PRP Therapy for Stress Urinary Incontinence and Pelvic Organ Prolapse: A New Frontier in Personalized Treatment?
Abstract
1. Introduction
2. Materials and Methods
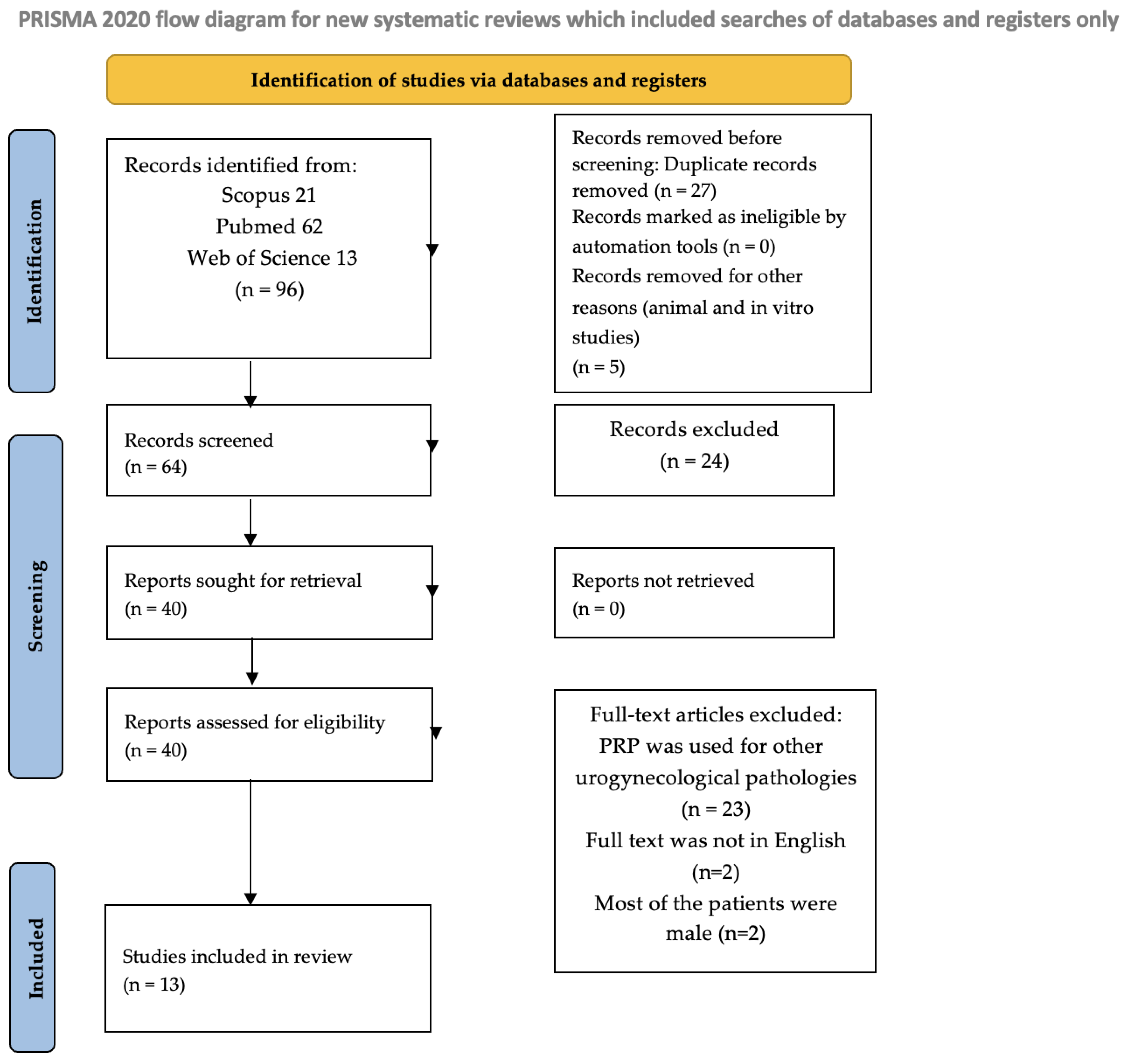
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Acquisition and Risk of Bias
2.4. Data Synthesis and Statistical Analysis
3. Results
3.1. Pelvic Organ Prolapse
3.1.1. Demographics and Participant Characteristics
3.1.2. Scores and Results
3.2. Stress Urinary Incontinence
3.2.1. Demographics and Participants Characteristics
3.2.2. Scores and Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| POP | Pelvic Organ Prolapse |
| SUI | Stress Urinary Incontinence |
| PRP | Platelet-rich plasma |
| RCT | Randomized Controlled Trial |
| FDA | Food and Drug Administration |
| ICS | International Continence Society |
| UI | Urinary Incontinence |
| PFMT | Pelvic Floor Muscle Training |
| BMI | Body Mass Index |
| POP-Q | Pelvic Organ Prolapse Quantification |
| APG | Autologous Platelet Gel |
| PGI-I | Patient’s Global Impression of Improvement |
| VAS | Visual Analogue Scale |
| P-QoL | Prolapse Quality of Life |
| ICIQ-FLUTS | Incontinence Questionnaire—Female Lower Urinary Tract Symptoms |
| KHQ | King’s Health Questionnaire |
| CST | Cough Stress Test |
| FSFI | Female Sexual Function Index |
| QUID | Questionnaire for Urinary Incontinence Diagnosis |
| I-QoL | Incontinence Quality of Life |
| PFMT | Pelvic Floor Muscle Training |
| UDI-6 | Urinary Distress Inventory |
| GRA | Global Response Assessment |
| IIQ-7 | Incontinence Impact Questionnaire |
| VUDS | Videourodynamic Study |
| ALPP | Abdominal Leak Point Pressure |
| APFQ | Australian Pelvic Floor Questionnaire |
| ICIQ-SF | Incontinence Questionnaire-Short Form |
| OABSS | Overactive Bladder Symptom Scores |
| POPDI-6 | Pelvic Organ Prolapse Distress Inventory 6 |
| FSFI | Female Sexual Function Index |
References
- Haylen, B.T.; Maher, C.F.; Barber, M.D.; Camargo, S.; Dandolu, V.; Digesu, A.; Goldman, H.B.; Huser, M.; Milani, A.L.; Moran, P.A.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) Joint Report on the Terminology for Female Pelvic Organ Prolapse (POP). Int. Urogynecol. J. 2016, 27, 165–194. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, A.Y.; Glinter, H.; Marcus-Braun, N. Narrative Review of the Epidemiology, Diagnosis and Pathophysiology of Pelvic Organ Prolapse. Int. Braz. J. Urol. 2020, 46, 5–14. [Google Scholar] [CrossRef]
- Nüssler, E.; Granåsen, G.; Bixo, M.; Löfgren, M. Long-Term Outcome after Routine Surgery for Pelvic Organ Prolapse—A National Register-Based Cohort Study. Int. Urogynecol. J. 2022, 33, 1863–1873. [Google Scholar] [CrossRef]
- Salvatore, S.; Siesto, G.; Serati, M. Risk Factors for Recurrence of Genital Prolapse. Curr. Opin. Obstet. Gynecol. 2010, 22, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Slack, M. Management of Prolapse of the Anterior Compartment. BJOG 2004, 111, 67–72. [Google Scholar] [CrossRef][Green Version]
- Taylor, D.W.; Petrera, M.; Hendry, M.; Theodoropoulos, J.S. A Systematic Review of the Use of Platelet-Rich Plasma in Sports Medicine as a New Treatment for Tendon and Ligament Injuries. Clin. J. Sport. Med. 2011, 21, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Chow, P.-M.; Chuang, Y.-C.; Hsu, K.C.P.; Shen, Y.-C.; Liu, S.-P. Impact of Female Stress Urinary Incontinence on Quality of Life, Mental Health, Work Limitation, and Healthcare Seeking in China, Taiwan, and South Korea (LUTS Asia): Results from a Cross-Sectional, Population-Based Study. Int. J. Womens Health 2022, 14, 1871–1880. [Google Scholar] [CrossRef]
- Vitale, S.G.; La Rosa, V.L.; Rapisarda, A.M.C.; Laganà, A.S. Sexual Life in Women with Stress Urinary Incontinence. Oman Med. J. 2017, 32, 174–175. [Google Scholar] [CrossRef]
- Haylen, B.T.; de Ridder, D.; Freeman, R.M.; Swift, S.E.; Berghmans, B.; Lee, J.; Monga, A.; Petri, E.; Rizk, D.E.; Sand, P.K.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) Joint Report on the Terminology for Female Pelvic Floor Dysfunction. Neurourol. Urodyn. 2010, 29, 4–20. [Google Scholar] [CrossRef]
