One-Stop Mitral Valve Transcatheter Edge-to-Edge Repair and Left Atrial Appendage Occlusion in Patients with Atrial Fibrillation and Mitral Regurgitation: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
- •
- Adult patients (≥18 years old) with concomitant indication for M-TEER (moderate-to-severe MR) and LAAO (high bleeding risk, contraindication for use of OAC, recurrent thromboembolism under treatment with OAC).
- •
- Randomized controlled trials (RCTs), observational cohort studies, and case series with a minimum of four patients reporting outcomes on the combined M-TEER/LAAO procedure with or without a control group (M-TEER only).
- •
- Records that provided data from the same registry (to assure independence of observations).
- •
- Narrative reviews, commentaries, expert opinions, case reports, case-control, and cross-sectional studies.
- •
- Clinical practice guidelines, conference abstracts, protocols, and dissertations.
- •
- Full text not retrievable (authors were contacted, and if a full text was not provided, the record was excluded).
2.3. Endpoints
- •
- Procedure-related outcomes (device success, procedural time, radiation time, volume of administered contrast, number of implanted mitral clips, residual LAAO device leak).
- •
- Short-term clinical outcomes (post-procedure MR > 2+, length of hospitalization, in-hospital death, acute kidney injury, bleeding, hematoma).
- •
- Long-term clinical outcomes (all-cause death, CV mortality, HF hospitalization, stroke, NYHA class reduction, device thrombosis, hematoma).
- •
- Anticoagulation and/or antithrombotic regimen on discharge.
2.4. Study Selection
2.5. Data Extraction
2.6. Quality Assessment
2.7. Data Analysis
3. Results
3.1. Study Selection and Characteristics of Included Studies
3.2. Single M-TEER/LAAO Intervention Outcomes
3.3. Comparative Intervention Outcomes
3.4. Subgroup Analysis
4. Discussion
4.1. Summary of Results in the Context of Existing Evidence
4.2. Implications for Clinical Practice and Future Research
4.3. Strengths and Limitations
4.4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Demir, O.M.; Bolland, M.; Curio, J.; Søndergaard, L.; Rodés-Cabau, J.; Redwood, S.; Prendergast, B.; Colombo, A.; Chau, M.; Latib, A. Transcatheter Mitral Valve Replacement: Current Evidence and Concepts. Interv. Cardiol. Rev. 2021, 16, e07. [Google Scholar] [CrossRef] [PubMed]
- Maisano, F.; Franzen, O.; Baldus, S.; Schäfer, U.; Hausleiter, J.; Butter, C.; Ussia, G.P.; Sievert, H.; Richardt, G.; Widder, J.D.; et al. Percutaneous mitral valve interventions in the real world: Early and 1-year results from the ACCESS-EU, a prospective, multicenter, nonrandomized post-approval study of the MitraClip therapy in Europe. J. Am. Coll. Cardiol. 