Antibiotic-Mixed Cement Filling for Chronic Osteomyelitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Operative Technique
2.3. Postoperative Care
2.4. Evaluation
2.4.1. Clinical Evaluation
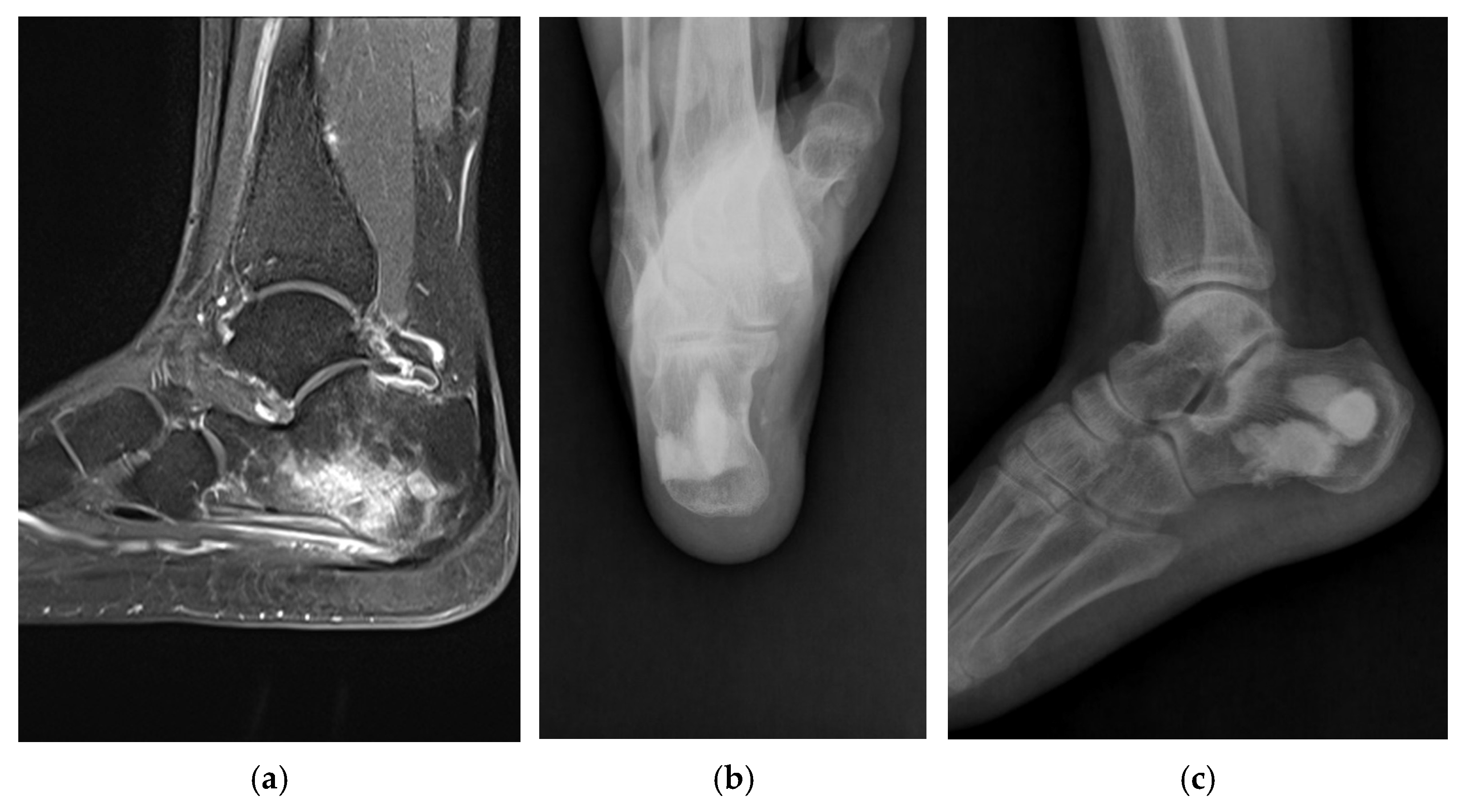
2.4.2. Radiographic Evaluation
2.4.3. Laboratory Evaluation
3. Results
3.1. Clinical Outcomes
3.2. Radiographic Outcomes
3.3. Laboratory Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buchholz, H.W.; Elson, R.A.; Heinert, K. Antibiotic-loaded acrylic cement: Current concepts. Clin. Orthop. Relat. Res. 1984, 190, 96–108. [Google Scholar] [CrossRef]
- Cho, S.H.; Song, H.R.; Koo, K.H.; Jeong, S.T.; Park, Y.J. Antibiotic-impregnated cement beads in the treatment of chronic osteomyelitis. Bull. Hosp. Jt. Dis. 1997, 56, 140–144. [Google Scholar]
- Evans, R.P.; Nelson, C.L. Gentamicin-impregnated polymethylmethacrylate beads compared with systemic antibiotic therapy in the treatment of chronic osteomyelitis. Clin. Orthop. Relat. Res. 1993, 295, 37–42. [Google Scholar] [CrossRef]
- Bowyer, G.W.; Cumberland, N. Antibiotic release from impregnated pellets and beads. J. Trauma 1994, 36, 331–335. [Google Scholar] [CrossRef]
- Kanellakopoulou, K.; Giamarellos-Bourboulis, E.J. Carrier systems for the local delivery of antibiotics in bone infections. Drugs 2000, 59, 1223–1232. [Google Scholar] [CrossRef]
- Patzakis, M.J.; Mazur, K.; Wilkins, J.; Sherman, R.; Holtom, P. Septopal beads and autogenous bone grafting for bone defects in patients with chronic osteomyelitis. Clin. Orthop. Relat. Res. 1993, 295, 112–118. [Google Scholar] [CrossRef]
- Patzakis, M.J.; Zalavras, C.G. Chronic posttraumatic osteomyelitis and infected nonunion of the tibia: Current management concepts. J. Am. Acad. Orthop. Surg. 2005, 13, 417–427. [Google Scholar] [CrossRef]
- Buchholz, H.W.; Engelbrecht, H. Depot effects of various antibiotics mixed with Palacos resins. Chir. Z. Fur Alle Geb. Operativen Medizen 1970, 41, 511–515. [Google Scholar]
- Klemm, K. Gentamicin-PMMA-beads in treating bone and soft tissue infections (author’s transl). Zentralblatt Chirurgie 1979, 104, 934–942. [Google Scholar]
- Mauffrey, C.; Barlow, B.T.; Smith, W. Management of segmental bone defects. J. Am. Acad. Orthop. Surg. 2015, 23, 143–153. [Google Scholar]