- Ostrzenski, A. The New Etiology and Surgical Therapy of Stress Urinary Incontinence in Women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 245, 26–34. [Google Scholar] [CrossRef]
- Kowalik, C.G.; Dmochowski, R.R.; De, E.J.B. Surgery for Female SUI: The ICI Algorithm. Neurourol. Urodyn. 2019, 38, S21–S27. [Google Scholar] [CrossRef]
- Capobianco, G.; Madonia, M.; Morelli, S.; Dessole, F.; De Vita, D.; Cherchi, P.L.; Dessole, S. Management of Female Stress Urinary Incontinence: A Care Pathway and Update. Maturitas 2018, 109, 32–38. [Google Scholar] [CrossRef]
- Kobashi, K.C.; Vasavada, S.; Bloschichak, A.; Hermanson, L.; Kaczmarek, J.; Kim, S.K.; Kirkby, E.; Malik, R. Updates to Surgical Treatment of Female Stress Urinary Incontinence (SUI): AUA/SUFU Guideline (2023). J. Urol. 2023, 209, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, A.K.; Arlandis, S.; Bø, K.; Cobussen-Boekhorst, H.; Costantini, E.; de Heide, M.; Farag, F.; Groen, J.; Karavitakis, M.; Lapitan, M.C.; et al. European Association of Urology Guidelines on the Diagnosis and Management of Female Non-Neurogenic Lower Urinary Tract Symptoms. Part 1: Diagnostics, Overactive Bladder, Stress Urinary Incontinence, and Mixed Urinary Incontinence. Eur. Urol. 2022, 82, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Kohler, N.; Lipton, A. Platelets as a Source of Fibroblast Growth-Promoting Activity. Exp. Cell Res. 1974, 87, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Cheng, L.; Cui, X.; Lei, X.; Tang, J.; Cheng, B. Application of Standardized Platelet-rich Plasma in Elderly Patients with Complex Wounds. Wound Repair. Regen. 2019, 27, 268–276. [Google Scholar] [CrossRef]
- Eppley, B.L.; Pietrzak, W.S.; Blanton, M. Platelet-Rich Plasma: A Review of Biology and Applications in Plastic Surgery. Plast. Reconstr. Surg. 2006, 118, 147e–159e. [Google Scholar] [CrossRef]
- Streit-Ciećkiewicz, D.; Kołodyńska, A.; Futyma-Gąbka, K.; Grzybowska, M.; Gołacki, J.; Futyma, K. Platelet Rich Plasma in Gynecology—Discovering Undiscovered—Review. Int. J. Environ. Res. Public Health 2022, 19, 5284. [Google Scholar] [CrossRef]
- Mehta, S.; Watson, J.T. Platelet Rich Concentrate: Basic Science and Current Clinical Applications. J. Orthop. Trauma. 2008, 22, 432–438. [Google Scholar] [CrossRef]
- Rutkowski, J.L.; Thomas, J.M.; Bering, C.L.; Speicher, J.L.; Radio, N.M.; Smith, D.M.; Johnson, D.A. Analysis of a Rapid, Simple, and Inexpensive Technique Used to Obtain Platelet-Rich Plasma for Use in Clinical Practice. J. Oral Implant. 2008, 34, 25–33. [Google Scholar] [CrossRef]
- Sampson, S.; Gerhardt, M.; Mandelbaum, B. Platelet Rich Plasma Injection Grafts for Musculoskeletal Injuries: A Review. Curr. Rev. Musculoskelet. Med. 2008, 1, 165–174. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-H.; Lee, P.-J.; Kuo, H.-C. Therapeutic Efficacy of Urethral Sphincter Injections of Platelet-Rich Plasma for the Treatment of Stress Urinary Incontinence Due to Intrinsic Sphincter Deficiency: A Proof-of-Concept Clinical Trial. Int. Neurourol. J. 2021, 25, 51–58. [Google Scholar] [CrossRef]
- Matz, E.L.; Pearlman, A.M.; Terlecki, R.P. Safety and Feasibility of Platelet Rich Fibrin Matrix Injections for Treatment of Common Urologic Conditions. Investig. Clin. Urol. 2018, 59, 61. [Google Scholar] [CrossRef] [PubMed]
- Medel, S.; Alarab, M.; Kufaishi, H.; Drutz, H.; Shynlova, O. Attachment of Primary Vaginal Fibroblasts to Absorbable and Nonabsorbable Implant Materials Coated With Platelet-Rich Plasma. Female Pelvic Med. Reconstr. Surg. 2015, 21, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Chrysanthopoulou, E.L.; Pergialiotis, V.; Perrea, D.; Κourkoulis, S.; Verikokos, C.; Doumouchtsis, S.K. Platelet Rich Plasma as a Minimally Invasive Approach to Uterine Prolapse. Med. Hypotheses 2017, 104, 97–100. [Google Scholar] [CrossRef]
- Nikolopoulos, K.I.; Chrysanthopoulou, E.; Pergialiotis, V.; Korrou, L.M.; Perrea, D.N.; Dimitroulis, D.; Doumouchtsis, S.K. An Animal Experimental Study on Pubourethral Ligament Restoration with Platelet Rich Plasma for the Treatment of Stress Urinary Incontinence. Cent. Eur. J. Urol. 2019, 72, 134–141. [Google Scholar] [CrossRef]
