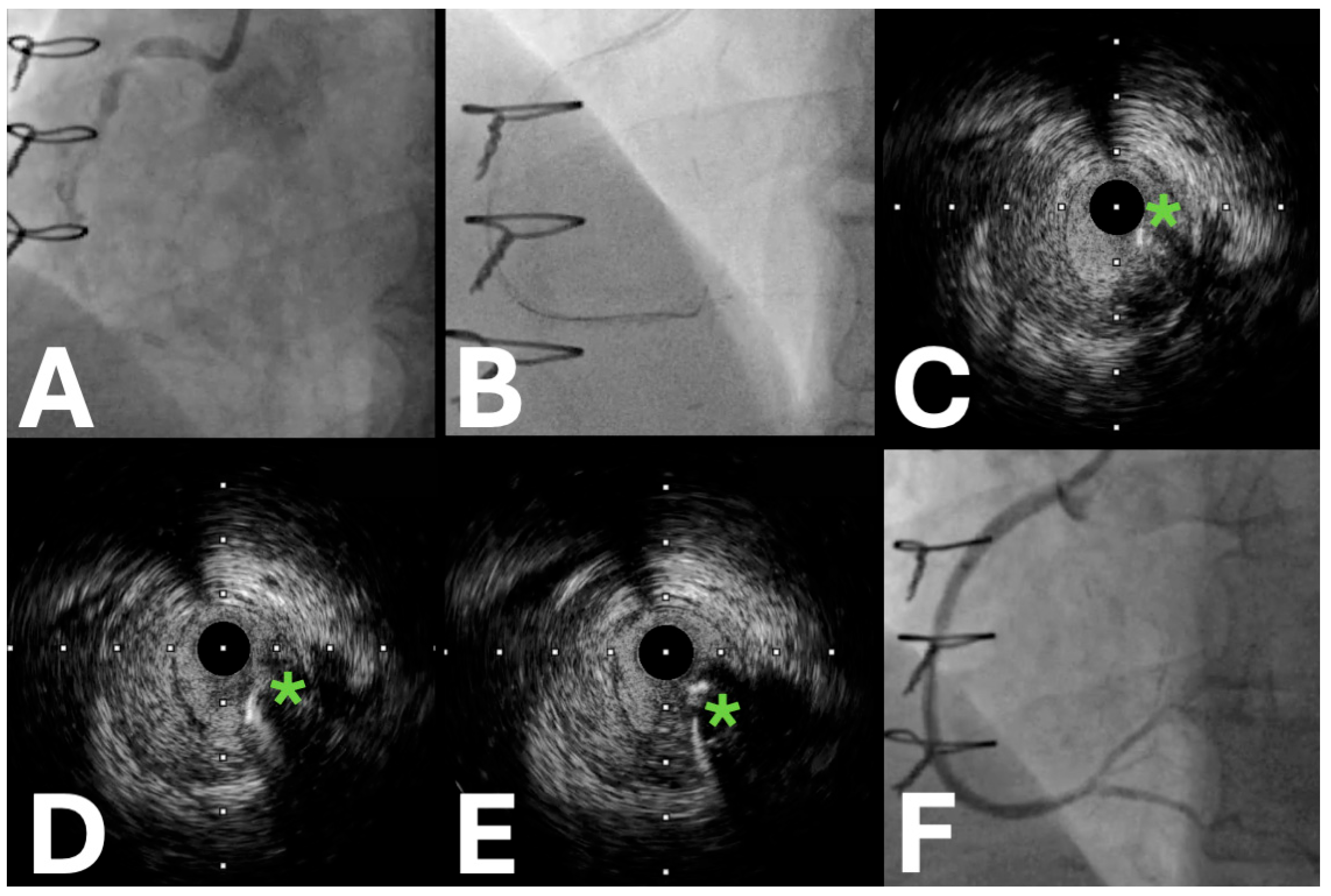
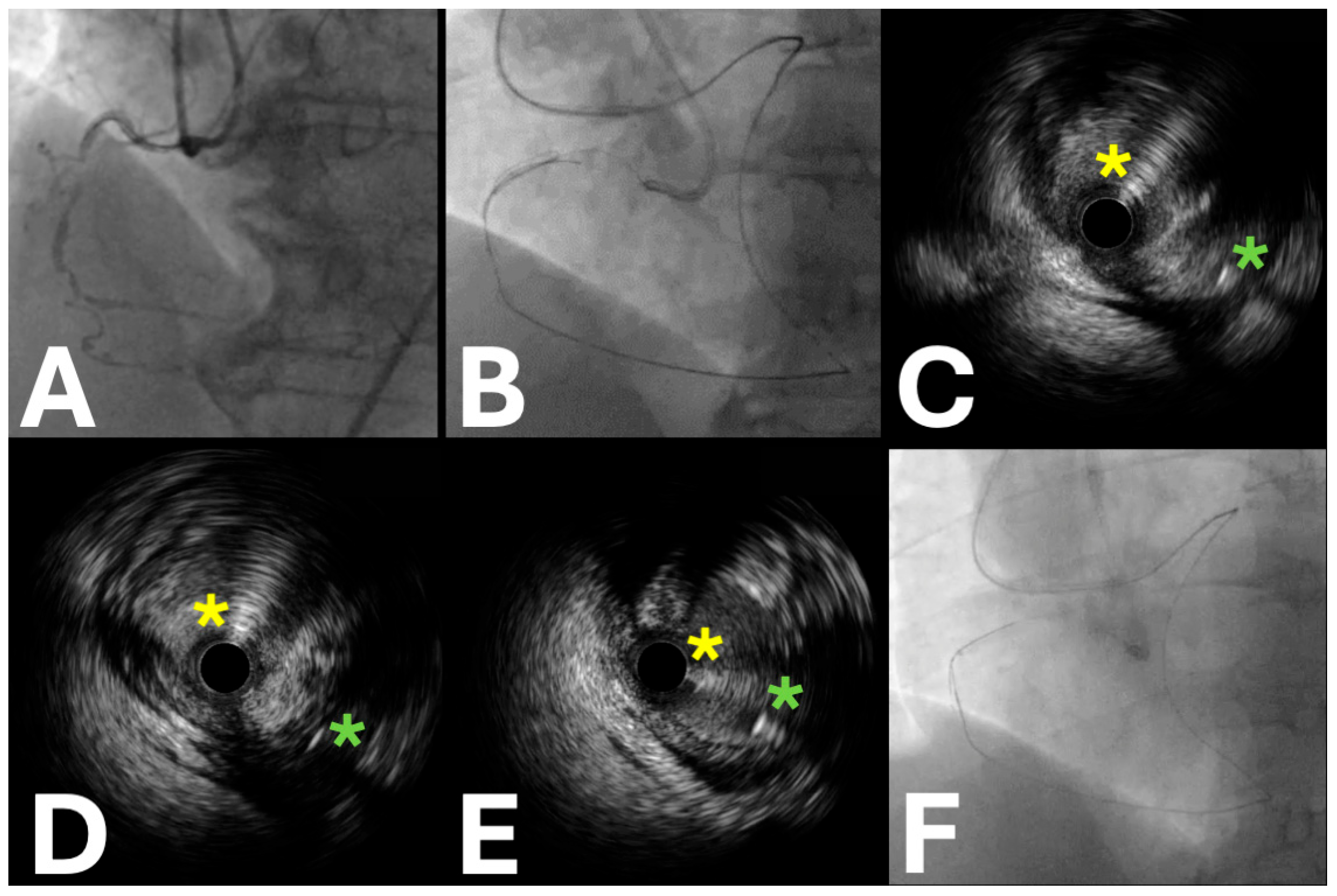
| Trial Name | Sample Size | Locations | Period | Primary Endpoint (s) | Secondary Endpoints | Key Inclusion Criteria | Study Design | Patient Population | Expected Clinical Impact |
|---|---|---|---|---|---|---|---|---|---|
| CRUISE-CTO | 1448 | China (45 centers) | 2022–2031 | MACE (death, MI, stent thrombosis, target vessel revascularization) at 12 months |
|
| Prospective, multicenter, open-label RCT | CTO lesions with moderate–high complexity |
|
| IMPROVE | 2500–3100 | US, Canada, Europe (120 centers) | 2020–2027 | 1. Minimum stent area by IVUS 2. Target vessel failure at 12 months |
|
| Prospective, single-blind RCT | Complex lesions, including CTO, bifurcations, calcified lesions, and long lesions |
|
| IVUS-CHIP | 2022 | 7 European countries (40 centers) | 2021–2025 | Target vessel failure (cardiac death, target vessel MI, target vessel revascularization) at 2 years |
|
| Randomized, controlled, multicenter | Complex coronary lesions including calcified, ostial, bifurcation, left main, CTO |
|
| Study Authors | Score Name | Design | Recruitment Period | Type of CT | No. of CTOs | Retrograde Approach | External Validation |
|---|---|---|---|---|---|---|---|
| Li et al. [48] | J-CTO CT | NR, retrospective | 2011–2014 | 64-slice dual source | 171 | NR | Yes |
| Fujino et al. [49] | J-CTO CT | Single-center, retrospective | 2012–2016 | 320-slice | 218 | 33% | Yes |
| Li et al. [50] | RECHARGE CT | Multicenter, retrospective | 2016–2019 | 64/128-slice dual source | 367 | 28% | Yes |
| Yu et al. [51] | KCCT | Multicenter, retrospective | 2007–2015 | 64-slice (including dual source) | 456 | 12% | No |