- McKee, M.D.; Li-Bland, E.A.; Wild, L.M.; Schemitsch, E.H. A prospective, randomized clinical trial comparing an antibiotic-impregnated bioabsorbable bone substitute with standard antibiotic-impregnated cement beads in the treatment of chronic osteomyelitis and infected nonunion. J. Orthop. Trauma 2010, 24, 483–490. [Google Scholar] [CrossRef] [PubMed]
- McNally, M.A.; Ferguson, J.Y.; Lau, A.C.K.; Diefenbeck, M.; Scarborough, M.; Ramsden, A.J.; Atkins, B.L. Single-stage treatment of chronic osteomyelitis with a new absorbable, gentamicin-loaded, calcium sulphate/hydroxyapatite biocomposite: A prospective series of 100 cases. Bone Jt. J. 2016, 98B, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.; Diefenbeck, M.; McNally, M. Ceramic Biocomposites as Biodegradable Antibiotic Carriers in the Treatment of Bone Infections. J. Bone Jt. Infect. 2017, 2, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Ferrao, P.; Myerson, M.S.; Schuberth, J.M.; McCourt, M.J. Cement spacer as definitive management for postoperative ankle infection. Foot Ankle Int. 2012, 33, 173–178. [Google Scholar] [CrossRef]
- Lee, H.S.; Ahn, J.Y.; Lee, J.S.; Lee, J.Y.; Jeong, J.J.; Choi, Y.R. Cement arthroplasty for ankle joint destruction. J. Bone Jt. Surg. Am. 2014, 96, 1468–1475. [Google Scholar] [CrossRef]
- Canavese, F.; Corradin, M.; Khan, A.; Mansour, M.; Rousset, M.; Samba, A. Successful treatment of chronic osteomyelitis in children with debridement, antibiotic-laden cement spacer and bone graft substitute. Eur. J. Orthop. Surg. Traumatol. 2017, 27, 221–228. [Google Scholar] [CrossRef]
- Dudareva, M.; Hotchen, A.J.; Ferguson, J.; Hodgson, S.; Scarborough, M.; Atkins, B.L.; McNally, M.A. The microbiology of chronic osteomyelitis: Changes over ten years. J. Infect. 2019, 79, 189–198. [Google Scholar] [CrossRef]
- Cierny, G.; Mader, J.T.; Pennick, J.J. A clinical staging system for adult osteomyelitis. Clin. Orthop. Relat. Res. 2003, 414, 7–24. [Google Scholar] [CrossRef]
- Cierny, G.; Mader, J.T. Adult chronic osteomyelitis. Orthopedics 1984, 7, 1557–1564. [Google Scholar] [CrossRef]
- Walenkamp, G.H.; Kleijn, L.L.; Leeuw, M.D. osteomyelitis treated with gentamicin-PMMA beads: 100 patients followed for 1-12 years. Acta Orthop. Scand. 1998, 69, 518–522. [Google Scholar] [CrossRef]
