Improvement of Fatigue and Body Composition in Women with Long COVID After Non-Aerobic Therapeutic Exercise Program
Abstract
1. Introduction
2. Methods
2.1. Design
2.2. Participants
2.3. Procedure
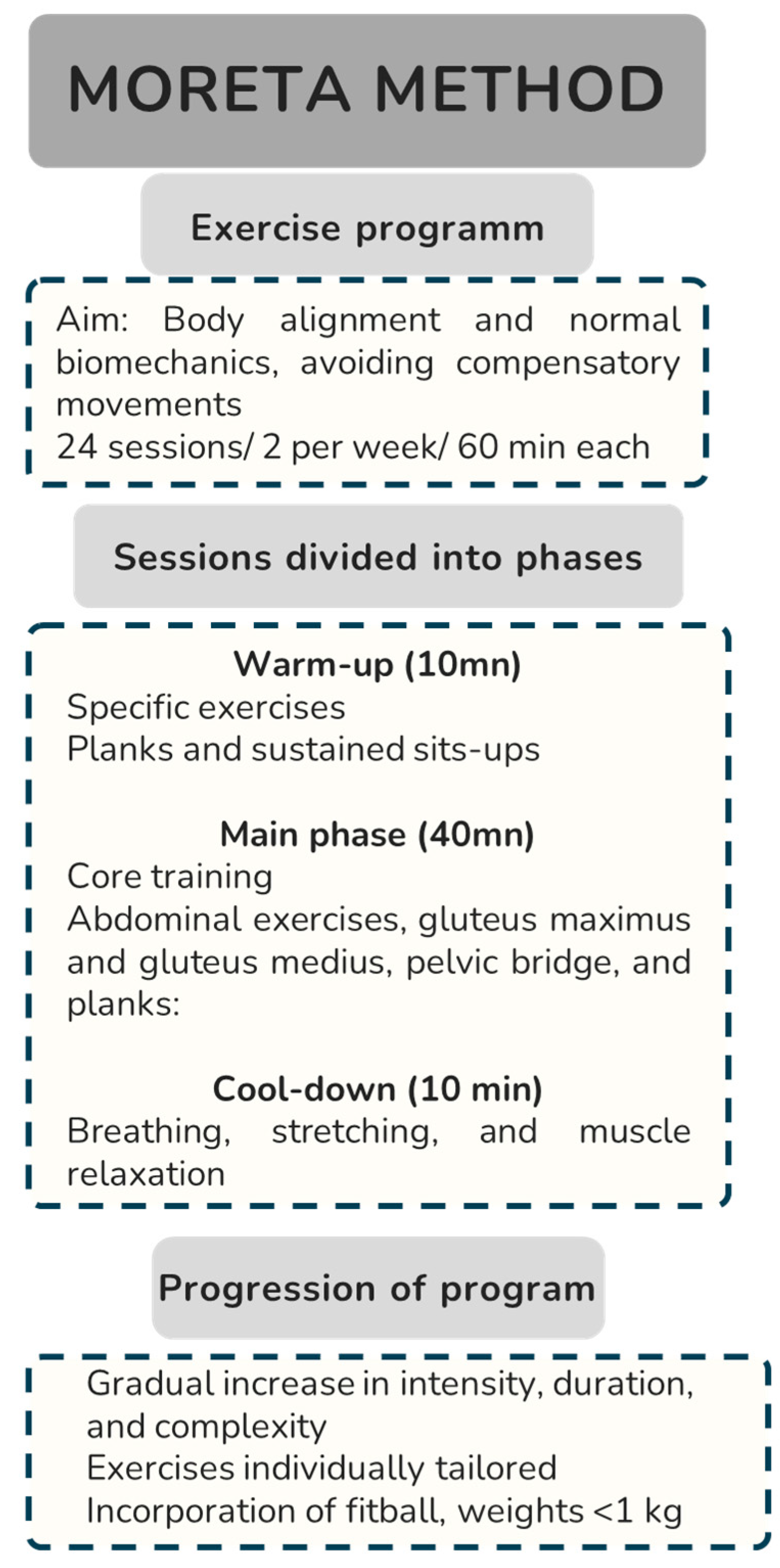
2.4. Intervention
2.5. Statistical Analysis
3. Results
3.1. Segmental Body Composition
3.2. Fatigue
4. Discussion
- Orthostatic Tachycardia: Patients with postural orthostatic tachycardia syndrome (POTS) experience significant increases in their heart rate upon standing, which can be exacerbated by upright aerobic exercise. The American College of Cardiology (ACC) notes that these patients often cannot tolerate upright exercise, such as power walking or jogging, as it can worsen symptoms like fatigue and post-exertional malaise [24].
