Study on the Epidemiological Characteristics, Treatment Patterns, and Factors Influencing the Timeliness of Treatment in Head and Neck Squamous Cell Carcinoma (HNSCC) in Stages III and IV: Experience of a Mexican Hospital
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Study Outcomes
2.4. Statistical Analysis
3. Results
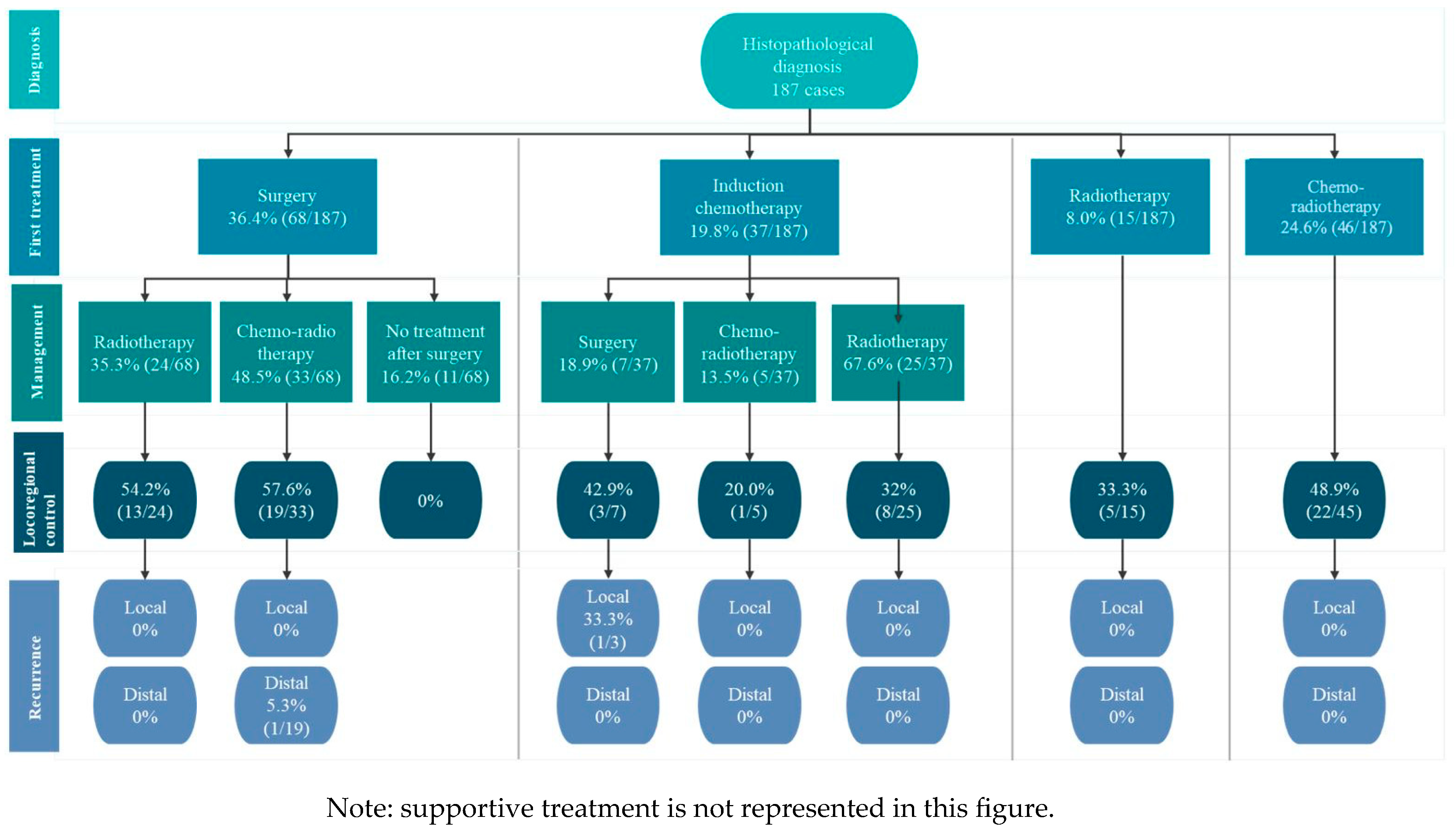
3.1. HNSCC Patient Journey and Treatment Flow
3.1.1. Flow 1: Histopathologic Diagnosis to Surgery
3.1.2. Flow 2: Histopathologic Diagnosis to Chemo-Radiotherapy
3.1.3. Flow 3: Histopathologic Diagnosis to Induction Chemotherapy
3.1.4. Flow 4: Histopathologic Diagnosis to Radiotherapy
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020; Available online: https://gco.iarc.fr/today (accessed on 5 May 2025).
