Molecular Biomarkers for the Diagnosis and Prognostication of Pancreatic Ductal Adenocarcinoma
Abstract
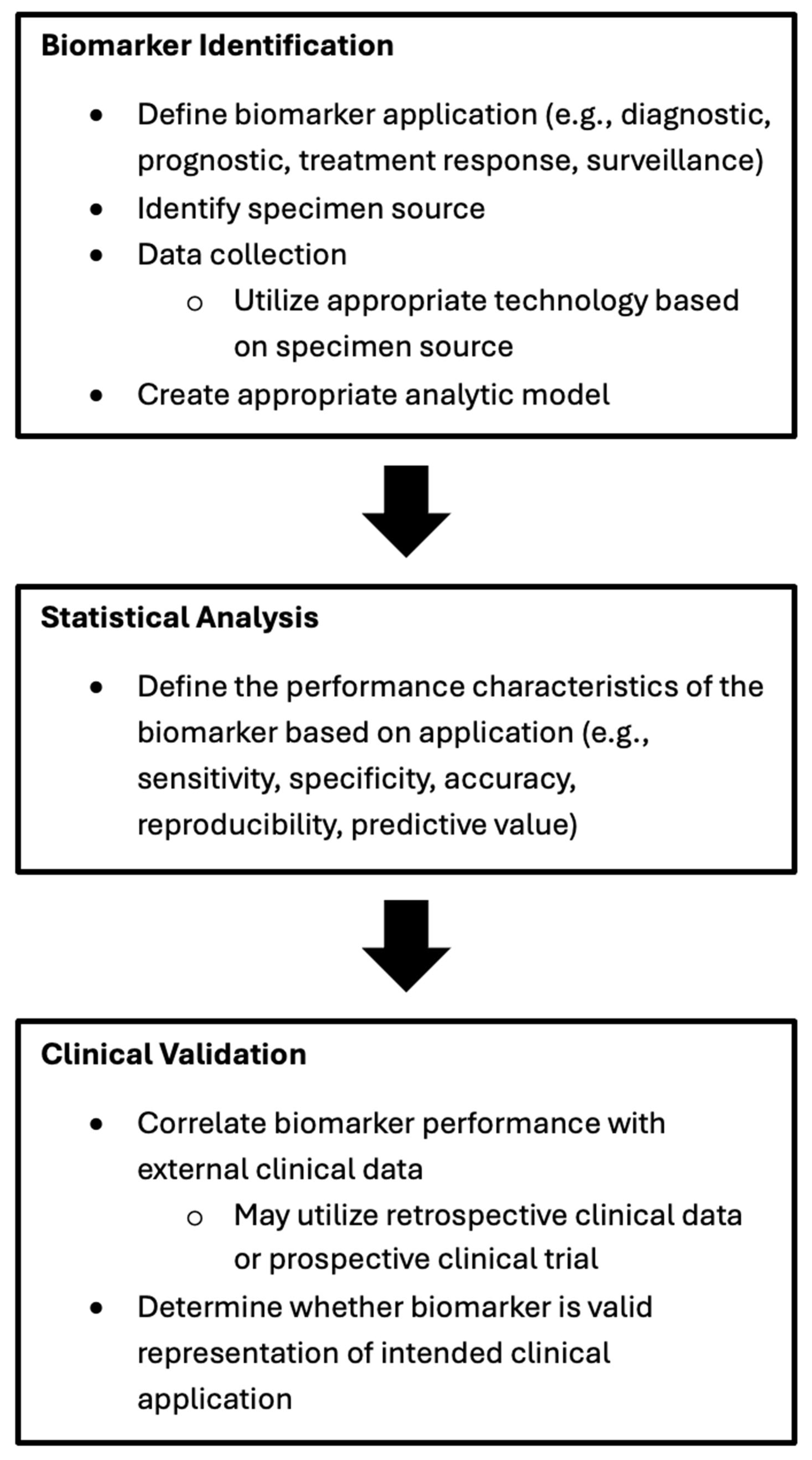
1. Introduction
2. Genetic Biomarkers
2.1. Oncogenes
2.2. Tumor Suppressor Genes
2.3. DNA Damage Repair Genes
2.4. DNA Mismatch Repair Genes
3. Liquid Biomarkers
3.1. Carbohydrate Antigen 19-9 (CA 19-9)
3.1.1. CA 19-9 for Pancreatic Cancer Screening
3.1.2. CA 19-9 for Pancreatic Cancer Prognostication
3.2. Human Epidermal Growth Factor Receptor 2 (HER2)
3.3. Claudin 18.2
3.3.1. Claudin 18.2 as a Therapeutic Target
3.3.2. Claudin 18.2 as a Pancreatic Cancer Biomarker
4. Circulating Tumor Cells
4.1. Circulating Tumor Cells for Pancreatic Cancer Diagnosis
4.2. Circulating Tumor Cells for Pancreatic Cancer Prognostication
5. Circulating Tumor DNA
5.1. Circulating Tumor DNA for Pancreatic Cancer Diagnosis
5.2. Circulating Tumor DNA for Pancreatic Cancer Prognostication
6. Exosomes
7. Cost-Effectiveness of Personalized Medicine
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| PDAC | Pancreatic ductal adenocarcinoma |
| CTCs | Circulating tumor cells |
| RNA | Ribonucleic acid |
| DNA | Deoxyribonucleic acid |
| OS | Overall survival |
| HR | Hazard ratio |
| MMR | Mismatch repair |
| MSI | Microsatellite instability |
| CA 19-9 | Carbohydrate antigen 19-9 |
| MCNs | Mucinous cystic neoplasms |
| IPMNs | Intraductal papillary mucinous neoplasms |
| PPV | Positive predictive value |
| NPV | Negative predictive value |
| AUROC | Area under receiver operating characteristic curve |
| IQR | Interquartile range |
| PCR | Polymerase chain reaction |
| NGS | Next generation sequencing |
| USD | US dollars |
| QALY | Quality-adjusted life-years |
References
- Conroy, T.; Bachet, J.B.; Ayav, A.; Huguet, F.; Lambert, A.; Caramella, C.; Maréchal, R.; Van Laethem, J.L.; Ducreux, M. Current Standards and New Innovative Approaches for Treatment of Pancreatic Cancer. Eur. J. Cancer 2016, 57, 10–22. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Chan-Seng-Yue, M.; Kim, J.C.; Wilson, G.W.; Ng, K.; Figueroa, E.F.; O’Kane, G.M.; Connor, A.A.; Denroche, R.E.; Grant, R.C.; McLeod, J.; et al. Transcription Phenotypes of Pancreatic Cancer Are Driven by Genomic Events during Tumor Evolution. Nat. Genet. 2020, 52, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Russano, M.; Napolitano, A.; Ribelli, G.; Iuliani, M.; Simonetti, S.; Citarella, F.; Pantano, F.; Dell’Aquila, E.; Anesi, C.; Silvestris, N.; et al. Liquid Biopsy and Tumor Heterogeneity in Metastatic Solid Tumors: The Potentiality of Blood Samples. J. Exp. Clin. Cancer Res. 2020, 39, 95. [Google Scholar] [CrossRef]
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer: A Review. JAMA 2021, 326, 851–862. [Google Scholar] [CrossRef]
- Blackford, A.L.; Canto, M.I.; Klein, A.P.; Hruban, R.H.; Goggins, M. Recent Trends in the Incidence and Survival of Stage 1A Pancreatic Cancer: A Surveillance, Epidemiology, and End Results Analysis. J. Natl. Cancer Inst. 2020, 112, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of Adjuvant Gemcitabine and Capecitabine with Gemcitabine Monotherapy in Patients with Resected Pancreatic Cancer (ESPAC-4): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef]
- Conroy, T.; Castan, F.; Lopez, A.; Turpin, A.; Ben Abdelghani, M.; Wei, A.C.; Mitry, E.; Biagi, J.J.; Evesque, L.; Artru, P.; et al. Five-Year Outcomes of FOLFIRINOX vs Gemcitabine as Adjuvant Therapy for Pancreatic Cancer: A Randomized Clinical Trial. JAMA Oncol. 2022, 8, 1571–1578. [Google Scholar] [CrossRef]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.-L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef]
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource 2016. Available online: https://pubmed.ncbi.nlm.nih.gov/27010052/ (accessed on 17 May 2025).
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology Pancreatic Adenocarcinoma Version 2. 2025. Available online: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf (accessed on 27 March 2025).