| Opolski et al. [52] | CT-RECTOR | Multicenter, retrospective | 2007–2013 | 64/128-slice dual source | 240 | 11% | Yes |
| Imaging Modality | Advantages | Disadvantages |
|---|---|---|
| IVUS |
|
|
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
|
|
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| ||
| CCTA Co-registration |
|
|
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sliman, H.; Kasem Ali Sliman, R.; Knaapen, P.; Nap, A.; Sobieszek, G.; Opolski, M.P. The Role of Intravascular Imaging in Coronary Chronic Total Occlusion PCI: Enhancing Procedural Success Through Real-Time Visualization. J. Pers. Med. 2025, 15, 318. https://doi.org/10.3390/jpm15070318
Sliman H, Kasem Ali Sliman R, Knaapen P, Nap A, Sobieszek G, Opolski MP. The Role of Intravascular Imaging in Coronary Chronic Total Occlusion PCI: Enhancing Procedural Success Through Real-Time Visualization. Journal of Personalized Medicine. 2025; 15(7):318. https://doi.org/10.3390/jpm15070318
Chicago/Turabian StyleSliman, Hussein, Rim Kasem Ali Sliman, Paul Knaapen, Alex Nap, Grzegorz Sobieszek, and Maksymilian P. Opolski. 2025. "The Role of Intravascular Imaging in Coronary Chronic Total Occlusion PCI: Enhancing Procedural Success Through Real-Time Visualization" Journal of Personalized Medicine 15, no. 7: 318. https://doi.org/10.3390/jpm15070318
APA StyleSliman, H., Kasem Ali Sliman, R., Knaapen, P., Nap, A., Sobieszek, G., & Opolski, M. P. (2025). The Role of Intravascular Imaging in Coronary Chronic Total Occlusion PCI: Enhancing Procedural Success Through Real-Time Visualization. Journal of Personalized Medicine, 15(7), 318. https://doi.org/10.3390/jpm15070318