- Gerullis, H.; Georgas, E.; Eimer, C.; Arndt, C.; Barski, D.; Lammers, B.; Klosterhalfen, B.; Borós, M.; Otto, T. Coating with Autologous Plasma Improves Biocompatibility of Mesh Grafts In Vitro: Development Stage of a Surgical Innovation. Biomed. Res. Int. 2013, 2013, 536814. [Google Scholar] [CrossRef][Green Version]
- Atılgan, A.E.; Aydın, A. Cystocele Repair with Platelet-Rich Plasma. Indian J. Surg. 2021, 83, 726–730. [Google Scholar] [CrossRef]
- Einarsson, J.I.; Jonsdottir, K.; Mandle, R. Use of Autologous Platelet Gel in Female Pelvic Organ Prolapse Surgery: A Feasibility Study. J. Minim. Invasive Gynecol. 2009, 16, 204–207. [Google Scholar] [CrossRef]
- Gorlero, F.; Glorio, M.; Lorenzi, P.; Bruno-Franco, M.; Mazzei, C. New Approach in Vaginal Prolapse Repair: Mini-Invasive Surgery Associated with Application of Platelet-Rich Fibrin. Int. Urogynecol. J. 2012, 23, 715–722. [Google Scholar] [CrossRef]
- Grigoriadis, T.; Kalantzis, C.; Zacharakis, D.; Kathopoulis, N.; Prodromidou, A.; Xadzilia, S.; Athanasiou, S. Platelet-Rich Plasma for the Treatment of Stress Urinary Incontinence—A Randomized Trial. Urogynecology 2023, 30, 42–49. [Google Scholar] [CrossRef]
- Athanasiou, S.; Kalantzis, C.; Zacharakis, D.; Kathopoulis, N.; Pontikaki, A.; Grigoriadis, T. The Use of Platelet-Rich Plasma as a Novel Nonsurgical Treatment of the Female Stress Urinary Incontinence: A Prospective Pilot Study. Female Pelvic Med. Reconstr. Surg. 2021, 27, e668–e672. [Google Scholar] [CrossRef]
- Saraluck, A.; Chinthakanan, O.; Kijmanawat, A.; Aimjirakul, K.; Wattanayingcharoenchai, R.; Manonai, J. Autologous Platelet Rich Plasma (A-PRP) Combined with Pelvic Floor Muscle Training for the Treatment of Female Stress Urinary Incontinence (SUI): A Randomized Control Clinical Trial. Neurourol. Urodyn. 2024, 43, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Long, C.-Y.; Lin, K.-L.; Shen, C.-R.; Ker, C.-R.; Liu, Y.-Y.; Loo, Z.-X.; Hsiao, H.-H.; Lee, Y.-C. A Pilot Study: Effectiveness of Local Injection of Autologous Platelet-Rich Plasma in Treating Women with Stress Urinary Incontinence. Sci. Rep. 2021, 11, 1584. [Google Scholar] [CrossRef] [PubMed]
- Ural, Ü.M. The Effect of Injectable Platelet Rich Fibrin as a Nonsurgical Treatment of the Female Stress Urinary Incontinence. Arch. Gynecol. Obstet. 2024, 309, 2229–2236. [Google Scholar] [CrossRef]
- Ashton, L.; Nakatsuka, H.; Johnson, C.M.; Kenne, K.; Kreder, K.J.; Kruse, R.; Wendt, L.; Takacs, E.B.; Vollstedt, A.J. A Single Injection of Platelet-Rich Plasma Injection for the Treatment of Stress Urinary Incontinence in Females: A Randomized Placebo-Controlled Trial. Urology 2024, 193, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Samy Tahoon, A.; El-Din Hussein Salem, H.; Anwar Abdo Mousa, A. The Role of Platelet Rich Plasma Injections in Cases of Stress Incontinence. 2022. Available online: https://www.qeios.com/read/KG77ZQ.2 (accessed on 10 May 2022).
- Behnia-Willison, F.; Nguyen, T.T.T.; Norbury, A.J.; Mohamadi, B.; Salvatore, S.; Lam, A. Promising Impact of Platelet Rich Plasma and Carbon Dioxide Laser for Stress Urinary Incontinence. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2020, 5, 100099. [Google Scholar] [CrossRef]
- Daneshpajooh, A.; Mirzaei, M.; Farsinejad, A.; Naghibzadeh-Tahami, A.; Eslami, A. The Effect of Periurethral Injection of Pure Platelet-Rich Plasma in the Treatment of Urinary Incontinence in Female Patients: A Randomized Clinical Trial. J. Kerman Univ. Med. Sci. 2021, 28, 330–337. [Google Scholar] [CrossRef]
- Chiang, C.-H.; Kuo, H.-C. The Efficacy and Mid-Term Durability of Urethral Sphincter Injections of Platelet-Rich Plasma in Treatment of Female Stress Urinary Incontinence. Front. Pharmacol. 2022, 13, 847520. [Google Scholar] [CrossRef]