- Lee, M.S.; Pant, S. Personalizing Medicine With Germline and Somatic Sequencing in Advanced Pancreatic Cancer: Current Treatments and Novel Opportunities. Am. Soc. Clin. Oncol. Educ. Book 2021, 41, e153–e165. [Google Scholar] [CrossRef]
- Pishvaian, M.J.; Blais, E.M.; Brody, J.R.; Lyons, E.; DeArbeloa, P.; Hendifar, A.; Mikhail, S.; Chung, V.; Sahai, V.; Sohal, D.P.S.; et al. Overall Survival in Patients with Pancreatic Cancer Receiving Matched Therapies Following Molecular Profiling: A Retrospective Analysis of the Know Your Tumor Registry Trial. Lancet Oncol. 2020, 21, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Crowley, F.; Gandhi, S.; Rudshteyn, M.; Sehmbhi, M.; Cohen, D.J. Adherence to NCCN Genetic Testing Guidelines in Pancreatic Cancer and Impact on Treatment. Oncologist 2023, 28, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Dbouk, M.; Katona, B.W.; Brand, R.E.; Chak, A.; Syngal, S.; Farrell, J.J.; Kastrinos, F.; Stoffel, E.M.; Blackford, A.L.; Rustgi, A.K.; et al. The Multicenter Cancer of Pancreas Screening Study: Impact on Stage and Survival. J. Clin. Oncol. 2022, 40, 28. [Google Scholar] [CrossRef] [PubMed]
- Luo, J. KRAS Mutation in Pancreatic Cancer. Semin. Oncol. 2021, 48, 10–18. [Google Scholar] [CrossRef]
- Knudsen, E.S.; O’Reilly, E.M.; Brody, J.R.; Witkiewicz, A.K. Genetic Diversity of Pancreatic Ductal Adenocarcinoma and Opportunities for Precision Medicine. Gastroenterology 2015, 150, 48. [Google Scholar] [CrossRef]
- Raphael, B.J.; Hruban, R.H.; Aguirre, A.J.; Moffitt, R.A.; Yeh, J.J.; Stewart, C.; Robertson, A.G.; Cherniack, A.D.; Gupta, M.; Getz, G.; et al. Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell 2017, 32, 185–203.e13. [Google Scholar] [CrossRef]
- Buscail, L.; Bournet, B.; Cordelier, P. Role of Oncogenic KRAS in the Diagnosis, Prognosis and Treatment of Pancreatic Cancer. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Ostrem, J.M.L.; Shokat, K.M. Direct Small-Molecule Inhibitors of KRAS: From Structural Insights to Mechanism-Based Design. Nat. Rev. Drug Discov. 2016, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Norton, C.; Shaw, M.S.; Rubnitz, Z.; Smith, J.; Soares, H.P.; Nevala-Plagemann, C.D.; Garrido-Laguna, I.; Florou, V. KRAS Mutation Status and Treatment Outcomes in Patients With Metastatic Pancreatic Adenocarcinoma. JAMA Netw. Open 2025, 8, e2453588. [Google Scholar] [CrossRef] [PubMed]
- Bournet, B.; Muscari, F.; Buscail, C.; Assenat, E.; Barthet, M.; Hammel, P.; Selves, J.; Guimbaud, R.; Cordelier, P.; Buscail, L. KRAS G12D Mutation Subtype Is A Prognostic Factor for Advanced Pancreatic Adenocarcinoma. Clin. Transl. Gastroenterol. 2016, 7, E157. [Google Scholar] [CrossRef]
- Liu, J.; Kang, R.; Tang, D. The KRAS-G12C Inhibitor: Activity and Resistance. Cancer Gene Ther. 2022, 29, 875–878. [Google Scholar] [CrossRef] [PubMed]
- Strickler, J.H.; Satake, H.; George, T.J.; Yaeger, R.; Hollebecque, A.; Garrido-Laguna, I.; Schuler, M.; Burns, T.F.; Coveler, A.L.; Falchook, G.S.; et al. Sotorasib in KRAS p.G12C–Mutated Advanced Pancreatic Cancer. N. Engl. J. Med. 2023, 388, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.Y.; Zhao, Y.; Aronowitz, J.; Mai, T.T.; Vides, A.; Qeriqi, B.; Kim, D.; Li, C.; de Stanchina, E.; Mazutis, L.; et al. Rapid Non-Uniform Adaptation to Conformation-Specific KRAS(G12C) Inhibition. Nature 2020, 577, 421–425. [Google Scholar] [CrossRef]
- Awad, M.M.; Liu, S.; Rybkin, I.I.; Arbour, K.C.; Dilly, J.; Zhu, V.W.; Johnson, M.L.; Heist, R.S.; Patil, T.; Riely, G.J.; et al. Acquired Resistance to KRAS G12C Inhibition in Cancer. N. Eng. J. Med. 2021, 384, 2382–2393. [Google Scholar] [CrossRef]
- Wang, X.; Allen, S.; Blake, J.F.; Bowcut, V.; Briere, D.M.; Calinisan, A.; Dahlke, J.R.; Fell, J.B.; Fischer, J.P.; Gunn, R.J.; et al. Identification of MRTX1133, a Noncovalent, Potent, and Selective KRASG12DInhibitor. J. Med. Chem. 2022, 65, 3123–3133. [Google Scholar] [CrossRef]
- Voutsadakis, I.A. Mutations of P53 Associated with Pancreatic Cancer and Therapeutic Implications. Ann. Hepatobiliary Pancreat. Surg. 2021, 25, 315. [Google Scholar] [CrossRef] [PubMed]
- Stefanoudakis, D.; Frountzas, M.; Schizas, D.; Michalopoulos, N.V.; Drakaki, A.; Toutouzas, K.G. Significance of TP53, CDKN2A, SMAD4 and KRAS in Pancreatic Cancer. Curr. Issues Mol. Biol. 2024, 46, 2827–2844. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Yang, L.V.; Abrams, S.L.; Steelman, L.S.; Follo, M.Y.; Cocco, L.; Ratti, S.; Martelli, A.M.; Augello, G.; Cervello, M. Effects of TP53 Mutations and MiRs on Immune Responses in the Tumor Microenvironment Important in Pancreatic Cancer Progression. Cells 2022, 11, 2155. [Google Scholar] [CrossRef]
- Gu, Y.; Ji, Y.; Jiang, H.; Qiu, G. Clinical Effect of Driver Mutations of KRAS, CDKN2A/P16, TP53, and SMAD4 in Pancreatic Cancer: A Meta-Analysis. Genet. Test. Mol. Biomark. 2020, 24, 777–788. [Google Scholar] [CrossRef]
- Dardare, J.; Witz, A.; Merlin, J.L.; Gilson, P.; Harlé, A. SMAD4 and the TGFβ Pathway in Patients with Pancreatic Ductal Adenocarcinoma. Int. J. Mol. Sci. 2020, 21, 3534. [Google Scholar] [CrossRef]
- Kimura, H.; Klein, A.P.; Hruban, R.H.; Roberts, N.J. The Role of Inherited Pathogenic CDKN2A Variants in Susceptibility to Pancreatic Cancer. Pancreas 2021, 50, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology Genetic/Familial High-Risk Assessment: Breast, Ovarian, Pancreatic, and Prostate Version 2. 2025. Available online: https://www.nccn.org/professionals/physician_gls/pdf/genetics_bopp.pdf (accessed on 27 March 2025).