- Kim, Y.H.; Inori, F.; Yamanaka, K.; Murakami, S.; Narita, E.; Yamamura, K.; Yasuda, H.; Fukuda, M.; Konishi, S.; Minoda, Y. A Case of Osteomyelitis after Calcaneal Fracture Treated by Antibiotic-Containing Calcium Phosphate Cements. Case Rep. Orthop. 2018, 2018, 9321830. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.; Liu, K.; Chen, W.; Wang, Z. Clinical observations of vancomycin-loaded calcium phosphate cement in the 1-stage treatment of chronic osteomyelitis: A randomized trial. Ann. Palliat. Med. 2021, 10, 6706–6714. [Google Scholar] [CrossRef] [PubMed]
- Gashegu, J.; Byamungu, T.; Ngarambe, C.; Bayisenga, J.; Kiswezi, A. Treatment of chronic osteomyelitis with locally made calcium sulfate bone cement pellets impregnated with antibiotics at University Teaching Hospital of Butare (CHUB), Rwanda. East Cent. Afr. J. Surg. 2018, 23, 3–8. [Google Scholar] [CrossRef][Green Version]


| Case | Sex/Age | Site | Cause of Osteomyelitis | Infecting Organism from Intraoperative Debrided Tissue | Antibiotics (Duration, Days) | Discharge from Wound (Post-Op Days) | Normalization of CRP (Post-Op Days) | Normalization of ESR (Post-Op Days) | |
|---|---|---|---|---|---|---|---|---|---|
| Intravenous | Oral | ||||||||
| 1 | Female/54 | Proximal tibia | Post-op (skin tumor excision) | No growth | Cefazolin (14) | Combined flap coverage | Normal, preoperative | Normal, preoperative | |
| 2 | Male/32 | Middle tibia | Open fracture | MRSA | Vancomycin (24) | 14 (redo surgery) | 18 | 25 | |
| 3 | Male/64 | Distal tibia | Post-op (SMO) | MRSA | Vancomycin, Levofloxacin, Metronidazole (27) | 20 | 30 | ||
| 4 | Male/46 | Distal tibia | Post-op (ORIF) | Streptococcus agalactiae | Cefazolin (7), Ampicillin (8) | Cephradine (14) | 15 | 69 | |
| 5 | Female/75 | Distal tibia | Unknown | Mycobacterium tuberculosis | Ceftezole (7) | HERZ (9 months) | 24 | 190 | |
| 6 | Female/41 | Distal tibia | Post-op (ORIF) | Burkholderia cepacia, Bacteroides and Prevotella | Piperacillin/Tazobactam (15), Ceftazidime (4) | 32 | Normal, preoperative | ||
| 7 | Male/39 | Distal tibia | Open fracture | Pseudomonas aeruginosa | Ceftezole (10), Piperacillin/Tazobactam (18) | Cephradine (10) | 27 | 49 | |
| 8 | Male/23 | Calcaneus | Laceration | Escherichia vulneris | Cefazolin (26) | Normal, preoperative | Normal, preoperative | ||
