Associations of PPARG and PPARGC1A Polymorphisms with Ritodrine-Induced Adverse Events in Patients with Preterm Labor
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Drug Administration
2.3. Data Collection and Outcomes
2.4. Genotyping
2.5. Statistical Analyses
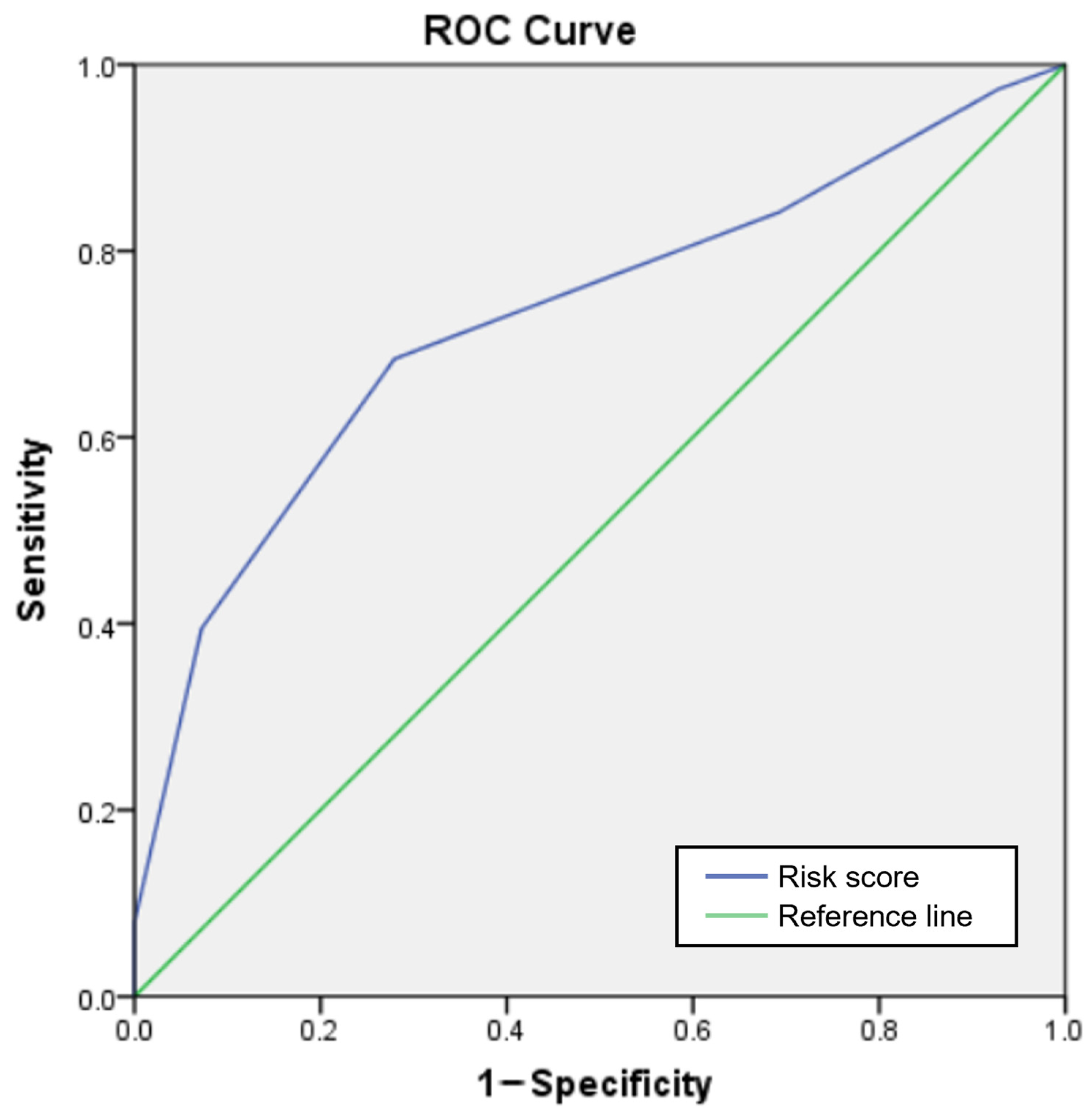
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rundell, K.; Panchal, B. Preterm labor: Prevention and management. Am. Fam. Physician 2017, 95, 366–372. [Google Scholar] [PubMed]
- Petrou, S.; Yiu, H.H.; Kwon, J. Economic consequences of preterm birth: A systematic review of the recent literature (2009–2017). Arch. Dis. Child. 2019, 104, 456–465. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Preterm Birth: Fact Sheet. 2020. Available online: https://www.who.int/news/item/06-10-2023-1-in-10-babies-worldwide-are-born-early--with-major-impacts-on-health-and-survival (accessed on 1 February 2025).
