Thromboembolic Risk and High Prothrombotic Factors in Childhood Acute Lymphoblastic Leukemia with Ischemic Stroke: A Literature Review of Personalized and Institutional Approaches to Prophylaxis
Abstract
1. Introduction
2. Materials and Methods
3. Case Presentation
4. Literature Review
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIEOP-BFM | International Collaborative Treatment Protocol for Children and Adolescents with Acute Lymphoblastic Leukemia Berlin-Frankfurt-Muenster |
| AlAT | Alanine aminotransferase |
| ALL | Acute lymphoblastic leukemia |
| APTT | Partial thromboplastin time |
| ASP | Asparaginase |
| AspAT | Aspartate aminotransferase |
| AT-III | Antithrombin III |
| CNS | Central nervous system |
| CRP | C-reactive protein |
| CSVT | Cerebral venous sinus thrombosis |
| CVC | Central venous catheter |
| CYP-C | Cancer in Young People Canada |
| DCOG | The Dutch Childhood Oncology Group |
| EFS | Event-free survival |
| F2 | Coagulation Factor II |
| F5 | Coagulation Factor V |
| HDL | High-density lipoprotein |
| Hg | Hemoglobin |
| Ht | Hematocrit |
| INR | International Normalized Ratio of Prothrombin |
| LDH | Lactate dehydrogenase |
| LDL | Low-density lipoprotein |
| LMWH | Low-molecular-weight heparin |
| NHL | Non-Hodgkin lymphoma |
| non-HDL | Non-high-density lipoprotein |
| PLT | Platelet |
| PT | Prothrombin time |
| TE | Thromboembolism |
| TT | Thrombin time |
| VTE | Venous thromboembolism |
| vWF | von Willebrand factor |
| WBC | White blood cell |
References
- Öztürk, A.P.; Koç, B.; Zülfikar, B. Acute complications and survival analysis of childhood acute lymphoblastic leukemia: A 15-year experience. Clin. Lymphoma Myeloma Leuk. 2021, 21, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Malard, F.; Mohty, M. Acute lymphoblastic leukaemia. Lancet 2020, 395, 1146–1162. [Google Scholar] [CrossRef] [PubMed]
- Kartal, V.; Zara, Z.; Yilmaz, S.; Ayhan, A.; Yoruk, A.; Timur, C. A thrombosis story and PRES. North. Clin. Istanb. 2014, 1, 49–52. [Google Scholar] [CrossRef]
- Páramo, J.A.; Marcos-Jubilar, M.; Lecumberri, R. Impact of the mutation profile on thrombotic risk in cancer patients. Rev. Clin. Esp. 2022, 222, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, V. Thrombosis complications in pediatric acute lymphoblastic leukemia: Risk factors, management, and prevention: Is there any role for pharmacologic prophylaxis? Front. Pediatr. 2022, 10, 828702. [Google Scholar] [CrossRef]
- Javed, I.; Sultan, T.; Rehman, Z.U.; Yaseen, M.R. Clinical spectrum and outcome of cerebral venous sinus thrombosis in children. J. Coll. Physicians Surg. Pak. 2018, 28, 390–393. [Google Scholar] [CrossRef]
- Güzelküçük, Z.; Karapınar, D.Y.; Gelen, S.A.; Tokgöz, H.; Özcan, A.; Ay, Y.; Bahadır, A.; Özbek, N.Y.; Ören, A.C.; Ayhan, A.C.; et al. Central nervous system thrombosis in pediatric acute lymphoblastic leukemia in Turkey: A multicenter study. Pediatr. Blood Cancer 2023, 70, e30425. [Google Scholar] [CrossRef]
- Ruiz-Llobet, A.; Gassiot, S.; Sarrate, E.; Zubicaray, J.; Dapena, J.L.; Rives, S.; Sevilla, J.; Menárguez López, Á.; Panesso Romero, M.; Montoya, C.; et al. Venous thromboembolism in pediatric patients with acute lymphoblastic leukemia under chemotherapy treatment. Risk factors and usefulness of thromboprophylaxis. Results of LAL-SEHOP-PETHEMA-2013. J. Thromb. Haemost. 2022, 20, 1390–1399. [Google Scholar] [CrossRef]
- Klukowska, A.H. Zmiany zakrzepowo-zatorowe u dzieci–kazuistyka? Pediatria po Dyplomie 2023. Available online: https://podyplomie.pl/pediatria/39134%2Czmiany-zakrzepowo-zatorowe-u-dzieci-kazuistyka (accessed on 10 March 2025).