- Post-Exertional Malaise: Post-exertional malaise (PEM) is a hallmark of LC, characterized by a significant worsening of symptoms following physical exertion. This can include increased fatigue, muscle pain, and cognitive difficulties, which can persist for days or even weeks after the activity. The National Institute for Health and Care Excellence (NICE) guidelines caution against graded exercise therapy in patients with LC, particularly those with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) [55].
- Exercise Intolerance: Studies have shown that patients with LC often have a reduced exercise capacity and chronotropic incompetence, which can lead to exercise intolerance. This is associated with a lower peak oxygen consumption and muscle strength, making it difficult for these patients to engage in and benefit from traditional aerobic exercise [56]. Given these factors, it is recommended that exercise regimens for these patients be tailored to their specific needs, starting with recumbent or semi-recumbent exercises (e.g., rowing, swimming, or cycling) and gradually progressing as tolerated. This approach helps to avoid exacerbating symptoms while still promoting physical activity [24].
- -
- In a safe and effective rehabilitation program, physical activity should be prescribed with a tailored approach and personalized based on individual characteristics.
- -
- The patient should be evaluated in his/her complexity to adapt an exercise program appropriate to their characteristics, health problems, symptoms, and consequences of COVID-19.
- -
- A multidisciplinary team should establish a general treatment plan adapted for each patient according to the clinical presentation [68].
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long Covid—Mechanisms, Risk Factors, and Management. BMJ 2021, n1648. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-Las-Peñas, C.; Martín-Guerrero, J.D.; Cancela-Cilleruelo, I.; Moro-López-Menchero, P.; Pellicer-Valero, O.J. Exploring the Recovery Curve for Long-Term Post-COVID Dyspnea and Fatigue. Eur. J. Intern. Med. 2022, 101, 120–123. [Google Scholar] [CrossRef]
- Fernández-de-Las-Peñas, C.; Torres-Macho, J.; Elvira-Martínez, C.M.; Molina-Trigueros, L.J.; Sebastián-Viana, T.; Hernández-Barrera, V. Obesity Is Associated with a Greater Number of Long-Term Post-COVID Symptoms and Poor Sleep Quality: A Multicentre Case-Control Study. Int. J. Clin. Pract. 2021, 75, e14917. [Google Scholar] [CrossRef]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A Clinical Case Definition of Post-COVID-19 Condition by a Delphi Consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef]
- Rao, S.; Benzouak, T.; Gunpat, S.; Burns, R.J.; Tahir, T.A.; Jolles, S.; Kisely, S. Fatigue Symptoms Associated With COVID-19 in Convalescent or Recovered COVID-19 Patients; a Systematic Review and Meta-Analysis. Ann. Behav. Med. 2022, 56, 219–234. [Google Scholar] [CrossRef]
- Malik, P.; Patel, K.; Pinto, C.; Jaiswal, R.; Tirupathi, R.; Pillai, S.; Patel, U. Post-Acute COVID-19 Syndrome (PCS) and Health-Related Quality of Life (HRQoL)-A Systematic Review and Meta-Analysis. J. Med. Virol. 2022, 94, 253–262. [Google Scholar] [CrossRef]
