Evaluation of Perioperative Risk Factors for Infection by Multidrug-Resistant Bacteria in Patients Undergoing Liver Transplantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study
2.2. Data Records
2.3. Colonization Record
2.4. Infection by Multi-Resistant Microorganism
2.5. Statistical Analysis
3. Results
3.1. Incidence
3.2. Colonization
3.3. Multi-Resistant Infection After Transplantation
3.4. Risk Factors for Multidrug-Resistant Infection
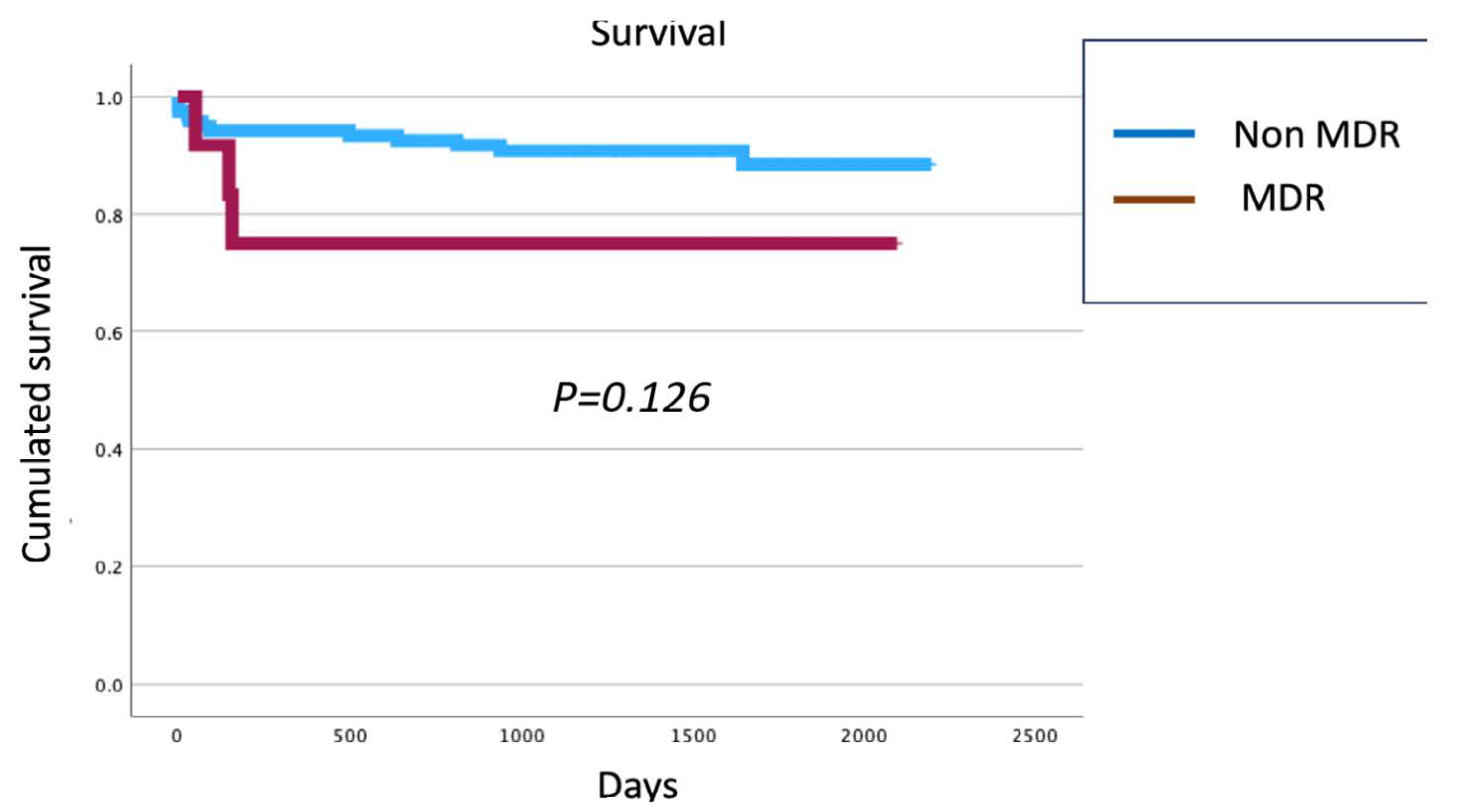
3.5. Survival
4. Discussion
5. Conclusions
- Infections caused by multidrug-resistant bacteria (MDRB) following liver transplantation are clinically significant and impact postoperative outcomes.
