Data-Driven Defragmentation: Achieving Value-Based Sarcoma and Rare Cancer Care Through Integrated Care Pathway Mapping
Abstract
1. Introduction
2. Theoretical and Practical Background
2.1. Fragmentation as a Systemic Challenge
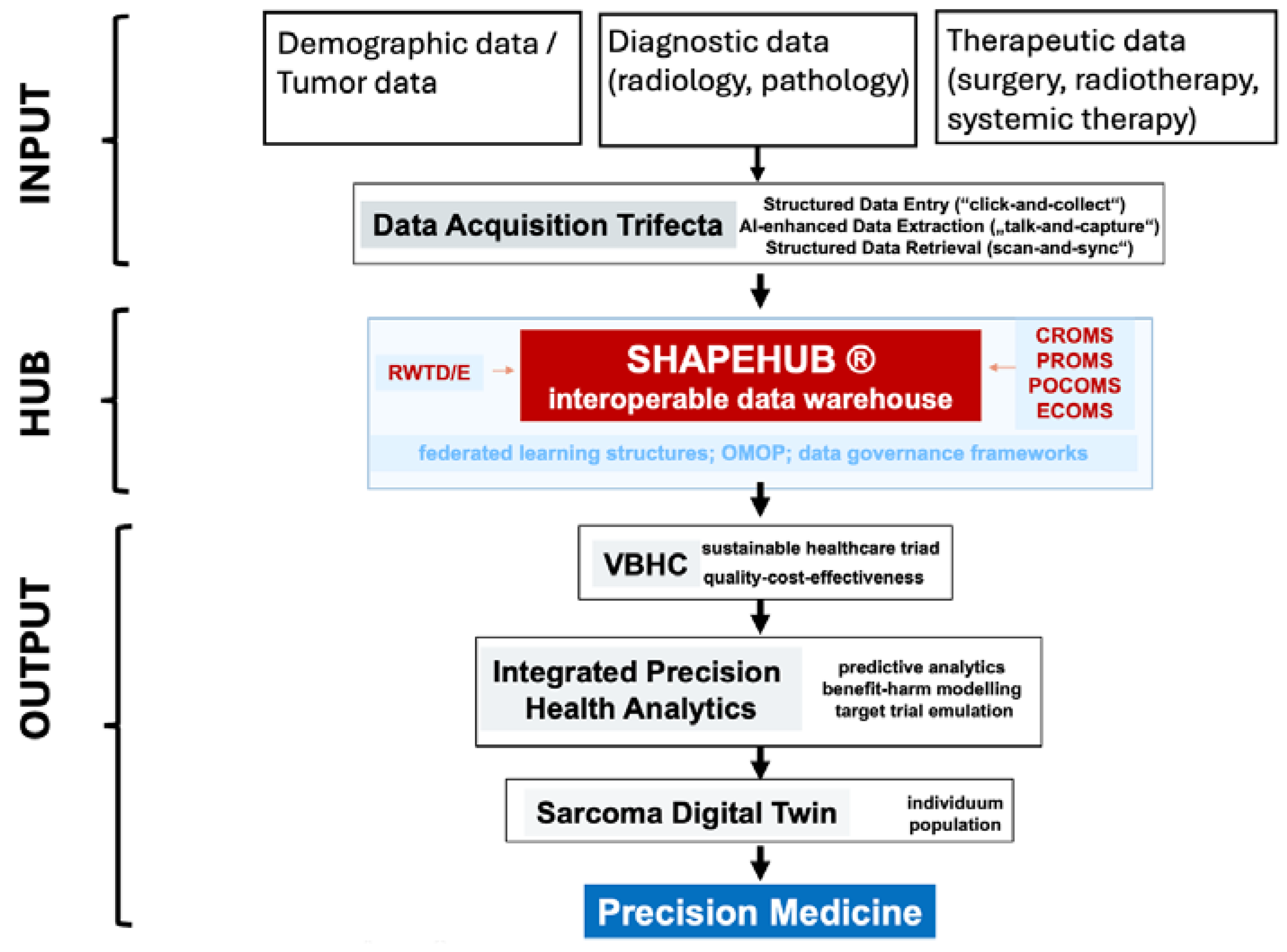
2.2. Digital Interoperable Platforms as Enablers
2.3. Related Work: Positioning ShapeHub Among Existing Digital Care Pathways
2.3.1. Integrated Practice Units and Value-Based Healthcare
2.3.2. Electronic Health Record (EHR) Enhancements and Learning Health Systems
2.3.3. National Interoperability Initiatives and Health Information Exchanges (HIEs)
2.3.4. Rare Disease Networks and European Reference Networks (ERNs)
2.3.5. AI-Driven Clinical Decision Support Systems
3. Case Study: ShapeHub’s Implementation in the Swiss Sarcoma Network (SSN)
3.1. Example 1: Refining Diagnostic Pathways
3.2. Example 2: Reducing Unplanned “Whoops” Surgeries
3.3. Example 3: Optimizing Radiotherapy and Surgical Protocols
4. Addressing Fragmentation: Care Pathway Mapping as a Solution to the Data Vortex
4.1. Care Pathway Mapping
4.2. Implementing Solutions to the Data Vortex
- AI and Blockchain Technologies: AI algorithms will structure data into actionable formats, while blockchain technology is planned to secure and authenticate each data entry, ensuring that information integrity is maintained across all points of care. Patient identification will be managed through systems like World ID [5], functioning as a trackable identification system that securely links patient data across institutions, ensuring that information is accessible to authorized healthcare providers and allowing for efficient tracking throughout the care pathway.