- Molnar, T.; Lehoczki, A.; Fekete, M.; Varnai, R.; Zavori, L.; Erdo-Bonyar, S.; Simon, D.; Berki, T.; Csecsei, P.; Ezer, E. Mitochondrial Dysfunction in Long COVID: Mechanisms, Consequences, and Potential Therapeutic Approaches. Geroscience 2024, 46, 5267–5286. [Google Scholar] [CrossRef]
- Elanwar, R.; Hussein, M.; Magdy, R.; Eid, R.A.; Yassien, A.; Abdelsattar, A.S.; Alsharaway, L.A.; Fathy, W.; Hassan, A.; Kamal, Y.S. Physical and Mental Fatigue in Subjects Recovered from COVID-19 Infection: A Case-Control Study. Neuropsychiatr. Dis. Treat. 2021, 17, 2063–2071. [Google Scholar] [CrossRef]
- Kamal, M.; Abo Omirah, M.; Hussein, A.; Saeed, H. Assessment and Characterisation of Post-COVID-19 Manifestations. Int. J. Clin. Pract. 2021, 75, e13746. [Google Scholar] [CrossRef]
- Díez-Cirarda, M.; Yus, M.; Gómez-Ruiz, N.; Polidura, C.; Gil-Martínez, L.; Delgado-Alonso, C.; Jorquera, M.; Gómez-Pinedo, U.; Matias-Guiu, J.; Arrazola, J.; et al. Multimodal Neuroimaging in Post-COVID Syndrome and Correlation with Cognition. Brain 2023, 146, 2142–2152. [Google Scholar] [CrossRef]
- Moore, D.; Jung, M.; Hillman, C.H.; Kang, M.; Loprinzi, P.D. Interrelationships between Exercise, Functional Connectivity, and Cognition among Healthy Adults: A Systematic Review. Psychophysiology 2022, 59, e14014. [Google Scholar] [CrossRef] [PubMed]
- Won, J.; Callow, D.D.; Pena, G.S.; Gogniat, M.A.; Kommula, Y.; Arnold-Nedimala, N.A.; Jordan, L.S.; Smith, J.C. Evidence for Exercise-Related Plasticity in Functional and Structural Neural Network Connectivity. Neurosci. Biobehav. Rev. 2021, 131, 923–940. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Al-Sultan, F.; Jamea, A.A.; Almousa, A.; Alnafisah, M.; Alzahrani, M.; Abualait, T.; Yoo, W.-K. Physical Exercise Keeps the Brain Connected by Increasing White Matter Integrity in Healthy Controls. Medicine 2021, 100, e27015. [Google Scholar] [CrossRef]
- Augusto-Oliveira, M.; Arrifano, G.P.; Leal-Nazaré, C.G.; Santos-Sacramento, L.; Lopes-Araújo, A.; Royes, L.F.F.; Crespo-Lopez, M.E. Exercise Reshapes the Brain: Molecular, Cellular, and Structural Changes Associated with Cognitive Improvements. Mol. Neurobiol. 2023, 60, 6950–6974. [Google Scholar] [CrossRef]
- Neva, J.L.; Brown, K.E.; Mang, C.S.; Francisco, B.A.; Boyd, L.A. An Acute Bout of Exercise Modulates Both Intracortical and Interhemispheric Excitability. Eur. J. Neurosci. 2017, 45, 1343–1355. [Google Scholar] [CrossRef]
- Durstenfeld, M.S.; Sun, K.; Tahir, P.; Peluso, M.J.; Deeks, S.G.; Aras, M.A.; Grandis, D.J.; Long, C.S.; Beatty, A.; Hsue, P.Y. Use of Cardiopulmonary Exercise Testing to Evaluate Long COVID-19 Symptoms in Adults: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2022, 5, e2236057. [Google Scholar] [CrossRef] [PubMed]
- Kahn, P.A.; Joseph, P.; Heerdt, P.M.; Singh, I. Differential Cardiopulmonary Haemodynamic Phenotypes in PASC-Related Exercise Intolerance. ERJ Open Res. 2024, 10, 00714–02023. [Google Scholar] [CrossRef]
- Weldon, E.J.; Hong, B.; Hayashi, J.; Goo, C.; Carrazana, E.; Viereck, J.; Liow, K. Mechanisms and Severity of Exercise Intolerance Following COVID-19 and Similar Viral Infections: A Comparative Review. Cureus 2023, 15, e39722. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Author Correction: Long COVID: Major Findings, Mechanisms and Recommendations. Nat. Rev. Microbiol. 2023, 21, 408. [Google Scholar] [CrossRef]