- Yamaji, N.; Suzuki, H.; Saito, K.; Swa, T.; Namba, F.; Vogel, J.P.; Ramson, J.A.; Cao, J.; Tina, L.; Ota, E. Tocolytic therapy inhibiting preterm birth in high-risk populations: A systematic review and meta-analysis. Children 2023, 10, 443. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, X. Use of Tocolytic Agents in Preterm Labor: A Cross-Sectional Analysis from a Chinese Real-World Study from 2016 to 2021. J. Clin. Pharm. Ther. 2024, 2024, 3206060. [Google Scholar] [CrossRef]
- Neilson, J.P.; West, H.M.; Dowswell, T. Betamimetics for inhibiting preterm labour. Cochrane Database Syst Rev. 2014, 2014, CD004352. [Google Scholar] [CrossRef]
- Lam, F.; Gill, P. Beta-agonist tocolytic therapy. Obstet. Gynecol. Clin. N. Am. 2005, 32, 457–484. [Google Scholar] [CrossRef]
- Driul, L.; Londero, A.P.; Adorati-Menegato, A.; Vogrig, E.; Bertozzi, S.; Fachechi, G.; Forzano, L.; Cacciaguerra, G.; Perin, E.; Miceli, A.; et al. Therapy side-effects and predictive factors for preterm delivery in patients undergoing tocolysis with atosiban or ritodrine for threatened preterm labour. J. Obstet. Gynaecol. 2014, 34, 684–689. [Google Scholar] [CrossRef]
- Lin, C.W.; Chan, K.A.; Chen, Y.Y.; Huang, W.I.; Chao, P.H.; Liang, H.Y.; Chen, W.; Hsiao, F. Risks of maternal cardiopulmonary events associated with ritodrine for tocolysis: A national database linkage study in 1 831 564 pregnant women. Int. J. Gynecol. Obstet. 2024, 167, 295–305. [Google Scholar] [CrossRef]
- Mori, C.; Yano, R.; Sakai, T.; Sakakibara, J.; Tanabe, K. Exploring risk factors that contribute to the onset of ritodrine-associated serious adverse drug reactions. J. Pharmacovigil 2016, 4, 224. [Google Scholar] [CrossRef]
- Chung, J.E.; Choi, S.A.; Hwang, H.S.; Park, J.Y.; Lee, K.E.; Yee, J.; Kim, Y.J.; Gwak, H.S. Association between ß2-adrenergic receptor gene polymorphisms and adverse events of ritodrine in the treatment of preterm labor: A prospective observational study. BMC Genet. 2017, 18, 96. [Google Scholar] [CrossRef]
- Jang, E.J.; Kim, Y.J.; Hwang, H.S.; Yee, J.; Gwak, H.S. Associations of GNAS and RGS gene polymorphisms with the risk of ritodrine-induced adverse events in Korean women with preterm labor: A cohort study. Pharmaceutics 2022, 14, 1220. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Quiles, M.; Broekema, M.F.; Kalkhoven, E. PPARgamma in metabolism, immunity, and cancer: Unified and diverse mechanisms of action. Front. Endocrinol. 2021, 12, 624112. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Ruíz, M.E.; Plata-Corona, J.C.; Soria-Castro, E.; Díaz-Juárez, J.A.; Sánchez-Aguilar, M. Pleiotropic Effects of Peroxisome Proliferator-Activated Receptor Alpha and Gamma Agonists on Myocardial Damage: Molecular Mechanisms and Clinical Evidence—A Narrative Review. Cells 2024, 13, 1488. [Google Scholar] [CrossRef] [PubMed]
- Naumenko, N.; Mutikainen, M.; Holappa, L.; Ruas, J.L.; Tuomainen, T.; Tavi, P. PGC-1α deficiency reveals sex-specific links between cardiac energy metabolism and EC-coupling during development of heart failure in mice. Cardiovasc. Res. 2022, 118, 1520–1534. [Google Scholar] [CrossRef]