- Al Baghdadi, T.; Halabi, S.; Garrett-Mayer, E.; Mangat, P.K.; Ahn, E.R.; Sahai, V.; Alvarez, R.H.; Kim, E.S.; Yost, K.J.; Rygiel, A.L.; et al. Palbociclib in Patients With Pancreatic and Biliary Cancer With CDKN2A Alterations: Results From the Targeted Agent and Profiling Utilization Registry Study. JCO Precis. Oncol. 2019, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kasuga, A.; Okamoto, T.; Udagawa, S.; Mori, C.; Mie, T.; Furukawa, T.; Yamada, Y.; Takeda, T.; Matsuyama, M.; Sasaki, T.; et al. Molecular Features and Clinical Management of Hereditary Pancreatic Cancer Syndromes and Familial Pancreatic Cancer. Int. J. Mol. Sci. 2022, 23, 1205. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Gong, Y.; Fan, Z.; Luo, G.; Huang, Q.; Deng, S.; Cheng, H.; Jin, K.; Ni, Q.; Yu, X.; et al. Molecular Alterations and Targeted Therapy in Pancreatic Ductal Adenocarcinoma. J. Hematol. Oncol. 2020, 13, 130. [Google Scholar] [CrossRef] [PubMed]
- Holter, S.; Borgida, A.; Dodd, A.; Grant, R.; Semotiuk, K.; Hedley, D.; Dhani, N.; Narod, S.; Akbari, M.; Moore, M.; et al. Germline BRCA Mutations in a Large Clinic-Based Cohort of Patients With Pancreatic Adenocarcinoma. J. Clin. Oncol. 2015, 33, 3124–3129. [Google Scholar] [CrossRef] [PubMed]
- Boursi, B.; Wileyto, E.P.; Mamtani, R.; Domchek, S.M.; Golan, T.; Hood, R.; Reiss, K.A. Analysis of BRCA1- and BRCA2-Related Pancreatic Cancer and Survival. JAMA Netw. Open 2023, 6, E2345013. [Google Scholar] [CrossRef]
- Jones, S.; Hruban, R.H.; Kamiyama, M.; Borges, M.; Zhang, X.; Parsons, D.W.; Lin, J.C.H.; Palmisano, E.; Brune, K.; Jaffee, E.M.; et al. Exomic Sequencing Identifies PALB2 as a Pancreatic Cancer Susceptibility Gene. Science 2009, 324, 217. [Google Scholar] [CrossRef]
- Ceccaldi, R.; Liu, J.C.; Amunugama, R.; Hajdu, I.; Primack, B.; Petalcorin, M.I.R.; O’Connor, K.W.; Konstantinopoulos, P.A.; Elledge, S.J.; Boulton, S.J.; et al. Homologous-Recombination-Deficient Tumours Are Dependent on Polθ-Mediated Repair. Nature 2015, 518, 258–262. [Google Scholar] [CrossRef]
- Gudmundsdottir, K.; Ashworth, A. The Roles of BRCA1 and BRCA2 and Associated Proteins in the Maintenance of Genomic Stability. Oncogene 2006, 25, 5864–5874. [Google Scholar] [CrossRef]
- Ashworth, A. A Synthetic Lethal Therapeutic Approach: Poly(ADP) Ribose Polymerase Inhibitors for the Treatment of Cancers Deficient in DNA Double-Strand Break Repair. J. Clin. Oncol. 2008, 26, 3785–3790. [Google Scholar] [CrossRef]
- van Gent, D.C.; Hoeijmakers, J.H.; Kanaar, R. Chromosomal Stability and the DNA Double-Stranded Break Connection. Nat. Rev. Genet. 2001, 2, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, F.; de La Rubia, G.; Ménissier-De Murcia, J.; Hostomsky, Z.; de Murcia, G.; Schreiber, V. Base Excision Repair Is Impaired in Mammalian Cells Lacking Poly(ADP-Ribose) Polymerase-1. Biochemistry 2000, 39, 7559–7569. [Google Scholar] [CrossRef]
- Rosen, M.N.; Goodwin, R.A.; Vickers, M.M. BRCA Mutated Pancreatic Cancer: A Change Is Coming. World J. Gastroenterol. 2021, 27, 1943–1958. [Google Scholar] [CrossRef]
- Golan, T.; Hammel, P.; Reni, M.; Van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.-O.; Hochhauser, D.; Arnold, D.; Oh, D.-Y.; et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019, 381, 317–327. [Google Scholar] [CrossRef]
- Kindler, H.L.; Hammel, P.; Reni, M.; Van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.O.; Hochhauser, D.; Arnold, D.; Oh, D.Y.; et al. Overall Survival Results from the POLO Trial: A Phase III Study of Active Maintenance Olaparib Versus Placebo for Germline BRCA-Mutated Metastatic Pancreatic Cancer. J. Clin. Oncol. 2022, 40, 3929–3939. [Google Scholar] [CrossRef]
- Kaufman, B.; Shapira-Frommer, R.; Schmutzler, R.K.; Audeh, M.W.; Friedlander, M.; Balmaña, J.; Mitchell, G.; Fried, G.; Stemmer, S.M.; Hubert, A.; et al. Olaparib Monotherapy in Patients with Advanced Cancer and a Germline BRCA1/2 Mutation. J. Clin. Oncol. 2015, 33, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Reiss, K.A.; Mick, R.; Teitelbaum, U.; O’Hara, M.; Schneider, C.; Massa, R.; Karasic, T.; Tondon, R.; Onyiah, C.; Gosselin, M.K.; et al. Niraparib plus Nivolumab or Niraparib plus Ipilimumab in Patients with Platinum-Sensitive Advanced Pancreatic Cancer: A Randomised, Phase 1b/2 Trial. Lancet Oncol. 2022, 23, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Reiss, K.A.; Mick, R.; O’Hara, M.H.; Teitelbaum, U.; Karasic, T.B.; Schneider, C.; Cowden, S.; Southwell, T.; Romeo, J.; Izgur, N.; et al. Phase II Study of Maintenance Rucaparib in Patients With Platinum-Sensitive Advanced Pancreatic Cancer and a Pathogenic Germline or Somatic Variant in BRCA1, BRCA2, or PALB2. J. Clin. Oncol. 2021, 39, 2497–2505. [Google Scholar] [CrossRef]
- O’Reilly, E.M.; Lee, J.W.; Zalupski, M.; Capanu, M.; Park, J.; Golan, T.; Tahover, E.; Lowery, M.A.; Chou, J.F.; Sahai, V.; et al. Randomized, Multicenter, Phase II Trial of Gemcitabine and Cisplatin With or Without Veliparib in Patients With Pancreas Adenocarcinoma and a Germline BRCA/PALB2 Mutation. J. Clin. Oncol. 2020, 38, 1378–1388. [Google Scholar] [CrossRef]
- Hsu, F.C.; Roberts, N.J.; Childs, E.; Porter, N.; Rabe, K.G.; Borgida, A.; Ukaegbu, C.; Goggins, M.G.; Hruban, R.H.; Zogopoulos, G.; et al. Risk of Pancreatic Cancer Among Individuals With Pathogenic Variants in the ATM Gene. JAMA Oncol. 2021, 7, 1664–1668. [Google Scholar] [CrossRef]
- Hannan, Z.; Yu, S.; Mamtani, R.; Reiss, K.A. Clinical Characteristics of Patients With Pancreatic Cancer and Pathogenic ATM Alterations. JNCI Cancer Spectr. 2021, 5, pkaa121. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Yao, W.; Ying, H.; Hua, S.; Liewen, A.; Wang, Q.; Zhong, Y.; Wu, C.-J.; Sadanandam, A.; Hu, B.; et al. Yap1 Activation Enables Bypass of Oncogenic Kras Addiction in Pancreatic Cancer. Cell 2014, 158, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Eso, Y.; Shimizu, T.; Takeda, H.; Takai, A.; Marusawa, H. Microsatellite Instability and Immune Checkpoint Inhibitors: Toward Precision Medicine against Gastrointestinal and Hepatobiliary Cancers. J. Gastroenterol. 2020, 55, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; de Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/ Mismatch Repair–Deficient Cancer: Results from the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Maio, M.; Ascierto, P.A.; Manzyuk, L.; Motola-Kuba, D.; Penel, N.; Cassier, P.A.; Bariani, G.M.; De Jesus Acosta, A.; Doi, T.; Longo, F.; et al. Pembrolizumab in Microsatellite Instability High or Mismatch Repair Deficient Cancers: Updated Analysis from the Phase II KEYNOTE-158 Study. Ann. Oncol. 2022, 33, 929–938. [Google Scholar] [CrossRef]
- Lee, T.; Teng, T.Z.J.; Shelat, V.G. Carbohydrate Antigen 19-9—Tumor Marker: Past, Present, and Future. World J. Gastrointest. Surg. 2020, 12, 468. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, K.T.; Lee, J.K.; Paik, S.W.; Rhee, J.C.; Choi, K.W. Clinical Usefulness of Carbohydrate Antigen 19-9 as a Screening Test for Pancreatic Cancer in an Asymptomatic Population. J. Gastroenterol. Hepatol. 2004, 19, 182–186. [Google Scholar] [CrossRef]