- Kerkhoff, T.J.; Charlton, B.T.; Appelman, B.; van Vugt, M.; Wüst, R.C.I. Post COVID-19 Condition: Critical Need for a Clear Definition and Detailed Pathophysiology. J. Cachexia Sarcopenia Muscle 2022, 13, 2754–2756. [Google Scholar] [CrossRef]
- Appelman, B.; Charlton, B.T.; Goulding, R.P.; Kerkhoff, T.J.; Breedveld, E.A.; Noort, W.; Offringa, C.; Bloemers, F.W.; Van Weeghel, M.; Schomakers, B.V.; et al. Muscle Abnormalities Worsen after Post-Exertional Malaise in Long COVID. Nat. Commun. 2024, 15, 17. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, H.; Kilby, L.; Kudiersky, N.; Copeland, R. Long COVID and the Role of Physical Activity: A Qualitative Study. BMJ Open 2021, 11, e047632. [Google Scholar] [CrossRef] [PubMed]
- Salman, D.; Vishnubala, D.; Le Feuvre, P.; Beaney, T.; Korgaonkar, J.; Majeed, A.; McGregor, A.H. Returning to Physical Activity after COVID-19. BMJ 2021, 372, m4721. [Google Scholar] [CrossRef]
- Writing Committee; Gluckman, T.J.; Bhave, N.M.; Allen, L.A.; Chung, E.H.; Spatz, E.S.; Ammirati, E.; Baggish, A.L.; Bozkurt, B.; Cornwell, W.K.; et al. 2022 ACC Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19 in Adults: Myocarditis and Other Myocardial Involvement, Post-Acute Sequelae of SARS-CoV-2 Infection, and Return to Play: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2022, 79, 1717–1756. [Google Scholar] [CrossRef]
- Yang, Y.; Ding, L.; Zou, X.; Shen, Y.; Hu, D.; Hu, X.; Li, Z.; Kamel, I.R. Visceral Adiposity and High Intramuscular Fat Deposition Independently Predict Critical Illness in Patients with SARS-CoV-2. Obesity 2020, 28, 2040–2048. [Google Scholar] [CrossRef]
- Watanabe, M.; Caruso, D.; Tuccinardi, D.; Risi, R.; Zerunian, M.; Polici, M.; Pucciarelli, F.; Tarallo, M.; Strigari, L.; Manfrini, S.; et al. Visceral Fat Shows the Strongest Association with the Need of Intensive Care in Patients with COVID-19. Metabolism 2020, 111, 154319. [Google Scholar] [CrossRef]
- Földi, M.; Farkas, N.; Kiss, S.; Dembrovszky, F.; Szakács, Z.; Balaskó, M.; Erőss, B.; Hegyi, P.; Szentesi, A. Visceral Adiposity Elevates the Risk of Critical Condition in COVID-19: A Systematic Review and Meta-Analysis. Obesity 2021, 29, 521–528. [Google Scholar] [CrossRef]
- Dietz, W.; Santos-Burgoa, C. Obesity and Its Implications for COVID-19 Mortality. Obesity 2020, 28, 1005. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, X.; Ye, S.; Lian, H.; Wang, H.; Ye, J. Obesity and COVID-19: Mechanistic Insights From Adipose Tissue. J. Clin. Endocrinol. Metab. 2022, 107, 1799–1811. [Google Scholar] [CrossRef]
- Chandarana, H.; Dane, B.; Mikheev, A.; Taffel, M.T.; Feng, Y.; Rusinek, H. Visceral Adipose Tissue in Patients with COVID-19: Risk Stratification for Severity. Abdom Radiol 2021, 46, 818–825. [Google Scholar] [CrossRef]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): A Systematic Review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Mantilla Toloza, S.C.; Gómez-Conesa, A. El Cuestionario Internacional de Actividad Física. Un instrumento adecuado en el seguimiento de la actividad física poblacional. Rev. Iberoam. Fisioter. Y Kinesiol. 2007, 10, 48–52. [Google Scholar] [CrossRef]
- Larson, R.D. Psychometric Properties of the Modified Fatigue Impact Scale. Int. J. MS Care 2013, 15, 15–20. [Google Scholar] [CrossRef]