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Michaud, D.S.; Langevin, S.M.; Eliot, M.; Nelson, H.H.; Pawlita, M.; McClean, M.D.; Kelsey, K.T. High-risk HPV types and head and neck cancer. Int. J. Cancer 2014, 135, 1653–1661. [Google Scholar] [CrossRef]
- Stein, A.P.; Saha, S.; Kraninger, J.L.; Swick, A.D.; Yu, M.; Lambert, P.F.; Kimple, R.J. Prevalence of Human Papillomavirus in Oropharyngeal Cancer: A Systematic Review. Cancer J. 2015, 21, 138–146. [Google Scholar] [CrossRef]
- Carrera-Cáceres, S.; Hernández-Hernández, D.M.; Apresa, T.; Gallegos-Hernández, J.F.; Guido-Jiménez, M.; García-Carrancá, A. Prevalence of human Papillomavirus in head and neck cancer in Mexico: A case-control study. BMC Cancer 2007, 7, A15. [Google Scholar] [CrossRef]
- Méndez-Matías, G.; Velázquez-Velázquez, C.; Castro-Oropeza, R.; Mantilla-Morales, A.; Ocampo-Sandoval, D.; Burgos-González, A.; Heredia-Gutiérrez, C.; Alvarado-Cabrero, I.; Sánchez-Sandoval, R.; Barco-Bazán, A.; et al. Prevalence of HPV in Mexican Patients with Head and Neck Squamous Carcinoma and Identification of Potential Prognostic Biomarkers. Cancers 2021, 13, 5602. [Google Scholar] [CrossRef]
- Orlandi, E.; Alfieri, S.; Simon, C.; Trama, A.; Licitra, L.; Hackl, M.; Van Eycken, E.; Henau, K.; Dimitrova, N.; Sekerija, M.; et al. Treatment challenges in and outside a network setting: Head and neck cancers. Eur. J. Surg. Oncol. 2019, 45, 40–45. [Google Scholar] [CrossRef]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef]
- Gebre-Medhin, M.; Brun, E.; Engström, P.; Cange, H.H.; Hammarstedt-Nordenvall, L.; Reizenstein, J.; Nyman, J.; Abel, E.; Friesland, S.; Sjödin, H.; et al. ARTSCAN III: A Randomized Phase III Study Comparing Chemoradiotherapy with Cisplatin Versus Cetuximab in Patients With Locoregionally Advanced Head and Neck Squamous Cell Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Trotti, A.M.; Harris, J.; Eisbruch, A.; Harari, P.M.; Adelstein, D.J.; Jordan, R.C.K.; Zhao, W.; Sturgis, E.M.; Burtness, B.; et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non-inferiority trial. Lancet 2019, 393, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Windon, M.J.; D’Souza, G.; Rettig, E.M.; Westra, W.H.; van Zante, A.; Wang, S.J.; Ryan, W.R.; Mydlarz, W.K.; Ha, P.K.; Miles, B.A.; et al. Increasing prevalence of human papillomavirus–positive oropharyngeal cancers among older adults. Cancer 2018, 124, 2993–2999. [Google Scholar] [CrossRef] [PubMed]
- Marta, G.N.; Silva, V.; De Andrade Carvalho, H.; de Arruda, F.F.; Hanna, S.A.; Gadia, R.; da Silva, J.L.F.; Correa, S.F.M.; Vita Abreu, C.E.C.; Riera, R. Intensity-modulated radiation therapy for head and neck cancer: Systematic review and meta-analysis. Radiother. Oncol. 2014, 110, 9–15. [Google Scholar] [CrossRef]
- Marta, G.N.; William, W.N.; Feher, O.; Carvalho, A.L.; Kowalski, L.P. Induction chemotherapy for oral cavity cancer patients: Current status and future perspectives. Oral Oncol. 2015, 51, 1069–1075. [Google Scholar] [CrossRef]
- Schoonbeek, R.C.; de Vries, J.; Bras, L.; Sidorenkov, G.; Plaat, B.E.C.; Witjes, M.J.H.; van der Laan, B.F.A.M.; Hoek, J.G.M.v.D.; van Dijk, B.A.C.; Langendijk, J.A.; et al. The effect of treatment delay on quality of life and overall survival in head and neck cancer patients. Eur. J. Cancer Care 2022, 31, e13589. [Google Scholar] [CrossRef]