- Zhu, X.; Shen, W.; Yao, K.; Wang, H.; Liu, B.; Li, T.; Song, L.; Diao, D.; Mao, G.; Huang, P.; et al. Fine-tuning of PGC1α expression regulates cardiac function and longevity. Circ. Res. 2019, 125, 707–719. [Google Scholar] [CrossRef]
- Chandra, M.; Miriyala, S.; Panchatcharam, M. PPARγ and its role in cardiovascular diseases. PPAR Res. 2017, 2017, 6404638. [Google Scholar] [CrossRef]
- Varillas-Delgado, D. Role of the PPARGC1A Gene and Its rs8192678 Polymorphism on Sport Performance, Aerobic Capacity, Muscle Adaptation and Metabolic Diseases: A Narrative Review. Genes 2024, 15, 1631. [Google Scholar] [CrossRef]
- Regieli, J.J.; Jukema, J.W.; Doevendans, P.A.; Zwinderman, A.H.; van der Graaf, Y.; Kastelein, J.J.; Grobbee, D.E. PPAR gamma variant influences angiographic outcome and 10-year cardiovascular risk in male symptomatic coronary artery disease patients. Diabetes Care 2009, 32, 839–844. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Xu, W.; Li, X.; Tang, Y.; Xie, P.; Ji, Y.; Fan, L.; Chen, Q. Association between PPARGC1A gene polymorphisms and coronary artery disease in a Chinese population. Clin. Exp. Pharmacol. Physiol. 2008, 35, 1172–1177. [Google Scholar] [CrossRef]
- Andersen, G.; Wegner, L.; Jensen, D.P.; Glümer, C.; Tarnow, L.; Drivsholm, T.; Poulsen, P.; Hansen, S.K.; Nielsen, E.-M.D.; Ek, J.; et al. PGC-1alpha Gly482Ser polymorphism associates with hypertension among Danish whites. Hypertension 2005, 45, 565–570. [Google Scholar] [CrossRef]
- Jang, E.J.; Lee, D.H.; Im, S.-S.; Yee, J.; Gwak, H.S. Correlation between PPARG Pro12Ala polymorphism and therapeutic responses to thiazolidinediones in patients with type 2 diabetes: A meta-analysis. Pharmaceutics 2023, 15, 1778. [Google Scholar] [CrossRef] [PubMed]
- Stage, T.B.; Christensen, M.M.; Feddersen, S.; Beck-Nielsen, H.; Brøsen, K. The role of genetic variants in CYP2C8, LPIN1, PPARGC1A and PPARγ on the trough steady-state plasma concentrations of rosiglitazone and on glycosylated haemoglobin A1c in type 2 diabetes. Pharmacogenet. Genom. 2013, 23, 219–227. [Google Scholar] [CrossRef]
- Franks, P.W.; Christophi, C.A.; Jablonski, K.A.; Billings, L.K.; Delahanty, L.M.; Horton, E.S.; Knowler, W.C.; Florez, J.C. Common variation at PPARGC1A/B and change in body composition and metabolic traits following preventive interventions: The Diabetes Prevention Program. Diabetologia 2014, 57, 485–490. [Google Scholar] [CrossRef]
- Chen, S.; Tsybouleva, N.; Ballantyne, C.M.; Gotto, A.M., Jr.; Marian, A. Effects of PPARα, γ and δ haplotypes on plasma levels of lipids, severity and progression of coronary atherosclerosis and response to statin therapy in the lipoprotein coronary atherosclerosis study. Pharmacogenetics Genom. 2004, 14, 61–71. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, J.W.; Yee, J.; Kim, S.J.; Chung, J.E.; Gwak, H.S. Interactive associations between PPARγ and PPARGC1A and bisphosphonate-related osteonecrosis of the jaw in patients with osteoporosis. Pharmaceuticals 2023, 16, 1035. [Google Scholar] [CrossRef]
- Bishop, E.H. Pelvic scoring for elective induction. Obstet. Gynecol. 1964, 24, 266–268. [Google Scholar]
- Sayers, E.W.; Bolton, E.E.; Brister, J.R.; Canese, K.; Chan, J.; Comeau, D.C.; Connor, R.; Funk, K.; Kelly, C.; Kim, S.; et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2022, 50, D20–D26. [Google Scholar] [CrossRef]
