Right Ventricular Strain by Echocardiography: Current Clinical Applications and Future Directions for Mechanics Assessment of the Forgotten Ventricle
Abstract
1. Introduction
2. Current Strain Applications for the Left Ventricle and Left Atrium
3. RV Strain Analysis
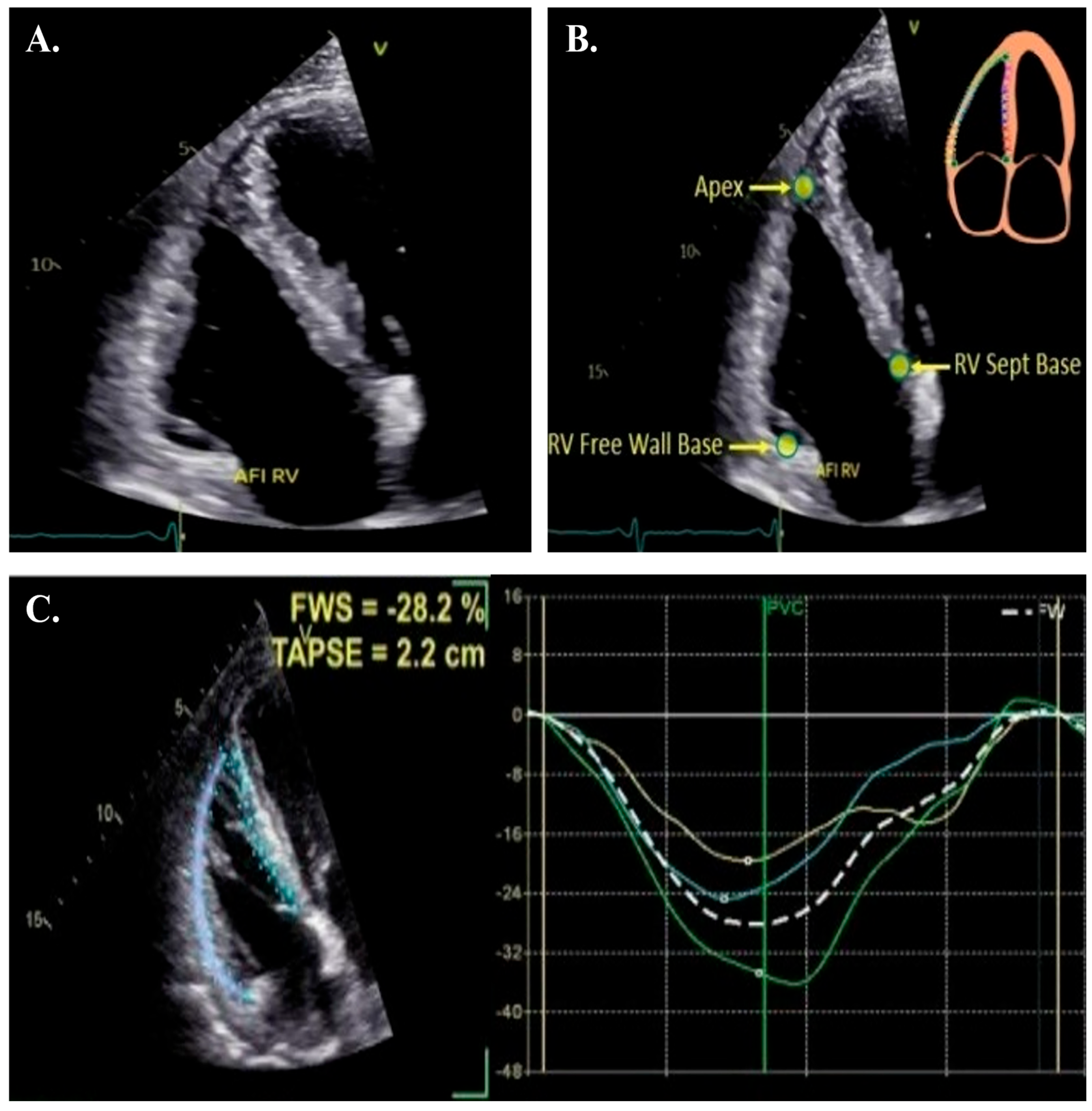
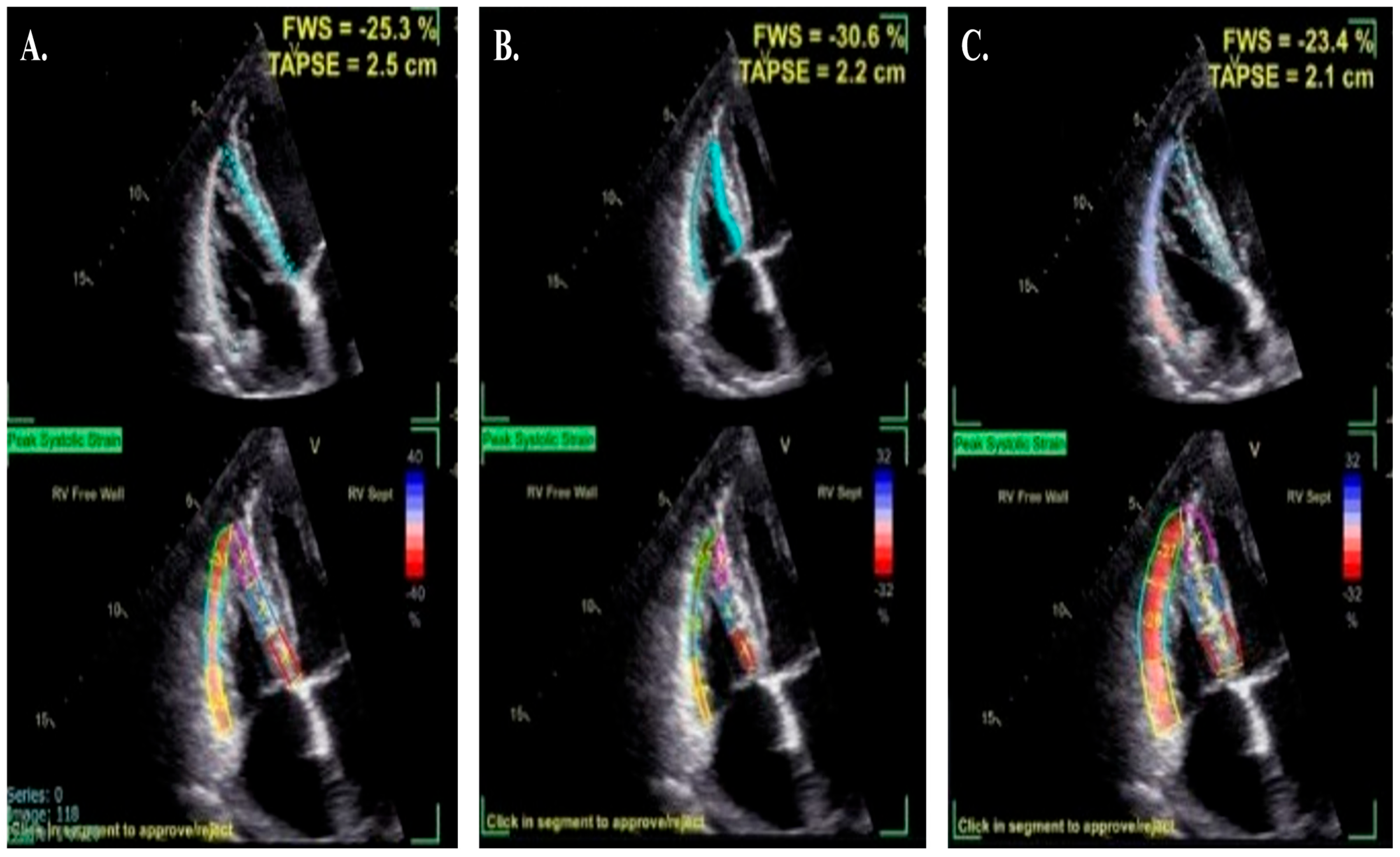
4. How to Acquire and Measure RV Strain
- 1.
- Image Acquisition:
- The patient should be in a steep lateral position with the probe moved laterally and tilted toward the liver to center the LV apex in the scanning sector. This ensures that only the interatrial septum is displayed, avoiding the LV outflow tract or coronary sinus, which enables clear visualization of the largest RV width, apex, and free wall throughout the cardiac cycle, facilitating optimal STE tracking. Optimal image quality requires adjustments to image depth (decreased), width (narrowed), sector angle, and gain (usually needs to be turned down), with a temporal resolution of 50–80 frames per second (higher for higher heart rates). The entire RV myocardium must be included in the image sector throughout the entire cardiac cycle.
- Respiratory maneuvers are often required to enhance spatial resolution, and three consecutive cardiac cycles should be acquired during a breath-hold. Acquiring 3–5 beat cycles is recommended for the optimal cycle to be chosen. Ectopic beats should be avoided.
- RV end-systole is identified using the pulmonary valve closure click (PVC) observed on the RV outflow tract Doppler tracing, which should be acquired immediately afterward to minimize variations between cardiac cycles. RV end-systole is identified via the pulmonary valve closure click (PVC) on RV outflow Doppler tracing, acquired immediately to reduce cycle variation.
