Fetal Thigh Circumference Nomograms Across Gestational Ages: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Ultrasound Examinations
2.3. Statistical Analysis
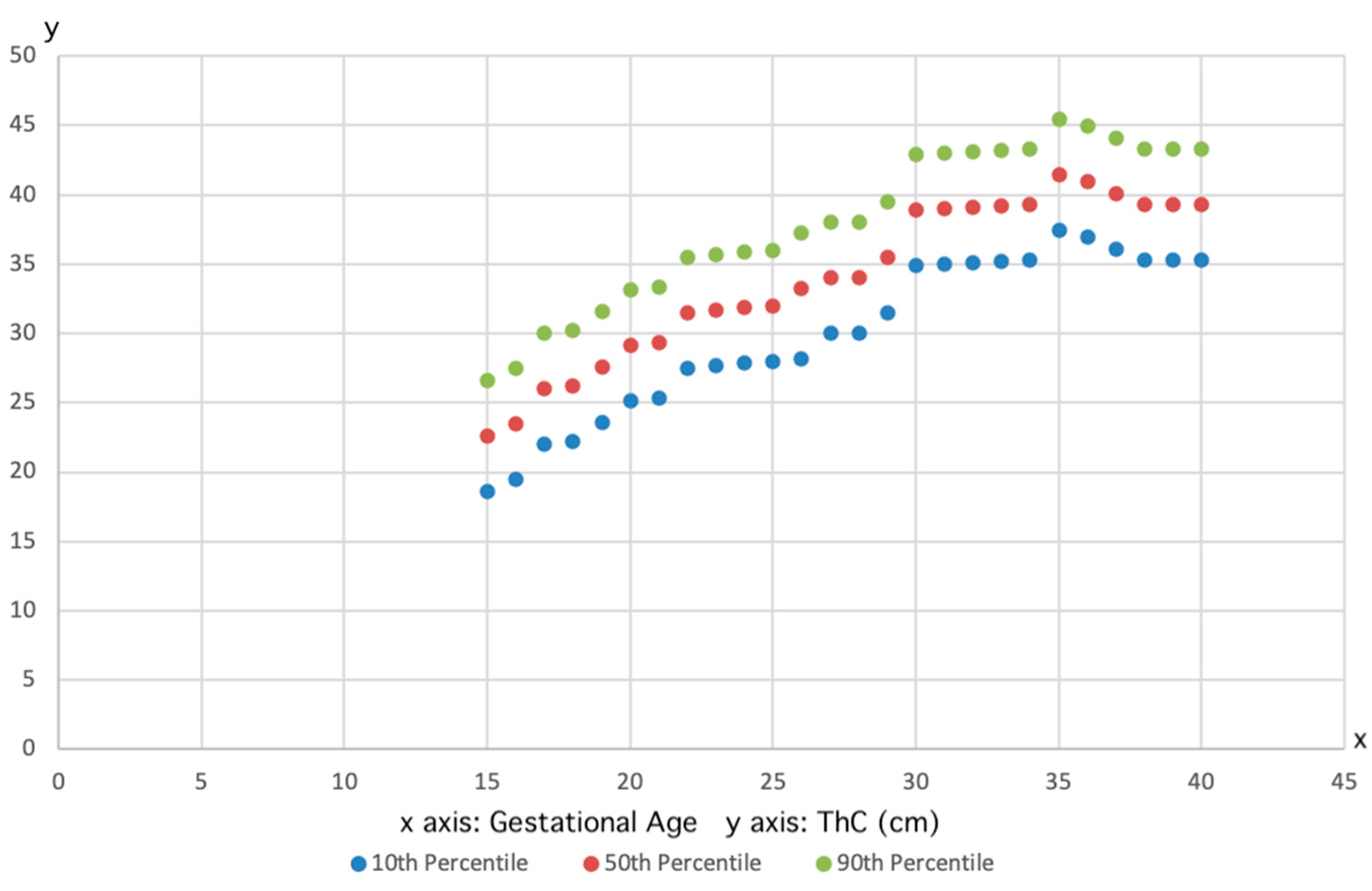
3. Results
4. Discussion
4.1. Summary and Interpretation of Results
4.2. Insights from the Existing Literature
4.3. Implications for Clinical Practice
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Granese, R.; Gulino, F.A.; Incognito, G.G.; Cianci, S.; Martinelli, C.; Ercoli, A. Ultrasonographic Prenatal Diagnosis: Unveiling the Path to Improved Antenatal Care. J. Clin. Med. 2023, 12, 4450. [Google Scholar] [CrossRef] [PubMed]
- Manzo, L.; Orlandi, G.; Gabrielli, O.; Toscano, P.; Di Lella, E.; Lettieri, A.; Mazzarelli, L.L.; Sica, G.; Di Meglio, L.; Di Meglio, L.; et al. Cerebellar Area: Ultrasound Reference Ranges at 13–39 Weeks of Gestation. J. Clin. Med. 2023, 12, 4080. [Google Scholar] [CrossRef] [PubMed]
- Tickle, C. How the embryo makes a limb: Determination, polarity and identity. J. Anat. 2015, 227, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Deter, R.L.; McNie, B.; Gonçalves, L.F.; Espinoza, J.; Chaiworapongsa, T.; Romero, R. Individualized growth assessment of fetal soft tissue using fractional thigh volume. Ultrasound Obs. Gynecol. 2004, 24, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Balasubramaniam, M.; Deter, R.L.; Yeo, L.; Hassan, S.S.; Gotsch, F.; Kusanovic, J.P.; Gonçalves, L.F.; Romero, R. New fetal weight estimation models using fractional limb volume. Ultrasound Obs. Gynecol. 2009, 34, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Stanirowski, P.J.; Majewska, A.; Lipa, M.; Bomba-Opoń, D.; Wielgoś, M. Ultrasound evaluation of the fetal fat tissue, heart, liver and umbilical cord measurements in pregnancies complicated by gestational and type 1 diabetes mellitus: Potential application in the fetal birth-weight estimation and prediction of the fetal macrosomia. Diabetol. Metab. Syndr. 