2013, 62, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Tamburino, C.; Ussia, G.P.; Maisano, F.; Capodanno, D.; La Canna, G.; Scandura, S.; Colombo, A.; Giacomini, A.; Michev, I.; Mangiafico, S.; et al. Percutaneous mitral valve repair with the MitraClip system: Acute results from a real world setting. Eur. Heart J. 2010, 31, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Grigioni, F.; Avierinos, J.-F.; Ling, L.H.; Scott, C.G.; Bailey, K.R.; Tajik, A.; Frye, R.L.; Enriquez-Sarano, M. Atrial fibrillation complicating the course of degenerative mitral regurgitation: Determinants and long-term outcome. J. Am. Coll. Cardiol. 2002, 40, 84–92. [Google Scholar] [CrossRef]
- Bin Wang, B.; Xu, Z.-Y.; Han, L.; Zhang, G.-X.; Lu, F.-L.; Song, Z.-G. Impact of preoperative atrial fibrillation on mortality and cardiovascular outcomes of mechanical mitral valve replacement for rheumatic mitral valve disease. Eur. J. Cardio Thoracic Surg. 2012, 43, 513–519. [Google Scholar] [CrossRef]
- Ngaage, D.L.; Schaff, H.V.; Mullany, C.J.; Barnes, S.; Dearani, J.A.; Daly, R.C.; Orszulak, T.A.; Sundt, T.M., III. Influence of preoperative atrial fibrillation on late results of mitral repair: Is concomitant ablation justified? Ann. Thorac. Surg. 2007, 84, 434–443. [Google Scholar] [CrossRef]
- Megaly, M.; Abraham, B.; Saad, M.; Omer, M.; Elbadawi, A.; Tawadros, M.; Khalil, C.; Nairoz, R.; Almomani, A.; Sengupta, J.; et al. Impact of Atrial Fibrillation on the Outcomes after MitraClip®: A Meta-Analysis. Struct. Heart 2018, 2, 531–537. [Google Scholar] [CrossRef]
- Kaur, S.; Sadana, D.; Patel, J.; Gad, M.; Sankaramangalam, K.; Krishnaswamy, A.; Miyasaka, R.; Harb, S.C.; Kapadia, S.R. Atrial Fibrillation and Transcatheter Repair of Functional Mitral Regurgitation: Evidence From a Meta-Regression. Cardiovasc. Interv. 2020, 13, 2374–2384. [Google Scholar] [CrossRef]
- De Stefano, F.; Benassi, A.; Cappelletti, A.M.; Donatelli, F.; Regazzoli, D.; Tolaro, S.; Perego, F.; Silverio, A.; Scatteia, A.; Guarini, P.; et al. Current Use of Oral Anticoagulation Therapy in Elderly Patients with Atrial Fibrillation: Results from an Italian Multicenter Prospective Study—The ISNEP Study. J. Pers. Med. 2022, 12, 1419. [Google Scholar] [CrossRef]
- Osmancik, P.; Herman, D.; Neuzil, P.; Hala, P.; Taborsky, M.; Kala, P.; Poloczek, M.; Stasek, J.; Haman, L.; Branny, M.; et al. Left Atrial Appendage Closure Versus Direct Oral Anticoagulants in High-Risk Patients With Atrial Fibrillation. J. Am. Coll. Cardiol. 2020, 75, 3122–3135. [Google Scholar] [CrossRef]
- Cochrane Handbook for Systematic Reviews of Interventions Version 6.5 (Updated August 2024). Cochrane, 2024. n.d. Available online: https://www.training.cochrane.org/handbook (accessed on 27 March 2025).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 29, 372. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Grainger, M.J.; Gray, C.T. Citationchaser: An R package and Shiny app for forward and backward citations chasing in academic searching. Zenodo 2021, 16. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.J.H.W. Cochrane Handbook for Systematic Reviews of Interventions|Cochrane Training n.d.; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar]