- Ballehaninna, U.K.; Chamberlain, R.S. The Clinical Utility of Serum CA 19-9 in the Diagnosis, Prognosis and Management of Pancreatic Adenocarcinoma: An Evidence Based Appraisal. J. Gastrointest. Oncol. 2012, 3, 105–119. [Google Scholar] [CrossRef]
- Scarà, S.; Bottoni, P.; Scatena, R. CA 19-9: Biochemical and Clinical Aspects. Adv. Exp. Med. Biol. 2015, 867, 247–260. [Google Scholar] [CrossRef]
- Poruk, K.E.; Gay, D.Z.; Brown, K.; Mulvihill, J.D.; Boucher, K.M.; Scaife, C.L.; Firpo, M.A.; Mulvihill, S.J. The Clinical Utility of CA 19-9 in Pancreatic Adenocarcinoma: Diagnostic and Prognostic Updates. Curr. Mol. Med. 2013, 13, 340. [Google Scholar] [CrossRef]
- Galli, C.; Basso, D.; Plebani, M. CA 19-9: Handle with Care. Clin. Chem. Lab. Med. 2013, 51, 1369–1383. [Google Scholar] [CrossRef]
- Goggins, M. The Role of Biomarkers in the Early Detection of Pancreatic Cancer. Fam. Cancer 2024, 23, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Dbouk, M.; Abe, T.; Koi, C.; Ando, Y.; Saba, H.; Diwan, E.A.; MacGregor-Das, A.; Blackford, A.L.; Mocci, E.; Beierl, K.; et al. Diagnostic Performance of a Tumor Marker Gene Test to Personalize Serum CA19–9 Reference Ranges. Clin. Cancer Res. 2023, 29, 4178. [Google Scholar] [CrossRef]
- Abe, T.; Koi, C.; Kohi, S.; Song, K.B.; Tamura, K.; Macgregor-Das, A.; Kitaoka, N.; Chuidian, M.; Ford, M.; Dbouk, M.; et al. Gene Variants That Affect Levels of Circulating Tumor Markers Increase Identification of Patients with Pancreatic Cancer. Clin. Gastroenterol. Hepatol. 2020, 18, 1161–1169.e5. [Google Scholar] [CrossRef] [PubMed]
- Metzgar, R.S.; Rodriguez, N.; Finn, O.J.; Lan, M.S.; Daasch, V.N.; Fernsten, P.D.; Meyers, W.C.; Sindelar, W.F.; Sandler, R.S.; Seigler, H.F. Detection of a Pancreatic Cancer-Associated Antigen (DU-PAN-2 Antigen) in Serum and Ascites of Patients with Adenocarcinoma. Proc. Natl. Acad. Sci. USA 1984, 81, 5242. [Google Scholar] [CrossRef] [PubMed]
- Kawa, S.; Oguchi, H.; Kobayashi, T.; Tokoo, M.; Furuta, S.; Kanai, M.; Homma, T. Elevated Serum Levels of Dupan-2 in Pancreatic Cancer Patients Negative for Lewis Blood Group Phenotype. Br. J. Cancer 1991, 64, 899. [Google Scholar] [CrossRef]
- Omiya, K.; Oba, A.; Inoue, Y.; Kobayashi, K.; Wu, Y.H.A.; Ono, Y.; Sato, T.; Sasaki, T.; Ozaka, M.; Sasahira, N.; et al. Serum DUPAN-2 Could Be an Alternative Biological Marker for CA19-9 Nonsecretors with Pancreatic Cancer. Ann. Surg. 2023, 277, E1278–E1283. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Dbouk, M.; Yoshida, T.; Saba, H.; Diwan, E.A.; Yoshida, K.; Dbouk, A.; Blackford, A.L.; Lin, M.-T.; Lennon, A.M.; et al. Using Tumor Marker Gene Variants to Improve the Diagnostic Accuracy of DUPAN-2 and Carbohydrate Antigen 19-9 for Pancreatic Cancer. J. Clin. Oncol. 2024, 42, 2196–2206. [Google Scholar] [CrossRef]
- Capello, M.; Bantis, L.E.; Scelo, G.; Zhao, Y.; Li, P.; Dhillon, D.S.; Patel, N.J.; Kundnani, D.L.; Wang, H.; Abbruzzese, J.L.; et al. Sequential Validation of Blood-Based Protein Biomarker Candidates for Early-Stage Pancreatic Cancer. J. Natl. Cancer Inst. 2017, 109, djw266. [Google Scholar] [CrossRef]
- Cai, D.; Chen, C.; Su, Y.; Tan, Y.; Lin, X.; Xing, R. LRG1 in Pancreatic Cancer Cells Promotes Inflammatory Factor Synthesis and the Angiogenesis of HUVECs by Activating VEGFR Signaling. J. Gastrointest. Oncol. 2022, 13, 400–412. [Google Scholar] [CrossRef]
- Furukawa, K.; Kawamoto, K.; Eguchi, H.; Tanemura, M.; Tanida, T.; Tomimaru, Y.; Akita, H.; Hama, N.; Wada, H.; Kobayashi, S.; et al. Clinicopathological Significance of Leucine-Rich A2-Glycoprotein-1 in Sera of Patients with Pancreatic Cancer. Pancreas 2015, 44, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ami, R.; Wang, Q.-L.; Zhang, J.; Supplee, J.G.; Fahrmann, J.F.; Lehmann-Werman, R.; Brais, L.K.; Nowak, J.; Yuan, C.; Loftus, M.; et al. Protein Biomarkers and Alternatively Methylated Cell-Free DNA Detect Early Stage Pancreatic Cancer. Gut 2024, 73, 639–648. [Google Scholar] [CrossRef]
- Barton, J.G.; Bois, J.P.; Sarr, M.G.; Wood, C.M.; Qin, R.; Thomsen, K.M.; Kendrick, M.L.; Farnell, M.B. Predictive and Prognostic Value of CA 19-9 in Resected Pancreatic Adenocarcinoma. J. Gastrointest. Surg. 2009, 13, 2050–2058. [Google Scholar] [CrossRef] [PubMed]
- van Manen, L.; Groen, J.V.; Putter, H.; Vahrmeijer, A.L.; Swijnenburg, R.J.; Bonsing, B.A.; Mieog, J.S.D. Elevated CEA and CA19-9 Serum Levels Independently Predict Advanced Pancreatic Cancer at Diagnosis. Biomarkers 2020, 25, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Murakami, Y.; Uemura, K.; Hayashidani, Y.; Sudo, T.; Hashimoto, Y.; Nakashima, A.; Sakabe, R.; Shigemoto, N.; Kato, Y.; et al. Prognostic Impact of Perioperative Serum CA 19-9 Levels in Patients with Resectable Pancreatic Cancer. Ann. Surg. Oncol. 2010, 17, 2321–2329. [Google Scholar] [CrossRef] [PubMed]
- Ferrone, C.R.; Finkelstein, D.M.; Thayer, S.P.; Muzikansky, A.; Fernandez-Del Castillo, C.; Warshaw, A.L. Perioperative CA19-9 Levels Can Predict Stage and Survival in Patients with Resectable Pancreatic Adenocarcinoma. J. Clin. Oncol. 2006, 24, 2897–2902. [Google Scholar] [CrossRef]
- Alexakis, N.; Gomatos, I.P.; Sbarounis, S.; Toutouzas, K.; Katsaragakis, S.; Zografos, G.; Konstandoulakis, M.M. High Serum CA 19-9 but Not Tumor Size Should Select Patients for Staging Laparoscopy in Radiological Resectable Pancreas Head and Peri-Ampullary Cancer. Eur. J. Surg. Oncol. 2015, 41, 265–269. [Google Scholar] [CrossRef]
- Maithel, S.K.; Maloney, S.; Winston, C.; Gönen, M.; D’Angelica, M.I.; Dematteo, R.P.; Jarnagin, W.R.; Brennan, M.F.; Allen, P.J. Preoperative CA 19-9 and the Yield of Staging Laparoscopy in Patients with Radiographically Resectable Pancreatic Adenocarcinoma. Ann. Surg. Oncol. 2008, 15, 3512–3520. [Google Scholar] [CrossRef]
- Al Abbas, A.I.; Zenati, M.; Reiser, C.J.; Hamad, A.; Jung, J.P.; Zureikat, A.H.; Zeh, H.J.; Hogg, M.E. Serum CA19-9 Response to Neoadjuvant Therapy Predicts Tumor Size Reduction and Survival in Pancreatic Adenocarcinoma. Ann. Surg. Oncol. 2020, 27, 2007. [Google Scholar] [CrossRef]
- Berger, A.C.; Garcia, M.; Hoffman, J.P.; Regine, W.F.; Abrams, R.A.; Safran, H.; Konski, A.; Benson, A.B.; MacDonald, J.; Willett, C.G. Postresection CA 19-9 Predicts Overall Survival in Patients with Pancreatic Cancer Treated with Adjuvant Chemoradiation: A Prospective Validation by RTOG 9704. J. Clin. Oncol. 2008, 26, 5918–5922. [Google Scholar] [CrossRef]
- Reni, M.; Cereda, S.; Balzano, G.; Passoni, P.; Rognone, A.; Fugazza, C.; Mazza, E.; Zerbi, A.; Di Carlo, V.; Villa, E. Carbohydrate Antigen 19-9 Change during Chemotherapy for Advanced Pancreatic Adenocarcinoma. Cancer 2009, 115, 2630–2639. [Google Scholar] [CrossRef] [PubMed]
- Hata, S.; Sakamoto, Y.; Yamamoto, Y.; Nara, S.; Esaki, M.; Shimada, K.; Kosuge, T. Prognostic Impact of Postoperative Serum CA 19-9 Levels in Patients with Resectable Pancreatic Cancer. Ann. Surg. Oncol. 2012, 19, 636–641. [Google Scholar] [CrossRef]