- Söderberg, M.W.; Falconer, C.; Byström, B.; Malmström, A.; Ekman, G. Young Women with Genital Prolapse Have a Low Collagen Concentration. Acta Obstet. Gynecol. Scand. 2004, 83, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.R.; Eckford, S.D.; Abrams, P.; Avery, N.C.; Tarlton, J.F.; Bailey, A.J. Changes in Metabolism of Collagen in Genitourinary Prolapse. Lancet 1996, 347, 1658–1661. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.L.; Izeta, A.; Herrera-Imbroda, B.; Amend, B.; Brinchmann, J.E. Cell Therapy for Stress Urinary Incontinence. Tissue Eng. Part. B Rev. 2015, 21, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Poulios, E.; Mykoniatis, I.; Pyrgidis, N.; Kalyvianakis, D.; Hatzichristou, D. Platelet-Rich Plasma for the Treatment of Erectile Dysfunction: A Systematic Review of Preclinical and Clinical Studies. Sex. Med. Rev. 2023, 11, 359–368. [Google Scholar] [CrossRef]
- Sukgen, G.; Ellibeş Kaya, A.; Karagün, E.; Çalışkan, E. Platelet-Rich Plasma Administration to the Lower Anterior Vaginal Wall to Improve Female Sexuality Satisfaction. J. Turk. Soc. Obstet. Gynecol. 2020, 16, 228–234. [Google Scholar] [CrossRef]
| Author | Study Type and Patients Number | Protocol | Scores | Results | Follow-Up from the Baseline (First Injection) and Adverse Events |
|---|---|---|---|---|---|
| Einarsson J.I. et al. [30] | Prospective cohort study n = 9 | A 6 mm punch biopsy was obtained from the anterior vaginal wall, approximately 4 cm posterior to the urethra and 2 cm lateral to the midline, at a single vaginal site. The cystocele repair followed standard surgical principles. The APG was applied after plication of the pubocervical fascia and before closure of the vaginal epithelium. Three months postoperatively, the patients returned for a follow-up examination and a second 6 mm punch biopsy. | POP-Q before surgery, 3 months after surgery, and at a mean of 20 months after surgery. Objective recurrence of anterior compartment prolapse was defined as point Aa at a position of −1 or greater. Subjective recurrence was defined as patient satisfaction less than 3 on a 5-point VAS. Collagen content was measured and compared. | VAS: Patient satisfaction mean at 3 months was 4.1 and at 20 months was 4.3. In patients who completed the follow-up (77.8%), at 20 months, the subjective failure rate was 12.5% and the objective failure rate was 66.7%. The reoperation rate was 12.5% for a recurrent and symptomatic stage II cystocele and enterocele. POP-Q showed statistical significance at 3 months at points Aa and Ba, but at 20 months follow-up, only a statistically significant difference at point Aa as compared with baseline was found. No significant differences existed between collagen content at 3 months (p = 0.63). The samples submitted during surgery were significantly heavier than samples taken during the follow-up (p = 0.004). | 3 and at a mean of 20 months |
| Gorlero F. et al. [31] | Prospective observational study n = 10 | Anterior repair was performed for transverse and midline defects plus application of PRF. Posterior repair was performed plus PRF after any posterior defects were identified. Perineorrhaphy was included when necessary. Apical repair was carried out using PRF in combination with reattachment of the vaginal cuff and the muscularis layer of the anterior vaginal wall to the uterosacral ligaments. Enterocele repair was performed concurrently with PRF application. In patients with occult stress urinary incontinence, plication of the endopelvic fascia at the mid-urethral level was performed in conjunction with PRF. | Italian version of the P-QoL (Version 4) Questionnaire, Scar quality and wound healing were assessed with the Vancouver Scar Scale. Prolapse repair was considered to be anatomically successful if the patient was asymptomatic and classified as stage 0 according to the POP-Q system. | POP-Q: The overall efficacy rate was 80% for stage 0 and 20% for stage I. P-QoL: when vaginal wall descent was repaired, the patients who had sexual activity increased by 20%, and no women had dyspareunia after surgery. Urinary and bowel symptoms had improved by 100% at 24 months. At follow-up, all patients exhibited normal scar formation (Vancouver Scar Scale). No keloid formation was observed. No patients reported pruritus or any type of pain in the surgical areas. There were no cases of wound infection. | 1, 6, 12, 18, and 24 months |