- Frazzetto, M.; Sanfilippo, C.; Costa, G.; Scandura, S.; Castania, G.; De Santis, J.; Sanfilippo, M.; Di Salvo, M.E.; Uccello, S.; Rugiano, G.; et al. Safety and Effectiveness of Concomitant Mitral Transcatheter Edge-to-Edge Repair and Left Atrial Appendage Closure. J. Clin. Med. 2023, 12, 4742. [Google Scholar] [CrossRef] [PubMed]
- Kuwata, S.; Taramasso, M.; Zuber, M.; Suetsch, G.; Attinger-Toller, A.; Wicki, D.; Maisano, F.; Nietlispach, F. Feasibility of concomitant MitraClip and left atrial appendage occlusion. EuroIntervention 2017, 12, 1940–1945. [Google Scholar] [CrossRef]
- D’amico, G.; Estèvez-Loureiro, R.; Rofastes, X.F.; Ronco, F.; Nombela-Franco, L.; Melica, B.; Bedogni, F.; Saia, F.; Cruz-Gonzalez, I.; Tarantini, G. Combined Procedure of Percutaneous Mitral Valve Repair and Left Atrial Appendage Occlusion: A Multicenter Study. JACC Cardiovasc. Interv. 2021, 14, 590–592. [Google Scholar] [CrossRef]
- Fukuda, N.; Imamura, T.; Tanaka, S.; Kataoka, N.; Ushijima, R.; Ueno, H.; Kinugawa, K. Feasibility of combined therapy: Percutaneous left atrial appendage closure and transcatheter edge-to-edge repair. Cardiovasc. Interv. Ther. 2024, 40, 400–413. [Google Scholar] [CrossRef]
- Al-Abcha, A.; Di Santo, P.; Rihal, C.S.; Simard, T.; Hibbert, B.; Alkhouli, M. Outcomes of Combined Left Atrial Appendage Occlusion and Transcatheter Mitral Edge-to-Edge Repair: The WATCH-TEER Study. JACC Adv. 2025, 4, 101541. [Google Scholar] [CrossRef] [PubMed]
- Tichelbäcker, T.; Puls, M.; Jacobshagen, C.; Hasenfuß, G.; Schillinger, W.; Hünlich, M.; Schroeter, M. MitraClip® and Amplatzer® cardiac plug implantation in a single procedure: A reasonable approach? Int. J. Cardiol. 2016, 220, 107–111. [Google Scholar] [CrossRef]
- Francisco, A.R.G.; de Oliveira, E.I.; Menezes, M.N.; Ferreira, P.C.; da Silva, P.C.; Nobre, Â.; Pinto, F.J. Combined MitraClip implantation and left atrial appendage occlusion using the Watchman device: A case series from a referral center. Rev. Port. Cardiol. 2017, 36, 525–532. [Google Scholar] [CrossRef]
- Salghetti, F.; Sieira, J.; Chierchia, G.-B.; Curnis, A.; de Asmundis, C. Recognizing and reacting to complications of trans-septal puncture. Expert Rev. Cardiovasc. Ther. 2017, 15, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Obadia, J.-F.; Messika-Zeitoun, D.; Leurent, G.; Iung, B.; Bonnet, G.; Piriou, N.; Lefèvre, T.; Piot, C.; Rouleau, F.; Carrié, D.; et al. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N. Engl. J. Med. 2018, 379, 2297–2306. [Google Scholar] [CrossRef]