- Zheng, Y.; Yang, W.; Estepp, J.; Pei, D.; Cheng, C.; Takemoto, C.M.; Inaba, H.; Jeha, S.; Pui, C.H.; Relling, M.V.; et al. Genomic analysis of venous thrombosis in children with acute lymphoblastic leukemia from diverse ancestries. Haematologica 2024, 109, 53–59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barzilai-Birenboim, S.; Nirel, R.; Arad-Cohen, N.; Avrahami, G.; Ben Harush, M.; Barg, A.A.; Bielorai, B.; Elhasid, R.; Gilad, G.; Toren, A.; et al. Venous thromboembolism and its risk factors in children with acute lymphoblastic leukemia in Israel: A population-based study. Cancers 2020, 12, 2759. [Google Scholar] [CrossRef]
- Elyamany, G.; Alzahrani, A.M.; Bukhary, E. Cancer-associated thrombosis: An overview. Clin. Med. Insights Oncol. 2014, 8, 129–137. [Google Scholar] [CrossRef]
- Rickles Falanga, A.; Marchetti, M.; Russo, L. Coagulation and cancer: Biological and clinical aspects. J. Thromb. Haemost. 2013, 11 (Suppl. 1), 223–233. [Google Scholar] [CrossRef]
- Adramerina, A.; Economou, M. Thrombotic Complications in Pediatric Cancer. Children 2024, 11, 1096. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Sabharwal, S. Risk factors for venous thromboembolism in hospitalized children and adolescents: A systemic review and pooled analysis. J. Pediatr. Orthop. B 2014, 23, 389–393. [Google Scholar] [CrossRef]
- Arpaia, G.G.; Caleffi, A.; Marano, G.; Laregina, M.; Erba, G.; Orlandini, F.; Cimminiello, C.; Boracchi, P. Padua prediction score and IMPROVE score do predict in-hospital mortality in Internal Medicine patients. Intern. Emerg. Med. 2020, 15, 997–1003. [Google Scholar] [CrossRef]
- Pelland-Marcotte, M.; Kulkarni, K.; Athale, U.H.; Pole, J.D.; Brandão, L.R.; Sung, L. Thrombosis is associated with worse survival in children with acute lymphoblastic leukemia: A report from CYP-C. Am. J. Hematol. 2021, 96, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, I.L.M.; Lauw, M.N.; Fiocco, M.; van der Sluis, I.M.; Pieters, R.; Middeldorp, S.; van de Wetering, M.D.; de Groot-Kruseman, H.A.; van Ommen, C.H. Venous thromboembolism in a large cohort of children with acute lymphoblastic leukemia: Risk factors and effect on prognosis. Res. Pract. Thromb. Haemost. 2019, 3, 234–241. [Google Scholar] [CrossRef]