- 2.
- Segmental Analysis:
- 3.
- Potential Errors and Adjustments:
5. Reference Ranges and Regional Differences of Right Ventricular Longitudinal Strain
6. Clinical Applications of RV Strain
7. Right Ventricular Strain in Heart Failure
- 1.
- Myocardial Injury: Comorbidities such as coronary artery disease, hypertension, diabetes mellitus, obesity, and chronic obstructive pulmonary disease, among others, contribute to systemic pathways of myocardial damage promoting RV myocardial hypertrophy and fibrosis resulting in RV dysfunction [41].
- 2.
- Atrial Fibrillation: Atrial fibrillation shortens ventricular filling time, elevates left atrial pressure, and increases pulmonary pressures, leading to greater RV afterload and dysfunction [41].
- 3.
- Interventricular Interaction: The functional interdependence between the LV and RV, mediated through the shared interventricular septum and LV contraction contributing 20–40% of RV contractile force, leads to RV dysfunction with LV dysfunction [42].
- 4.
- Pulmonary Hypertension: Chronic pulmonary venous hypertension due to LV systolic dysfunction increases RV afterload, impairing its function [41]. The RV may be affected in all types of pulmonary hypertension.
- Acute heart failure
- Heart failure with reduced ejection fraction
- Heart failure with preserved ejection fraction
- Left Ventricular Assist Devices
- Cardiac Transplantation
8. Right Ventricular Strain in Cardiomyopathies
- 1.
- Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC)
- 2.
- Hypertrophic Cardiomyopathy (HCM)
- 3.
- Nonischemic dilated cardiomyopathy (NIDCM)
- 4.
- Amyloidosis
9. Right Ventricular Strain in Acute Myocardial Infarction and Coronary Artery Disease
10. Right Ventricular Strain in Pulmonary Embolism
11. Right Ventricular Strain in Pulmonary Hypertension
12. Right Ventricular Strain in Cardio-Oncology
13. Right Ventricular Strain in Valvular Heart Diseases
- 1.
- Aortic Stenosis
- 2.
- Mitral Regurgitation
- 3.
- Tricuspid regurgitation
14. Right Ventricular Strain in Congenital Heart Diseases
15. Future Directions
15.1. Three-Dimensional Echocardiography
15.2. Cardiac Magnetic Resonance
15.3. Artificial Intelligence (AI)
16. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.; Sánchez-Quintana, D.; Bossone, E.; Bogaard, H.J.; Naeije, R. Anatomy, function, and dysfunction of the right ventricle: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2019, 73, 1463–1482. [Google Scholar] [CrossRef]
- Ji, M.; Wu, W.; He, L.; Gao, L.; Zhang, Y.; Lin, Y.; Qian, M.; Wang, J.; Zhang, L.; Xie, M. Right ventricular longitudinal strain in patients with heart failure. Diagnostics 2022, 12, 445. [Google Scholar] [CrossRef]
- Smiseth, O.A.; Rider, O.; Cvijic, M.; Valkovič, L.; Remme, E.W.; Voigt, J.-U. Myocardial strain imaging: Theory, current practice, and the future. JACC Cardiovasc. Imaging 2024, 18, 340–381. [Google Scholar] [CrossRef]
- Mertens, L.; Singh, G.; Armenian, S.; Chen, M.-H.; Dorfman, A.L.; Garg, R.; Husain, N.; Joshi, V.; Leger, K.J.; Lipshultz, S.E. Multimodality imaging for cardiac surveillance of cancer treatment in children: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2023, 36, 1227–1253. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Kokkinidis, D.G.; Kampaktsis, P.N.; Amir, E.A.; Marwick, T.H.; Gupta, D.; Thavendiranathan, P. Assessment of prognostic value of left ventricular global longitudinal strain for early prediction of chemotherapy-induced cardiotoxicity: A systematic review and meta-analysis. JAMA Cardiol. 2019, 4, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Negishi, T.; Thavendiranathan, P.; Penicka, M.; Lemieux, J.; Murbraech, K.; Miyazaki, S.; Shirazi, M.; Santoro, C.; Cho, G.-Y.; Popescu, B.A. Cardioprotection using strain-guided management of potentially cardiotoxic cancer therapy: 3-year results of the SUCCOUR trial. Cardiovasc. Imaging 2023, 16, 269–278. [Google Scholar]
- Zeppenfeld, K.; Tfelt-Hansen, J.; De Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; De Chillou, C. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Developed by the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC) Endorsed by the Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar]
- Halliday, B.P.; Senior, R.; Pennell, D.J. Assessing left ventricular systolic function: From ejection fraction to strain analysis. Eur. Heart J. 2021, 42, 789–797. [Google Scholar] [CrossRef]
- Cuddy, S.A.; Chetrit, M.; Jankowski, M.; Desai, M.; Falk, R.H.; Weiner, R.B.; Klein, A.L.; Phelan, D.; Grogan, M. Practical points for echocardiography in cardiac amyloidosis. J. Am. Soc. Echocardiogr. 2022, 35, A31–A40. [Google Scholar] [CrossRef]
- Negri, F.; Muser, D.; Driussi, M.; Sanna, G.D.; Masè, M.; Cittar, M.; Poli, S.; De Bellis, A.; Fabris, E.; Puppato, M. Prognostic role of global longitudinal strain by feature tracking in patients with hypertrophic cardiomyopathy: The STRAIN-HCM study. Int. J. Cardiol. 2021, 345, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Pozios, I.; Haileselassie, B.; Nowbar, A.; Sorensen, L.L.; Phillip, S.; Lu, D.-Y.; Ventoulis, I.; Luo, H.; Abraham, M.R. Role of global longitudinal strain in predicting outcomes in hypertrophic cardiomyopathy. Am. J. Cardiol. 2017, 120, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Barki, M.; Losito, M.; Caracciolo, M.M.; Sugimoto, T.; Rovida, M.; Viva, T.; Arosio, R.; Alfonzetti, E.; Bandera, F.; Moroni, A. Left atrial strain in acute heart failure: Clinical and prognostic insights. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.S.; Jin, X.; Oon, Y.Y.; Chan, S.P.; Gong, L.; Lunaria, J.B.; Liew, O.-W.; Chong, J.P.-C.; Tay, E.L.; Soo, W.M. Prognostic value of left atrial strain in aortic stenosis: A competing risk analysis. J. Am. Soc. Echocardiogr. 2023, 36, 29–37.e25. [Google Scholar] [CrossRef]
- Hirose, K.; Nakanishi, K.; Daimon, M.; Yoshida, Y.; Ishiwata, J.; Nakao, T.; Morita, H.; Di Tullio, M.R.; Homma, S.; Komuro, I. Prevalence, Determinants, and Prognostic Value of Left Atrial Dysfunction in Patients with Chronic Coronary Syndrome and Normal Left Ventricular Ejection Fraction. Am. J. Cardiol. 2023, 187, 30–37. [Google Scholar] [CrossRef]
- Raafs, A.G.; Vos, J.L.; Henkens, M.T.; Slurink, B.O.; Verdonschot, J.A.; Bossers, D.; Roes, K.; Gerretsen, S.; Knackstedt, C.; Hazebroek, M.R. Left atrial strain has superior prognostic value to ventricular function and delayed-enhancement in dilated cardiomyopathy. Cardiovasc. Imaging 2022, 15, 1015–1026. [Google Scholar] [CrossRef]
- Leng, S.; Ge, H.; He, J.; Kong, L.; Yang, Y.; Yan, F.; Xiu, J.; Shan, P.; Zhao, S.; Tan, R.-S. Long-term prognostic value of cardiac MRI left atrial strain in ST-segment elevation myocardial infarction. Radiology 2020, 296, 299–309. [Google Scholar] [CrossRef]
- Voigt, J.-U.; Cvijic, M. 2-and 3-dimensional myocardial strain in cardiac health and disease. JACC Cardiovasc. Imaging 2019, 12, 1849–1863. [Google Scholar] [CrossRef]
- Carlsson, M.; Ugander, M.; Heiberg, E.; Arheden, H. The quantitative relationship between longitudinal and radial function in left, right, and total heart pumping in humans. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H636–H644. [Google Scholar] [CrossRef]
- Amsallem, M.; Mercier, O.; Kobayashi, Y.; Moneghetti, K.; Haddad, F. Forgotten no more: A focused update on the right ventricle in cardiovascular disease. JACC Heart Fail. 2018, 6, 891–903. [Google Scholar] [CrossRef]