- Both preoperative and intraoperative risk factors contribute to the development of these infections.
- Pre-transplant colonization with MDRB is a well-established and independent risk factor.
- Perioperative cardiac and renal function are modifiable factors that influence infection risk.
- Identifying these modifiable risk factors may help define specific patient phenotypes who could benefit from personalized preventive or therapeutic strategies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
- This is a study of general public interest and social value in the field of biomedical research.
- This is a case registry in which the collection of patient personal and health data is minimized; it is maintained in a system with restricted access exclusively to researchers at the participating hospital and the study coordinators.
- Obtaining informed consent for this registry may be impractical in many cases, due to the patient’s acute or serious condition, or death. Additionally, obtaining informed consent would be a limitation for the proper execution of the project, which requires a comprehensive record of all known cases.
- Finally, it is considered that collecting cases with the established confidentiality guarantees does not pose any risk to patients.
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lucey, M.R.; Furuya, K.N.; Foley, D.P. Liver Transplantation. N. Engl. J. Med. 2023, 389, 1888–1900. [Google Scholar] [CrossRef] [PubMed]
- Ferrarese, A.; Senzolo, M.; Sasset, L.; Bassi, D.; Cillo, U.; Burra, P. Multidrug-resistant bacterial infections in the liver transplant setting. Updates Surg. 2024, 76, 2521–2529. [Google Scholar] [CrossRef] [PubMed]
- Righi, E. Management of bacterial and fungal infections in end stage liver disease and liver transplantation: Current options and future directions. World J. Gastroenterol. 2018, 24, 4311–4329. [Google Scholar] [CrossRef] [PubMed]
- Shbaklo, N.; Tandoi, F.; Lupia, T.; Corcione, S.; Romagnoli, R.; De Rosa, F.G. Bacterial and Viral Infections in Liver Transplantation: New Insights from Clinical and Surgical Perspectives. Biomedicines 2022, 10, 1561. [Google Scholar] [CrossRef]
- Huang, Y.S.; Lai, L.C.; Chen, Y.A.; Lin, K.Y.; Chou, Y.H.; Chen, H.C.; Wang, S.-S.; Wang, J.-T.; Chang, S.-C. Colonization with multidrug-resistant organisms among healthy adults in the community setting: Prevalence, risk factors, and composition of gut microbiome. Front. Microbiol. 2020, 11, 1402. [Google Scholar] [CrossRef]
- Woerther, P.L.; Angebault, C.; Jacquier, H.; Clermont, O.; El Mniai, A.; Moreau, B.; Djossou, F.; Peroz, G.; Catzeflis, F.; Denamur, E.; et al. Characterization of fecal extended-spectrum-b-lactamase-producing Escherichia coli in a remote community during a long time period. Antimicrob. Agents Chemother. 2013, 57, 5060e6. [Google Scholar] [CrossRef]