- Ocak, Ö.; Şahin, E.M. Fatigue in Long-COVID; Frequency, Severity and Impact on Quality of Life. Troia Med. J. 2024, 2, 74–81. [Google Scholar] [CrossRef]
- Amiri, B.; Sahebozamani, M.; Sedighi, B. The Effects of 10-Week Core Stability Training on Balance in Women with Multiple Sclerosis According to Expanded Disability Status Scale: A Single-Blinded Randomized Controlled Trial. Eur. J. Phys. Rehabil. Med. 2019, 55, 199–208. [Google Scholar] [CrossRef]
- Nambi, G.; Abdelbasset, W.K.; Alrawaili, S.M.; Elsayed, S.H.; Verma, A.; Vellaiyan, A.; Eid, M.M.; Aldhafian, O.R.; Nwihadh, N.B.; Saleh, A.K. Comparative Effectiveness Study of Low versus High-Intensity Aerobic Training with Resistance Training in Community-Dwelling Older Men with Post-COVID 19 Sarcopenia: A Randomized Controlled Trial. Clin. Rehabil. 2022, 36, 59–68. [Google Scholar] [CrossRef]
- Tabacof, L.; Tosto-Mancuso, J.; Wood, J.; Cortes, M.; Kontorovich, A.; McCarthy, D.; Rizk, D.; Rozanski, G.; Breyman, E.; Nasr, L.; et al. Post-Acute COVID-19 Syndrome Negatively Impacts Physical Function, Cognitive Function, Health-Related Quality of Life, and Participation. Am. J. Phys. Med. Rehabil. 2022, 101, 48–52. [Google Scholar] [CrossRef]
- Twomey, R.; DeMars, J.; Franklin, K.; Culos-Reed, S.N.; Weatherald, J.; Wrightson, J.G. Chronic Fatigue and Postexertional Malaise in People Living With Long COVID: An Observational Study. Phys. Ther. 2022, 102, pzac005. [Google Scholar] [CrossRef]
- Santana, K.; França, E.; Sato, J.; Silva, A.; Queiroz, M.; de Farias, J.; Rodrigues, D.; Souza, I.; Ribeiro, V.; Caparelli-Dáquer, E.; et al. Non-Invasive Brain Stimulation for Fatigue in Post-Acute Sequelae of SARS-CoV-2 (PASC). Brain Stimul. 2023, 16, 100–107. [Google Scholar] [CrossRef]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and Cognitive Impairment in Post-COVID-19 Syndrome: A Systematic Review and Meta-Analysis. Brain, Behavior, and Immunity 2022, 101, 93–135. [Google Scholar] [CrossRef]
- Douaud, G.; Lee, S.; Alfaro-Almagro, F.; Arthofer, C.; Wang, C.; McCarthy, P.; Lange, F.; Andersson, J.L.R.; Griffanti, L.; Duff, E.; et al. SARS-CoV-2 Is Associated with Changes in Brain Structure in UK Biobank. Nature 2022, 604, 697–707. [Google Scholar] [CrossRef]
- Ortelli, P.; Quercia, A.; Cerasa, A.; Dezi, S.; Ferrazzoli, D.; Sebastianelli, L.; Saltuari, L.; Versace, V.; Quartarone, A. Lowered Delta Activity in Post-COVID-19 Patients with Fatigue and Cognitive Impairment. Biomedicines 2023, 11, 2228. [Google Scholar] [CrossRef]
- Ortelli, P.; Ferrazzoli, D.; Sebastianelli, L.; Maestri, R.; Dezi, S.; Spampinato, D.; Saltuari, L.; Alibardi, A.; Engl, M.; Kofler, M.; et al. Altered Motor Cortex Physiology and Dysexecutive Syndrome in Patients with Fatigue and Cognitive Difficulties after Mild COVID-19. Eur. J. Neurol. 2022, 29, 1652–1662. [Google Scholar] [CrossRef]
- Casula, E.P.; Esposito, R.; Dezi, S.; Ortelli, P.; Sebastianelli, L.; Ferrazzoli, D.; Saltuari, L.; Pezzopane, V.; Borghi, I.; Rocchi, L.; et al. Reduced TMS-Evoked EEG Oscillatory Activity in Cortical Motor Regions in Patients with Post-COVID Fatigue. Clin. Neurophysiol. 2024, 165, 26–35. [Google Scholar] [CrossRef]
- Teo, W.-P.; Goodwill, A.M. Can Exercise Attenuate the Negative Effects of Long COVID Syndrome on Brain Health? Front. Immunol. 2022, 13, 986950. [Google Scholar] [CrossRef]