- Muraru, D.; Haugaa, K.; Donal, E.; Stankovic, I.; Voigt, J.U.; Petersen, S.E.; Popescu, B.A.; Marwick, T. Right ventricular longitudinal strain in the clinical routine: A state-of-the-art review. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 898–912. [Google Scholar] [CrossRef] [PubMed]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, M.; Rudski, L.G.; Addetia, K.; Afilalo, J.; D’Alto, M.; Freed, B.H.; Friend, L.B.; Gargani, L.; Grapsa, J.; Hassoun, P.M. Guidelines for the echocardiographic assessment of the right heart in adults and special considerations in pulmonary hypertension: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2025, 38, 141–186. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Muraru, D.; Onciul, S.; Peluso, D.; Soriani, N.; Cucchini, U.; Aruta, P.; Romeo, G.; Cavalli, G.; Iliceto, S.; Badano, L.P. Sex-and method-specific reference values for right ventricular strain by 2-dimensional speckle-tracking echocardiography. Circ. Cardiovasc. Imaging 2016, 9, e003866. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Genovese, D.; Mor-Avi, V.; Palermo, C.; Muraru, D.; Volpato, V.; Kruse, E.; Yamat, M.; Aruta, P.; Addetia, K.; Badano, L.P. Comparison between four-chamber and right ventricular–focused views for the quantitative evaluation of right ventricular size and function. J. Am. Soc. Echocardiogr. 2019, 32, 484–494. [Google Scholar] [CrossRef]
- Unlu, S.; Mirea, O.; Bezy, S. Eacvi-Ase-Industry Standardization Task Force Chair by and Participating Companies. Inter-vendor variability in strain measurements depends on software rather than image characteristics. Int. J. Cardiovasc. Imaging 2021, 37, 1689–1697. [Google Scholar] [CrossRef]
- Il’Giovine, Z.J.; Mulder, H.; Chiswell, K.; Arges, K.; Tomfohr, J.; Hashmi, A.; Velazquez, E.J.; Kisslo, J.A.; Samad, Z.; Rajagopal, S. Right ventricular longitudinal strain reproducibility using vendor-dependent and vendor-independent software. J. Am. Soc. Echocardiogr. 2018, 31, 721–732.e725. [Google Scholar] [CrossRef]
- Morris, D.A.; Krisper, M.; Nakatani, S.; Köhncke, C.; Otsuji, Y.; Belyavskiy, E.; Radha Krishnan, A.K.; Kropf, M.; Osmanoglou, E.; Boldt, L.-H. Normal range and usefulness of right ventricular systolic strain to detect subtle right ventricular systolic abnormalities in patients with heart failure: A multicentre study. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 212–223. [Google Scholar] [CrossRef]
- Addetia, K.; Miyoshi, T.; Citro, R.; Daimon, M.; Fajardo, P.G.; Kasliwal, R.R.; Kirkpatrick, J.N.; Monaghan, M.J.; Muraru, D.; Ogunyankin, K.O. Two-dimensional echocardiographic right ventricular size and systolic function measurements stratified by sex, age, and ethnicity: Results of the world alliance of societies of echocardiography study. J. Am. Soc. Echocardiogr. 2021, 34, 1148–1157.e1141. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.K.M.; Grimm, R.A.; Rodriguez, L.L.; Collier, P.; Griffin, B.P.; Popović, Z.B. Defining the reference range for right ventricular systolic strain by echocardiography in healthy subjects: A meta-analysis. PLoS ONE 2021, 16, e0256547. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, L.; Gao, Y.; Wan, X.; Xiao, Q.; Zhang, Y.; Sun, W.; Xie, Y.; Zeng, Q.; Chen, Y. Comprehensive assessment of right ventricular function by three-dimensional speckle-tracking echocardiography: Comparisons with cardiac magnetic resonance imaging. J. Am. Soc. Echocardiogr. 2021, 34, 472–482. [Google Scholar] [CrossRef]
- Fine, N.M.; Shah, A.A.; Han, I.-Y.; Yu, Y.; Hsiao, J.-f.; Koshino, Y.; Saleh, H.K.; Miller, F.A.; Oh, J.K.; Pellikka, P.A. Left and right ventricular strain and strain rate measurement in normal adults using velocity vector imaging: An assessment of reference values and intersystem agreement. Int. J. Cardiovasc. Imaging 2013, 29, 571–580. [Google Scholar] [CrossRef]
- Chia, E.-M.; Hsieh, C.H.; Boyd, A.; Pham, P.; Vidaic, J.; Leung, D.; Thomas, L. Effects of age and gender on right ventricular systolic and diastolic function using two-dimensional speckle-tracking strain. J. Am. Soc. Echocardiogr. 2014, 27, 1079–1086.e1071. [Google Scholar] [CrossRef]
- McGhie, J.S.; Menting, M.E.; Vletter, W.B.; Frowijn, R.; Roos-Hesselink, J.W.; van der Zwaan, H.B.; Soliman, O.I.; Geleijnse, M.L.; van den Bosch, A.E. Quantitative assessment of the entire right ventricle from one acoustic window: An attractive approach. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 754–762. [Google Scholar] [CrossRef]
- Park, J.-H.; Choi, J.-O.; Park, S.W.; Cho, G.-Y.; Oh, J.K.; Lee, J.-H.; Seong, I.-W. Normal references of right ventricular strain values by two-dimensional strain echocardiography according to the age and gender. Int. J. Cardiovasc. Imaging 2018, 34, 177–183. [Google Scholar] [CrossRef]
- Espersen, C.; Skaarup, K.G.; Lassen, M.C.H.; Johansen, N.D.; Hauser, R.; Olsen, F.J.; Jensen, G.B.; Schnohr, P.; Møgelvang, R.; Biering-Sørensen, T. Normal age-and sex-based values of right ventricular free wall and four-chamber longitudinal strain by speckle-tracking echocardiography: From the Copenhagen City heart study. Clin. Res. Cardiol. 2024, 113, 456–468. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar]
- Lam, C.S.; Yancy, C. Universal definition and classification of heart failure: Is it universal? Does it define heart failure? J. Card. Fail. 2021, 27, 509–511. [Google Scholar] [CrossRef]
- Ghio, S.; Guazzi, M.; Scardovi, A.B.; Klersy, C.; Clemenza, F.; Carluccio, E.; Temporelli, P.L.; Rossi, A.; Faggiano, P.; Traversi, E. Different correlates but similar prognostic implications for right ventricular dysfunction in heart failure patients with reduced or preserved ejection fraction. Eur. J. Heart Fail. 2017, 19, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Iacoviello, M.; Citarelli, G.; Antoncecchi, V.; Romito, R.; Monitillo, F.; Leone, M.; Puzzovivo, A.; Lattarulo, M.S.; Rizzo, C.; Caldarola, P. Right ventricular longitudinal strain measures independently predict chronic heart failure mortality. Echocardiography 2016, 33, 992–1000. [Google Scholar] [CrossRef]
- Verhaert, D.; Mullens, W.; Borowski, A.; Popović, Z.B.; Curtin, R.J.; Thomas, J.D.; Tang, W.W. Right ventricular response to intensive medical therapy in advanced decompensated heart failure. Circ. Heart Fail. 2010, 3, 340–346. [Google Scholar] [CrossRef]
- Hamada-Harimura, Y.; Seo, Y.; Ishizu, T.; Nishi, I.; Machino-Ohtsuka, T.; Yamamoto, M.; Sugano, A.; Sato, K.; Sai, S.; Obara, K. Incremental prognostic value of right ventricular strain in patients with acute decompensated heart failure. Circ. Cardiovasc. Imaging 2018, 11, e007249. [Google Scholar] [CrossRef] [PubMed]
- Borovac, J.A.; Glavas, D.; Susilovic Grabovac, Z.; Supe Domic, D.; Stanisic, L.; D’Amario, D.; Duplancic, D.; Bozic, J. Right ventricular free wall strain and congestive hepatopathy in patients with acute worsening of chronic heart failure: A CATSTAT-HF echo substudy. J. Clin. Med. 2020, 9, 1317. [Google Scholar] [CrossRef]
- Bleakley, C.; De Marvao, A.; Morosin, M.; Androulakis, E.; Russell, C.; Athayde, A.; Cannata, A.; Passariello, M.; Ledot, S.; Singh, S. Utility of echocardiographic right ventricular subcostal strain in critical care. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 820–828. [Google Scholar] [CrossRef]
- Houard, L.; Benaets, M.-B.; de Meester de Ravenstein, C.; Rousseau, M.F.; Ahn, S.A.; Amzulescu, M.-S.; Roy, C.; Slimani, A.; Vancraeynest, D.; Pasquet, A. Additional prognostic value of 2D right ventricular speckle-tracking strain for prediction of survival in heart failure and reduced ejection fraction: A comparative study with cardiac magnetic resonance. JACC Cardiovasc. Imaging 2019, 12, 2373–2385. [Google Scholar] [CrossRef] [PubMed]