| Atilgan et al. [29] | Randomized Control Clinical Trial—single blind n = 28 patients treated with PRP injection into the pubocervical fascia and colporraphy—cases n = 28 with colporraphy alone—control group | Anterior repair was performed and 4 mL PRP was injected into the pubocervical fascia. | POP-Q system to detect anatomic recurrence, PFDI questionnaire for assessment of symptomatic recurrence and PGI-I scale for assessment of subjective success. Anatomical success was considered when point Aa or Ba was less than −1 according to POP-Q system. Subjective success rate was assessed with PGI-I scale. The main outcome was low recurrence rate according POP-Q. | The Aa and Ba points’ means were significant lower in cases than in controls at 48 months follow-up (p = 0.001 and p = 0.002, respectively). Symptomatic (PFDI) and anatomic recurrence (POP-Q > 1) and reoperation rate were significantly lower in cases than in controls (p = 0.008, p = 0.001 and p = 0.001, respectively). Only 3.8% of the patients in the cases group had a symptomatic anatomic cystocele recurrence and was reoperated on. Subjective success (PGI-I) was significantly higher in cases, with a rate of 89% (p = 0.012). | 1, 6 months and then annually for 48 months No adverse events were observed. |
| Author | Study Type and Patients (n) | Protocol | Scores | Results | Follow-Up from the Baseline (First Injection) and Adverse Events |
|---|---|---|---|---|---|
| Grigoriadis T. et al. [32] | Randomized Control Clinical Trial—Double blind n = 25 patients treated with PRP—cases n = 25 with sham (sodium chloride 0.9%)—control group | 2 injection sessions—at baseline and 4–6 weeks later. The injections were performed peri-urethrally at the 4-, 6-, and 8-o’clock positions in 3 different levels of the urethra 1–2 cm apart (distal, mid, and proximal). | At the baseline, 3 and 6 months follow-up: 1 h pad test, ICIQ-FLUTS, and KHQ questionnaire. At 3 and 6 months: PGI-I Primary outcome was the subjective evaluation of reported SUI symptoms, indicated by question 11a of the ICIQ-FLUTS questionnaire. The level of discomfort during the PRP injections: 10 cm VAS score. | 11a question of the ICIQ-FLUTS, incontinence and total ICIQ-FLUTS mean score, PGI-I and 1 h pad test were significantly improved at 3 months and 6 months in cases and not in the controls (p < 0.001). At 6 months follow-up, the subjective cure rate was significantly higher in the PRP group (32%) compared to the sham group (4%) (p < 0.01). No patient achieved objective cure during the 6-month follow-up, as defined by urine leakage of less than 1 g during the 1 h pad test. Filling and voiding symptom scores (ICIQ-FLUTS) did not show significant changes over the follow-up period in either treatment group. No statistically significant differences were observed between the groups in any domain of the King’s Health Questionnaire (KHQ). | 3 and 6 months No adverse effects were noted after completion of the study |
| Ashton L. et al. [37] | Randomized Control Clinical Trial—single blind n = 25 patients treated with PRP—cases n = 25 with sham (sodium chloride 0.9%)—control group | Injections were administered into the anterior vaginal mucosa surrounding the mid-urethral region, approximately 1 cm inferior to the urethral meatus, at a depth of approximately 1.5 cm. A total of 2 mL was injected beneath the mid-urethral area, with an additional 1.5 mL administered on each side of the urethra. | FSFI, the I-QOL survey, and the QUID survey. VAS for pain/discomfort for the patients and VAS for injector difficulty for the injector. Primary outcomes were as follows: Subjective improvement on the PGI-I. A negative CST at a bladder volume of 300 mL Secondary outcomes: improvements in the FSFI scores, I-QOL scores, QUID, VAS for pain, VAS for injector difficulty. | There was no statistically significant difference in the primary outcomes (PGI-I improvement and CST). FSFI, I-QOL, and QUID showed no differences between the 2 groups at any of the follow-up Intervals. The average VAS for patients and injector for placebo and treatment groups were similar. | 1, 3, and 6 months The most common adverse events were vaginal spotting and vaginal discomfort. There was no difference between the placebo and treatment groups |