- Hendrikse, J.; Kandola, A.; Coxon, J.; Rogasch, N.; Yücel, M. Combining Aerobic Exercise and Repetitive Transcranial Magnetic Stimulation to Improve Brain Function in Health and Disease. Neurosci. Biobehav. Rev. 2017, 83, 11–20. [Google Scholar] [CrossRef]
- Hendy, A.M.; Andrushko, J.W.; Della Gatta, P.A.; Teo, W.-P. Acute Effects of High-Intensity Aerobic Exercise on Motor Cortical Excitability and Inhibition in Sedentary Adults. Front. Psychol. 2022, 13, 814633. [Google Scholar] [CrossRef]
- Colas, C.; Le Berre, Y.; Fanget, M.; Savall, A.; Killian, M.; Goujon, I.; Labeix, P.; Bayle, M.; Féasson, L.; Roche, F.; et al. Physical Activity in Long COVID: A Comparative Study of Exercise Rehabilitation Benefits in Patients with Long COVID, Coronary Artery Disease and Fibromyalgia. Int. J. Environ. Res. Public Health 2023, 20, 6513. [Google Scholar] [CrossRef]
- Sandler, C.X.; Wyller, V.B.B.; Moss-Morris, R.; Buchwald, D.; Crawley, E.; Hautvast, J.; Katz, B.Z.; Knoop, H.; Little, P.; Taylor, R.; et al. Long COVID and Post-Infective Fatigue Syndrome: A Review. Open Forum Infect. Dis. 2021, 8, ofab440. [Google Scholar] [CrossRef]
- Di Profio, E.; Leone, A.; Vizzuso, S.; Fiore, G.; Pascuzzi, M.C.; Agostinelli, M.; Dilillo, D.; Mannarino, S.; Fiori, L.; D’Auria, E.; et al. Longitudinal Anthropometry and Body Composition in Children With SARS-CoV-2-Associated Multisystem Inflammatory Syndrome. J. Pediatr. Gastroenterol. Nutr. 2023, 76, 505–511. [Google Scholar] [CrossRef]
- Vasylieva, N.O.; Koziy, T.P.; Lavrykova, O.V.; Karpukhina, Y.V. Influence of Therapeutic Gymnastics on Body Weight Composition, Anthropometric Parameters and Quality of Life of Women with Obesity in the Conditions of Quarantine Restrictions in the Context of the COVID-19 Pandemic. Wiad. Lek. 2023, 76, 90–96. [Google Scholar] [CrossRef]
- Garbsch, R.; Schäfer, H.; Mooren, F.C.; Schmitz, B. Analysis of Fat Oxidation Capacity during Cardiopulmonary Exercise Testing Indicates Long-Lasting Metabolic Disturbance in Patients with Post-COVID-19 Syndrome. Clin. Nutr. 2024, 43, 26–35. [Google Scholar] [CrossRef]
- Ramírez-Vélez, R.; Legarra-Gorgoñon, G.; Oscoz-Ochandorena, S.; García-Alonso, Y.; García-Alonso, N.; Oteiza, J.; Ernaga Lorea, A.; Correa-Rodríguez, M.; Izquierdo, M. Reduced Muscle Strength in Patients with Long-COVID-19 Syndrome Is Mediated by Limb Muscle Mass. J. Appl. Physiol. 2023, 134, 50–58. [Google Scholar] [CrossRef]
- Al-Hakeim, H.K.; Al-Rubaye, H.T.; Al-Hadrawi, D.S.; Almulla, A.F.; Maes, M. Long-COVID Post-Viral Chronic Fatigue and Affective Symptoms Are Associated with Oxidative Damage, Lowered Antioxidant Defenses and Inflammation: A Proof of Concept and Mechanism Study. Mol. Psychiatry 2023, 28, 564–578. [Google Scholar] [CrossRef]
- Faghy, M.A.; Duncan, R.; Hume, E.; Gough, L.; Roscoe, C.; Laddu, D.; Arena, R.; Asthon, R.E.M.; Dalton, C. Developing Effective Strategies to Optimize Physical Activity and Cardiorespiratory Fitness in the Long Covid Population- The Need for Caution and Objective Assessment. Prog. Cardiovasc. Dis. 2024, 83, 62–70. [Google Scholar] [CrossRef]
- Durstenfeld, M.S.; Peluso, M.J.; Kaveti, P.; Hill, C.; Li, D.; Sander, E.; Swaminathan, S.; Arechiga, V.M.; Lu, S.; Goldberg, S.A.; et al. Reduced Exercise Capacity, Chronotropic Incompetence, and Early Systemic Inflammation in Cardiopulmonary Phenotype Long Coronavirus Disease 2019. J. Infect Dis. 2023, 228, 542–554. [Google Scholar] [CrossRef]