- Motoki, H.; Borowski, A.G.; Shrestha, K.; Hu, B.; Kusunose, K.; Troughton, R.W.; Tang, W.W.; Klein, A.L. Right ventricular global longitudinal strain provides prognostic value incremental to left ventricular ejection fraction in patients with heart failure. J. Am. Soc. Echocardiogr. 2014, 27, 726–732. [Google Scholar] [CrossRef]
- Nguyen, K.-L.; Hu, P.; Finn, J.P. Cardiac magnetic resonance quantification of structure-function relationships in heart failure. Heart Fail. Clin. 2021, 17, 9–24. [Google Scholar] [CrossRef]
- Cho, D.-H.; Yoo, B.-S. Current prevalence, incidence, and outcomes of heart failure with preserved ejection fraction. Heart Fail. Clin. 2021, 17, 315–326. [Google Scholar] [CrossRef]
- Puwanant, S.; Priester, T.C.; Mookadam, F.; Bruce, C.J.; Redfield, M.M.; Chandrasekaran, K. Right ventricular function in patients with preserved and reduced ejection fraction heart failure. Eur. J. Echocardiogr. 2009, 10, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, Y.; Ran, H.; Wang, Z. Assessment of right ventricular function by two-dimensional speckle tracking echocardiography in heart failure patients with different left ventricular diastolic dysfunction grade. Chin. J. Ultrason. 2020, 12, 564–570. [Google Scholar]
- Morris, D.A.; Gailani, M.; Pérez, A.V.; Blaschke, F.; Dietz, R.; Haverkamp, W.; Özcelik, C. Right ventricular myocardial systolic and diastolic dysfunction in heart failure with normal left ventricular ejection fraction. J. Am. Soc. Echocardiogr. 2011, 24, 886–897. [Google Scholar] [CrossRef]
- Lejeune, S.; Roy, C.; Ciocea, V.; Slimani, A.; de Meester, C.; Amzulescu, M.; Pasquet, A.; Vancraeynest, D.; Beauloye, C.; Vanoverschelde, J.-L. Right ventricular global longitudinal strain and outcomes in heart failure with preserved ejection fraction. J. Am. Soc. Echocardiogr. 2020, 33, 973–984.e972. [Google Scholar] [CrossRef]
- Gumus, F.; Sarıcaoglu, C.; Inan, M.B.; Akar, A.R. Right ventricular strain to assess early right heart failure in the left ventricular assist device candidate. Curr. Heart Fail. Rep. 2019, 16, 212–219. [Google Scholar] [CrossRef]
- Bellavia, D.; Iacovoni, A.; Agnese, V.; Falletta, C.; Coronnello, C.; Pasta, S.; Novo, G.; di Gesaro, G.; Senni, M.; Maalouf, J. Usefulness of regional right ventricular and right atrial strain for prediction of early and late right ventricular failure following a left ventricular assist device implant: A machine learning approach. Int. J. Artif. Organs 2020, 43, 297–314. [Google Scholar] [CrossRef]
- Magunia, H.; Dietrich, C.; Langer, H.F.; Schibilsky, D.; Schlensak, C.; Rosenberger, P.; Nowak-Machen, M. 3D echocardiography derived right ventricular function is associated with right ventricular failure and mid-term survival after left ventricular assist device implantation. Int. J. Cardiol. 2018, 272, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Dufendach, K.A.; Zhu, T.; Diaz Castrillon, C.; Hong, Y.; Countouris, M.E.; Hickey, G.; Keebler, M.; Thoma, F.W.; Kilic, A. Pre-implant right ventricular free wall strain predicts post-LVAD right heart failure. J. Card. Surg. 2021, 36, 1996–2003. [Google Scholar] [CrossRef]
- Grant, A.D.; Smedira, N.G.; Starling, R.C.; Marwick, T.H. Independent and incremental role of quantitative right ventricular evaluation for the prediction of right ventricular failure after left ventricular assist device implantation. J. Am. Coll. Cardiol. 2012, 60, 521–528. [Google Scholar] [CrossRef]
- Bellavia, D.; Iacovoni, A.; Scardulla, C.; Moja, L.; Pilato, M.; Kushwaha, S.S.; Senni, M.; Clemenza, F.; Agnese, V.; Falletta, C. Prediction of right ventricular failure after ventricular assist device implant: Systematic review and meta-analysis of observational studies. Eur. J. Heart Fail. 2017, 19, 926–946. [Google Scholar] [CrossRef]
- Barakat, A.F.; Sperry, B.W.; Starling, R.C.; Mentias, A.; Popovic, Z.B.; Griffin, B.P.; Desai, M.Y. Prognostic utility of right ventricular free wall strain in low risk patients after orthotopic heart transplantation. Am. J. Cardiol. 2017, 119, 1890–1896. [Google Scholar] [CrossRef]
- Ji, X.; Zhang, Y.; Xie, Y.; Wang, W.; Tan, Y.; Xie, M.; Zhang, L. Feasibility value of right ventricular longitudinal shortening fraction and the prognostic implications in patients with heart transplantation. J. Am. Heart Assoc. 2024, 13, e032402. [Google Scholar] [CrossRef]
- Tian, F.; LI, Y.; Zhang, Y.; Gu, Y.; Zhang, B.; Xie, M. The relationship between three-dimensional right ventricular longitudinal deformation and myocardial fibrosis in patients with end-stage heart failure. Chin. J. Ultrason. 2021, 376–381. [Google Scholar]
- Venner, C.; Selton-Suty, C.; Huttin, O.; Erpelding, M.-L.; Aliot, E.; Juillière, Y. Right ventricular dysfunction in patients with idiopathic dilated cardiomyopathy: Prognostic value and predictive factors. Arch. Cardiovasc. Dis. 2016, 109, 231–241. [Google Scholar] [CrossRef]
- Kawata, T.; Daimon, M.; Kimura, K.; Nakao, T.; Lee, S.L.; Hirokawa, M.; Kato, T.S.; Watanabe, M.; Yatomi, Y.; Komuro, I. Echocardiographic assessment of right ventricular function in routine practice: Which parameters are useful to predict one-year outcome in advanced heart failure patients with dilated cardiomyopathy? J. Cardiol. 2017, 70, 316–322. [Google Scholar] [CrossRef]
- Prati, G.; Vitrella, G.; Allocca, G.; Muser, D.; Buttignoni, S.C.; Piccoli, G.; Morocutti, G.; Delise, P.; Pinamonti, B.; Proclemer, A. Right ventricular strain and dyssynchrony assessment in arrhythmogenic right ventricular cardiomyopathy: Cardiac magnetic resonance feature-tracking study. Circ. Cardiovasc. Imaging 2015, 8, e003647. [Google Scholar] [CrossRef]
- Heermann, P.; Hedderich, D.M.; Paul, M.; Schülke, C.; Kroeger, J.R.; Baeßler, B.; Wichter, T.; Maintz, D.; Waltenberger, J.; Heindel, W. Biventricular myocardial strain analysis in patients with arrhythmogenic right ventricular cardiomyopathy (ARVC) using cardiovascular magnetic resonance feature tracking. J. Cardiovasc. Magn. Reson. 2014, 16, 75. [Google Scholar] [CrossRef]
- Dong, Z.; Ma, X.; Wang, J.; Yang, S.; Yu, S.; Song, Y.; Tang, Y.; Xiang, X.; Yang, K.; Zhao, K. Incremental Diagnostic Value of Right Ventricular Strain Analysis in Arrhythmogenic Right Ventricular Cardiomyopathy. J. Am. Heart Assoc. 2024, 13, e031403. [Google Scholar] [CrossRef]
- Vigneault, D.M.; Te Riele, A.S.; James, C.A.; Zimmerman, S.L.; Selwaness, M.; Murray, B.; Tichnell, C.; Tee, M.; Noble, J.A.; Calkins, H. Right ventricular strain by MR quantitatively identifies regional dysfunction in patients with arrhythmogenic right ventricular cardiomyopathy. J. Magn. Reson. Imaging 2016, 43, 1132–1139. [Google Scholar] [CrossRef]
- Prakasa, K.R.; Wang, J.; Tandri, H.; Dalal, D.; Bomma, C.; Chojnowski, R.; James, C.; Tichnell, C.; Russell, S.; Judge, D. Utility of tissue Doppler and strain echocardiography in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Am. J. Cardiol. 2007, 100, 507–512. [Google Scholar] [CrossRef]
- Malik, N.; Win, S.; James, C.A.; Kutty, S.; Mukherjee, M.; Gilotra, N.A.; Tichnell, C.; Murray, B.; Agafonova, J.; Tandri, H. Right ventricular strain predicts structural disease progression in patients with arrhythmogenic right ventricular cardiomyopathy. J. Am. Heart Assoc. 2020, 9, e015016. [Google Scholar] [CrossRef]