- Han, J.H.; Nachamkin, I.; Zaoutis, T.E.; Coffin, S.E.; Linkin, D.R.; Fishman, N.O.; Weiner, M.G.; Hu, B.; Tolomeo, P.; Lautenbach, E. Risk factors for gastrointestinal tract colonization with extended-spectrum b-lactamase (ESBL)-producing Escherichia coli and Klebsiella species in hospitalized patients. Infect. Control Hosp. Epidemiol. 2012, 33, 1242e5. [Google Scholar] [CrossRef]
- Hong Nguyen, M.; Shields, R.K.; Chen, L.; William Pasculle, A.; Hao, B.; Cheng, S.; Sun, J.; Kline, E.G.; Kreiswirth, B.N.; Clancy, C.J. Molecular Epidemiology, Natural History, and Long-Term Outcomes of Multidrug-Resistant Enterobacterales Colonization and Infections Among Solid Organ Transplant Recipients. Clin. Infect. Dis. 2022, 74, 395–406. [Google Scholar] [CrossRef]
- Fernández, A.; Díez-Picazo, C.; Iglesias Sobrino, C.; Trueba Collado, C.; Romero Cristóbal, M.; Díaz Fontenla, F.; Marcos, A.C.; Valerio, M.; Olmedo, M.; Rangel, T.V.; et al. Implementation and impact of an antibiotic control program and multidrug-resistant bacterial colonization in a liver transplant unit. Rev. Esp. Enferm. Dig. 2023, 115, 357–361. [Google Scholar] [CrossRef]
- Ferstl, P.G.; Filmann, N.; Heilgenthal, E.M.; Schnitzbauer, A.A.; Bechstein, W.O.; Kempf, V.A.J.; Villinger, D.; Schultze, T.G.; Hogardt, M.; Stephan, C.; et al. Colonization with multidrug-resistant organisms is associated with in increased mortality in liver transplant candidates. PLoS ONE 2021, 16, e0245091. [Google Scholar] [CrossRef]
- Campos-Madueno, E.I.; Moradi, M.; Eddoubaji, Y.; Shahi, F.; Moradi, S.; Bernasconi, O.J.; Moser, A.I.; Endimiani, A. Intestinal colonization with multidrug-resistant Enterobacterales: Screening, epidemiology, clinical impact, and strategies to decolonize carriers. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 229–254. [Google Scholar] [CrossRef]
- Fernández-Martínez, M.; González-Rico, C.; Gozalo-Margüello, M.; Marco, F.; Gracia-Ahufinger, I.; Aranzamendi, M.; Sánchez-Díaz, A.M.; Vicente-Rangel, T.; Chaves, F.; Calvo Montes, J.; et al. Molecular characterization of multidrug resistant Enterobacterales strains isolated from liver and kidney transplant recipients in Spain. Sci. Rep. 2021, 11, 11875. [Google Scholar] [CrossRef] [PubMed]
- Giannella, M.; Bartoletti, M.; Campoli, C.; Rinaldi, M.; Coladonato, S.; Pascale, R.; Tedeschi, S.; Ambretti, S.; Cristini, F.; Tumietto, F.; et al. The impact of carbapenemase-producing Enterobacteriaceae colonization on infection risk after liver transplantation: A prospective observational cohort study. Clin. Microbiol. Infect. 2019, 25, 1525–1531. [Google Scholar] [CrossRef]