2021, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Wu, Q.Q.; Sun, L.J.; Gao, F.Y.; Wang, J.J. Predicting fetal weight by three-dimensional limb volume ultrasound (AVol/TVol) and abdominal circumference. Chin. Med. J. Engl. 2021, 134, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Kandil, N.M.; Fatouh, M.M.; Elagamy, A.A.; Hashem, A.B. Ultrasonographic fetal thigh measurement in the estimation of fetal weight based on Isobe’s formula in women with an engaged fetal head in the pelvis: A comparative study. J. Ultrasound 2022, 25, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.A.; Ali, A.E.; Mohamed, M.S.; Abd El-Rahman, M.M. Role of thigh circumference in predicting the fetal weight: Comparison with other ultrasound methods-A prospective observational study. J. Obs. Gynaecol. Res. 2021, 47, 4210–4215. [Google Scholar] [CrossRef] [PubMed]
- Salomon, L.J.; Alfirevic, Z.; Da Silva Costa, F.; Deter, R.L.; Figueras, F.; Ghi, T.; Khalil, A.; Papageorghiou, A.T.; Sotiriadis, A.; Yeo, G.; et al. ISUOG Practice Guidelines: Performance of fetal biometry and growth assessment in pregnancy. Ultrasound Obstet Gynecol. 2019, 53, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Maruotti, G.M.; Saccone, G.; Martinelli, P. Third trimester ultrasound soft-tissue measurements accurately predicts macrosomia. J. Matern. Fetal Neo. Med. 2017, 30, 972–976. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, C.; Farah, N.; O’Higgins, A.; Segurado, R.; Fitzpatrick, C.; Turner, M.J.; Stuart, B.; Kennelly, M.M. Longitudinal measurement of fetal thigh soft tissue parameters and its role in the prediction of birth weight. Prenat. Diagn. 2013, 33, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, L.F.; Rojas, M.V.; Vitorello, D.; Pereira, E.T.; Pereima, M.; Saab Neto, J.A. Klippel-Trenaunay-Weber syndrome presenting as massive lymphangiohemangioma of the thigh: Prenatal diagnosis. Ultrasound Obs. Gynecol. 2000, 15, 537–541. [Google Scholar] [CrossRef] [PubMed]
| Number of Cases | Gestational Age (Weeks) | 10th Percentile (cm) | 50th Percentile (cm) | 90th Percentile (cm) |
|---|---|---|---|---|
| 2600 | 12 | 4.6 | 6.8 | 9 |
| 1250 | 13 | 4.7 | 6.9 | 9.1 |
| 365 | 15 | 7.9 | 10.1 | 12.3 |
| 720 | 16 | 11.3 | 13.5 | 15.7 |
| 770 | 17 | 11.9 | 14.1 | 16.3 |