5. Transdisciplinary Collaboration: The Key to Overcoming the Data Vortex
5.1. Unified Protocols and Shared Data
5.2. Impact on Clinical Decision-Making
6. Economic Impact and Cost Efficiency
6.1. Comprehensive Cost Mapping
6.2. Benchmarking and Efficiency Gains
7. Future Vision: Precision Medicine and Digital Twins
7.1. The Role of Digital Twins
7.2. Scalability Beyond Sarcoma
8. Implications
8.1. Health System Integration and Learning Health Systems
8.2. Policy and Reimbursement Models Aligned with VBHC
8.3. Clinical Standardization Across Decentralized Networks
8.4. Enhancing Patient Empowerment and Safety
8.5. Scalable Infrastructure for Digital Twin and Precision Medicine
9. Conclusions: Call to Action
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Radaelli, S.; Merlini, A.; Khan, M.; Gronchi, A. Progress in Histology Specific Treatments in Soft Tissue Sarcoma. Expert Rev. Anticancer Ther. 2024, 24, 845–868. [Google Scholar] [CrossRef]
- Blay, J.-Y.; Penel, N.; Gouin, F.; Le Cesne, A.; Toulmonde, M. Improving at a Nationwide Level the Management of Patients with Sarcomas with an Expert Network. Ann. Oncol. 2022, 33, 659–661. [Google Scholar] [CrossRef]
- Elyes, M.; Heesen, P.; Schelling, G.; Bode-Lesniewska, B.; Studer, G.; Fuchs, B. Swiss Sarcoma Network Enhancing Healthcare for Sarcoma Patients: Lessons from a Diagnostic Pathway Efficiency Analysis. Cancers 2023, 15, 4892. [Google Scholar] [CrossRef]
- Borghi, A.; Gronchi, A. Extremity and Truncal Soft Tissue Sarcoma: Risk Assessment and Multidisciplinary Management. Semin. Radiat. Oncol. 2024, 34, 147–163. [Google Scholar] [CrossRef]
- World Whitepaper. Available online: https://whitepaper.world.org/ (accessed on 15 November 2024).
- Fanconi, C.; van Buchem, M.; Hernandez-Boussard, T. Natural Language Processing Methods to Identify Oncology Patients at High Risk for Acute Care with Clinical Notes. AMIA Summits Transl. Sci. Proc. 2023, 2023, 138–147. [Google Scholar]
- Vorisek, C.N.; Lehne, M.; Klopfenstein, S.A.I.; Mayer, P.J.; Bartschke, A.; Haese, T.; Thun, S. Fast Healthcare Interoperability Resources (FHIR) for Interoperability in Health Research: Systematic Review. JMIR Med. Inform. 2022, 10, e35724. [Google Scholar] [CrossRef]
- Guerrazzi, C.; Feldman, S.S. Health Information Exchange: What Matters at the Organizational Level? J. Biomed. Inform. 2020, 102, 103375. [Google Scholar] [CrossRef]
- Payne, T.H.; Lovis, C.; Gutteridge, C.; Pagliari, C.; Natarajan, S.; Yong, C.; Zhao, L.-P. Status of Health Information Exchange: A Comparison of Six Countries. J. Glob. Health 2019, 9, 0204279. [Google Scholar] [CrossRef]
- Rajkomar, A.; Dean, J.; Kohane, I. Machine Learning in Medicine. N. Engl. J. Med. 2019, 380, 1347–1358. [Google Scholar] [CrossRef]
- Topol, E.J. High-Performance Medicine: The Convergence of Human and Artificial Intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef]
- Rieke, N.; Hancox, J.; Li, W.; Milletarì, F.; Roth, H.R.; Albarqouni, S.; Bakas, S.; Galtier, M.N.; Landman, B.A.; Maier-Hein, K.; et al. The Future of Digital Health with Federated Learning. NPJ Digit. Med. 2020, 3, 119. [Google Scholar] [CrossRef] [PubMed]
- Corrigan-Curay, J.; Sacks, L.; Woodcock, J. Real-World Evidence and Real-World Data for Evaluating Drug Safety and Effectiveness. JAMA 2018, 320, 867–868. [Google Scholar] [CrossRef] [PubMed]
- Hernán, M.A.; Robins, J.M. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. Am. J. Epidemiol. 2016, 183, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, B.; Borrebaeck, C.; Elander, N.; Gasslander, T.; Gawel, D.R.; Gustafsson, M.; Jörnsten, R.; Lee, E.J.; Li, X.; Lilja, S.; et al. Digital Twins to Personalize Medicine. Genome Med. 2019, 12, 4. [Google Scholar] [CrossRef]
- Harvard University. Harvard Business Review; Harvard University: Cambridge, MA, USA, 2013. [Google Scholar]
- Ma, X.; Long, L.; Moon, S.; Adamson, B.J.S.; Baxi, S.S. Comparison of Population Characteristics in Real-World Clinical Oncology Databases in the US: Flatiron Health, SEER, and NPCR. bioRxiv 2020. 2020.03.16.20037143. [Google Scholar]
- Page d’accueil. Available online: https://www.health-data-hub.fr (accessed on 7 May 2025).