- Cullaro, G.; Verna, E.C.; Lee, B.P.; Lai, J.C. Chronic Kidney Disease in Liver Transplant Candidates: A Rising Burden Impacting Post-Liver Transplant Outcomes. Liver Transpl. 2020, 26, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Dong, V.; Nadim, M.K.; Karvellas, C.J. Post-Liver Transplant Acute Kidney Injury. Liver Transpl. 2021, 27, 1653–1664. [Google Scholar] [CrossRef]
- Su, G.; Xu, H.; Riggi, E.; He, Z.; Lu, L.; Lindholm, B.; Marrone, G.; Wen, Z.; Liu, X.; Johnson, D.W.; et al. Association of Kidney Function with Infections by Multidrug-Resistant Organisms: An Electronic Medical Record Analysis. Sci. Rep. 2018, 8, 13372. [Google Scholar] [CrossRef]
- Calfee, D.P. Multidrug-resistant organisms within the dialysis population: A potentially preventable perfect storm. Am. J. Kidney Dis. 2015, 65, 3–5. [Google Scholar] [CrossRef] [PubMed]
- AbuTaha, S.A.; Al-Kharraz, T.; Belkebir, S.; Abu Taha, A.; Zyoud, S.H. Patterns of microbial resistance in bloodstream infections of hemodialysis patients: A cross-sectional study from Palestine. Sci. Rep. 2022, 12, 18003. [Google Scholar] [CrossRef]
- Iglesias, J.I.; DePalma, J.A.; Levine, J.S. Risk factors for acute kidney injury following orthotopic liver transplantation: The impact of changes in renal function while patients await transplantation. BMC Nephrol. 2010, 11, 30. [Google Scholar] [CrossRef]
- Humble, C.A.S.; Huang, S.; Jammer, I.; Björk, J.; Chew, M.S. Prognostic performance of preoperative cardiac troponin and perioperative changes in cardiac troponin for the prediction of major adverse cardiac events and mortality in noncardiac surgery: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0215094. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Y.; Zeng, W.; Ye, L.; Yu, C.; Shi, F. Myocardial injury before noncardiac surgery. Front. Cardiovasc. Med. 2023, 10, 1207124. [Google Scholar] [CrossRef] [PubMed]
- Landesberg, G.; Beattie, W.S.; Mosseri, M.; Jaffe, A.S.; Alpert, J.S. Perioperative myocardial infarction. Circulation 2009, 119, 2936–2944. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, G.; Yokoyama, Y.; Ebata, T.; Igami, T.; Yamaguchi, J.; Mizuno, T.; Onoe, S.; Watanabe, N.; Nagino, M. Postoperative infectious complications caused by multidrug-resistant pathogens in patients undergoing major hepatectomy with extrahepatic bile duct resection. Surgery 2020, 167, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Men, T.Y.; Li, H.; Peng, Z.H.; Gu, Y.; Ding, X.; Xing, T.H.; Fan, J.W. Multidrug-resistant gram-negative bacterial infections after liver transplantation-spectrum and risk factors. J. Infect. 2012, 64, 299–310. [Google Scholar] [CrossRef]
- Li, C. Analysis of infections in the first 3 months after living donor liver transplantation. World J. Gastroenterol. 2012, 18, 1975–1983. [Google Scholar] [CrossRef]