- Stone, G.W.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Grayburn, P.A.; Rinaldi, M.; Kapadia, S.R.; et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N. Engl. J. Med. 2018, 379, 2307–2318. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Friede, T.; von Bardeleben, R.-S.; Butler, J.; Khan, M.-S.; Diek, M.; Heinrich, J.; Geyer, M.; Placzek, M.; Ferrari, R.; et al. Transcatheter Valve Repair in Heart Failure with Moderate to Severe Mitral Regurgitation. N. Engl. J. Med. 2024, 391, 1799–1809. [Google Scholar] [CrossRef]
- Isigi, S.S.; Parsa, A.D.; Alasqah, I.; Mahmud, I.; Kabir, R. Predisposing Factors of Nosocomial Infections in Hospitalized Patients in the United Kingdom: Systematic Review. JMIR Public Health Surveill. 2023, 9, e43743. [Google Scholar] [CrossRef]
- Holmes, D.R.; Reddy, V.Y.; Turi, Z.G.; Doshi, S.K.; Sievert, H.; Buchbinder, M.; Mullin, C.M.; Sick, P. PROTECT AF Investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: A randomised non-inferiority trial. Lancet 2009, 374, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Attizzani, G.F.; Capodanno, D.; Barbanti, M.; Cannata, S.; Dipasqua, F.; Immé, S.; Ministeri, M.; Caggegi, A.; Pistritto, A.M.; et al. Impact of chronic kidney disease on outcomes after percutaneous mitral valve repair with the MitraClip system: Insights from the GRASP registry. EuroIntervention 2016, 11, e1649–e1657. [Google Scholar] [CrossRef]
- Kefer, J.; Tzikas, A.; Freixa, X.; Shakir, S.; Gafoor, S.; Nielsen-Kudsk, J.E.; Berti, S.; Santoro, G.; Aminian, A.; Landmesser, U.; et al. Impact of chronic kidney disease on left atrial appendage occlusion for stroke prevention in patients with atrial fibrillation. Int. J. Cardiol. 2016, 207, 335–340. [Google Scholar] [CrossRef]
- Armijo, G.; Estevez-Loureiro, R.; Carrasco-Chinchilla, F.; Arzamendi, D.; Fernández-Vázquez, F.; Jimenez-Quevedo, P.; Freixa, X.; Pascual, I.; Serrador, A.M.; Mesa, D.; et al. Acute kidney injury after percutaneous Edge-to-Edge mitral repair. J. Am. Coll. Cardiol. 2020, 76, 2463–2473. [Google Scholar] [CrossRef]
- Baturina, O.; Chashkina, M.; Andreev, D.; Mirzaev, K.; Bykova, A.; Suvorov, A.; Yeryshova, D.; Suchkova, S.; Sychev, D.; Syrkin, A. Pharmacokinetic and Pharmacogenetic Predictors of Major Bleeding Events in Patients with an Acute Coronary Syndrome and Atrial Fibrillation Receiving Combined Antithrombotic Therapy. J. Pers. Med. 2023, 13, 1371. [Google Scholar] [CrossRef]
- Kornej, J.; Börschel, C.S.; Benjamin, E.J.; Schnabel, R.B. Epidemiology of Atrial fibrillation in the 21st century. Circ. Res. 2020, 127, 4–20. [Google Scholar] [CrossRef]
- Tsampasian, V.; Militaru, C.; Parasuraman, S.K.; Loudon, B.L.; Lowery, C.; Rudd, A.; Srinivasan, J.; Singh, S.; Dwivedi, G.; Mahadavan, G.; et al. Prevalence of asymptomatic valvular heart disease in the elderly population: A community-based echocardiographic study. Eur. Hear. J. Cardiovasc. Imaging 2024, 25, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Passiatore, M.; Imberti, J.F.; Valenti, A.C.; Leo, G.; Vitolo, M.; Coppi, F.; Sgura, F.A.; Boriani, G. Ventricular and Atrial Remodeling after Transcatheter Edge-to-Edge Repair: A Pilot Study. J. Pers. Med. 2022, 12, 1916. [Google Scholar] [CrossRef]