- Dutta, N.; Ingraham, N.E.; Usher, M.G.; Fox, C.; Tignanelli, C.J.; Bramante, C.T. We Should Do More to Offer Evidence-Based Treatment for an Important Modifiable Risk Factor for COVID-19: Obesity. J. Prim. Care Community Health 2021, 12, 2150132721996283. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, Y.; Huang, Y.-M.; Wang, M.; Ling, W.; Sui, Y.; Zhao, H.-L. Obesity in Patients with COVID-19: A Systematic Review and Meta-Analysis. Metabolism 2020, 113, 154378. [Google Scholar] [CrossRef]
- Brito, D.; Meester, S.; Yanamala, N.; Patel, H.B.; Balcik, B.J.; Casaclang-Verzosa, G.; Seetharam, K.; Riveros, D.; Beto, R.J.; Balla, S.; et al. High Prevalence of Pericardial Involvement in College Student Athletes Recovering From COVID-19. JACC Cardiovasc. Imaging 2021, 14, 541–555. [Google Scholar] [CrossRef]
- Maglietta, G.; Diodati, F.; Puntoni, M.; Lazzarelli, S.; Marcomini, B.; Patrizi, L.; Caminiti, C. Prognostic Factors for Post-COVID-19 Syndrome: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 1541. [Google Scholar] [CrossRef]
- Edward, J.A.; Peruri, A.; Rudofker, E.; Shamapant, N.; Parker, H.; Cotter, R.; Sabin, K.; Lawley, J.; Cornwell, W.K. Characteristics and Treatment of Exercise Intolerance in Patients With Long COVID. J. Cardiopulm. Rehabil. Prev. 2023, 43, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Barz, A.; Berger, J.; Speicher, M.; Morsch, A.; Wanjek, M.; Rissland, J.; Jäger, J. Effects of a Symptom-Titrated Exercise Program on Fatigue and Quality of Life in People with Post-COVID Condition—A Randomized Controlled Trial. Sci. Rep. 2024, 14, 30511. [Google Scholar] [CrossRef]
- Ostrowska, M.; Rzepka-Cholasińska, A.; Pietrzykowski, Ł.; Michalski, P.; Kosobucka-Ozdoba, A.; Jasiewicz, M.; Kasprzak, M.; Kryś, J.; Kubica, A. Effects of Multidisciplinary Rehabilitation Program in Patients with Long COVID-19: Post-COVID-19 Rehabilitation (PCR SIRIO 8) Study. J. Clin. Med. 2023, 12, 420. [Google Scholar] [CrossRef]
- Ohkawara, K.; Tanaka, S.; Miyachi, M.; Ishikawa-Takata, K.; Tabata, I. A Dose–Response Relation between Aerobic Exercise and Visceral Fat Reduction: Systematic Review of Clinical Trials. Int J Obes 2007, 31, 1786–1797. [Google Scholar] [CrossRef]
- Lakhdar, N.; Denguezli, M.; Zaouali, M.; Zbidi, A.; Tabka, Z.; Bouassida, A. Diet and Diet Combined with Chronic Aerobic Exercise Decreases Body Fat Mass and Alters Plasma and Adipose Tissue Inflammatory Markers in Obese Women. Inflammation 2013, 36, 1239–1247. [Google Scholar] [CrossRef]
- Constantin-Teodosiu, D.; Constantin, D. Molecular Mechanisms of Muscle Fatigue. Int. J. Mol. Sci. 2021, 22, 11587. [Google Scholar] [CrossRef]
- Santos, M.; Dorna, M.; Franco, E.; Geronutti, J.; Brizola, L.; Ishimoto, L.; Barros, Y.; Costa, A.; Breda, C.; Marin, C.; et al. Clinical and Physiological Variables in Patients with Post-COVID-19 Condition and Persistent Fatigue. J. Clin. Med. 2024, 13, 3876. [Google Scholar] [CrossRef]
- Cavigli, L.; Fusi, C.; Focardi, M.; Mandoli, G.E.; Pastore, M.C.; Cameli, M.; Valente, S.; Zorzi, A.; Bonifazi, M.; D’Andrea, A.; et al. Post-Acute Sequelae of COVID-19: The Potential Role of Exercise Therapy in Treating Patients and Athletes Returning to Play. J. Clin. Med. 2022, 12, 288. [Google Scholar] [CrossRef]