- Nacif, L.S.; Zanini, L.Y.; Pinheiro, R.S.; Waisberg, D.R.; Rocha-Santos, V.; Andraus, W.; Carrilho, F.J.; Carneiro-D’Albuquerque, L. Portal vein surgical treatment on non-tumoral portal vein thrombosis in liver transplantation: Systematic Review and Meta-Analysis. Clinics 2021, 76, e2184. [Google Scholar] [CrossRef]
- Chow, J.K.L.; Ganz, T.; Ruthazer, R.; Simpson, M.A.; Pomfret, E.A.; Gordon, F.D.; Westerman, M.E.; Snydman, D.R. Iron-related markers are associated with infection after liver transplantation. Liver Transpl. 2017, 23, 1541–1552. [Google Scholar] [CrossRef]
- Remy, K.E.; Hall, M.W.; Cholette, J.; Juffermans, N.P.; Nicol, K.; Doctor, A.; Blumberg, N.; Spinella, P.C.; Norris, P.J.; Dahmer, M.K.; et al. Mechanisms of red blood cell transfusion-related immunomodulation. Transfusion 2018, 58, 804–815. [Google Scholar] [CrossRef]
- Cinar, G.; Kalkan, İ.A.; Azap, A.; Kirimker, O.E.; Balci, D.; Keskin, O.; Yuraydin, C.; Ormeci, N.; Dokmeci, A. Carbapenemase-producing bacterial infections in patients with liver transplant. Transplant. Proc. 2019, 51, 2461–2465. [Google Scholar] [CrossRef]
- Martin-Mateos, R.; Martínez-Arenas, L.; Carvalho-Gomes, Á.; Aceituno, L.; Cadahía, V.; Salcedo, M.; Arias, A.; Lorente, S.; Odriozola, A.; Zamora, J.; et al. Multidrug-resistant bacterial infections after liver transplantation: Prevalence, impact, and risk factors. J. Hepatol. 2024, 80, 904–912. [Google Scholar] [CrossRef]
| NO-MDRB (n = 120) Median (IQ25–IQ75) | MDRB (n = 13) Median (IQ25–IQ75) | p Value | |
|---|---|---|---|
| Age (y) | 58 (50–63) | 61 (56–63) | 0.410 |
| Sex (male) (n, %) | 87 (70.2) | 7 (53.8) | 0.228 |
| Weight (kg) | 75 (65–87) | 70 (60–72) | 0.253 |
| Height (cm) | 168 (164–174) | 162 (159–174) | 0.217 |
| Hemoglobin (g/dL) | 12.2 (10.4–13.7) | 11.2 (10.2–13.3) | 0.347 |
| Platelets per microliter ×103 | 79 (55–109) | 69 (66–134) | 0.783 |
| INR | 1.28 (1.16–1.56) | 1.35 (1.29–1.62) | 0.082 |
| aPTT (s) | 34.1 (31.6–38.5) | 37.9 (33.2–40.4) | 0.444 |
| Hs-cTnI before surgery (ng/mL) | 3.4 (1.9–5.9) | 3.1 (1.7–3.9) | 0.790 |
| D-dimer (µg/mL) | 195 (129–793) | 500 (203–797) | 0.430 |
| Preoperative proBNP (pg/mL) | 133 (73–318) | 141 (50–276) | 0.024 |
| Bilirubin (mg/dL) | 2.1 (1–4.3) | 2.5 (1.4–11.7) | 0.297 |
| Creatinine (mg/dL) | 0.8 (0.71–0.98) | 0.9 (0.84–1.09) | 0.334 |
| Child (points) | 8 (6–10) | 9 (8–11) | 0.076 |
| MELD (points) | 13.2 (9.6–17) | 16.3 (11.1–19.3) | 0.069 |
| Portal Thrombosis (n, %) | 26 (32.9) | 2 (22.2) | 0.407 |
| ETIOLOGY | 0.742 | ||
| 40 (33.3) | 4 (30.8) | |
| 38 (31.6) | 3 (23.1) | |
| 11 (9.2) | 2 (15.4) | |
| 7 (5.8) | 1 (7.7) | |
| 24 (20) | 3 (23.1) | |
| MDRB Colonization Before Transplantation | MDRB Colonization After Transplantation | ||
|---|---|---|---|
| Bacteria | Isolates n = 32 | Bacteria | Isolates n = 45 |