| Saraluck A. et al. [34] | Randomized Phase II Clinical Trial—single blind—blinded investigator n = 31 cases treated with PRP + PFMT n = 29 controls treated with PFMT alone | 2 injection sessions—at baseline and 4 weeks later. Into the anterior vaginal mucosa surrounding the mid-urethral region, approximately 1 cm inferior to the urethral meatus, at a depth of approximately 1.5 cm. A total of 2 mL was injected beneath the mid-urethral area, with an additional 1.5 mL administered on each side of the urethra. All participants were instructed to perform pelvic floor muscle training (PFMT) three times daily—morning, noon, and evening—completing 8 to 12 contractions and relaxations per session, each lasting 4 to 6 s. | Primary outcome: objective 1 h pad test at the baseline and at 5 months follow-up. Secondary subjective outcomes: I-QoL questionnaire score, ICIQ-FLUTS question 11a and 11b, PGI-I, and the percentage subjective improvement score. | 1 h pad test after 5 months was statistically significant (p < 0.05). I-QoL scores and 11a and 11b in the ICIQ FLUTS were statistically significantly better in the cases group (p < 0.001) and the same was reported for PGI-I scores (p < 0.05). Self-reported percentage of subjective improvement indicated that 90% of patients in the cases group experienced an improvement in SUI of at least 50%. | 5 months No adverse effects were noted after completion of the study. |
| Athanasiou et al. [33] | Prospective observational pilot study n = 20 | 2 injection sessions—at baseline (T0) and 4–6 weeks later. The injections were performed into the lower one third of the anterior vaginal wall at 10, 12, and 2 o’clock in 3 different levels of the urethra 1–2 cm apart (distal, mid, and proximal) | At baseline: urodynamic studies, a ICS-standardized 1 h pad test, and ICIQ-FLUTS and KHQ questionnaire. Primary outcome: question 11a of the ICIQ-FLUTS questionnaire. At follow-up visits: 1 h pad test, ICIQ-FLUTS and KHQ, and PGI-I Scale of Improvement. The level of discomfort during the PRP injections as recorded by the 10-cm VAS score. | The 11a question score, filling, incontinence, and total mean score of the ICIQ-FLUTS questionnaire decreased significantly at 3 and 6 months follow-up (p < 0.001). Subjective cure rate (11a questione ICIQ-FLUTS) was 10%, whereas 80% of the patients subjectively improved. The voiding symptoms score of the ICIQ-FLUTS did not change significantly throughout the follow-up period. The PGI-I showed a significant improvement in 80% of the patients throughout the follow-up period. A mean reduction of 50.2% in urine loss was observed in the 1 h pad test at the 6 months follow-up and 10% of participants were considered objectively cured (defined as urine leakage of less than 1 g during the 1 h pad test). No statistical significance was observed for KHQ domain, with the exception of incontinence impact domain. | 6 months No adverse effects were noted after completion of the study |
| Chiang et al. [41] | Prospective observational study n = 26 | Every 1 month for a total of 4 treatments within 3 months. One ml of PRP was injected in the urethral sphincter at 5 sites around the urethral meatus (2, 5, 7, 10, and 12 o’ clock positions). | Primary treatment outcomes: GRA score (categorized from −3 to +3). GRA score of ≥2 was considered a success. Secondary endpoints: Subjective parameters: the dry rate after PRP treatments, changes in SUI VAS, UDI-6 and IIQ-7 from baseline to 3 months after the fourth PRP treatment. SUI VAS followed up at 12 months after the fourth PRP treatment. Objective urodynamic parameters (VUDS and ALPP) at 3 months after the fourth PRP injection (6 months from the baseline). | The GRA mean after treatment was 1.5. After 4 PRP 80.8% reported a positive response in alleviating SUI, including 50% that achieved a GRA ≥ 2. Dry rate, 46.2% at 3 months and 26.9% at 