- Śliwa-Tytko, P.; Kaczmarska, A.; Lejman, M.; Zawitkowska, J. Neurotoxicity associated with treatment of acute lymphoblastic leukemia chemotherapy and immunotherapy. Int. J. Mol. Sci. 2022, 23, 5515. [Google Scholar] [CrossRef] [PubMed]
- Santoro, N.; Giordano, P.; Del Vecchio, G.C.; Guido, G.; Rizzari, C.; Varotto, S.; Masera, G.; De Mattia, D. Ischemic stroke in children treated for acute lymphoblastic leukemia: A retrospective study. J. Pediatr. Hematol. Oncol. 2005, 27, 153–157. [Google Scholar] [CrossRef]
- Bowers, D.C.; Liu, Y.; Leisenring, W.; McNeil, E.; Stovall, M.; Gurney, J.G.; Robison, L.L.; Packer, R.J.; Oeffinger, K.C. Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors: A report from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2006, 24, 5277–5282. [Google Scholar] [CrossRef]
- Noje, C.; Cohen, K.; Jordan, L.C. Hemorrhagic and ischemic stroke in children with cancer. Pediatr. Neurol. 2013, 49, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Musgrave, K.M.; van Delft, F.W.; Avery, P.J.; Clack, R.M.; Chalmers, E.A.; Qureshi, A.; Vora, A.J.; Biss, T.T. Cerebral sinovenous thrombosis in children and young adults with acute lymphoblastic leukaemia—A cohort study from the United Kingdom. Br. J. Haematol. 2017, 179, 667–669. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Xu, Y.; Zhou, G.; Chen, F.; Li, C.; Ma, L.; Wen, F. Case Report: A successful outcome of nadroparin calcium therapy for cerebral venous sinus thrombosis in a child with acute lymphoblastic leukemia. Front. Pediatr. 2024, 12, 1448445. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boersma, R.S.; Hamulyak, K.; van Oerle, R.; Tuinenburg, A.; Ten Cate-Hoek, A.J.; Schouten, H.C. Biomarkers for prediction of central venous catheter-related thrombosis in patients with hematological malignancies. Clin. Appl. Thromb. Hemost. 2016, 22, 779–784. [Google Scholar] [CrossRef]