- Namasivayam, M.; Bertrand, P.B.; Bernard, S.; Churchill, T.W.; Khurshid, S.; Marcus, F.I.; Mestroni, L.; Saffitz, J.E.; Towbin, J.A.; Zareba, W. Utility of left and right ventricular strain in arrhythmogenic right ventricular cardiomyopathy: A prospective multicenter registry. Circ. Cardiovasc. Imaging 2023, 16, e015671. [Google Scholar] [CrossRef]
- Jacquemyn, X.; Van den Eynde, J.; Zhan, J.; Doshi, A.N.; Ravekes, W.J.; Gilotra, N.A.; Scheel, P.; Wu, K.C.; Gasperetti, A.; James, C.A. Impaired Atrial and Ventricular Strain Predicts Heart Failure in Arrhythmogenic Right Ventricular Cardiomyopathy. Can. J. Cardiol. 2024, 41, 215–223. [Google Scholar] [CrossRef]
- Anwer, S.; Stollenwerk, L.; Winkler, N.E.; Guastafierro, F.; Hebeisen, M.; Akdis, D.; Saguner, A.M.; Brunckhorst, C.; Duru, F.; Tanner, F.C. Right heart strain in arrhythmogenic right ventricular cardiomyopathy: Implications for cardiovascular outcome. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 1061–1068. [Google Scholar] [CrossRef]
- D’Andrea, A.; Limongelli, G.; Baldini, L.; Verrengia, M.; Carbone, A.; Di Palma, E.; Vastarella, R.; Masarone, D.; Tagliamonte, G.; Riegler, L. Exercise speckle-tracking strain imaging demonstrates impaired right ventricular contractile reserve in hypertrophic cardiomyopathy. Int. J. Cardiol. 2017, 227, 209–216. [Google Scholar] [CrossRef]
- Hiemstra, Y.L.; Debonnaire, P.; Bootsma, M.; Schalij, M.J.; Bax, J.J.; Delgado, V.; Marsan, N.A. Prevalence and prognostic implications of right ventricular dysfunction in patients with hypertrophic cardiomyopathy. Am. J. Cardiol. 2019, 124, 604–612. [Google Scholar] [CrossRef]
- Mahmod, M.; Raman, B.; Chan, K.; Sivalokanathan, S.; Smillie, R.W.; Abd Samat, A.H.; Ariga, R.; Dass, S.; Ormondroyd, E.; Watkins, H. Right ventricular function declines prior to left ventricular ejection fraction in hypertrophic cardiomyopathy. J. Cardiovasc. Magn. Reson. 2022, 24, 36. [Google Scholar] [CrossRef]
- Qian, Y.; Zhao, X.; Chen, B.-H.; An, D.-A.; Wu, R.; Shi, R.-Y.; Zhang, C.; Ma, X.; Zhou, Y.; Zhao, L. Right ventricular global strain in patients with hypertrophic cardiomyopathy with and without right ventricular hypertrophy. Eur. J. Radiol. 2023, 169, 111148. [Google Scholar] [CrossRef]
- Vîjîiac, A.; Onciul, S.; Guzu, C.; Verinceanu, V.; Bătăilă, V.; Deaconu, S.; Scărlătescu, A.; Zamfir, D.; Petre, I.; Onuţ, R. The prognostic value of right ventricular longitudinal strain and 3D ejection fraction in patients with dilated cardiomyopathy. Int. J. Cardiovasc. Imaging 2021, 37, 3233–3244. [Google Scholar] [CrossRef]
- Ishiwata, J.; Daimon, M.; Nakanishi, K.; Sugimoto, T.; Kawata, T.; Shinozaki, T.; Nakao, T.; Hirokawa, M.; Sawada, N.; Yoshida, Y. Combined evaluation of right ventricular function using echocardiography in non-ischaemic dilated cardiomyopathy. ESC Heart Fail. 2021, 8, 3947–3956. [Google Scholar] [CrossRef]
- Palomero, V.M.; Durante-Lopez, A.; Sanabria, M.T.; Cubero, J.S.; González-Mirelis, J.; Lopez-Ibor, J.V.; Rico, S.M.N.; Krsnik, I.; Dominguez, F.; Mingo, A.M. Role of right ventricular strain measured by two-dimensional echocardiography in the diagnosis of cardiac amyloidosis. J. Am. Soc. Echocardiogr. 2019, 32, 845–853.e841. [Google Scholar] [CrossRef]
- Uzan, C.; Lairez, O.; Raud-Raynier, P.; Garcia, R.; Degand, B.; Christiaens, L.P.; Rehman, M.B. Right ventricular longitudinal strain: A tool for diagnosis and prognosis in light-chain amyloidosis. Amyloid 2018, 25, 18–25. [Google Scholar] [CrossRef]
- Istratoaie, S.; Bourg, C.; Lee, K.C.; Marut, B.; Antonelli, J.; L’official, G.; Wazzan, A.A.; Donal, E. Right ventricular free wall strain predicts transthyretin amyloidosis prognosis as well as biomarker-based staging systems. Eur. Heart J. Cardiovasc. Imaging 2025, 26, 239–248. [Google Scholar] [CrossRef]
- Tjahjadi, C.; Fortuni, F.; Stassen, J.; Debonnaire, P.; Lustosa, R.P.; Marsan, N.A.; Delgado, V.; Bax, J.J. Prognostic implications of right ventricular systolic dysfunction in cardiac amyloidosis. Am. J. Cardiol. 2022, 173, 120–127. [Google Scholar] [CrossRef]
- Ozbay, B.; Satyavolu, B.S.; Rearick, C.; Soman, P.; Katz, W.E.; Sezer, A.; Sade, L.E. Right ventricular strain improves the echocardiographic diagnosis and risk stratification of transthyretin cardiac amyloidosis among other phenotypes of left ventricular hypertrophy. J. Am. Soc. Echocardiogr. 2024, 37, 947–959. [Google Scholar] [CrossRef]
- Risum, N.; Valeur, N.; Søgaard, P.; Hassager, C.; Køber, L.; Ersbøll, M. Right ventricular function assessed by 2D strain analysis predicts ventricular arrhythmias and sudden cardiac death in patients after acute myocardial infarction. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 800–807. [Google Scholar] [CrossRef]
- Backmann, V.; Dykun, I.; Kampf, J.; Balcer, B.; Roggel, A.; Totzeck, M.; Rassaf, T.; Mahabadi, A.A. Comprehensive strain assessment and mortality after acute myocardial infarction: A retrospective observational study based on the Essen Coronary Artery Disease registry. Heart 2024, 110, 1408–1415. [Google Scholar] [CrossRef]
- Sonmez, O.; Kayrak, M.; Altunbas, G.; Abdulhalikov, T.; Alihanoglu, Y.; Bacaksiz, A.; Ozdemir, K.; Gok, H. Right ventricular involvement in anterior myocardial infarction: A tissue Doppler-derived strain and strain rate study. Clinics 2013, 68, 1225–1230. [Google Scholar] [CrossRef]
- Sevimli, S.; Gundogdu, F.; Aksakal, E.; Arslan, S.; Tas, H.; Islamoglu, Y.; Buyukkaya, E.; Gurlertop, H.Y.; Senocak, H. Right ventricular strain and strain rate properties in patients with right ventricular myocardial infarction. Echocardiography 2007, 24, 732–738. [Google Scholar] [CrossRef]
- Radwan, H.I.; Alhoseeny, A.M.A.; Ghoniem, S.M.; Nashy, B.N.E.; Shehata, I.E. Early right ventricular dysfunction after primary percutaneous coronary intervention in anterior versus isolated inferior myocardial infarction assessed by tissue Doppler imaging and speckle tracking echocardiography. Heart Fail. Rev. 2023, 28, 407–417. [Google Scholar] [CrossRef]
- Chang, W.-T.; Tsai, W.-C.; Liu, Y.-W.; Lee, C.-H.; Liu, P.-Y.; Chen, J.-Y.; Li, Y.-H.; Tsai, L.-M. Changes in right ventricular free wall strain in patients with coronary artery disease involving the right coronary artery. J. Am. Soc. Echocardiogr. 2014, 27, 230–238. [Google Scholar] [CrossRef]
- Chang, W.-T.; Liu, Y.-W.; Liu, P.-Y.; Chen, J.-Y.; Lee, C.-H.; Li, Y.-H.; Tsai, L.-M.; Tsai, W.-C. Association of decreased right ventricular strain with worse survival in non–acute coronary syndrome angina. J. Am. Soc. Echocardiogr. 2016, 29, 350–358.e354. [Google Scholar] [CrossRef]
- Pellikka, P.A.; Arruda-Olson, A.; Chaudhry, F.A.; Chen, M.H.; Marshall, J.E.; Porter, T.R.; Sawada, S.G. Guidelines for performance, interpretation, and application of stress echocardiography in ischemic heart disease: From the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2020, 33, 1–41.e48. [Google Scholar] [CrossRef]
- Platz, E.; Hassanein, A.H.; Shah, A.; Goldhaber, S.Z.; Solomon, S.D. Regional right ventricular strain pattern in patients with acute pulmonary embolism. Echocardiography 2012, 29, 464–470. [Google Scholar] [CrossRef]
- Vitarelli, A.; Barilla, F.; Capotosto, L.; D’Angeli, I.; Truscelli, G.; De Maio, M.; Ashurov, R. Right ventricular function in acute pulmonary embolism: A combined assessment by three-dimensional and speckle-tracking echocardiography. J. Am. Soc. Echocardiogr. 2014, 27, 329–338. [Google Scholar] [CrossRef]