- Ward, L.D.; Kellis, M. HaploReg v4: Systematic mining of putative causal variants, cell types, regulators and target genes for human complex traits and disease. Nucleic Acids Res. 2016, 44, D877–D881. [Google Scholar] [CrossRef]
- De Silva, T.M.; Li, Y.; Kinzenbaw, D.A.; Sigmund, C.D.; Faraci, F.M. Endothelial PPARγ (Peroxisome Proliferator-Activated Receptor-γ) is essential for preventing endothelial dysfunction with aging. Hypertension 2018, 72, 227–234. [Google Scholar] [CrossRef]
- Duan, S.Z.; Ivashchenko, C.Y.; Russell, M.W.; Milstone, D.S.; Mortensen, R.M. Cardiomyocyte-specific knockout and agonist of peroxisome proliferator-activated receptor-gamma both induce cardiac hypertrophy in mice. Circ Res. 2005, 97, 372–379. [Google Scholar] [CrossRef]
- Son, N.H.; Park, T.S.; Yamashita, H.; Yokoyama, M.; Huggins, L.A.; Okajima, K.; Homma, S.; Szabolcs, M.J.; Huang, L.-S.; Goldberg, I.J. Cardiomyocyte expression of PPARgamma leads to cardiac dysfunction in mice. J. Clin. Investig. 2007, 117, 2791–2801. [Google Scholar] [CrossRef] [PubMed]
- Carithers, L.J.; Ardlie, K.; Barcus, M.; Branton, P.A.; Britton, A.; Buia, S.A.; Compton, C.C.; DeLuca, D.S.; Peter-Demchok, J.; Gelfand, E.T.; et al. A novel approach to high-quality postmortem tissue procurement: The GTEx Project. Biopreserv. Biobank. 2015, 13, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Redondo, V.; Pettersson, A.T.; Ruas, J.L. The hitchhiker’s guide to PGC-1α isoform structure and biological functions. Diabetologia 2015, 58, 1969–1977. [Google Scholar] [CrossRef] [PubMed]
- Valle, I.; Alvarez-Barrientos, A.; Arza, E.; Lamas, S.; Monsalve, M. PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc. Res. 2005, 66, 562–573. [Google Scholar] [CrossRef]
- Rius-Pérez, S.; Torres-Cuevas, I.; Millán, I.; Ortega, Á.L.; Pérez, S. PGC-1α, inflammation, and oxidative stress: An integrative view in metabolism. Oxid. Med. Cell. Longev. 2020, 2020, 1452696. [Google Scholar] [CrossRef]
- Sharma, R.; Matharoo, K.; Kapoor, R.; Bhanwer, A.J.S. Association of PGC-1α gene with type 2 diabetes in three unrelated endogamous groups of North-West India (Punjab): A case-control and meta-analysis study. Mol. Genet. Genom. 2018, 293, 317–329. [Google Scholar] [CrossRef]
- McIlleron, H.; Rustomjee, R.; Vahedi, M.; Mthiyane, T.; Denti, P.; Connolly, C.; Rida, W.; Pym, A.; Smith, P.J.; Onyebujoh, P.C. Reduced antituberculosis drug concentrations in HIV-infected patients who are men or have low weight: Implications for international dosing guidelines. Antimicrob. Agents Chemother. 2012, 56, 3232–3238. [Google Scholar] [CrossRef]

| Characteristic | Total | Adverse Event | No Adverse Event | p-Value |
|---|---|---|---|---|
| (n = 149) | (n = 38) | (n = 111) | ||
| Age (years) | 0.555 | |||
| <35 | 126 (84.6%) | 31 (81.6%) | 95 (85.6%) | |
| ≥35 | 23 (15.4%) | 7 (18.4%) | 16 (14.4%) | |
| Gestational age (weeks) | 0.470 | |||
| <30 | 67(45.0%) | 19 (50.0%) | 48 (43.2%) | |
| ≥30 | 82 (55.0%) | 19 (50.0%) | 63 (56.8%) | |
| Weight (kg) | 0.008 | |||
| <60 | 59 (39.6%) | 22 (57.9%) | 37 (33.3%) | |
| ≥60 | 90 (60.4%) | 16 (42.1%) | 74 (66.7%) | |