| E. coli ESBLP | 31.25% (10 isolates) | E. coli ESBLP | 22.2% (10 isolates) |
| K. pneumoniae ESBLP | 9.3% (3 isolates) | K. pneumoniae ESBLP | 8.8% (4 isolates) |
| K. pneumoniae CBP | 15.6% (5 isolates) | K. pneumoniae CBP | 8.8 % (4 isolates) |
| E. coli CBP | 6.25% (2 isolates) | E. coli CBP | 13.3% (6 isolates) |
| C. freundii CBP | 6.25% (2 isolates) | En. cloacae CBP | 15.5% (7 isolates) |
| MRSA | 15.6% (5 isolates) | C. freundii ESBLP | 4.4% (2 isolates) |
| VRE | 6.26% (2 isolates) | VRE | 15.5% (7 isolates) |
| Others (P. mirabilis ESBLP. E. cloacae CBP, and S. maltophilia) | 9.3% (3 isolates) | Others (C. amalonaticus ESBLP. K. oxytoca CBP. A. baumanii, MRSA and S. maltophilia) | 11.1% (5 isolates) |
| Total | Non-Infected Patients (n = 120) | Infected Patients (n = 13) | p Value | |
|---|---|---|---|---|
| Blood transfusion during LT | 64 (46.7) | 55 (44.4) | 9 (69.2) | 0.078 |
| Concentrated red blood cells units | 0 (0–3) | 0 (0–2) | 3 (1–5) | 0.018 |
| Transfusion of more than 4 units of blood | 16 (11.7)% | 12 (9.7) | 4 (30.8) | 0.036 |
| Platelets units | 0 (0–0) | 0 (0–0) | 0 (0–1) | 0.049 |
| Fibrinogen (gr) | 0 (0–4) | 0 (0–3) | 4 (0–4) | 0.048 |
| Fresh frozen plasma units | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.322 |
| Total crystalloids (mL) | 2325 (2000–2500) | 2300 (2000–2500) | 3000 (2400–3000) | 0.322 |
| Hs-cTnI before surgery (ng/mL) | 3.4 (1.8–5.8) | 3.4 (1.9–5.9) | 3.1 (1.7–3.9) | 0.790 |
| Hs-cTnI 60 min after reperfusion (ng/mL) | 19.6 (12.4–35.1) | 18.8 (12.4–34.7) | 31.3 (23.5–49.7) | 0.033 |
| Preoperative proBNP (pg/mL) | 132(73–283) | 133 (73–318) | 141 (50–276) | 0.024 |
| Donor age (y) | 62 (51–73) | 62 (51–73) | 69 (60–74) | 0.238 |
| Graft Ischemia length (min) | 357 (312–397) | 357 (315–396) | 358 (308–420) | 0.919 |
| Hepatic Flow artery (mL/min) | 200 (140–300) | 200 (145–300) | 165 (128–265) | 0.311 |
| Portal Flow venous (mL/min) | 1115 (765–1600) | 1200 (850–1600) | 950 (685–1627) | 0.443 |
| Surgery duration (min) | 200 (178–232) | 200 (180–231) | 233 (170–260) | 0.699 |
| PDR 60 min after reperfusion (%) | 15.2 (11.2–19) | 15 (11–19.2) | 17.1 (12.4–18.1) | 0.649 |
| Lower hemoglobin during LT (gr/dL) | 7.6 (6.8–9.8) | 7.6 (6.8–9.8) | 7.3 (6.5–8.4) | 0.722 |
| Hypotension Phase I | 37 (27.2) | 33 (26.8) | 4 (30.8) | 0.493 |
| Hypotension Phase II | 33 (24.3) | 29 (23.6) | 4 (30.8) | 0.39 |
| Hypotension Phase III | 53 (39) | 49 (39.8) | 4 (30.8) | 0.374 |
| PRS | 33 (24.3) | 27 (22) | 6 (46.2) | 0.053 |
| Use Norepinephrine during surgery | 59 (43.4) | 51 (41.5) | 8 (61.5) | 0.165 |
| HR_baseline (bpm) | 68 (59–79) | 68 (59–78) | 66 (63–82) | 0.773 |
| MAP_baseline (mmHg) | 75 (66–87) | 74 (66–87) | 80 (74–91) | 0.743 |
| CI baseline (L/min/m2) | 3.1 (2.67–3.66) | 3.1 (2.7–3.7) | 3.17 (2.6–3.35) | 0.363 |