| 9 | Male/53 | Calcaneus | Open fracture | MRSA | Vancomycin, Rifampicin (15) | Ciprofloxacin (14) | 6 | 22 | |
| 10 | Female/49 | Calcaneus | Post-op (ORIF) | MSSA | Cefazolin, Rifampicin (8), Nafcillin (5) | Amoxicillin/Clavulanate, Ciprofloxacin (14) | Normal, preoperative | 57 | |
| 11 | Male/57 | Calcaneus | Post-op (ORIF) | MSSA | Ceftriaxone (6), Cefazolin (16) | Cephradine (10), Amoxicillin/Clavulanate, Ciprofloxacin (28) | 28 (redo surgery) | 14 | 14 |
| 12 | Female/57 | Calcaneus | Unknown | Salmonella typhi | Ceftriaxone (11) | Ciprofloxacin (14) | 14 | 23 | |
| 13 | Male/43 | Calcaneus | Post-op (ORIF) | MRSA | Ceftriaxone, Linezolid (8), Linezolid (4) | Linezolid (26) | 38 | 56 | |
| 14 | Male/61 | Calcaneus | Post-op (ORIF) | Pseudomonas aeruginosa | Ceftezole (5) | Cefditoren (7) | Normal, preoperative | 30 | |
| 15 | Male/63 | Cuboid | Puncture wound | Achromobacter xyloxidans | Ceftazidime (11) | Ciprofloxacin (14) | 30 (healed with simple dressing) | 3 | 27 |
| 16 | Male/43 | Medial cuneiform | Post-op (ORIF) | No growth | Amoxicillin/Sulbactam, Amikacin (29) | Amoxicillin/Sulbactam (7) | Normal, preoperative | 12 | |
| 17 | Female/67 | 1st metatarsal bone | Post-op (HV) | MSSA | Nafcillin (21) | Cephradine (15) | 74 (conversion into arthrodesis) | Normal, preoperative | 45 |
| 18 | Male/79 | 1st metatarsal bone | Puncture wound | No growth | Cefotiam (13) | Normal, preoperative | Normal, preoperative | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-H.; Choi, Y.R.; Jeong, I.; Lee, H.S. Antibiotic-Mixed Cement Filling for Chronic Osteomyelitis. J. Pers. Med. 2025, 15, 187. https://doi.org/10.3390/jpm15050187
Park S-H, Choi YR, Jeong I, Lee HS. Antibiotic-Mixed Cement Filling for Chronic Osteomyelitis. Journal of Personalized Medicine. 2025; 15(5):187. https://doi.org/10.3390/jpm15050187
Chicago/Turabian StylePark, Seung-Hwan, Young Rak Choi, Inyong Jeong, and Ho Seong Lee. 2025. "Antibiotic-Mixed Cement Filling for Chronic Osteomyelitis" Journal of Personalized Medicine 15, no. 5: 187. https://doi.org/10.3390/jpm15050187
APA StylePark, S.-H., Choi, Y. R., Jeong, I., & Lee, H. S. (2025). Antibiotic-Mixed Cement Filling for Chronic Osteomyelitis. Journal of Personalized Medicine, 15(5), 187. https://doi.org/10.3390/jpm15050187