- Vormittag, R.; Simanek, R.; Ay, C.; Dunkler, D.; Quehenberger, P.; Marosi, C.; Zielinski, C.; Pabinger, I. High factor VIII levels independently predict venous thromboembolism in cancer patients: The cancer and thrombosis study. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 2176–2181. [Google Scholar] [CrossRef] [PubMed]
- Elmoamly, S.; Mattar, M.; Yacoub, M.F.; Afif, A. Can biomarkers of coagulation, platelet activation, and inflammation predict venous thromboembolism in patients with haematological malignancies? Acta Haematol. 2019, 141, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, N.A.; Takemoto, C.M.; Yee, D.L.; Kittelson, J.M.; Massicotte, M.P. Improving evidence on anticoagulant therapies for venous thromboembolism in children: Key challenges and opportunities. Blood 2015, 126, 2541–2547. [Google Scholar] [CrossRef]
- Klaassen, I.L.M.; Lauw, M.N.; van de Wetering, M.D.; Biemond, B.J.; Middeldorp, S.; Abbink, F.C.H.; Bierings, M.; Te Loo, D.M.M.W.; Pieters, R.; van der Sluis, I.M.; et al. TropicALL study: Thromboprophylaxis in children treated for acute lymphoblastic leukemia with low-molecular-weight heparin: A multicenter randomized controlled trial. BMC Pediatr. 2017, 17, 122. [Google Scholar] [CrossRef]
- Goyal, G.; Bhatt, V.R. L-asparaginase and venous thromboembolism in acute lymphocytic leukemia. Future Oncol. 2015, 11, 2459–2470. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Greiner, J.; Schrappe, M.; Claviez, A.; Zimmermann, M.; Niemeyer, C.; Kolb, R.; Eberl, W.; Berthold, F.; Bergsträsser, E.; Gnekow, A.; et al. THROMBOTECT—A randomized study comparing low molecular weight heparin, antithrombin and unfractionated heparin for thromboprophylaxis during induction therapy of acute lymphoblastic leukemia in children and adolescents. Haematologica 2019, 104, 756–765. [Google Scholar] [CrossRef]
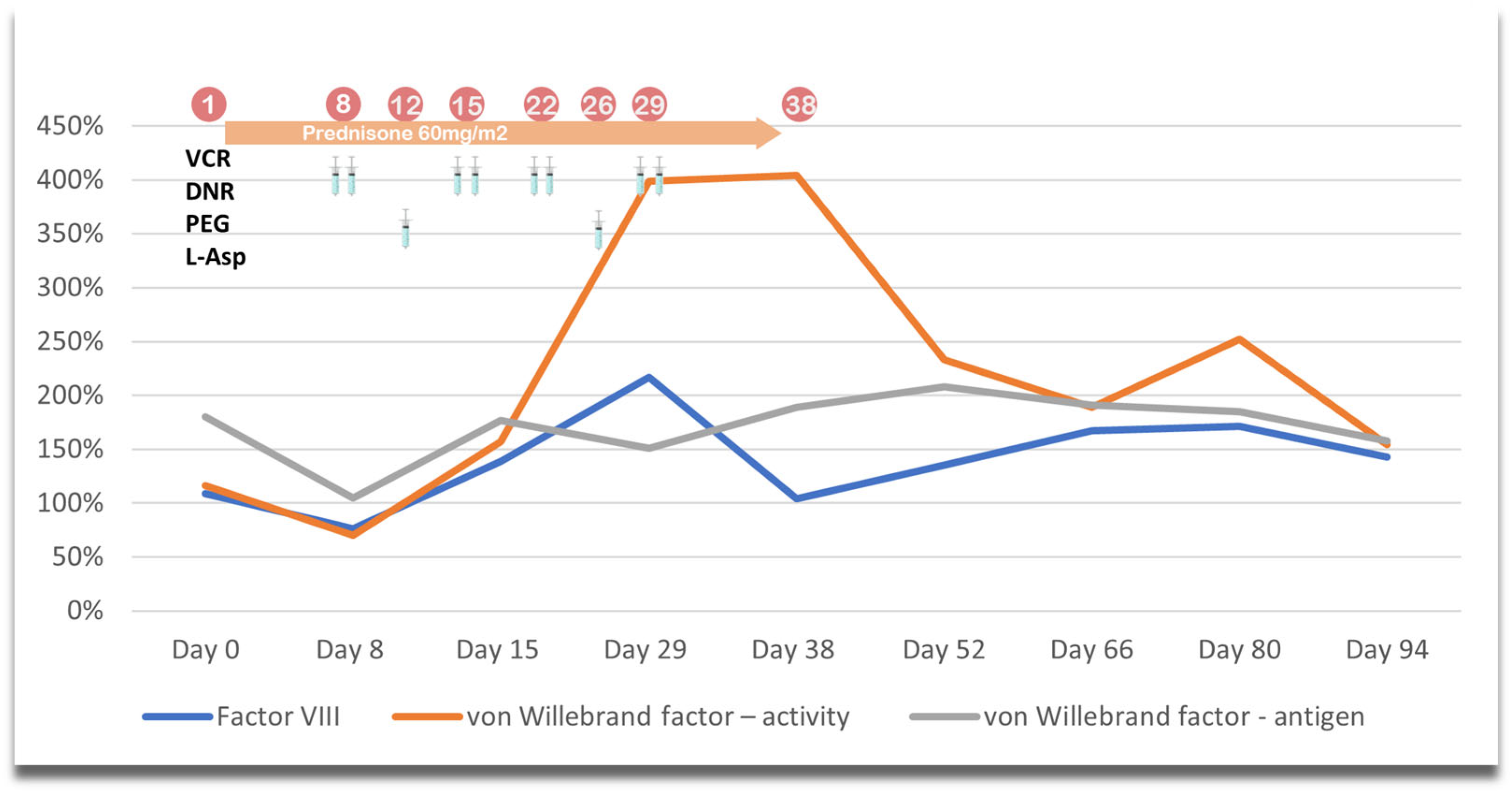

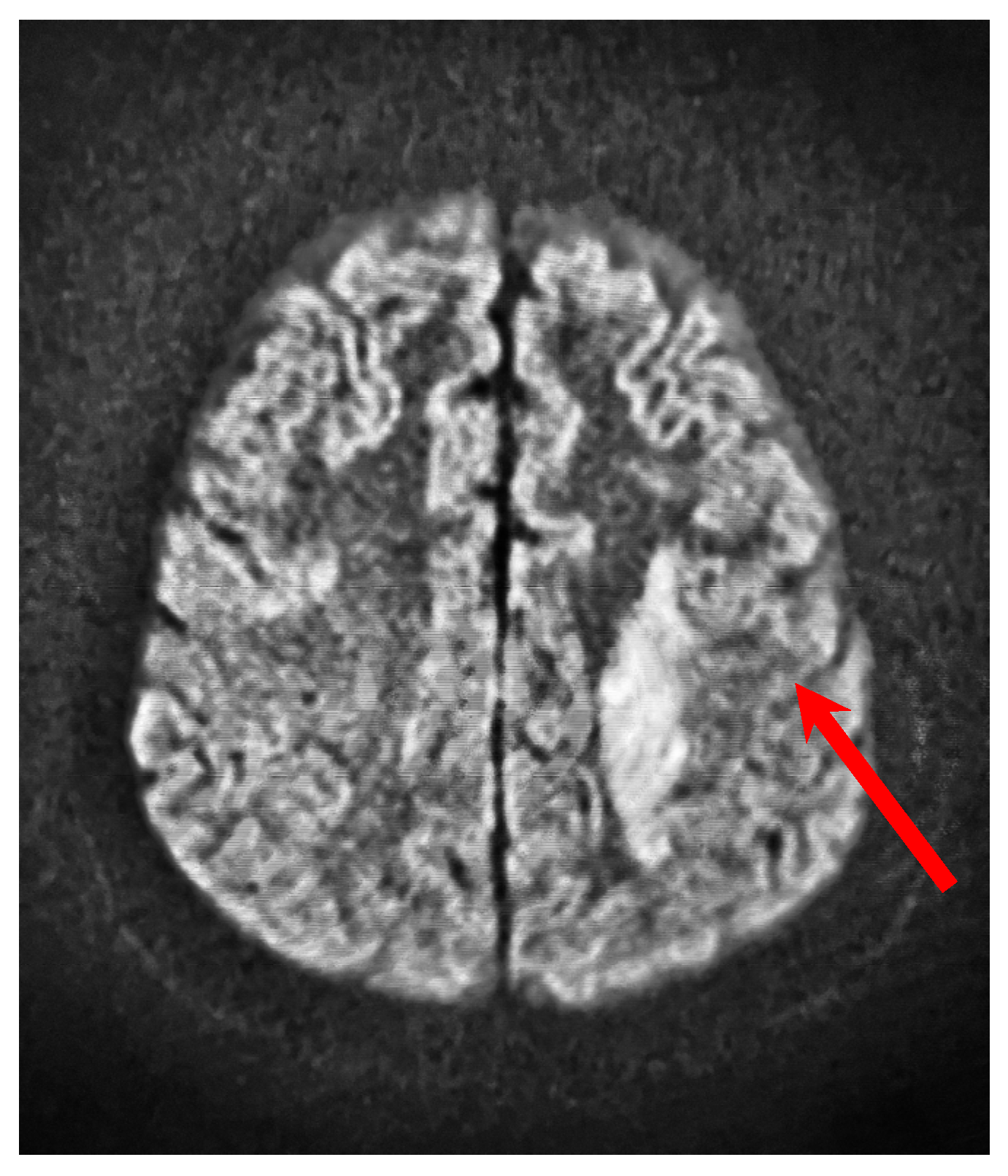
| Parameter | Results |
|---|---|
| WBC * (×103/uL) | 171,370 |
| Neutrophils (×103/uL) | 1050 |