- Azizian, A.; Rühlmann, F.; Krause, T.; Bernhardt, M.; Jo, P.; König, A.; Kleiß, M.; Leha, A.; Ghadimi, M.; Gaedcke, J. CA19-9 for Detecting Recurrence of Pancreatic Cancer. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Rieser, C.J.; Zenati, M.; Hamad, A.; Al Abbas, A.I.; Bahary, N.; Zureikat, A.H.; Zeh, H.J.; Hogg, M.E. CA19-9 on Postoperative Surveillance in Pancreatic Ductal Adenocarcinoma: Predicting Recurrence and Changing Prognosis over Time. Ann. Surg. Oncol. 2018, 25, 3483–3491. [Google Scholar] [CrossRef]
- Vreeland, T.J.; McAllister, F.; Javadi, S.; Prakash, L.R.; Fogelman, D.R.; Ho, L.; Varadhachary, G.; Aloia, T.A.; Vauthey, J.N.; Lee, J.E.; et al. Benefit of Gemcitabine/Nab-Paclitaxel Rescue of Patients with Borderline Resectable or Locally Advanced Pancreatic Adenocarcinoma after Early Failure of FOLFIRINOX. Pancreas 2019, 48, 837–843. [Google Scholar] [CrossRef]
- Alva-Ruiz, R.; Yohanathan, L.; Yonkus, J.A.; Abdelrahman, A.M.; Gregory, L.A.; Halfdanarson, T.R.; Mahipal, A.; McWilliams, R.R.; Ma, W.W.; Hallemeier, C.L.; et al. Neoadjuvant Chemotherapy Switch in Borderline Resectable/Locally Advanced Pancreatic Cancer. Ann. Surg. Oncol. 2022, 29, 1579–1591. [Google Scholar] [CrossRef] [PubMed]
- Thalji, S.Z.; Kamgar, M.; George, B.; Aldakkak, M.; Christians, K.K.; Clarke, C.N.; Erickson, B.A.; Hall, W.A.; Tolat, P.P.; Smith, Z.L.; et al. CA19-9 Response to First-Line Neoadjuvant FOLFIRINOX and Second-Line Gemcitabine/Nab-Paclitaxel for Patients with Operable Pancreatic Cancer. Ann. Surg. Oncol. 2023, 30, 3013–3021. [Google Scholar] [CrossRef] [PubMed]
- Moasser, M.M. The Oncogene HER2: Its Signaling and Transforming Functions and Its Role in Human Cancer Pathogenesis. Oncogene 2007, 26, 6469–6487. [Google Scholar] [CrossRef]
- Yoon, J.; Oh, D.Y. HER2-Targeted Therapies beyond Breast Cancer—An Update. Nat. Rev. Clin. Oncol. 2024, 21, 675–700. [Google Scholar] [CrossRef]
- Han, S.-H.; Ryu, K.H.; Kwon, A.-Y. The Prognostic Impact of HER2 Genetic and Protein Expression in Pancreatic Carcinoma-HER2 Protein and Gene in Pancreatic Cancer. Diagnostics 2021, 11, 653. [Google Scholar] [CrossRef]
- Shibata, W.; Kinoshita, H.; Hikiba, Y.; Sato, T.; Ishii, Y.; Sue, S.; Sugimori, M.; Suzuki, N.; Sakitani, K.; Ijichi, H.; et al. Overexpression of HER2 in the Pancreas Promotes Development of Intraductal Papillary Mucinous Neoplasms in Mice. Sci. Rep. 2018, 8, 6150. [Google Scholar] [CrossRef] [PubMed]
- King, D.A.; Smith, A.R.; Pineda, G.; Nakano, M.; Michelini, F.; Goedegebuure, S.P.; Thyparambil, S.; Liao, W.-L.; McCormick, A.; Ju, J.; et al. Complete Remission of Widely Metastatic Human Epidermal Growth Factor Receptor 2–Amplified Pancreatic Adenocarcinoma After Precision Immune and Targeted Therapy with Description of Sequencing and Organoid Correlates. JCO Precis. Oncol. 2023, 7, e2100489. [Google Scholar] [CrossRef] [PubMed]
- Randall, J.; Hunt, A.L.; Nutcharoen, A.; Johnston, L.; Chouraichi, S.; Wang, H.; Winer, A.; Wadlow, R.; Huynh, J.; Davis, J.; et al. Quantitative Proteomic Analysis of HER2 Protein Expression in PDAC Tumors. Clin. Proteom. 2024, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bradley, R.; Braybrooke, J.; Gray, R.; Hills, R.; Liu, Z.; Peto, R.; Davies, L.; Dodwell, D.; McGale, P.; Pan, H.; et al. Trastuzumab for Early-Stage, HER2-Positive Breast Cancer: A Meta-Analysis of 13,864 Women in Seven Randomised Trials. Lancet Oncol. 2021, 22, 1139. [Google Scholar] [CrossRef]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in Combination with Chemotherapy versus Chemotherapy Alone for Treatment of HER2-Positive Advanced Gastric or Gastro-Oesophageal Junction Cancer (ToGA): A Phase 3, Open-Label, Randomised Controlled Trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA Grants Accelerated Approval to Fam-Trastuzumab Deruxtecan-Nxki for Unresectable or Metastatic HER2-Positive Solid Tumors. 2024. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-fam-trastuzumab-deruxtecan-nxki-unresectable-or-metastatic-her2 (accessed on 7 May 2025).
- Meric-Bernstam, F.; Makker, V.; Oaknin, A.; Oh, D.Y.; Banerjee, S.; González-Martín, A.; Jung, K.H.; Ługowska, I.; Manso, L.; Manzano, A.; et al. Efficacy and Safety of Trastuzumab Deruxtecan in Patients With HER2-Expressing Solid Tumors: Primary Results From the DESTINY-PanTumor02 Phase II Trial. J. Clin. Oncol. 2024, 42, 47–58. [Google Scholar] [CrossRef]
- Smit, E.F.; Felip, E.; Uprety, D.; Nagasaka, M.; Nakagawa, K.; Paz-Ares Rodríguez, L.; Pacheco, J.M.; Li, B.T.; Planchard, D.; Baik, C.; et al. Trastuzumab Deruxtecan in Patients with Metastatic Non-Small-Cell Lung Cancer (DESTINY-Lung01): Primary Results of the HER2-Overexpressing Cohorts from a Single-Arm, Phase 2 Trial. Lancet Oncol 2024, 25, 439–454. [Google Scholar] [CrossRef]
- Raghav, K.; Siena, S.; Takashima, A.; Kato, T.; Van den Eynde, M.; Pietrantonio, F.; Komatsu, Y.; Kawakami, H.; Peeters, M.; Andre, T.; et al. Trastuzumab Deruxtecan in Patients with HER2-Positive Advanced Colorectal Cancer (DESTINY-CRC02): Primary Results from a Multicentre, Randomised, Phase 2 Trial. Lancet Oncol. 2024, 25, 1147–1162. [Google Scholar] [CrossRef]
- Cao, W.; Xing, H.; Li, Y.; Tian, W.; Song, Y.; Jiang, Z.; Yu, J. Claudin18.2 Is a Novel Molecular Biomarker for Tumor-Targeted Immunotherapy. Biomark. Res. 2022, 10, 38. [Google Scholar] [CrossRef]
- Park, S.; Shin, K.; Kim, I.H.; Hong, T.; Kim, Y.; Suh, J.; Lee, M. Clinicopathological Features and Prognosis of Resected Pancreatic Ductal Adenocarcinoma Patients with Claudin-18 Overexpression. J. Clin. Med. 2023, 12, 5394. [Google Scholar] [CrossRef]
- Lyu, S.I.; Fretter, C.; Simon, A.G.; Spielmann, S.M.; Damanakis, A.I.; Zhao, Y.; Bruns, C.J.; Schmidt, T.; Popp, F.C.; Waldschmidt, D.; et al. Extent and Clinical Significance of the Therapy-Relevant Tight Junction Protein Claudin 18.2 in Pancreatic Ductal Adenocarcinoma—Real-World Evidence. Transl. Oncol. 2024, 47, 102044. [Google Scholar] [CrossRef] [PubMed]
- Niimi, T.; Nagashima, K.; Ward, J.M.; Minoo, P.; Zimonjic, D.B.; Popescu, N.C.; Kimura, S. Claudin-18, a Novel Downstream Target Gene for the T/EBP/NKX2.1 Homeodomain Transcription Factor, Encodes Lung- and Stomach-Specific Isoforms through Alternative Splicing. Mol. Cell. Biol. 2001, 21, 7380–7390. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Koslowski, M.; Dhaene, K.; Usener, D.; Brandenburg, G.; Seitz, G.; Huber, C.; Turecil, O. Claudin-18 Splice Variant 2 Is a Pan-Cancer Target Suitable for Therapeutic Antibody Development. Clin. Cancer Res. 2008, 14, 7624–7634. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA Approves Zolbetuximab-Clzb with Chemotherapy for Gastric or Gastroesophageal Junction Adenocarcinoma. 2024. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-zolbetuximab-clzb-chemotherapy-gastric-or-gastroesophageal-junction-adenocarcinoma (accessed on 13 May 2025).