- Descotes-Genon, V.; Chopard, R.; Morel, M.; Meneveau, N.; Schiele, F.; Bernard, Y. Comparison of right ventricular systolic function in patients with low risk and intermediate-to-high risk pulmonary embolism: A two-dimensional strain imaging study. Echocardiography 2013, 30, 301–308. [Google Scholar] [CrossRef]
- Wilson, R.; Eguchi, S.; Orihara, Y.; Pfeiffer, M.; Peterson, B.; Ruzieh, M.; Gao, Z.; Gorcsan III, J.; Boehmer, J. Association between right ventricular global longitudinal strain and mortality in intermediate-risk pulmonary embolism. Echocardiography 2024, 41, e15815. [Google Scholar] [CrossRef]
- Li, Y.; Wang, T.; Haines, P.; Li, M.; Wu, W.; Liu, M.; Chen, Y.; Jin, Q.; Xie, Y.; Wang, J. Prognostic value of right ventricular two-dimensional and three-dimensional speckle-tracking strain in pulmonary arterial hypertension: Superiority of longitudinal strain over circumferential and radial strain. J. Am. Soc. Echocardiogr. 2020, 33, 985–994.e981. [Google Scholar] [CrossRef]
- Smith, B.C.; Dobson, G.; Dawson, D.; Charalampopoulos, A.; Grapsa, J.; Nihoyannopoulos, P. Three-dimensional speckle tracking of the right ventricle: Toward optimal quantification of right ventricular dysfunction in pulmonary hypertension. J. Am. Coll. Cardiol. 2014, 64, 41–51. [Google Scholar] [CrossRef]
- Shukla, M.; Park, J.-H.; Thomas, J.D.; Delgado, V.; Bax, J.J.; Kane, G.C.; Howlett, J.G.; White, J.A.; Fine, N.M. Prognostic value of right ventricular strain using speckle-tracking echocardiography in pulmonary hypertension: A systematic review and meta-analysis. Can. J. Cardiol. 2018, 34, 1069–1078. [Google Scholar] [CrossRef]
- Hardegree, E.L.; Sachdev, A.; Villarraga, H.R.; Frantz, R.P.; McGoon, M.D.; Kushwaha, S.S.; Hsiao, J.-F.; McCully, R.B.; Oh, J.K.; Pellikka, P.A. Role of serial quantitative assessment of right ventricular function by strain in pulmonary arterial hypertension. Am. J. Cardiol. 2013, 111, 143–148. [Google Scholar] [CrossRef]
- Filusch, A.; Mereles, D.; Gruenig, E.; Buss, S.; Katus, H.A.; Meyer, F.J. Strain and strain rate echocardiography for evaluation of right ventricular dysfunction in patients with idiopathic pulmonary arterial hypertension. Clin. Res. Cardiol. 2010, 99, 491–498. [Google Scholar] [CrossRef]
- Sachdev, A.; Villarraga, H.R.; Frantz, R.P.; McGoon, M.D.; Hsiao, J.-F.; Maalouf, J.F.; Ammash, N.M.; McCully, R.B.; Miller, F.A.; Pellikka, P.A. Right ventricular strain for prediction of survival in patients with pulmonary arterial hypertension. Chest 2011, 139, 1299–1309. [Google Scholar] [CrossRef]
- Ünlü, S.; Bézy, S.; Cvijic, M.; Duchenne, J.; Delcroix, M.; Voigt, J.-U. Right ventricular strain related to pulmonary artery pressure predicts clinical outcome in patients with pulmonary arterial hypertension. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 635–642. [Google Scholar] [CrossRef]
- Lindholm, A.; Hesselstrand, R.; Rådegran, G.; Arheden, H.; Ostenfeld, E. Decreased biventricular longitudinal strain in patients with systemic sclerosis is mainly caused by pulmonary hypertension and not by systemic sclerosis per se. Clin. Physiol. Funct. Imaging 2019, 39, 215–225. [Google Scholar] [CrossRef]
- Gami, A.; Jani, V.P.; Mombeini, H.; Osgueritchian, R.; Salazar, I.M.C.; Kauffman, M.; Simpson, C.E.; Damico, R.L.; Kolb, T.M.; Shah, A.A. Prognostic Value of Echocardiographic Coupling Metrics in Systemic Sclerosis–Associated Pulmonary Vascular Disease. J. Am. Soc. Echocardiogr. 2024, 38, 115–126. [Google Scholar] [CrossRef]
- Baldassarre, L.A.; Ganatra, S.; Lopez-Mattei, J.; Yang, E.H.; Zaha, V.G.; Wong, T.C.; Ayoub, C.; DeCara, J.M.; Dent, S.; Deswal, A. Advances in multimodality imaging in cardio-oncology: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2022, 80, 1560–1578. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Munoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.; Lyon, A.R. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef]
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.; Lancellotti, P. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 2020, 31, 171–190. [Google Scholar] [CrossRef]
- Negishi, K.; Negishi, T.; Hare, J.L.; Haluska, B.A.; Plana, J.C.; Marwick, T.H. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J. Am. Soc. Echocardiogr. 2013, 26, 493–498. [Google Scholar] [CrossRef]
- Keramida, K.; Farmakis, D.; Bingcang, J.; Sulemane, S.; Sutherland, S.; Bingcang, R.A.; Ramachandran, K.; Tzavara, C.; Charalampopoulos, G.; Filippiadis, D. Longitudinal changes of right ventricular deformation mechanics during trastuzumab therapy in breast cancer patients. Eur. J. Heart Fail. 2019, 21, 529–535. [Google Scholar] [CrossRef]
- Wang, B.; Yu, Y.; Zhang, Y.; Hao, X.; Yang, S.; Zhao, H.; Sun, Q.; Wang, Y. Right ventricular dysfunction in patients with diffuse large B-cell lymphoma undergoing anthracycline-based chemotherapy: A 2D strain and 3D echocardiography study. Int. J. Cardiovasc. Imaging 2021, 37, 1311–1319. [Google Scholar] [CrossRef]
- Faggiano, A.; Gherbesi, E.; Giordano, C.; Gamberini, G.; Vicenzi, M.; Cuspidi, C.; Carugo, S.; Cipolla, C.M.; Cardinale, D.M. Anthracycline-Induced Subclinical Right Ventricular Dysfunction in Breast Cancer Patients: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 3883. [Google Scholar] [CrossRef]
- Kitano, T.; Nabeshima, Y.; Negishi, K.; Takeuchi, M. Prognostic value of automated longitudinal strain measurements in asymptomatic aortic stenosis. Heart 2021, 107, 578–584. [Google Scholar] [CrossRef]
- Dahou, A.; Clavel, M.-A.; Capoulade, R.; Bartko, P.E.; Magne, J.; Mundigler, G.; Bergler-Klein, J.; Burwash, I.; Mascherbauer, J.; Ribeiro, H.B. Right ventricular longitudinal strain for risk stratification in low-flow, low-gradient aortic stenosis with low ejection fraction. Heart 2016, 102, 548–554. [Google Scholar] [CrossRef]
- Holmberg, E.; Tamás, É.; Nylander, E.; Engvall, J.; Granfeldt, H. Right ventricular function in severe aortic stenosis assessed by echocardiography and MRI. Clin. Physiol. Funct. Imaging 2024, 44, 211–219. [Google Scholar] [CrossRef]
- Duncan, A.E.; Sarwar, S.; Kashy, B.K.; Sonny, A.; Sale, S.; Alfirevic, A.; Yang, D.; Thomas, J.D.; Gillinov, M.; Sessler, D.I. Early left and right ventricular response to aortic valve replacement. Anesth. Analg. 2017, 124, 406–418. [Google Scholar] [CrossRef]
- Posada-Martinez, E.L.; Fritche-Salazar, J.F.; Arias-Godinez, J.A.; Ortiz-Leon, X.A.; Balderas-Muñoz, K.; Ruiz-Esparza, M.E.; Sánchez, E.A.; Sandoval, J.P.; Morales, A.K.T.; Rodriguez-Zanella, H. Right ventricular longitudinal strain predicts low-cardiac-output syndrome after surgical aortic valve replacement in patients with preserved and mid-range ejection fraction. J. Cardiothorac. Vasc. Anesth. 2021, 35, 1638–1645. [Google Scholar] [CrossRef]
- Medvedofsky, D.; Koifman, E.; Jarrett, H.; Miyoshi, T.; Rogers, T.; Ben-Dor, I.; Satler, L.F.; Torguson, R.; Waksman, R.; Asch, F.M. Association of right ventricular longitudinal strain with mortality in patients undergoing transcatheter aortic valve replacement. J. Am. Soc. Echocardiogr. 2020, 33, 452–460. [Google Scholar] [CrossRef]
- Schueler, R.; Öztürk, C.; Laser, J.V.; Wirth, F.; Werner, N.; Welz, A.; Nickenig, G.; Sinning, J.M.; Hammerstingl, C. Right ventricular assessment in patients undergoing transcatheter or surgical aortic valve replacement. Catheter. Cardiovasc. Interv. 2020, 96, E711–E722. [Google Scholar] [CrossRef]