| Lymphocytes (×103/uL) | 137,660 |
| Monocytes (×103/uL)) | 32,500 |
| Blasts (%) | 89 |
| Hg (g/L) | 7.4 |
| PLT (×103/uL) | 20,000 |
| AspAT(U/L) | 14 |
| AlAT (U/L) | 14 |
| LDH (U/L) | 471 |
| CRP (mg/dL) | 1.39 |
| Uric acid (mmol/L) | 0.20 |
| Creatinine (mg/dL) | 0.77 |
| Urea (mg/dL) | 34.9 |
| IgG (mg/dL) | 1155 |
| PT (s) | 14.3 |
| INR | 1.21 |
| APTT (s) | 30.3 |
| TT (s) | 17.3 |
| AT-III (%) | 96 |
| Fibrinogen (g/L) | 2.91 |
| Protein C (%) | 67 |
| Protein S (%) | 41.2 |
| Factor V Leiden | 3.05 |
| Factor VIII (%) | 109 |
| von Willebrand factor—activity (%) | 116 |
| von Willebrand factor—antigen (%) | 180 |
| Course of the Protocol | Drugs |
|---|---|
| Protocol I | Prednisone, vincristine, daunorubicin, and Escherichia coli PEG-asparaginase, |
| Consolidation A and B | Cytarabine, 6-mercaptopurine, and cyclophosphamide |
| Protocol M | 6-Mercaptopurine and high-dose methotrexate |
| Protocol II | Dexamethasone, vincristine, doxorubicin, Escherichia coli PEG-asparaginase, cyclophosphamide, cytarabine, and 6-thioguanine |
| Maintenance Therapy | 6-Mercaptopurine and methotrexate |
| Day 0 | Day 21 | Day 28 | Day 35 | Day 49 | Day of the Stroke | |
|---|---|---|---|---|---|---|
| Factor VIII (%) | 143 | 301 | 323 | 200 | 74 | 103 |
| von Willebrand factor—activity (%) | 235 | 508 | 511 | 319 | 112 | 159 |
| von Willebrand factor—antigen (%) | 171 | 757 | 769 | 183 | 157 | 172 |
| AT-III * (%) | 120 | 63 | 83 | 86 | 105 | 85% |
| Fibrinogen (g/L) | 2.47 | 0.55 | 0.49 | 3.46 | 5.01 | 6.25 |
| Parameter | Results |
|---|---|
| WBC * (×103/uL) | 0.73 |
| Neutrophils (×103/uL) | 0.59 |
| Lymphocytes (×103/uL) | 0.13 |
| Monocytes (×103/uL) | 0.01 |
| Hg (g/L) | 9.2 |
| Ht (%) | 25.7 |
| Reticulocytes (‰) | 1.9 |
| PLT (×103/uL) | 22,000 |
| AspAT(U/L) | 51 |
| AlAT (U/L) | 18 |
| LDH (U/L) | 181 |
| CRP (mg/dL) | 1.66 |
| Creatinine (mg/dL) | 0.30 |
| Urea (mg/dL) | 26 |
| IgG (mg/dL) | 813 |
| Total cholesterol (mg/dL) | 117 |
| Cholesterol LDL (mg/dL) | 68 |
| Cholesterol HDL (mg/dL) | 35 |