15 months achieved complete continence and pad-free. 53.8% remained mild SUI with acceptable outcome. VAS of SUI was significantly better immediately after the first PRP injection and consolidated by the following repeated injections. The therapeutic effect persisted throughout the 1-year period (p < 0.001). At 3 months follow-up, UDI-6 and IIQ-7 significantly decreased (p < 0.001). VUDS parameters showed no significant difference between baseline and after PRP treatment. Only ALPP was significantly increased (p = 0.045). | 1, 2, 3, 4, 6, 9, 12, and 15 months Adverse events: only 3.8% reported straining of urination after PRP injection, that resolved after intermittent self-catheterization for several days. |
| Behnia-Willson et al. [39] | Prospective observational pilot study n = 62 at the baseline and at 3 months follow-up. 37 patients at 1224 months follow-up. | Patients received three treatments, 4 to 6 weeks apart (within 2–3 months) with a 90° vaginal laser-emitting probe inserted up to the level of the bladder neck, rotated and withdrawn, exposing the anterior lower one third of the vagina to the laser. Participants were also subjected to total vaginal length laser treatments with a 360° probe. Participants also received the same amount of PRP immediately after each vaginal laser treatment. PRP was injected into the anterior lower one third of the vagina and peri-urethral area. | APFQ Questionnaire: at baseline, 3 months after the third treatment (5–6 months from the baseline), and at a 12–24 months after treatment. Primary treatment outcome: question 6 of the APFQ, which indicates Stress Incontinence. The secondary treatment outcomes: relevant items of the APFQ. | Question 6 APFQ: At the baseline, 0% reported either occasional or no stress urinary symptoms and 100% reported frequently or daily symptoms. At 3 months, 66.2% reported occasional or no symptoms. At 12–24 months, 62.1% of the patients report either occasional or no symptoms. The improvement was statistically significant (p < 0.001) All the symptoms (APFQ) improved significantly (p < 0.001) within the baseline and at 3 months follow-up and then from 3 to 12–24 months, except for pad usage, which did not improve from 3 to 12–24 follow-up (p = 0.073). | 5/6 and 12/24 months No adverse effects were noted after completion of the study. |
| Long CY et al. [35] | Prospective interventional pilot study n = 20 | Injections were administered into the anterior vaginal mucosa surrounding the mid-urethral region, approximately 1 cm inferior to the urethral meatus, at a depth of approximately 1.5 cm. A total of 2 mL was injected beneath the mid-urethral area, with an additional 1.5 mL administered on each side of the urethra. | Primary outcome: ICIQ-SF, UDI-6, IIQ-7, OABSS, and POPDI-6. Only 40% of the women completed the urodynmaic studies before and 6 months after intervention. Secondary outcome: sexual function investigated before and after the treatment by FSFI questionnaire. | ICIQ-SF, UDI-6, and IIQ-7 showed significant incontinence improvement at both 1 month and 6 months post-treatment. OABSS had an improvement only at 1 month follow-up. POPDI-6 did not show statistically significant improvement. ICIQ-SF particularly demonstrated an efficacy of 60% in symptoms improvement. In the urodynamic studies, residual urine and bladder volume at first sensation to void increased significantly. All other urodynamic parameters showed no significant differences following treatment. No significant changes were reported in the FSFI questionnaire. | 1 and 6 months No adverse effects were noted after completion of the study. |