| GEDI_baseline (mL/m2) | 639 (543–725) | 639 (539–725) | 610 (581–670) | 0.823 |
| HR_end of surgery (bpm) | 82 (70–94) | 80 (70–95) | 82 (71–86) | 0.773 |
| MAP_end of surgery (mmHg) | 63 (55–70) | 62 (51–68) | 61 (55–70) | 0.594 |
| GEDI_end of surgery (mL/m2) | 609 (508–691) | 677 (585–830) | 799 (668–821) | 0.517 |
| CI_end of surgery (L/min/m2) | 4.27 (3.2–5.1) | 3.68 (3.1–4.4) | 3.14 (2.3–4.7) | 0.037 |
| Total (n = 133) | Non-MDRB Infection (n = 120) | MDRB Infection (n = 13) | p Value | |
|---|---|---|---|---|
| Hb (g/dL) PICU arrival | 10.1 (8.8–11.75) | 10.05 (8.8–11.7) | 10.3 (9–11.95) | 0.589 |
| Highest creatinine in the first 5 days PO | 1.43 (1.01–1.94) | 1.39 (1.01–1.9) | 2.03 (1.1–2.46) | 0.152 |
| AST day 1 after LT | 144 (85–262) | 133 (81–230) | 326 (163–631) | 0.002 |
| AST day 2 after LT | 69.2 (36.2–169) | 66 (35–152) | 166 (56–546) | 0.018 |
| INR day 1 after LT | 1.93 (1.58–2.51) | 1.91 (1.56–2.42) | 2.74 (1.83–4.57) | 0.009 |
| INR day 2 after LT | 1.51 (1.28–1.89) | 1.5 (1.28–1.87) | 1.98 (1.24–3.01) | 0.215 |
| Bilirubin day 1 after LT | 3.6 (2.2–6.8) | 3.2 (1.9–8.1) | 3.65 (2.15–6.75) | 0.806 |
| Bilirubin day 2 after LT | 1.9 (1–3.2) | 1.9 (1.1–3.4) | 2.15 (0.8–4.75) | 0.898 |
| Troponin I day 1 after LT | 66.2 (30.4–156) | 59 (27.8–123) | 138 (86.4–225) | 0.024 |
| Troponin I day 2 after LT (ng/mL) | 87.9 (32.9–253) | 77 (31–249) | 161 (118–265) | 0.238 |
| ProBNP on 1st day PICU | 226 (102–384) | 219 (98–365) | 334 (208–589) | 0.225 |
| PICU stay (days) | 2 (1–3) | 2 (1–3) | 3 (2–7) | 0.075 |
| Reintubation (Yes) | 11 (8) | 9 (7.3) | 2 (15.4) | 0.305 |
| Pneumonia in PICU (Yes) | 1 (0.7) | 0 (0) | 1 (7.7) | 0.95 |
| Respiratory failure (Yes) | 43 (32.3) | 35 (29.1) | 8 (61.5) | 0.034 |
| Length Mechanical Ventilation (days) | 0 (0–1) | 0 (0–1) | 1 (0–2) | 0.077 |
| Length intubation (days) | 0 (0–1) | 0 (0–1) | 1 (0–2) | 0.081 |
| Reintervention (Yes) | 37 (27.8) | 32 (26.7) | 5 (38.5) | 0.274 |
| Retransplantation (Yes) | 15 (11.3) | 12 (10) | 3 (23.1) | 0.165 |
| Length intravenous central line (days) | 2 (1–3) | 2 (1–3) | 3(2–7) | 0.072 |
| Hospital stay length (days) | 16 (13–23) | 15 (13–14) | 28 (16–48) | 0.004 |
| PICU stay length (days) | 3 (2–4) | 3 (2–4) | 4 (3–7) | 0.021 |
| Postoperative Graft Function 72 h (I vs. II, III, or IV grades) | 36 (29) | 36 (26.3) | 0 (0) | 0.021 |
| Extubated patient in the operating room | 89 (66.9) | 84 (70) | 5 (38.5) | 0.057 |
| Norepinephrine need in PICU | 19 (14.3) | 17 (14,2) | 2 (15.4) | 0.598 |
| Reintervention in first week | 22 (23.7) | 19 (22.6) | 3 (23.1) | 0.36 |
| Died in first week post LT | 2 (7.1) | 2 (7.7) | 0 (0) | 0.86 |
| Death in PICU | 4 (3.2) | 4 (2.9) | 0 (0) | 0.651 |
| Died in first year post LT | 10 (7.5) | 7 (5.8) | 3 (23.1) | 0.059 |