- Shitara, K.; Lordick, F.; Bang, Y.J.; Enzinger, P.; Ilson, D.; Shah, M.A.; Van Cutsem, E.; Xu, R.H.; Aprile, G.; Xu, J.; et al. Zolbetuximab plus MFOLFOX6 in Patients with CLDN18.2-Positive, HER2-Negative, Untreated, Locally Advanced Unresectable or Metastatic Gastric or Gastro-Oesophageal Junction Adenocarcinoma (SPOTLIGHT): A Multicentre, Randomised, Double-Blind, Phase 3 Trial. Lancet 2023, 401, 1655–1668. [Google Scholar] [CrossRef]
- Shah, M.A.; Shitara, K.; Ajani, J.A.; Bang, Y.J.; Enzinger, P.; Ilson, D.; Lordick, F.; Van Cutsem, E.; Gallego Plazas, J.; Huang, J.; et al. Zolbetuximab plus CAPOX in CLDN18.2-Positive Gastric or Gastroesophageal Junction Adenocarcinoma: The Randomized, Phase 3 GLOW Trial. Nat. Med. 2023, 29, 2133–2141. [Google Scholar] [CrossRef]
- Park, W.; O’Reilly, E.M.; Furuse, J.; Li, C.-P.; Oh, D.-Y.; Garcia-Carbonero, R.; Roth, G.; Lee, H.-J.; Kunieda, F. Zolbetuximab plus Gemcitabine and Nab-Paclitaxel (GN) in First-Line Treatment of Claudin 18.2-Positive Metastatic Pancreatic Cancer (MPC): Phase 2, Open-Label, Randomized Study. J. Clin. Oncol. 2022, 40 (Suppl. 16), TPS4186. [Google Scholar] [CrossRef]
- Wöll, S.; Schlitter, A.M.; Dhaene, K.; Roller, M.; Esposito, I.; Sahin, U.; Türeci, Ö. Claudin 18.2 Is a Target for IMAB362 Antibody in Pancreatic Neoplasms. Int. J. Cancer 2014, 134, 731–739. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.S.; Dong, X.Y.; Hu, Y.; Duan, B.J.; Bai, J.; Wu, Y.Y.; Fan, L.; Liao, X.H.; Kang, Y.; et al. Claudin 18.2 Is a Potential Therapeutic Target for Zolbetuximab in Pancreatic Ductal Adenocarcinoma. World J. Gastrointest. Oncol. 2022, 14, 1252–1264. [Google Scholar] [CrossRef]
- Jasani, B.; Taniere, P.; Schildhaus, H.U.; Blighe, K.; Parry, S.; Wilkinson, D.; Atkey, N.; Clare-Antony, S.; McCabe, C.; Quinn, C.; et al. Global Ring Study to Investigate the Comparability of Total Assay Performance of Commercial Claudin 18 Antibodies for Evaluation in Gastric Cancer. Lab. Investig. 2024, 104, 100284. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Park, S.S.; Lee, Y.K.; Norton, J.A.; Jeffrey, S.S. Liquid Biopsy in Pancreatic Ductal Adenocarcinoma: Current Status of Circulating Tumor Cells and Circulating Tumor DNA. Mol. Oncol. 2019, 13, 1623–1650. [Google Scholar] [CrossRef]
- Hou, J.; Li, X.T.; Xie, K.P. Coupled Liquid Biopsy and Bioinformatics for Pancreatic Cancer Early Detection and Precision Prognostication. Mol. Cancer 2021, 20, 34. [Google Scholar] [CrossRef] [PubMed]
- Stosic, K.; Senar, O.A.; Tarfouss, J.; Bouchart, C.; Navez, J.; Van Laethem, J.L.; Arsenijevic, T. A Comprehensive Review of the Potential Role of Liquid Biopsy as a Diagnostic, Prognostic, and Predictive Biomarker in Pancreatic Ductal Adenocarcinoma. Cells 2023, 13, 3. [Google Scholar] [CrossRef]
- Yeo, D.; Bastian, A.; Strauss, H.; Saxena, P.; Grimison, P.; Rasko, J.E.J. Exploring the Clinical Utility of Pancreatic Cancer Circulating Tumor Cells. Int. J. Mol. Sci. 2022, 23, 1671. [Google Scholar] [CrossRef] [PubMed]
- Rhim, A.D.; Thege, F.I.; Santana, S.M.; Lannin, T.B.; Saha, T.N.; Tsai, S.; Maggs, L.R.; Kochman, M.L.; Ginsberg, G.G.; Lieb, J.G.; et al. Detection of Circulating Pancreas Epithelial Cells in Patients with Pancreatic Cystic Lesions. Gastroenterology 2014, 146, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Ankeny, J.S.; Court, C.M.; Hou, S.; Li, Q.; Song, M.; Wu, D.; Chen, J.F.; Lee, T.; Lin, M.; Sho, S.; et al. Circulating Tumour Cells as a Biomarker for Diagnosis and Staging in Pancreatic Cancer. Br. J. Cancer 2016, 114, 1367–1375. [Google Scholar] [CrossRef]
- Buscail, E.; Alix-Panabières, C.; Quincy, P.; Cauvin, T.; Chauvet, A.; Degrandi, O.; Caumont, C.; Verdon, S.; Lamrissi, I.; Moranvillier, I.; et al. High Clinical Value of Liquid Biopsy to Detect Circulating Tumor Cells and Tumor Exosomes in Pancreatic Ductal Adenocarcinoma Patients Eligible for Up-Front Surgery. Cancers 2019, 11, 1656. [Google Scholar] [CrossRef]
- Court, C.M.; Ankeny, J.S.; Sho, S.; Winograd, P.; Hou, S.; Song, M.; Wainberg, Z.A.; Girgis, M.D.; Graeber, T.G.; Agopian, V.G.; et al. Circulating Tumor Cells Predict Occult Metastatic Disease and Prognosis in Pancreatic Cancer. Ann. Surg. Oncol. 2018, 25, 1000–1008. [Google Scholar] [CrossRef]
- Bissolati, M.; Sandri, M.T.; Burtulo, G.; Zorzino, L.; Balzano, G.; Braga, M. Portal Vein-Circulating Tumor Cells Predict Liver Metastases in Patients with Resectable Pancreatic Cancer. Tumour Biol. 2015, 36, 991–996. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Ning, N.; Chen, Q.; Yang, Z.; Guo, Y.; Xu, D.; Zhang, D.; Zhan, T.; Cui, W. Patterns of Circulating Tumor Cells Identified by CEP8, CK and CD45 in Pancreatic Cancer. Int. J. Cancer 2015, 136, 1228–1233. [Google Scholar] [CrossRef]
- Poruk, K.E.; Blackford, A.L.; Weiss, M.J.; Cameron, J.L.; He, J.; Goggins, M.; Rasheed, Z.A.; Wolfgang, C.L.; Wood, L.D. Circulating Tumor Cells Expressing Markers of Tumor-Initiating Cells Predict Poor Survival and Cancer Recurrence in Patients with Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2017, 23, 2681–2690. [Google Scholar] [CrossRef]
- Poruk, K.E.; Valero, V.; Saunders, T.; Blackford, A.L.; Griffin, J.F.; Poling, J.; Hruban, R.H.; Anders, R.A.; Herman, J.; Zheng, L.; et al. Circulating Tumor Cell Phenotype Predicts Recurrence and Survival in Pancreatic Adenocarcinoma. Ann. Surg. 2016, 264, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhu, Y.; Zhang, Z.; Zhang, C.; Huang, X.; Yuan, Z. Clinical Significance of Pancreatic Circulating Tumor Cells Using Combined Negative Enrichment and Immunostaining-Fluorescence in Situ Hybridization. J. Exp. Clin. Cancer Res. 2016, 35, 66. [Google Scholar] [CrossRef]
- Gemenetzis, G.; Groot, V.P.; Yu, J.; Ding, D.; Teinor, J.A.; Javed, A.A.; Wood, L.D.; Burkhart, R.A.; Cameron, J.L.; Makary, M.A.; et al. Circulating Tumor Cells Dynamics in Pancreatic Adenocarcinoma Correlate With Disease Status: Results of the Prospective CLUSTER Study. Ann. Surg. 2018, 268, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Semenkovich, N.P.; Szymanski, J.J.; Earland, N.; Chauhan, P.S.; Pellini, B.; Chaudhuri, A.A. Genomic Approaches to Cancer and Minimal Residual Disease Detection Using Circulating Tumor DNA. J. Immunother. Cancer 2023, 11, e006284. [Google Scholar] [CrossRef] [PubMed]
- Sausen, M.; Phallen, J.; Adleff, V.; Jones, S.; Leary, R.J.; Barrett, M.T.; Anagnostou, V.; Parpart-Li, S.; Murphy, D.; Li, Q.K.; et al. Clinical Implications of Genomic Alterations in the Tumour and Circulation of Pancreatic Cancer Patients. Nat. Commun. 2015, 6, 7686. [Google Scholar] [CrossRef]