- Hung, A.; Yang, J.; Wallace, M.; Zwischenberger, B.A.; Vemulapalli, S.; Mentz, R.J.; Thoma, E.; Goates, S.; Lewis, J.; Strong, S.; et al. Patient Risk-Benefit Preferences for Transcatheter Versus Surgical Mitral Valve Repair. J. Am. Hear. Assoc. 2024, 13, e032807. [Google Scholar] [CrossRef] [PubMed]
- Martsevich, S.; Lukina, Y.; Kutishenko, N. Primary Non-adherence to Treatment with New Oral Anticoagulants: The Results of a Prospective Observational Study «ANTEY». Open Cardiovasc. Med. J. 2021, 15, 56–61. [Google Scholar] [CrossRef]
- Wegner, F.K.; Radke, R.M.; Ellermann, C.; Wolfes, J.; Willy, K.; Lange, P.S.; Frommeyer, G.; Baumgartner, H.; Eckardt, L.; Diller, G.-P.; et al. Incidence and Predictors of Left Atrial Appendage Thrombus before Catheter Ablation of Thrombogenic Arrhythmias. J. Pers. Med. 2022, 12, 460. [Google Scholar] [CrossRef]
- Harrington, D.J.; Gorska, R.; Wheeler, R.; Davidson, S.; Murden, S.; Morse, C.; Shearer, M.J.; Mumford, A.D. Pharmacodynamic resistance to warfarin is associated with nucleotide substitutions in VKORC1. J. Thromb. Haemost. 2008, 6, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Kanuri, S.H.; Kreutz, R.P. Pharmacogenomics of Novel Direct Oral Anticoagulants: Newly Identified Genes and Genetic Variants. J. Pers. Med. 2019, 9, 7. [Google Scholar] [CrossRef]
- Ragia, G.; Thomopoulos, T.; Chalikias, G.; Trikas, A.; Tziakas, D.N.; Manolopoulos, V.G. Circulating microRNAs and DNA Methylation as Regulators of Direct Oral Anticoagulant Response in Atrial Fibrillation and Key Elements for the Identification of the Mechanism of Action (miR-CRAFT): Study Design and Patient Enrolment. J. Pers. Med. 2024, 14, 562. [Google Scholar] [CrossRef]
- Rivera-Caravaca, J.M.; Hendriks, J.M. Progressions in Cardiac Arrhythmia: Specific Populations and the Need for Precision Medicine. J. Pers. Med. 2023, 13, 1122. [Google Scholar] [CrossRef]
- Karakasis, P.; Pamporis, K.; Siontis, K.C.; Theofilis, P.; Samaras, A.; Patoulias, D.; Stachteas, P.; Karagiannidis, E.; Stavropoulos, G.; Tzikas, A.; et al. Major clinical outcomes in symptomatic vs. asymptomatic atrial fibrillation: A meta-analysis. Eur. Heart J. 2024, 46, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Pamporis, K.; Karakasis, P.; Sagris, M.; Theofilis, P.; Milaras, N.; Pantelidaki, A.; Mourouzis, I.; Fragakis, N.; Vlachos, K.; Kordalis, A.; et al. Prevalence of asymptomatic atrial fibrillation and risk factors associated with asymptomatic status: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2025, zwaf138. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Theofilis, P.; Sagris, M.; Pamporis, K.; Stachteas, P.; Sidiropoulos, G.; Vlachakis, P.K.; Patoulias, D.; Antoniadis, A.P.; Fragakis, N. Artificial Intelligence in Atrial Fibrillation: From Early Detection to Precision Therapy. J. Clin. Med. 2025, 14, 2627. [Google Scholar] [CrossRef] [PubMed]