- Lauritzen, B.B.; Schmidt, J.J.; Christian, G.; Irene, W.; von Buchwald, C. Impact of delay in diagnosis and treatment-initiation on disease stage and survival in oral cavity cancer: A systematic review. Acta Oncol. 2021, 60, 1083–1090. [Google Scholar] [CrossRef]
- Chang, A.K.; Kruglik, C.P.; Valentin, G.F.S.; Barry, M.M.; Brundage, W.J.; Devenney, B.; Gagne, H.M.; Nelson, C.J.; Silverman, D.; Sajisevi, M.B. Factors associated with radiation treatment delay in head and neck squamous cell carcinoma. J. Radiother. Pract. 2024, 23, e22. [Google Scholar] [CrossRef]
- Lalango, F.; Kabagenyi, F.; Seguya, A.; Byaruhanga, R.; Otiti, J. A descriptive study on diagnostic timelines, and factors influencing delayed diagnosis among adult head and neck cancer patients at Uganda cancer institute. World J. Surg. Oncol. 2024, 22, 130. [Google Scholar] [CrossRef]
- Rygalski, C.J.; Zhao, S.; Eskander, A.; Zhan, K.Y.; Mroz, E.A.; Brock, G.; Silverman, D.A.; Blakaj, D.; Bonomi, M.R.; Carrau, R.L.; et al. Time to Surgery and Survival in Head and Neck Cancer. Ann. Surg. Oncol. 2021, 28, 877–885. [Google Scholar] [CrossRef]
- Liao, D.Z.; Schlecht, N.F.; Rosenblatt, G.; Kinkhabwala, C.M.; Leonard, J.A.; Ference, R.S.; Prystowsky, M.B.; Ow, T.J.; Schiff, B.A.; Smith, R.V.; et al. Association of Delayed Time to Treatment Initiation With Overall Survival and Recurrence Among Patients with Head and Neck Squamous Cell Carcinoma in an Underserved Urban Population. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 1001–1009. [Google Scholar] [CrossRef]
- Mack, D.P.; Spencer, H.; Wang, K.; Lewis, G.D. The Effects of the COVID-19 Pandemic on Cancer Staging in Patients Diagnosed With Head and Neck Cancer. Cureus 2023, 15, e34190. [Google Scholar] [CrossRef] [PubMed]
- O’Rorke, M.A.; Ellison, M.V.; Murray, L.J.; Moran, M.; James, J.; Anderson, L.A. Human papillomavirus related head and neck cancer survival: A systematic review and meta-analysis. Oral Oncol. 2012, 48, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Parsons, J.L. The radiobiology of HPV-positive and HPV-negative head and neck squamous cell carcinoma. Expert Rev. Mol. Med. 2020, 22, e3. [Google Scholar] [CrossRef] [PubMed]
- Linton, O.R.; Moore, M.G.; Brigance, J.S.; Gordon, C.A.; Summerlin, D.-J.; McDonald, M.W. Prognostic significance of basaloid squamous cell carcinoma in head and neck cancer. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 1306–1311. [Google Scholar] [CrossRef]
- Cheraghlou, S.; Torabi, S.J.; Husain, Z.A.; Otremba, M.D.; Osborn, H.A.; Mehra, S.; Yarbrough, W.G.; Burtness, B.A.; Judson, B.L. HPV status in unknown primary head and neck cancer: Prognosis and treatment outcomes. Laryngoscope 2019, 129, 684–691. [Google Scholar] [CrossRef]
- Rosenberg, A.J.; Vokes, E.E. Optimizing Treatment De-Escalation in Head and Neck Cancer: Current and Future Perspectives. Oncologist 2021, 26, 40–48. [Google Scholar] [CrossRef]
| Female | Male | Overall | |
|---|---|---|---|
| Patients, n (%) | 32 (17.1) | 155 (82.9) | 187 (100) |
| Age at diagnosis, years ± SD | 59.7 ± 11.9 | 59.6 ± 11.8 | 59.6 ± 11.7 |
| BMI * at baseline, kg/m2 ± SD | 24.3 ± 5.2 | 24.3 ± 5.2 | 24.3 ± 5.2 |
| Tobacco use, n (%) | 7 (21.9) | 107 (69.0) | 114 (61.0) |
| Alcohol consumption, n (%) | 9 (28.1) | 96 (62.0) | 105 (56.1) |
| Hypertension, n (%) | 5 (15.6) | 36 (23.2) | 41 (21.9) |