| Body mass index (kg/m2) | 0.515 | |||
| <23 | 60 (40.3%) | 17 (44.7%) | 43 (38.7%) | |
| ≥23 | 89 (59.7%) | 21 (55.3%) | 68 (61.3%) | |
| Maximum infusion rate (mL/h) | 60.0 ± 33.6 | 68.7 ± 38.4 | 57.1 ± 31.3 | 0.067 |
| Modified Bishop score a | 0.431 | |||
| <2 | 78 (52.3%) | 22 (73.3%) | 57 (65.5%) | |
| ≥2 | 69 (47.7%) | 8 (26.7%) | 30 (34.5%) |
| Gene Polymorphism a | Grouped Genotypes | Adverse Event | No Adverse Event | p-Value |
|---|---|---|---|---|
| (n = 38) | (n = 111) | |||
| PPARG | ||||
| rs6808179 (T > G) | TT | 6 (15.8%) | 38 (34.2%) | 0.031 |
| TG, GG | 32 (84.2%) | 73 (65.8%) | ||
| PPARGC1A | ||||
| rs2946385 (G > T) | GG, GT | 27 (71.1%) | 95 (88.0%) | 0.016 |
| TT | 11 (28.9%) | 13 (12.0%) | ||
| rs35523565 (C > T) | CC | 9 (24.3%) | 50 (45.0%) | 0.026 |
| CT, TT | 28 (75.7%) | 61 (55.0%) | ||
| rs2240748 (G > A) | GG | 13 (35.1%) | 64 (57.7%) | 0.018 |
| GA, AA | 24 (64.9%) | 47 (42.3%) | ||
| rs10002521 (C > T) | CC, CT | 17 (44.7%) | 71 (64.0%) | 0.037 |
| TT | 21 (55.3%) | 40 (36.0%) |
| Predictors | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | p-Value | Score |
|---|---|---|---|---|
| Age (years) ≥ 35 | 1.34 (0.51–3.56) | |||
| Gestational age (weeks) < 30 | 2.44 (0.62–9.62) | |||
| Weight (kg) < 60 | 2.75 (1.29–5.85) | 2.99 (1.26–7.04) | 0.013 | 1 |
| Maximum infusion rate (mL/h) | 1.01 (1.00–1.02) | |||
| PPARG | ||||
| rs6808179 TG/GG | 2.78 (1.07–7.22) | |||
| PPARGC1A | ||||
| rs2946385 TT | 2.98 (1.20–7.39) | 4.07 (1.43–11.58) | 0.008 | 1 |
| rs35523565 CT/TT | 2.55 (1.10–5.90) | 4.68 (1.76–12.44) | 0.002 | 2 |
| rs2240748 GA/AA | 2.51 (1.16–5.45) | 3.27 (1.36–7.87) | 0.008 | 1 |
| rs10002521 TT | 2.19 (1.04–4.63) |
| Score | Patients with Adverse Events (n) | Total Patients (n) | Observed Adverse Event Risk (%) | Predicted Adverse Event Risk (%) |
|---|---|---|---|---|
| 0 | 1 | 9 | 11.1 | 4.1 |
| 1 | 5 | 31 | 16.1 | 9.1 |
| 2 | 6 | 45 | 13.3 | 18.7 |
| 3 | 11 | 34 | 32.3 | 34.8 |
| 4 | 12 | 20 | 60.0 | 55.2 |
| 5 | 3 | 3 | 100.0 | 74.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, E.J.; Lee, D.H.; Song, Y.; Kim, J.S.; Kim, Y.J.; Yee, J.; Gwak, H.S. Associations of PPARG and PPARGC1A Polymorphisms with Ritodrine-Induced Adverse Events in Patients with Preterm Labor. J. Pers. Med. 2025, 15, 212. https://doi.org/10.3390/jpm15050212
Jang EJ, Lee DH, Song Y, Kim JS, Kim YJ, Yee J, Gwak HS. Associations of PPARG and PPARGC1A Polymorphisms with Ritodrine-Induced Adverse Events in Patients with Preterm Labor. Journal of Personalized Medicine. 2025; 15(5):212. https://doi.org/10.3390/jpm15050212
Chicago/Turabian StyleJang, Eun Jeong, Da Hoon Lee, Yubin Song, Jung Sun Kim, Young Ju Kim, Jeong Yee, and Hye Sun Gwak. 2025. "Associations of PPARG and PPARGC1A Polymorphisms with Ritodrine-Induced Adverse Events in Patients with Preterm Labor" Journal of Personalized Medicine 15, no. 5: 212. https://doi.org/10.3390/jpm15050212
APA StyleJang, E. J., Lee, D. H., Song, Y., Kim, J. S., Kim, Y. J., Yee, J., & Gwak, H. S. (2025). Associations of PPARG and PPARGC1A Polymorphisms with Ritodrine-Induced Adverse Events in Patients with Preterm Labor. Journal of Personalized Medicine, 15(5), 212. https://doi.org/10.3390/jpm15050212