| First Author, Year | Follow-Up (Months) | Population | Intervention | Control | Study Type | Operator’s Experience Level | Number of Participants (n) |
|---|---|---|---|---|---|---|---|
| Francisco ARG, 2017 [22] | 1 (0) | Inoperable severe MR and permanent AF | M-TEER (MitraClip) + LAAO (Watchman) | N/C | Case series | High | 5 |
| D’ Amico G, 2021 [18] | 15 (15.5) | Severe MR and permanent AF | M-TEER (MitraClip) + LAAO (Amplatzer or Watchman) | N/C | Cohort (prospective) | N/R | 30 |
| Kuwata S, 2017 [17] | 12 | Severe MR and AF | M-TEER (MitraClip) + LAAO (Amplatzer or Amulet) | M-TEER (MitraClip) | Cohort (retrospective) | N/R | 50 |
| Tichelbäcker T, 2016 [21] | 18 (0) | Severe MR and AF | M-TEER (MitraClip) + LAAO (ProGlide) | N/C | Case series | High | 4 |
| Frazzetto M, 2023 [16] | 12 | Severe MR and AF | M-TEER (MitraClip or PASCAL) + LAAO (Watchman FLX or ProGlide) | M-TEER (MitraClip or PASCAL) | Cohort (retrospective) | N/R | 99 |
| Fukuda N, 2024 [19] | 12 | Moderate-to-severe MR and AF (high bleeding risk, OA contraindication) | M-TEER (MitraClip) + LAAO (Watchman) | N/C | Cohort (retrospective) | N/R | 11 |
| Al-Abcha A, 2025 [20] | 12 | Moderate-to-severe MR and AF | M-TEER (MitraClip or PASCAL) + LAAO (Watchman FLX) | N/C | Cohort (prospective) | N/R | 24 |
| First Author, Year | Number of Participants (n) | Age in Years, Mean (SD) | Males, n (%) | DM, n (%) | CAD, n (%) | MI, n (%) | Permanent AF | Functional/Mixed MR, n (%) | Hypertension, n (%) | CKD, n (%) | LVEF in %, Mean (SD) | CHA2DS2-VASC Score, Mean (SD) | HAS-BLED Score, Mean (SD) | EuroSCORE II | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | I | C | I | C | I | C | I | C | I | C | I | C | I | C | I | C | I | C | I | C | I | C | I | C | I | C | I | C | |
| Francisco ARG, 2017 [22] | 5 | 5 | 0 | 70.8 (8.2) | 3 (60) | 1 (20) | 2 (40) | N/R | 5 (100) | 5 (100) | 5 (100) | 5 (100) | 26.4 (6) | 4.8 (0.8) | 3.2 (0.45) | N/R | |||||||||||||
| D’Amico G, 2021 [18] | 30 | 30 | 0 | 74 (9.1) | 21 (70) | N/R | N/R | 16 (53) | N/R | 21 (70) | N/R | 22 (73) | 42.7 (19.8) | 4.4 (0.7) | 3.5 (0.8) | 8.5 (9.1) | |||||||||||||
| Kuwata S, 2017 [17] | 50 | 25 | 25 | 79.7 (7) | 78.8 (8.6) | 16 (66) | 15 (60) | 3 (12) | 7 (28) | 10 (40) | 10 (40) | 5 (20) | 9 (36) | 18 (72) | 14 (56) | 18 (72) | 17 (68) | 21 (84) | 24 (96) | N/R | N/R | 54.1 (12.2) | 47.6 (23.2) | 4.2 (2) | 4 (1.57) | 3 (1.57) | 3.3 (0.8) | 3.3 (2.4) | 3.68 (3) |
| Tichelbäcker T, 2016 [21] | 4 | 4 | 0 | 82 (6.48) | 2 (50) | N/R | 1 (25) | N/R | N/R | 3 (75) | N/R | N/R | 41.5 (23.2) | 5 (0.8) | 3.25 (0.5) | N/R | |||||||||||||
| Frazzetto M, 2023 [16] | 99 | 41 | 58 | 77.3 (7.68) | 78.3 (6) | 24 (58.6) | 30 (51.8) | 16 (39) | 16 (27.5) | 20 (48.7) | 18 (31) | 10 (24.3) | 12 (20.6) | N/R | N/R | 32 (78) | 39 (67.2) | 36 (87.8) | 52 (89.6) | 30 (73.1) | 31 (53.4) | 43.58 (18.43) | 41.1 (17.48) | 4.64 (0.76) | 4 (1.5) | 3.35 (0.76) | 2.85 (1.5) | 6.79 (2.9) | 5.6 (4.3) |