| 1150 | 18 | 12.9 | 15.1 | 17.3 |
| 8060 | 19 | 15.1 | 17.3 | 19.5 |
| 14,060 | 20 | 16.8 | 19 | 21.2 |
| 7630 | 21 | 17.7 | 19.9 | 22.1 |
| 2340 | 22 | 19.8 | 22 | 24.2 |
| 910 | 23 | 20.2 | 22.4 | 24.6 |
| 560 | 24 | 21.8 | 24 | 26.2 |
| 570 | 25 | 23.5 | 25.7 | 27.9 |
| 610 | 26 | 26.5 | 28.7 | 30.9 |
| 540 | 27 | 26.6 | 28.8 | 31 |
| 550 | 28 | 26.7 | 28.9 | 31.1 |
| 670 | 29 | 28.8 | 31 | 33.2 |
| 920 | 30 | 31.6 | 33.8 | 36 |
| 1030 | 31 | 31.6 | 33.8 | 36 |
| 966 | 32 | 31.7 | 33.9 | 36.1 |
| 820 | 33 | 31.4 | 33.6 | 35.8 |
| 650 | 34 | 32.9 | 35.1 | 37.3 |
| 540 | 35 | 34 | 36.2 | 38.4 |
| 420 | 36 | 36.3 | 38.5 | 40.7 |
| 140 | 38 | 36.3 | 38.5 | 40.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulino, F.A.; Arcarese, G.; Incognito, G.G.; Orlandi, G.; Gabrielli, O.; Lettieri, A.; Manzo, L.; Mazzarelli, L.L.; Sica, G.; Di Meglio, L.; et al. Fetal Thigh Circumference Nomograms Across Gestational Ages: A Retrospective Study. J. Pers. Med. 2025, 15, 265. https://doi.org/10.3390/jpm15070265
Gulino FA, Arcarese G, Incognito GG, Orlandi G, Gabrielli O, Lettieri A, Manzo L, Mazzarelli LL, Sica G, Di Meglio L, et al. Fetal Thigh Circumference Nomograms Across Gestational Ages: A Retrospective Study. Journal of Personalized Medicine. 2025; 15(7):265. https://doi.org/10.3390/jpm15070265
Chicago/Turabian StyleGulino, Ferdinando Antonio, Giorgio Arcarese, Giosuè Giordano Incognito, Giuliana Orlandi, Olimpia Gabrielli, Antonia Lettieri, Luigi Manzo, Laura Letizia Mazzarelli, Giordana Sica, Letizia Di Meglio, and et al. 2025. "Fetal Thigh Circumference Nomograms Across Gestational Ages: A Retrospective Study" Journal of Personalized Medicine 15, no. 7: 265. https://doi.org/10.3390/jpm15070265
APA StyleGulino, F. A., Arcarese, G., Incognito, G. G., Orlandi, G., Gabrielli, O., Lettieri, A., Manzo, L., Mazzarelli, L. L., Sica, G., Di Meglio, L., Di Meglio, L., Tuscano, A., Occhipinti, S., Guida, M., & Di Meglio, A. (2025). Fetal Thigh Circumference Nomograms Across Gestational Ages: A Retrospective Study. Journal of Personalized Medicine, 15(7), 265. https://doi.org/10.3390/jpm15070265