- Homepage van Stichting. Available online: https://www.medmij.nl/ (accessed on 7 May 2025).
- European Reference Networks. Available online: https://health.ec.europa.eu/european-reference-networks/overview_en (accessed on 7 May 2025).
- Esteva, A.; Robicquet, A.; Ramsundar, B.; Kuleshov, V.; DePristo, M.; Chou, K.; Cui, C.; Corrado, G.; Thrun, S.; Dean, J. A Guide to Deep Learning in Healthcare. Nat. Med. 2019, 25, 24–29. [Google Scholar] [CrossRef]
- Obergfell, T.T.A.F.; Nydegger, K.N.; Heesen, P.; Schelling, G.; Bode-Lesniewska, B.; Studer, G.; Fuchs, B. SwissSarcomaNetwork Improving Sarcoma Outcomes: Target Trial Emulation to Compare the Impact of Unplanned and Planned Resections on the Outcome. Cancers 2024, 16, 2443. [Google Scholar] [CrossRef]
- Mattmann, A.; Glanzmann, C.; Fuchs, B.; Bode, B.; Studer, G. Swiss Sarcoma Network Preoperative Ultrahypofractionated Radiation Therapy for Soft Tissue Sarcomas: Low Rate of Wound Complications. Adv. Radiat. Oncol. 2024, 9, 101562. [Google Scholar] [CrossRef]
- Parsai, S.; Lawrenz, J.; Kilpatrick, S.; Rubin, B.; Hymes, C.; Gray, M.; Mesko, N.; Shah, C.; Nystrom, L.; Scott, J.G. Early Outcomes of Preoperative 5-Fraction Radiation Therapy for Soft Tissue Sarcoma Followed by Immediate Surgical Resection. Adv. Radiat. Oncol. 2020, 5, 1274–1279. [Google Scholar]
- Porter, M.E.; Lee, T.H. From Volume to Value in Health Care: The Work Begins. JAMA 2016, 316, 1047–1048. [Google Scholar] [CrossRef]
- Sager, S. Digital Twins in Oncology. J. Cancer Res. Clin. Oncol. 2023, 149, 5475–5477. [Google Scholar] [CrossRef]
- Ellis, L.A.; Sarkies, M.; Churruca, K.; Dammery, G.; Meulenbroeks, I.; Smith, C.L.; Pomare, C.; Mahmoud, Z.; Zurynski, Y.; Braithwaite, J. The Science of Learning Health Systems: Scoping Review of Empirical Research. JMIR Med. Inform. 2022, 10, e34907. [Google Scholar] [CrossRef] [PubMed]
- Global Patient Safety Action Plan 2021–2030. Available online: https://www.who.int/publications/i/item/9789240032705 (accessed on 7 May 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuchs, B.; Heesen, P. Data-Driven Defragmentation: Achieving Value-Based Sarcoma and Rare Cancer Care Through Integrated Care Pathway Mapping. J. Pers. Med. 2025, 15, 203. https://doi.org/10.3390/jpm15050203
Fuchs B, Heesen P. Data-Driven Defragmentation: Achieving Value-Based Sarcoma and Rare Cancer Care Through Integrated Care Pathway Mapping. Journal of Personalized Medicine. 2025; 15(5):203. https://doi.org/10.3390/jpm15050203
Chicago/Turabian StyleFuchs, Bruno, and Philip Heesen. 2025. "Data-Driven Defragmentation: Achieving Value-Based Sarcoma and Rare Cancer Care Through Integrated Care Pathway Mapping" Journal of Personalized Medicine 15, no. 5: 203. https://doi.org/10.3390/jpm15050203
APA StyleFuchs, B., & Heesen, P. (2025). Data-Driven Defragmentation: Achieving Value-Based Sarcoma and Rare Cancer Care Through Integrated Care Pathway Mapping. Journal of Personalized Medicine, 15(5), 203. https://doi.org/10.3390/jpm15050203