| Stage | III | IVA | IVB | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| HNSCC Site | n | % | n | % | n | % | n | % | |
| Oropharynx | 6 | 3.2 | 20 | 10.7 | 7 | 3.7 | 33 | 17.6 | |
| Oral cavity | 13 | 7.0 | 36 | 19.3 | 20 | 10.7 | 69 | 36.9 | |
| Larynx | 24 | 12.8 | 41 | 21.9 | 4 | 2.1 | 69 | 36.9 | |
| Hypopharynx | 1 | 0.5 | 5 | 2.7 | 8 | 4.3 | 14 | 7.5 | |
| Unknown primary tumor | 0 | 0 | 1 | 0.5 | 1 | 0.5 | 2 | 1.1 | |
| Total | 44 | 23.5 | 103 | 55.1 | 40 | 21.4 | 187 | 100.0 | |
| p-16 Test | Clinical Stage | Oropharynx | Oral Cavity | Total | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Negative | III | 3 | 37.5% | 5 | 62.5% | 8 | 20.5% |
| IVA | 6 | 26.1% | 17 | 73.9% | 23 | 59.0% | |
| IVB | 1 | 12.5% | 7 | 87.5% | 8 | 20.5% | |
| Subtotal | 10 | 25.6% | 29 | 74.4% | 39 | 100.0% | |
| Positive | III | 3 | 30.0% | 7 | 70.0% | 10 | 27.8% |
| IVA | 13 | 86.7% | 2 | 13.3% | 15 | 41.7% | |
| IVB | 5 | 45.5% | 6 | 54.5% | 11 | 30.6% | |
| Subtotal | 21 | 58.3% | 15 | 41.7% | 36 | 100.0% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyervides Juarez, V.M.; Ruiz Sanchez, D.; De Leon Cruz, A.; Ceceñas Falcon, L.A.; Mendez Saenz, M.; Gomez de la Cruz, C.A.; Campos Coy, M.A.; Sánchez Castillo, J.M.; Vidal Gutierrez, O.; Manzo Merino, J.; et al. Study on the Epidemiological Characteristics, Treatment Patterns, and Factors Influencing the Timeliness of Treatment in Head and Neck Squamous Cell Carcinoma (HNSCC) in Stages III and IV: Experience of a Mexican Hospital. J. Pers. Med. 2025, 15, 193. https://doi.org/10.3390/jpm15050193
Oyervides Juarez VM, Ruiz Sanchez D, De Leon Cruz A, Ceceñas Falcon LA, Mendez Saenz M, Gomez de la Cruz CA, Campos Coy MA, Sánchez Castillo JM, Vidal Gutierrez O, Manzo Merino J, et al. Study on the Epidemiological Characteristics, Treatment Patterns, and Factors Influencing the Timeliness of Treatment in Head and Neck Squamous Cell Carcinoma (HNSCC) in Stages III and IV: Experience of a Mexican Hospital. Journal of Personalized Medicine. 2025; 15(5):193. https://doi.org/10.3390/jpm15050193
Chicago/Turabian StyleOyervides Juarez, Victor Manuel, Daneli Ruiz Sanchez, Alejandro De Leon Cruz, Luis Angel Ceceñas Falcon, Marco Mendez Saenz, Carlos Alfredo Gomez de la Cruz, Mario Alberto Campos Coy, Juan Manuel Sánchez Castillo, Oscar Vidal Gutierrez, Joaquin Manzo Merino, and et al. 2025. "Study on the Epidemiological Characteristics, Treatment Patterns, and Factors Influencing the Timeliness of Treatment in Head and Neck Squamous Cell Carcinoma (HNSCC) in Stages III and IV: Experience of a Mexican Hospital" Journal of Personalized Medicine 15, no. 5: 193. https://doi.org/10.3390/jpm15050193
APA StyleOyervides Juarez, V. M., Ruiz Sanchez, D., De Leon Cruz, A., Ceceñas Falcon, L. A., Mendez Saenz, M., Gomez de la Cruz, C. A., Campos Coy, M. A., Sánchez Castillo, J. M., Vidal Gutierrez, O., Manzo Merino, J., Peralonso Bombin, S., Rodríguez Rosales, Y. E., Lugo Martinez, G., Iglesias, J. M., Medina Gonzalez, S., & Beltran Rodriguez, C. C. (2025). Study on the Epidemiological Characteristics, Treatment Patterns, and Factors Influencing the Timeliness of Treatment in Head and Neck Squamous Cell Carcinoma (HNSCC) in Stages III and IV: Experience of a Mexican Hospital. Journal of Personalized Medicine, 15(5), 193. https://doi.org/10.3390/jpm15050193