- Singh, K.; Pruski, M.; Bland, R.; Younes, M.; Guha, S.; Thosani, N.; Maitra, A.; Cash, B.D.; McAllister, F.; Logsdon, C.D.; et al. Kras Mutation Rate Precisely Orchestrates Ductal Derived Pancreatic Intraepithelial Neoplasia and Pancreatic Cancer. Lab. Investig. 2021, 101, 177–192. [Google Scholar] [CrossRef]
- Dubrovsky, G.; Ross, A.; Jalali, P.; Lotze, M. Liquid Biopsy in Pancreatic Ductal Adenocarcinoma: A Review of Methods and Applications. Int. J. Mol. Sci. 2024, 25, 11013. [Google Scholar] [CrossRef]
- Cox, M.; Vitello, D.J.; Chawla, A. The Current Role of Circulating Tumor DNA in the Management of Pancreatic Cancer. J. Gastrointest. Cancer 2025, 56, 44. [Google Scholar] [CrossRef]
- Chawla, A.; Qadan, M.; Castillo, C.F.; Wo, J.Y.; Allen, J.N.; Clark, J.W.; Murphy, J.E.; Catalano, O.A.; Ryan, D.P.; Ting, D.T.; et al. Prospective Phase II Trials Validate the Effect of Neoadjuvant Chemotherapy on Pattern of Recurrence in Pancreatic Adenocarcinoma. Ann. Surg. 2022, 276, E502–E509. [Google Scholar] [CrossRef]
- Theparee, T.; Akroush, M.; Sabatini, L.M.; Wang, V.; Mangold, K.A.; Joseph, N.; Stocker, S.J.; Freedman, A.; Helseth, D.L.; Talamonti, M.S.; et al. Cell Free DNA in Patients with Pancreatic Adenocarcinoma: Clinicopathologic Correlations. Sci. Rep. 2024, 14, 15744. [Google Scholar] [CrossRef]
- Cox, M.; Vitello, D.; Chawla, A. Translating the Multifaceted Use of Liquid Biopsy to Management of Early Disease in Pancreatic Adenocarcinoma. Front. Oncol. 2025, 15, 1520717. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.D.; Li, L.; Wang, Y.; Thoburn, C.; Afsari, B.; Danilova, L.; Douville, C.; Javed, A.A.; Wong, F.; Mattox, A.; et al. Detection and Localization of Surgically Resectable Cancers with a Multi-Analyte Blood Test. Science 2018, 359, 926–930. [Google Scholar] [CrossRef]
- Lennon, A.M.; Buchanan, A.H.; Kinde, I.; Warren, A.; Honushefsky, A.; Cohain, A.T.; Ledbetter, D.H.; Sanfilippo, F.; Sheridan, K.; Rosica, D.; et al. Feasibility of Blood Testing Combined with PET-CT to Screen for Cancer and Guide Intervention. Science 2020, 369, eabb9601. [Google Scholar] [CrossRef]
- Schrag, D.; Beer, T.M.; McDonnell, C.H.; Nadauld, L.; Dilaveri, C.A.; Reid, R.; Marinac, C.R.; Chung, K.C.; Lopatin, M.; Fung, E.T.; et al. Blood-Based Tests for Multicancer Early Detection (PATHFINDER): A Prospective Cohort Study. Lancet 2023, 402, 1251–1260. [Google Scholar] [CrossRef]
- Hussung, S.; Akhoundova, D.; Hipp, J.; Follo, M.; Klar, R.F.U.; Philipp, U.; Scherer, F.; von Bubnoff, N.; Duyster, J.; Boerries, M.; et al. Longitudinal Analysis of Cell-Free Mutated KRAS and CA 19-9 Predicts Survival Following Curative Resection of Pancreatic Cancer. BMC Cancer 2021, 21, 49. [Google Scholar] [CrossRef]
- Groot, V.P.; Mosier, S.; Javed, A.A.; Teinor, J.A.; Gemenetzis, G.; Ding, D.; Haley, L.M.; Yu, J.; Burkhart, R.A.; Hasanain, A.; et al. Circulating Tumor DNA as a Clinical Test in Resected Pancreatic Cancer. Clin. Cancer Res. 2019, 25, 4973–4984. [Google Scholar] [CrossRef]
- Guo, S.; Shi, X.; Shen, J.; Gao, S.; Wang, H.; Shen, S.; Pan, Y.; Li, B.; Xu, X.; Shao, Z.; et al. Preoperative Detection of KRAS G12D Mutation in CtDNA Is a Powerful Predictor for Early Recurrence of Resectable PDAC Patients. Br. J. Cancer 2020, 122, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Rhee, T.M.; Pietrasz, D.; Bachet, J.B.; Laurent-Puig, P.; Kong, S.Y.; Takai, E.; Yachida, S.; Shibata, T.; Lee, J.W.; et al. Circulating Tumor DNA as a Prognostic Indicator in Resectable Pancreatic Ductal Adenocarcinoma: A Systematic Review and Meta-Analysis. Sci. Rep. 2019, 9, 16971. [Google Scholar] [CrossRef] [PubMed]
- Pietrasz, D.; Pécuchet, N.; Garlan, F.; Didelot, A.; Dubreuil, O.; Doat, S.; Imbert-Bismut, F.; Karoui, M.; Vaillant, J.C.; Taly, V.; et al. Plasma Circulating Tumor DNA in Pancreatic Cancer Patients Is a Prognostic Marker. Clin. Cancer Res. 2017, 23, 116–123. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.; Park, M.R.; Choi, Z.; Han, S.J.; Kim, D.; Shin, S.; Lee, S.-T.; Choi, J.R.; Park, S.W. Prognostic Value of Residual Circulating Tumor DNA in Metastatic Pancreatic Ductal Adenocarcinoma. Ann. Lab. Med. 2025, 45, 199–208. [Google Scholar] [CrossRef]
- Hálková, T.; Bunganič, B.; Traboulsi, E.; Minárik, M.; Zavoral, M.; Benešová, L. Prognostic Role of Specific KRAS Mutations Detected in Aspiration and Liquid Biopsies from Patients with Pancreatic Cancer. Genes 2024, 15, 1302. [Google Scholar] [CrossRef] [PubMed]
- Vitello, D.J.; Shah, D.; Wells, A.; Masnyk, L.; Cox, M.; Janczewski, L.M.; Abad, J.; Dawravoo, K.; D’Souza, A.; Suh, G.; et al. Mutant KRAS in Circulating Tumor DNA as a Biomarker in Localized Pancreatic Cancer in Patients Treated with Neoadjuvant Chemotherapy. Ann. Surg. 2024. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Löhr, J.M.; Nilsson, M.; Segersvärd, R.; Matsson, H.; Verbeke, C.; Heuchel, R.; Kere, J.; Iafrate, A.J.; Zheng, Z.; et al. Variant Profiling of Candidate Genes in Pancreatic Ductal Adenocarcinoma. Clin. Chem. 2015, 61, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.A.; Desai, N.N.; Qureshi, M.Z.; Librelotto, D.R.N.; Gasparri, M.L.; Bishayee, A.; Nabavi, S.M.; Curti, V.; Daglia, M. Exosome Biogenesis, Bioactivities and Functions as New Delivery Systems of Natural Compounds. Biotechnol. Adv. 2018, 36, 328–334. [Google Scholar] [CrossRef]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA Delivery by Extracellular Vesicles in Mammalian Cells and Its Applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef]
- Zhang, H.; Xing, J.; Dai, Z.; Wang, D.; Tang, D. Exosomes: The Key of Sophisticated Cell-Cell Communication and Targeted Metastasis in Pancreatic Cancer. Cell Commun. Signal. 2022, 20, 9. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, K.; Chen, Y.; Wu, X.; Chen, Z.; Cao, K.; Tao, Y.; Chen, X.; Liao, J.; Zhou, J. Exosomes and Their Role in Cancer Progression. Front. Oncol. 2021, 11, 639159. [Google Scholar] [CrossRef]
- Fang, X.; Lan, H.; Jin, K.; Qian, J. Pancreatic Cancer and Exosomes: Role in Progression, Diagnosis, Monitoring, and Treatment. Front. Oncol. 2023, 13, 1149551. [Google Scholar] [CrossRef]
- Xu, B.; Chen, Y.; Peng, M.; Zheng, J.H.; Zuo, C. Exploring the Potential of Exosomes in Diagnosis and Drug Delivery for Pancreatic Ductal Adenocarcinoma. Int. J. Cancer 2022, 152, 110. [Google Scholar] [CrossRef]