- Rodríguez Ledo, P.; Armenteros Del Olmo, L.; Rodríguez Rodríguez, E.; Gómez Acebo, F.; en representación de Sociedad Española de Médicos Generales y de Familia (SEMG) y colectivo Long COVID ACTS. Descripción de Los 201 Síntomas de La Afectación Multiorgánica Producida En Los Pacientes Afectados Por La COVID-19 Persistente. Med. Gen. Fam. 2021, 10, 60–68. [Google Scholar] [CrossRef]
| PRE n (%) | POST n (%) | |
|---|---|---|
| Underweight | 2 (11.8) | - |
| Normal range | 3 (17.6) | 6 (35.3) |
| Overweight/pre-obesity | 8 (47.0) | 8 (47.0) |
| Obese class I | 4 (23.5) | 3 (17.6) |
| PRE (mean ± sd) | POST (mean ± sd) | Paired Differences (mean ± sd) | 95% CI | t | df | p-Value | r | |
|---|---|---|---|---|---|---|---|---|
| Total Body Fat (%) | 37.18 ± 7.08 | 35.46 ± 7.71 | −1.72 ± 1.67 | −2.57/ −0.86 | 4.24 | 16 | 0.001 | 0.73 |
| Right Arm | 37.22 ± 8.36 | 35.71 ± 8.53 | −1.51 ± 1.47 | −2.26/ −0.75 | 4.23 | 16 | 0.001 | 0.73 |
| Left Arm | 37.71 ± 7.93 | 36.38 ± 8.32 | −1.34 ± 1.48 | −2.10/ −0.58 | 3.73 | 16 | 0.002 | 0.68 |
| Right leg | 39.45 ± 5.49 | 38.24 ± 6.76 | −1.21 ± 2.01 | −2.24/ −0.18 | 2.49 | 16 | 0.000 | 0.53 |
| Left leg | 38.97 ± 5.93 | 37.59 ± 6.73 | −1.38 ± 2.24 | −2.53/ −0.23 | 2.54 | 16 | 0.024 | 0.54 |
| Trunk | 35.86 ± 8.15 | 33.78 ± 8.75 | −2.09 ± 1.98 | −3.10/ −1.07 | 4.35 | 16 | 0.022 | 0.74 |
| PRE (mean ± sd) | POST (mean ± sd) | Paired Differences (mean ± sd) | 95% CI | t | df | p-Value | r | |
|---|---|---|---|---|---|---|---|---|
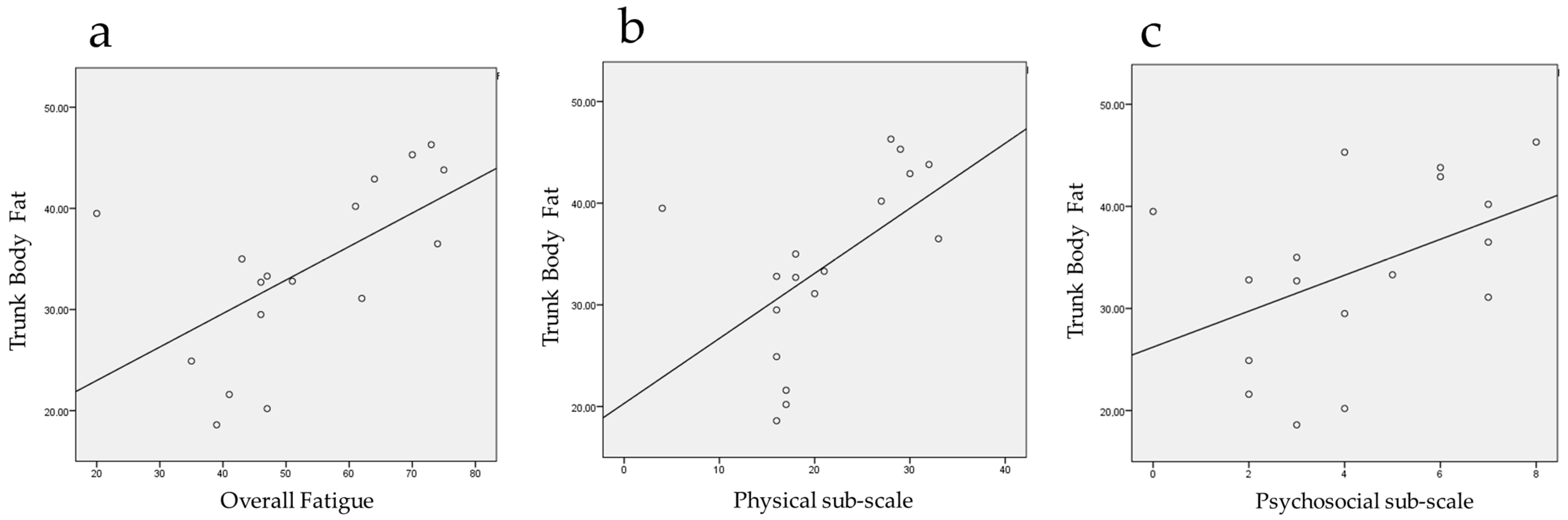
| Overall fatigue | 66.59 ± 9.27 | 52.59 ± 15.62 | −14.00 ± 14.95 | −21.69/ −6.31 | 3.86 | 16 | 0.001 | 0.69 |
| Physical sub-scale | 29.71 ± 4.91 | 21.06 ± 7.64 | −8.65 ± 7.2 | −12.35/ −4.94 | 4.94 | 16 | 0.000 | 0.78 |
| Cognitive sub-scale | 30.88 ± 4.57 | 27.24 ± 7.1 | −3.65 ± 7.85 | −7.68/ 0.39 | 1.91 | 16 | 0.073 | - |
| Psychosocial sub-scale | 6.00 ± 1.73 | 4.29 ± 2.26 | −1.70 ± 1.76 | −2.61/ −0.80 | 4.00 | 16 | 0.001 | 0.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miana, M.; Moreta-Fuentes, R.; Jiménez-Antona, C.; Moreta-Fuentes, C.; Laguarta-Val, S. Improvement of Fatigue and Body Composition in Women with Long COVID After Non-Aerobic Therapeutic Exercise Program. J. Pers. Med. 2025, 15, 217. https://doi.org/10.3390/jpm15060217
Miana M, Moreta-Fuentes R, Jiménez-Antona C, Moreta-Fuentes C, Laguarta-Val S. Improvement of Fatigue and Body Composition in Women with Long COVID After Non-Aerobic Therapeutic Exercise Program. Journal of Personalized Medicine. 2025; 15(6):217. https://doi.org/10.3390/jpm15060217
Chicago/Turabian StyleMiana, María, Ricardo Moreta-Fuentes, Carmen Jiménez-Antona, César Moreta-Fuentes, and Sofía Laguarta-Val. 2025. "Improvement of Fatigue and Body Composition in Women with Long COVID After Non-Aerobic Therapeutic Exercise Program" Journal of Personalized Medicine 15, no. 6: 217. https://doi.org/10.3390/jpm15060217
APA StyleMiana, M., Moreta-Fuentes, R., Jiménez-Antona, C., Moreta-Fuentes, C., & Laguarta-Val, S. (2025). Improvement of Fatigue and Body Composition in Women with Long COVID After Non-Aerobic Therapeutic Exercise Program. Journal of Personalized Medicine, 15(6), 217. https://doi.org/10.3390/jpm15060217