| Fukuda N, 2024 [19] | 11 | 11 | 0 | 79.3 (8.5) | 9 (82) | 4 (36) | 5 (55) | 3 (27) | N/R | N/R | 2 (18) | N/R | 52.1 (11.7) | 4.5 (1.7) | 2.9 (1.1) | N/R | |||||||||||||
| Al-Abcha A, 2025 [20] | 24 | 24 | 0 | 79.5 (6.3) | 20 (83) | N/R | N/R | N/R | 8 (32) | 18 (75) | 24 (100) | 19 (79) | 45.8 (12.3) | 2.8 (1.3) | 3.3 (1.5) | N/R | |||||||||||||
| Outcome | Number of Patients | Number of Studies | Point Estimate (95% CI) * | I2 | pheterogeneity |
|---|---|---|---|---|---|
| Procedural success (%) | 223 | 7 | 89.5 (73.40, 96.30) | 0.73 | 0 |
| Technical success (%) | 179 | 3 | 96.2 (89.60, 98.70) | 0 | 0.82 |
| Vascular complications (%) | 184 | 4 | 8.50 (2.80, 23) | 0.43 | 0.15 |
| Procedural time (min) | 223 | 7 | 101.6 (85.06, 118.13) | 0.92 | <0.001 |
| Radiation time (min) | 223 | 7 | 29.97 (23.85, 36.09) | 0.9 | <0.001 |
| Contrast (ml) | 194 | 5 | 88.29 (65.02, 111.56) | 0.81 | 0.001 |
| Number of clips (n) | 208 | 5 | 1.64 (1.31, 1.97) | 0.91 | 0.001 |
| Post-procedural MR > 2+ (%) | 83 | 4 | 14.8 (3.6, 44.5) | 0.63 | 0.06 |
| Length of stay (days) | 177 | 4 | 5.21 (3.31, 7.12) | 0.78 | 0.004 |
| All-cause death (%) | 168 | 5 | 11.9 (6.9, 19.6) | 0 | 0.81 |
| In-hospital death (%) | 183 | 4 | 5 (1.6, 14.9) | 0.22 | 0.28 |
| Stroke (%) | 218 | 6 | 3.5 (1.3, 8.9) | 0 | 0.91 |
| Acute kidney injury (%) | 160 | 3 | 3 (0.7, 11.2) | 0 | 0.75 |
| Bleeding (%) | 219 | 6 | 7.5 (2.7, 19.5) | 0.49 | 0.08 |
| HF hospitalization (%) | 129 | 2 | 9.1 (0.6, 63.4) | 0.76 | 0.04 |
| Myocardial Infarction (%) | 34 | 2 | 3.9 (0.5, 23.6) | 0 | 0.35 |
| NYHA reduction ≥ 1 class (%) | 5 | 1 | 91.7 (37.8, 99.5) | N/A | N/A |
| Any procedural complication (%) | 46 | 3 | 7.5 (2.7, 19.5) | 0 | 0.96 |
| CV mortality (%) | 30 | 1 | 1.6 (0.1, 21.1) | N/A | N/A |
| LAAO residual leak (%) | 139 | 4 | 3.7 (1.1, 12) | 0 | 0.79 |
| LAAO device thrombosis (%) | 108 | 3 | 4.5 (0.9, 19.9) | 0 | 0.48 |
| Embolism (%) | 149 | 5 | 5.2 (1.4, 17.3) | 0.1 | 0.35 |
| Hematoma (%) | 99 | 1 | 12.2 (5.2, 26.1) | N/A | N/A |
| Tamponade (%) | 168 | 5 | 3.2 (1, 9.4) | 0 | 0.86 |
| DAPT at discharge (%) | 140 | 3 | 34.2 (2.8, 90.4) | 0.94 | 0.001 |
| SAPT at discharge (%) | 140 | 3 | 11.7 (1, 63.3) | 0.88 | 0.001 |
| Anticoagulation at discharge (%) | 140 | 3 | 20.2 (7.5, 44.3) | 0.7 | 0.04 |
| SAPT + anticoagulation at discharge (%) | 140 | 3 | 11.1 (0.9, 62.3) | 0.86 | 0.001 |
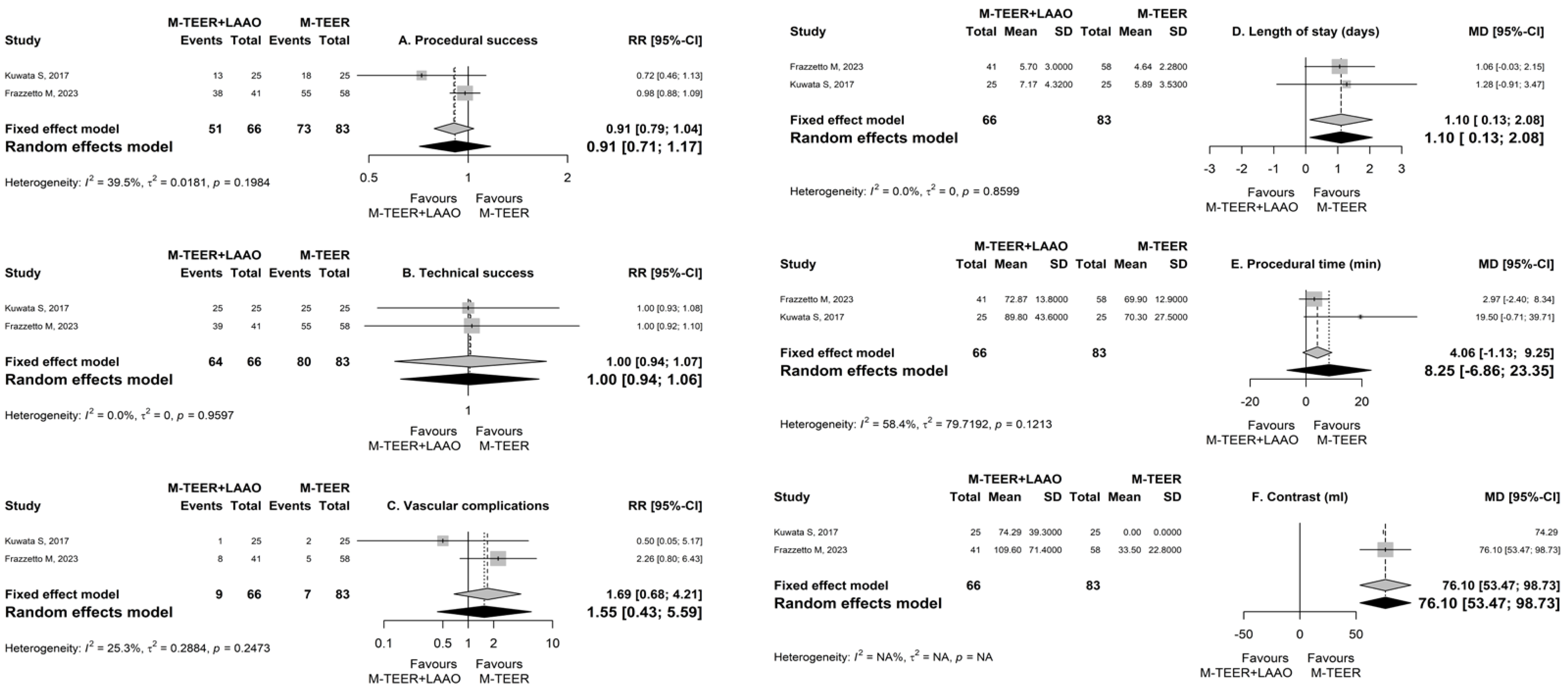
| Outcome | Number of Patients | Number of Studies | Point Estimate (95% CI) * | p-Value | I2 | pheterogeneity |
|---|---|---|---|---|---|---|
| Procedural success | 149 | 2 | 0.91 (0.71, 1.17) | 0.468 | 0.4 | 0.2 |
| Technical success | 149 | 2 | 1 (0.94, 1.06) | 0.966 | 0 | 0.96 |
| Vascular complications | 149 | 2 | 1.55 (0.43, 5.59) | 0.503 | 0.25 | 0.25 |