| Cholesterol non-HDL (mg/dL) | 82 |
| Triglycerides (mg/dL) | 69 |
| PT (s) | 12.5 |
| INR | 1.06 |
| APTT (s) | 32.4 |
| AT-III (%) | 85 |
| Fibrinogen (g/L) | 6.25 |
| D-dimers (ng/mL) | 536 |
| Protein C (%) | 72 |
| Protein S (%) | 90.5 |
| Factor V Leiden | 3.02 |
| Factor VIII (%) | 103 |
| von Willebrand factor—activity (%) | 159 |
| von Willebrand factor—antigen (%) | 172 |
| Lupus anticoagulant (total ratio) | 1.10 |
| Beta-2 glycoprotein antibody IgM | <2.00 |
| Beta-2 glycoprotein antibody IgG | <2.00 |
| Anti-cardiolipin antibodies IgM | <2.00 |
| Anti-cardiolipin antibodies IgG | <2.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malczewska, M.; Dudkiewicz, E.; Zawitkowska, J.; Lejman, M. Thromboembolic Risk and High Prothrombotic Factors in Childhood Acute Lymphoblastic Leukemia with Ischemic Stroke: A Literature Review of Personalized and Institutional Approaches to Prophylaxis. J. Pers. Med. 2025, 15, 228. https://doi.org/10.3390/jpm15060228
Malczewska M, Dudkiewicz E, Zawitkowska J, Lejman M. Thromboembolic Risk and High Prothrombotic Factors in Childhood Acute Lymphoblastic Leukemia with Ischemic Stroke: A Literature Review of Personalized and Institutional Approaches to Prophylaxis. Journal of Personalized Medicine. 2025; 15(6):228. https://doi.org/10.3390/jpm15060228
Chicago/Turabian StyleMalczewska, Marta, Ewa Dudkiewicz, Joanna Zawitkowska, and Monika Lejman. 2025. "Thromboembolic Risk and High Prothrombotic Factors in Childhood Acute Lymphoblastic Leukemia with Ischemic Stroke: A Literature Review of Personalized and Institutional Approaches to Prophylaxis" Journal of Personalized Medicine 15, no. 6: 228. https://doi.org/10.3390/jpm15060228
APA StyleMalczewska, M., Dudkiewicz, E., Zawitkowska, J., & Lejman, M. (2025). Thromboembolic Risk and High Prothrombotic Factors in Childhood Acute Lymphoblastic Leukemia with Ischemic Stroke: A Literature Review of Personalized and Institutional Approaches to Prophylaxis. Journal of Personalized Medicine, 15(6), 228. https://doi.org/10.3390/jpm15060228