| Ural et al. [36] | Prospective interventional study n = 34 | i-PRF was injected 3 times, separated by 1 month, into the anterior vaginal mucosa under the mid-urethral, 1–2 cm below the urethra meatus. Half of the dose was administered under the mid-urethra, and the remaining quarter was administered into the right and left sides of the mid-urethra. | ICIQ-SF, UDI-6, IIQ-7, and POPDI-6 questionnaires. Primary outcome: ICIQ-SF. If a woman felt no symptoms of SUI after surgery, she was considered cured. Secondary outcomes included UDI-6, IIQ-7, and POPDI-6. The results of the study were not evaluated with clinical parameters such as urodynamics and pad test. | According to ICIQ-SF, the rate of clinical improvement was 79.4% at 1 month and 64.7% at 6 months after treatment. ICIQ-SF, UDI-6, IIQ-7, and POPDI-6 scores at 1 and 6 months post-treatment were significantly lower than pre-treatment values (p < 0.001). No significant changes were observed in the scores between 1 and 6 months following treatment. | 1 and 6 months No adverse effects were noted after completion of the study. |
| Daneshpajooh et al. [40] | Prospective randomized controlled clinical trial. n = 10 patients received PRP—case group n = 10 patients received mid-urethral sling procedure—control group. | 3 mL of PRP was injected via cystourethroscopy at four sites along the mid-urethra using endoscopic needles. Three patients in the treatment group consented to receive a subsequent dose. The second injection was administered one month after the initial injection, and in one case, a third injection was performed one month after the second. | ICIQ, I-QOL, UDI-6, and cough stress test. | In PRP group cough stress test at 1 month, results negative for 70% of the patients. In the control group, the results were negative for 80%. The results were not significantly different between 1 month and 3 months follow-up. ICIQ, I-QoL, and UDI-6 results were significantly different before and after treatments in both groups. However, the response to the mid-urethral sling procedure was better and the difference was statistically significant. | 1 and 3 months |
| Tahoon A.S. et al. [38] | Prospective observational study n = 26 | One injection was administered into the anterior vaginal wall, near the external urethral sphincter, at three sites on each side. An additional injection was performed in the paraurethral area, with a depth of 10 mm from lateral to the external urethral orifice. | Urodynamics study changes before and three months after treatment. 1 h pad test before and after PRP injection. ICIQ-SF, UDI-6 IIQ-7, OBSS, and FSFI after 1 and 3 months. | 50% of the women completed the urodynamics studies before and 3 months after intervention. Residual urine and bladder volume at first sensation to void increased significantly. All other urodynamics parameters showed no significant differences. After the PRP treatment, the average 1 h pad test was 2.55 g (−2.8 g compared to initial value). ICIQ-SF, UDI-6 IIQ-7, OBSS, and FSFI showed significant incontinence improvement at both 1 and 3 months. According to ICIQ-SF, 30% were cured, 25% reported mild symptoms, 40% reported moderate and 5% severe diseases. Improvement occurred in 54% patients and no change in 46%. No significant changes before and after the treatment were reported by FSFI | 1 and 3 months No adverse effects were noted after completion of the study. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitsillidi, A.; Vona, L.; Bettocchi, S.; Schiermeier, S.; Noé, G.K. PRP Therapy for Stress Urinary Incontinence and Pelvic Organ Prolapse: A New Frontier in Personalized Treatment? J. Pers. Med. 2025, 15, 214. https://doi.org/10.3390/jpm15060214
Pitsillidi A, Vona L, Bettocchi S, Schiermeier S, Noé GK. PRP Therapy for Stress Urinary Incontinence and Pelvic Organ Prolapse: A New Frontier in Personalized Treatment? Journal of Personalized Medicine. 2025; 15(6):214. https://doi.org/10.3390/jpm15060214
Chicago/Turabian StylePitsillidi, Anna, Laura Vona, Stefano Bettocchi, Sven Schiermeier, and Günter Karl Noé. 2025. "PRP Therapy for Stress Urinary Incontinence and Pelvic Organ Prolapse: A New Frontier in Personalized Treatment?" Journal of Personalized Medicine 15, no. 6: 214. https://doi.org/10.3390/jpm15060214
APA StylePitsillidi, A., Vona, L., Bettocchi, S., Schiermeier, S., & Noé, G. K. (2025). PRP Therapy for Stress Urinary Incontinence and Pelvic Organ Prolapse: A New Frontier in Personalized Treatment? Journal of Personalized Medicine, 15(6), 214. https://doi.org/10.3390/jpm15060214