| Procedural time (min) | 149 | 2 | 8.25 (−6.86, 23.35) | 0.285 | 0.58 | 0.12 |
| Radiation time (min) | 149 | 2 | 5.1 (−7.77, 17.97) | 0.437 | 0.89 | 0.002 |
| Contrast (ml) | 99 | 1 | 76.1 (53.47, 98.73) | <0.001 | N/A | N/A |
| Acute kidney injury | 149 | 2 | 1 (0.07, 15.12) | 1 | N/A | N/A |
| Bleeding | 149 | 2 | 0.4 (0.01, 18.06) | 0.637 | 0.69 | 0.07 |
| Number of clips | 149 | 2 | 0.26 (−0.27, 0.79) | 0.328 | 0.81 | 0.02 |
| HF hospitalization | 99 | 1 | 1.09 (0.53, 2.24) | 0.818 | N/A | N/A |
| Length of stay (days) | 149 | 2 | 1.1 (0.13, 2.08) | 0.027 | 0 | 0.86 |
| In-hospital death | 149 | 2 | 3 (0.13, 70.23) | 0.495 | N/A | N/A |
| All-cause death | 99 | 1 | 0.59 (0.22, 1.54) | 0.282 | N/A | N/A |
| Stroke | 149 | 2 | 3 (0.13, 70.23) | 0.495 | N/A | N/A |
| Post-procedure MR > 2+ | 50 | 1 | 1.67 (0.71, 3.89) | 0.237 | N/A | N/A |
| Device success | 149 | 2 | 0.91 (0.71, 1.17) | 0.468 | 0.4 | 0.2 |
| Hematoma | 99 | 1 | 1.77 (0.51, 6.19) | 0.372 | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pamporis, K.; Tsiachris, D.; Grigoriou, K.; Karakasis, P.; Doundoulakis, I.; Theofilis, P.; Kouvatsos, P.; Saplaouras, A.; Kordalis, A.; Karanikola, A.-E.; et al. One-Stop Mitral Valve Transcatheter Edge-to-Edge Repair and Left Atrial Appendage Occlusion in Patients with Atrial Fibrillation and Mitral Regurgitation: A Systematic Review and Meta-Analysis. J. Pers. Med. 2025, 15, 197. https://doi.org/10.3390/jpm15050197
Pamporis K, Tsiachris D, Grigoriou K, Karakasis P, Doundoulakis I, Theofilis P, Kouvatsos P, Saplaouras A, Kordalis A, Karanikola A-E, et al. One-Stop Mitral Valve Transcatheter Edge-to-Edge Repair and Left Atrial Appendage Occlusion in Patients with Atrial Fibrillation and Mitral Regurgitation: A Systematic Review and Meta-Analysis. Journal of Personalized Medicine. 2025; 15(5):197. https://doi.org/10.3390/jpm15050197
Chicago/Turabian StylePamporis, Konstantinos, Dimitrios Tsiachris, Konstantinos Grigoriou, Paschalis Karakasis, Ioannis Doundoulakis, Panagiotis Theofilis, Panagiotis Kouvatsos, Athanasios Saplaouras, Athanasios Kordalis, Aikaterini-Eleftheria Karanikola, and et al. 2025. "One-Stop Mitral Valve Transcatheter Edge-to-Edge Repair and Left Atrial Appendage Occlusion in Patients with Atrial Fibrillation and Mitral Regurgitation: A Systematic Review and Meta-Analysis" Journal of Personalized Medicine 15, no. 5: 197. https://doi.org/10.3390/jpm15050197
APA StylePamporis, K., Tsiachris, D., Grigoriou, K., Karakasis, P., Doundoulakis, I., Theofilis, P., Kouvatsos, P., Saplaouras, A., Kordalis, A., Karanikola, A.-E., Goutis, P. A., & Tsioufis, K. (2025). One-Stop Mitral Valve Transcatheter Edge-to-Edge Repair and Left Atrial Appendage Occlusion in Patients with Atrial Fibrillation and Mitral Regurgitation: A Systematic Review and Meta-Analysis. Journal of Personalized Medicine, 15(5), 197. https://doi.org/10.3390/jpm15050197