- Pardo Sanz, A.; Santoro, C.; Hinojar, R.; Salido, L.; Rajjoub, E.A.; Monteagudo, J.M.; García, A.; González, A.; Hernández-Antolín, R.; Sánchez Recalde, Á. Right ventricle assessment in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation. Echocardiography 2020, 37, 586–591. [Google Scholar] [CrossRef]
- Kempny, A.; Diller, G.-P.; Kaleschke, G.; Orwat, S.; Funke, A.; Schmidt, R.; Kerckhoff, G.; Ghezelbash, F.; Rukosujew, A.; Reinecke, H. Impact of transcatheter aortic valve implantation or surgical aortic valve replacement on right ventricular function. Heart 2012, 98, 1299–1304. [Google Scholar] [CrossRef]
- Vizzardi, E.; Gavazzoni, M.; Sciatti, E.; Dallapellegrina, L.; Bernardi, N.; Raddino, R.; Fiorina, C.; Adamo, M.; Metra, M. Right ventricular deformation and right ventricular-arterial coupling in patients with heart failure due to severe aortic stenosis undergoing TAVI: Long-term results. Am. J. Cardiovasc. Dis. 2020, 10, 150. [Google Scholar]
- Di Franco, A.; Kim, J.; Rodriguez-Diego, S.; Khalique, O.; Siden, J.Y.; Goldburg, S.R.; Mehta, N.K.; Srinivasan, A.; Ratcliffe, M.B.; Levine, R.A. Multiplanar strain quantification for assessment of right ventricular dysfunction and non-ischemic fibrosis among patients with ischemic mitral regurgitation. PLoS ONE 2017, 12, e0185657. [Google Scholar] [CrossRef]
- Le Tourneau, T.; Deswarte, G.; Lamblin, N.; Foucher-Hossein, C.; Fayad, G.; Richardson, M.; Polge, A.-S.; Vannesson, C.; Topilsky, Y.; Juthier, F. Right ventricular systolic function in organic mitral regurgitation: Impact of biventricular impairment. Circulation 2013, 127, 1597–1608. [Google Scholar] [CrossRef]
- Hyllen, S.; Nozohoor, S.; Ingvarsson, A.; Meurling, C.; Wierup, P.; Sjögren, J. Right ventricular performance after valve repair for chronic degenerative mitral regurgitation. Ann. Thorac. Surg. 2014, 98, 2023–2030. [Google Scholar] [CrossRef]
- Iacuzio, L.; Essayagh, B.; Civaia, F.; Schouver, E.D.; Rusek, S.; Dommerc, C.; Tribouilloy, C.; Dreyfus, G.; Levy, F. Right-sided heart structural and functional remodeling in mitral regurgitation secondary to mitral valve prolapse. Am. J. Cardiol. 2018, 122, 2095–2103. [Google Scholar] [CrossRef]
- Hungerford, S.; Bart, N.; Jansz, P.; Kay, S.; Emmanuel, S.; Namasivayam, M.; Dahle, G.; Duncan, A.; Hayward, C.; Muller, D.W. Improved right ventricular function following transapical transcatheter mitral valve implantation for severe mitral regurgitation. IJC Heart Vasc. 2021, 32, 100687. [Google Scholar] [CrossRef]
- Elgharably, H.; Javadikasgari, H.; Koprivanac, M.; Lowry, A.M.; Sato, K.; Blackstone, E.H.; Klein, A.L.; Gillinov, A.M.; Svensson, L.G.; Navia, J.L. Right versus left heart reverse remodelling after treating ischaemic mitral and tricuspid regurgitation. Eur. J. Cardio-Thorac. Surg. 2021, 59, 442–450. [Google Scholar] [CrossRef]
- Vitarelli, A.; Mangieri, E.; Capotosto, L.; Tanzilli, G.; D’Angeli, I.; Viceconte, N.; Placanica, A.; Placanica, G.; Cocco, N.; Ashurov, R. Assessment of biventricular function by three-dimensional speckle-tracking echocardiography in secondary mitral regurgitation after repair with the MitraClip system. J. Am. Soc. Echocardiogr. 2015, 28, 1070–1082. [Google Scholar] [CrossRef]
- Chang, W.-T.; Wu, N.-C.; Shih, J.-Y.; Hsu, C.-H.; Chen, Z.-C.; Cheng, B.-C. Right ventricular reserve post mitral valve repair is associated with heart failure hospitalization. Pulm. Circ. 2020, 10, 2045894020943858. [Google Scholar] [CrossRef] [PubMed]
- Prihadi, E.A.; van der Bijl, P.; Dietz, M.; Abou, R.; Vollema, E.M.; Marsan, N.A.; Delgado, V.; Bax, J.J. Prognostic implications of right ventricular free wall longitudinal strain in patients with significant functional tricuspid regurgitation. Circ. Cardiovasc. Imaging 2019, 12, e008666. [Google Scholar] [CrossRef]
- Romano, S.; Dell’Atti, D.; Judd, R.M.; Kim, R.J.; Weinsaft, J.W.; Kim, J.; Heitner, J.F.; Hahn, R.T.; Farzaneh-Far, A. Prognostic value of feature-tracking right ventricular longitudinal strain in severe functional tricuspid regurgitation: A multicenter study. Cardiovasc. Imaging 2021, 14, 1561–1568. [Google Scholar] [CrossRef]
- Hinojar, R.; Zamorano, J.L.; Gómez, A.G.; García-Martin, A.; Monteagudo, J.M.; Lunar, I.G.; Recalde, A.S.; Fernández-Golfín, C. Prognostic impact of right ventricular strain in isolated severe tricuspid regurgitation. J. Am. Soc. Echocardiogr. 2023, 36, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Ancona, F.; Melillo, F.; Calvo, F.; Attalla El Halabieh, N.; Stella, S.; Capogrosso, C.; Ingallina, G.; Tafciu, E.; Pascaretta, A.; Ancona, M.B. Right ventricular systolic function in severe tricuspid regurgitation: Prognostic relevance of longitudinal strain. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 868–875. [Google Scholar] [CrossRef]
- Bannehr, M.; Kahn, U.; Liebchen, J.; Okamoto, M.; Haehnel, V.; Georgi, C.; Dworok, V.; Edlinger, C.; Lichtenauer, M.; Kuecken, T. Right ventricular longitudinal strain predicts survival in patients with functional tricuspid regurgitation. Can. J. Cardiol. 2021, 37, 1086–1093. [Google Scholar] [CrossRef]
- Kim, M.; Lee, H.J.; Park, J.B.; Kim, J.; Lee, S.P.; Kim, Y.J.; Chang, S.A.; Kim, H.K. Preoperative right ventricular free-wall longitudinal strain as a prognosticator in isolated surgery for severe functional tricuspid regurgitation. J. Am. Heart Assoc. 2021, 10, e019856. [Google Scholar] [CrossRef] [PubMed]
- Van De Bruaene, A.; Buys, R.; Vanhees, L.; Delcroix, M.; Voigt, J.-U.; Budts, W. Regional right ventricular deformation in patients with open and closed atrial septal defect. Eur. J. Echocardiogr. 2011, 12, 206–213. [Google Scholar] [CrossRef]
- Moceri, P.; Duchateau, N.; Gillon, S.; Jaunay, L.; Baudouy, D.; Squara, F.; Ferrari, E.; Sermesant, M. Three-dimensional right ventricular shape and strain in congenital heart disease patients with right ventricular chronic volume loading. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 1174–1181. [Google Scholar] [CrossRef]
- Almeida-Morais, L.; Pereira-da-Silva, T.; Branco, L.; Timoteo, A.T.; Agapito, A.; de Sousa, L.; Oliveira, J.A.; Thomas, B.; Jalles-Tavares, N.; Soares, R. The value of right ventricular longitudinal strain in the evaluation of adult patients with repaired tetralogy of Fallot: A new tool for a contemporary challenge. Cardiol. Young 2017, 27, 498–506. [Google Scholar] [CrossRef]
- van Rosendael, P.J.; Taha, K.; Guglielmo, M.; Teske, A.J.; van der Harst, P.; Sieswerda, G.; Cramer, M.J.; van der Zwaan, H.B. Prognostic significance of echocardiographic deformation imaging in adult congenital heart disease. Eur. J. Clin. Investig. 2024, 54, e14200. [Google Scholar] [CrossRef]
- Kempny, A.; Diller, G.-P.; Orwat, S.; Kaleschke, G.; Kerckhoff, G.; Bunck, A.C.; Maintz, D.; Baumgartner, H. Right ventricular–left ventricular interaction in adults with tetralogy of Fallot: A combined cardiac magnetic resonance and echocardiographic speckle tracking study. Int. J. Cardiol. 2012, 154, 259–264. [Google Scholar] [CrossRef]
- Chowdhury, S.M.; Hijazi, Z.M.; Fahey, J.T.; Rhodes, J.F.; Kar, S.; Makkar, R.; Mullen, M.; Cao, Q.-L.; Shirali, G.S. Speckle-tracking echocardiographic measures of right ventricular function correlate with improvement in exercise function after percutaneous pulmonary valve implantation. J. Am. Soc. Echocardiogr. 2015, 28, 1036–1044. [Google Scholar] [CrossRef]
- Seo, Y.; Ishizu, T.; Atsumi, A.; Kawamura, R.; Aonuma, K. Three-Dimensional Speckle Tracking Echocardiography–A Promising Tool for Cardiac Functional Analysis–. Circ. J. 2014, 78, 1290–1301. [Google Scholar] [CrossRef]