- Monguió-Tortajada, M.; Gálvez-Montón, C.; Bayes-Genis, A.; Roura, S.; Borràs, F.E. Extracellular Vesicle Isolation Methods: Rising Impact of Size-Exclusion Chromatography. Cell. Mol. Life Sci. 2019, 76, 2369–2382. [Google Scholar] [CrossRef]
- Nakamura, S.; Sadakari, Y.; Ohtsuka, T.; Okayama, T.; Nakashima, Y.; Gotoh, Y.; Saeki, K.; Mori, Y.; Nakata, K.; Miyasaka, Y.; et al. Pancreatic Juice Exosomal MicroRNAs as Biomarkers for Detection of Pancreatic Ductal Adenocarcinoma. Ann. Surg. Oncol. 2019, 26, 2104–2111. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, S.; Iinuma, H.; Wada, K.; Takahashi, K.; Minezaki, S.; Kainuma, M.; Shibuya, M.; Miura, F.; Sano, K. Exosome-Encapsulated MicroRNA-4525, MicroRNA-451a and MicroRNA-21 in Portal Vein Blood Is a High-Sensitive Liquid Biomarker for the Selection of High-Risk Pancreatic Ductal Adenocarcinoma Patients. J. Hepatobiliary Pancreat. Sci. 2019, 26, 63–72. [Google Scholar] [CrossRef]
- Goto, T.; Fujiya, M.; Konishi, H.; Sasajima, J.; Fujibayashi, S.; Hayashi, A.; Utsumi, T.; Sato, H.; Iwama, T.; Ijiri, M.; et al. An Elevated Expression of Serum Exosomal MicroRNA-191, - 21, -451a of Pancreatic Neoplasm Is Considered to Be Efficient Diagnostic Marker. BMC Cancer 2018, 18, 116. [Google Scholar] [CrossRef]
- Xu, Y.-F.; Hannafon, B.N.; Zhao, Y.D.; Postier, R.G.; Ding, W.-Q. Plasma Exosome MiR-196a and MiR-1246 Are Potential Indicators of Localized Pancreatic Cancer. Oncotarget 2017, 8, 77028–77040. [Google Scholar] [CrossRef] [PubMed]
- Reese, M.; Flammang, I.; Yang, Z.; Dhayat, S.A. Potential of Exosomal MicroRNA-200b as Liquid Biopsy Marker in Pancreatic Ductal Adenocarcinoma. Cancers 2020, 12, 197. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Qin, H.; Liu, K.; Wang, H.; Bai, S.; Liu, S.; Shao, Z.; Zhang, Y.; Song, B.; Xu, X.; et al. Blood Small Extracellular Vesicles Derived MiRNAs to Differentiate Pancreatic Ductal Adenocarcinoma from Chronic Pancreatitis. Clin. Transl. Med. 2021, 11, e520. [Google Scholar] [CrossRef]
- National Human Genome Research Institute. DNA Sequencing Costs: Data. 2023. Available online: https://www.genome.gov/about-genomics/fact-sheets/DNA-Sequencing-Costs-Data (accessed on 13 May 2025).
- Pennell, N.A.; Mutebi, A.; Zhou, Z.-Y.; Ricculli, M.L.; Tang, W.; Wang, H.; Guerin, A.; Arnhart, T.; Dalal, A.; Sasane, M.; et al. Economic Impact of Next-Generation Sequencing Versus Single-Gene Testing to Detect Genomic Alterations in Metastatic Non–Small-Cell Lung Cancer Using a Decision Analytic Model. JCO Precis. Oncol. 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Mirza, M.; Goerke, L.; Anderson, A.; Wilsdon, T. Assessing the Cost-Effectiveness of Next-Generation Sequencing as a Biomarker Testing Approach in Oncology and Policy Implications: A Literature Review. Value Health 2024, 27, 1300–1309. [Google Scholar] [CrossRef]
- Bayle, A.; Bonastre, J.; Chaltiel, D.; Latino, N.; Rouleau, E.; Peters, S.; Galotti, M.; Bricalli, G.; Besse, B.; Giuliani, R. ESMO Study on the Availability and Accessibility of Biomolecular Technologies in Oncology in Europe. Ann. Oncol. 2023, 34, 934–945. [Google Scholar] [CrossRef]
- Pruneri, G.; De Braud, F.; Sapino, A.; Aglietta, M.; Vecchione, A.; Giusti, R.; Marchiò, C.; Scarpino, S.; Baggi, A.; Bonetti, G.; et al. Next-Generation Sequencing in Clinical Practice: Is It a Cost-Saving Alternative to a Single-Gene Testing Approach? Pharmacoecon. Open 2021, 5, 285–298. [Google Scholar] [CrossRef]
- Vanderpoel, J.; Stevens, A.L.; Emond, B.; Lafeuille, M.H.; Hilts, A.; Lefebvre, P.; Morrison, L. Total Cost of Testing for Genomic Alterations Associated with Next-Generation Sequencing versus Polymerase Chain Reaction Testing Strategies among Patients with Metastatic Non-Small Cell Lung Cancer. J. Med. Econ. 2022, 25, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Acosta, R.H.P.; Fuentes, M.D.C.D.; Pena, N.G.; Rojas, P.S.; Zambrano, C.B. Therapeutic Impact and Routine Application of Next-Generation Sequencing: A Single Institute Study. Biomed. Rep. 2022, 16, 33. [Google Scholar] [CrossRef] [PubMed]
- Ree, A.H.; Mælandsmo, G.M.; Flatmark, K.; Russnes, H.G.; Gómez Castañeda, M.; Aas, E. Cost-Effectiveness of Molecularly Matched off-Label Therapies for End-Stage Cancer–the MetAction Precision Medicine Study. Acta Oncol. 2022, 61, 955–962. [Google Scholar] [CrossRef]
- Christofyllakis, K.; Bittenbring, J.T.; Thurner, L.; Ahlgrimm, M.; Stilgenbauer, S.; Bewarder, M.; Kaddu-Mulindwa, D. Cost-Effectiveness of Precision Cancer Medicine-Current Challenges in the Use of next Generation Sequencing for Comprehensive Tumour Genomic Profiling and the Role of Clinical Utility Frameworks (Review). Mol. Clin. Oncol. 2022, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.A.; Deverka, P.A.; Hooker, G.W.; Douglas, M.P. Genetic Test Availability and Spending: Where Are We Now? Where Are We Going? Health Aff. 2018, 37, 710–716. [Google Scholar] [CrossRef]
| Type | Biomarker | Significance | Uses | Limitations | Clinical Trials |
|---|---|---|---|---|---|
| Oncogene | KRAS |
|
|
|
|
| Tumor suppressor genes | TP53 |
|
|
|
|
| SMAD4 |
|
|
|
| |
| CDKN2A |
|
|
|
| |
| DNA damage repair | BRCA 1/2 |
|
|
|
|
| Serum protein | CA 19-9 |
|
|
|
|
| Membrane tyrosine kinase, Oncogene | HER2 |
|
|
|
|
| Transmembrane tight junction protein | Claudin 18.2 |
|
|
|
|
| Tumor epithelial cell | Circulating Tumor Cell (CTC) |
|
|
|
|
| Tumor extracellular DNA | ctDNA |
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Awad, M.A.; Hwang, J.; Villano, A.M. Molecular Biomarkers for the Diagnosis and Prognostication of Pancreatic Ductal Adenocarcinoma. J. Pers. Med. 2025, 15, 236. https://doi.org/10.3390/jpm15060236
Sun J, Awad MA, Hwang J, Villano AM. Molecular Biomarkers for the Diagnosis and Prognostication of Pancreatic Ductal Adenocarcinoma. Journal of Personalized Medicine. 2025; 15(6):236. https://doi.org/10.3390/jpm15060236
Chicago/Turabian StyleSun, James, Morcos A. Awad, Jennifer Hwang, and Anthony M. Villano. 2025. "Molecular Biomarkers for the Diagnosis and Prognostication of Pancreatic Ductal Adenocarcinoma" Journal of Personalized Medicine 15, no. 6: 236. https://doi.org/10.3390/jpm15060236
APA StyleSun, J., Awad, M. A., Hwang, J., & Villano, A. M. (2025). Molecular Biomarkers for the Diagnosis and Prognostication of Pancreatic Ductal Adenocarcinoma. Journal of Personalized Medicine, 15(6), 236. https://doi.org/10.3390/jpm15060236