| Bacteremia in PICU | 1 (0.7) | 1 (0.8) | 0 (0) | 0.137 |
| Other infection in PICU | 26 (19.7) | 21 (17.5) | 5 (41.7) | 0.059 |
| Sepsis | 7 (5.3) | 3 (2.5) | 4 (30.7) | <0.001 |
| PICU respiratory failure | 31 (23.5) | 25 (20.8) | 6 (46.1) | 0.035 |
| Hemodialysis | 17 (12.9) | 12 (10) | 5 (38.4) | 0.009 |
| Readmission PICU | 5 (3.8) | 3 (2.5) | 2 (16.7) | 0.065 |
| Arterial thrombosis | 8 (6.1) | 6 (5) | 2 (16.7) | 0.156 |
| Portal thrombosis | 6 (4.5) | 4 (3.3) | 2 (16.7) | 0.035 |
| Bile leakage | 15 (11.4) | 11 (9.2) | 4 (33.3) | 0.031 |
| Cholangitis | 5 (3.8) | 3 (2.5) | 2 (16.7) | 0.065 |
| Corticosteroids | 130 (96.3) | 117 (95.2) | 13 (100) | 0.598 |
| Tacrolimus | 69 (87.3) | 63 (87.5) | 6 (46.2) | 0.892 |
| Basiliximab | 98 (71.5) | 87 (70.2) | 11 (84.6) | 0.272 |
| Mycophenolate mofetil | 101 (73.7) | 91 (73.4) | 10 (76.9) | 0.54 |
| MDRB colonization before LT | 22 (16.5) | 17 (14.2) | 5 (38.5) | 0.028 |
| MDRB colonization at PICU | 28 (21) | 21 (17.5) | 7 (53.8) | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos Fernández, R.; Calvo García, A.; Fernández Yunkera, A.; Ramos Cerro, S.; Garutti, I.; Hortal Iglesias, J.; Muñoz García, P.; García Ramos, S.; Elvira Rodríguez, A.; Power Esteban, M.; et al. Evaluation of Perioperative Risk Factors for Infection by Multidrug-Resistant Bacteria in Patients Undergoing Liver Transplantation. J. Pers. Med. 2025, 15, 240. https://doi.org/10.3390/jpm15060240
Ramos Fernández R, Calvo García A, Fernández Yunkera A, Ramos Cerro S, Garutti I, Hortal Iglesias J, Muñoz García P, García Ramos S, Elvira Rodríguez A, Power Esteban M, et al. Evaluation of Perioperative Risk Factors for Infection by Multidrug-Resistant Bacteria in Patients Undergoing Liver Transplantation. Journal of Personalized Medicine. 2025; 15(6):240. https://doi.org/10.3390/jpm15060240
Chicago/Turabian StyleRamos Fernández, Rafael, Alberto Calvo García, Ainhoa Fernández Yunkera, Silvia Ramos Cerro, Ignacio Garutti, Javier Hortal Iglesias, Patricia Muñoz García, Sergio García Ramos, Adoración Elvira Rodríguez, Mercedes Power Esteban, and et al. 2025. "Evaluation of Perioperative Risk Factors for Infection by Multidrug-Resistant Bacteria in Patients Undergoing Liver Transplantation" Journal of Personalized Medicine 15, no. 6: 240. https://doi.org/10.3390/jpm15060240
APA StyleRamos Fernández, R., Calvo García, A., Fernández Yunkera, A., Ramos Cerro, S., Garutti, I., Hortal Iglesias, J., Muñoz García, P., García Ramos, S., Elvira Rodríguez, A., Power Esteban, M., Duque González, P., & Piñeiro, P. (2025). Evaluation of Perioperative Risk Factors for Infection by Multidrug-Resistant Bacteria in Patients Undergoing Liver Transplantation. Journal of Personalized Medicine, 15(6), 240. https://doi.org/10.3390/jpm15060240