- Lv, Q.; Sun, W.; Wang, J.; Wu, C.; Li, H.; Shen, X.; Liang, B.; Dong, N.; Li, Y.; Zhang, L. Evaluation of biventricular functions in transplanted hearts using 3-dimensional speckle-tracking echocardiography. J. Am. Heart Assoc. 2020, 9, e015742. [Google Scholar] [CrossRef]
- Sato, T.; Calderon, R.J.; Klas, B.; Pedrizzetti, G.; Banerjee, A. Simultaneous volumetric and functional assessment of the right ventricle in hypoplastic left heart syndrome after Fontan palliation, utilizing 3-dimensional speckle-tracking echocardiography. Circ. J. 2020, 84, 235–244. [Google Scholar] [CrossRef]
- Geva, T. Is MRI the preferred method for evaluating right ventricular size and function in patients with congenital heart disease? MRI is the preferred method for evaluating right ventricular size and function in patients with congenital heart disease. Circ. Cardiovasc. Imaging 2014, 7, 190–197. [Google Scholar] [CrossRef]
- Xu, J.; Yang, W.; Zhao, S.; Lu, M. State-of-the-art myocardial strain by CMR feature tracking: Clinical applications and future perspectives. Eur. Radiol. 2022, 32, 5424–5435. [Google Scholar] [CrossRef]
- Rajiah, P.S.; Kalisz, K.; Broncano, J.; Goerne, H.; Collins, J.D.; François, C.J.; Ibrahim, E.-S.; Agarwal, P.P. Myocardial strain evaluation with cardiovascular MRI: Physics, principles, and clinical applications. Radiographics 2022, 42, 968–990. [Google Scholar] [CrossRef]
- Bourfiss, M.; Prakken, N.; James, C.A.; Planken, R.; Boekholdt, S.; Ahmetagic, D.; Van Den Berg, M.; Tichnell, C.; Van der Heijden, J.; Loh, P. Prognostic value of strain by feature-tracking cardiac magnetic resonance in arrhythmogenic right ventricular cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 98–107. [Google Scholar] [CrossRef]
- Fogel, M.A.; Anwar, S.; Broberg, C.; Browne, L.; Chung, T.; Johnson, T.; Muthurangu, V.; Taylor, M.; Valsangiacomo-Buechel, E.; Wilhelm, C. Society for Cardiovascular Magnetic Resonance/European Society of Cardiovascular Imaging/American Society of Echocardiography/Society for Pediatric Radiology/North American Society for Cardiovascular Imaging Guidelines for the Use of Cardiac Magnetic Resonance in Pediatric Congenital and Acquired Heart Disease: Endorsed by The American Heart Association. Circ. Cardiovasc. Imaging 2022, 15, e014415. [Google Scholar] [PubMed]
- Johannesen, J.; Fukuda, R.; Zhang, D.T.; Tak, K.; Meier, R.; Agoglia, H.; Horn, E.; Devereux, R.B.; Weinsaft, J.W.; Kim, J. Direct comparison of echocardiography speckle tracking and cardiac magnetic resonance feature tracking for quantification of right ventricular strain: A prospective intermodality study in functional mitral regurgitation. Echo Res. Pract. 2022, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Pryds, K.; Larsen, A.H.; Hansen, M.S.; Grøndal, A.Y.K.; Tougaard, R.S.; Hansson, N.H.; Clemmensen, T.S.; Løgstrup, B.B.; Wiggers, H.; Kim, W.Y. Myocardial strain assessed by feature tracking cardiac magnetic resonance in patients with a variety of cardiovascular diseases–A comparison with echocardiography. Sci. Rep. 2019, 9, 11296. [Google Scholar] [CrossRef]
- Maceira, A.M.; Tuset-Sanchis, L.; López-Garrido, M.; San Andres, M.; López-Lereu, M.P.; Monmeneu, J.V.; García-González, M.P.; Higueras, L. Feasibility and reproducibility of feature-tracking-based strain and strain rate measures of the left ventricle in different diseases and genders. J. Magn. Reson. Imaging 2018, 47, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Bose, R.; Ahmed, A.; Maslow, A.; Feng, Y.; Sharkey, A.; Baribeau, Y.; Mahmood, F.; Matyal, R.; Khabbaz, K. Artificial intelligence-based assessment of indices of right ventricular function. J. Cardiothorac. Vasc. Anesth. 2020, 34, 2698–2702. [Google Scholar] [CrossRef]
- Penso, M.; Ranalletta, R.A.; Pepi, M.; Garlaschè, A.; Ali, S.G.; Fusini, L.; Mantegazza, V.; Muratori, M.; Maragna, R.; Tamborini, G. Comparison between automatic and semiautomatic system for the 3D echocardiographic multiparametric evaluation of RV function and dimension. J. Clin. Med. 2022, 11, 4528. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Bao, Y.; Zheng, K.; Zhou, W.; Zhang, J.; Sun, R.; Deng, Y.; Xia, L.; Liu, Y. Quantitative assessment of right ventricular size and function with multiple parameters from artificial intelligence-based three-dimensional echocardiography: A comparative study with cardiac magnetic resonance. Echocardiography 2022, 39, 223–232. [Google Scholar] [CrossRef]
- Shad, R.; Quach, N.; Fong, R.; Kasinpila, P.; Bowles, C.; Castro, M.; Guha, A.; Suarez, E.E.; Jovinge, S.; Lee, S. Predicting post-operative right ventricular failure using video-based deep learning. Nat. Commun. 2021, 12, 5192. [Google Scholar] [CrossRef]



| Study | Study Type | Sample Size | Ultrasound Device (s) | Strain Analysis Software | RVLS% | RVGLS% | ||
|---|---|---|---|---|---|---|---|---|
| Average | LLN | Average | LLN | |||||
| Fine 2013 [34] | Prospective Cohort | 186 | Philips iE33, GE Vivid 7, Siemens Sequoia C512 | Syngo VVI | −21.7 | −13.3 | −13.3 | −14.0 |
| Chia 2014 [35] | Prospective Cohort | 142 | GE Vivid 7 | EchoPac | −27.3 | −20.7 | −22.4 | −17.6 |
| Morris 2016 [30] | Prospective Cohort | 238 | GE Vivid 7 | EchoPac | −28.5 | −18.9 | −24.5 | −16.9 |
| Muraru 2016 [25] | Prospective Cohort | 250 | GE Vivid E9 | EchoPac | −30.5 | −22.7 | −25.8 | −19.8 |
| McGhie 2017 [36] | Prospective Cohort | 147 | Philips iE33 or EPIQ7 | TomTec | −25.4 | −15.4 | - | - |
| Park 2018 [37] | Prospective Cohort | 493 | GE | EchoPac | −26.4 | −18.0 | −21.5 | −15.1 |
| Addetia 2021 [31] | Retrospective Cohort | 1913 | Philips, Siemens, GE | TomTec | −28.3 | −20.0 | −25.4 | −18.2 |
| Wang 2021 [32] | Meta-analysis | 3673 | - | - | −26.9 | −18.0 | −23.4 | −16.4 |
| Espersen 2024 [38] | Prospective Cohort | 2951 | GE Vivid 9 | EchoPac | −26.7 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelnabi, M.; Ibrahim, R.; Pham, H.N.; Heon, B.J.; Bcharah, G.; Pathangey, G.; Pereyra Pietri, M.; Farina, J.M.; Chang, I.C.; Arsanjani, R.; et al. Right Ventricular Strain by Echocardiography: Current Clinical Applications and Future Directions for Mechanics Assessment of the Forgotten Ventricle. J. Pers. Med. 2025, 15, 224. https://doi.org/10.3390/jpm15060224
Abdelnabi M, Ibrahim R, Pham HN, Heon BJ, Bcharah G, Pathangey G, Pereyra Pietri M, Farina JM, Chang IC, Arsanjani R, et al. Right Ventricular Strain by Echocardiography: Current Clinical Applications and Future Directions for Mechanics Assessment of the Forgotten Ventricle. Journal of Personalized Medicine. 2025; 15(6):224. https://doi.org/10.3390/jpm15060224
Chicago/Turabian StyleAbdelnabi, Mahmoud, Ramzi Ibrahim, Hoang Nhat Pham, Bobbi Jo Heon, George Bcharah, Girish Pathangey, Milagros Pereyra Pietri, Juan M. Farina, Ian C. Chang, Reza Arsanjani, and et al. 2025. "Right Ventricular Strain by Echocardiography: Current Clinical Applications and Future Directions for Mechanics Assessment of the Forgotten Ventricle" Journal of Personalized Medicine 15, no. 6: 224. https://doi.org/10.3390/jpm15060224
APA StyleAbdelnabi, M., Ibrahim, R., Pham, H. N., Heon, B. J., Bcharah, G., Pathangey, G., Pereyra Pietri, M., Farina, J. M., Chang, I. C., Arsanjani, R., & Ayoub, C. (2025). Right Ventricular Strain by Echocardiography: Current Clinical Applications and Future Directions for Mechanics Assessment of the Forgotten Ventricle. Journal of Personalized Medicine, 15(6), 224. https://doi.org/10.3390/jpm15060224