Perioperative Coronavirus Disease 2019 Infection and Its Impact on Postoperative Outcomes: Pulmonary Complications and Mortality Based on Korean National Health Insurance Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Variables and Outcomes
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- COVIDSurg Collaborative. Elective surgery cancellations due to the COVID-19 pandemic: Global predictive modelling to inform surgical recovery plans. Br. J. Surg. 2020, 107, 1440–1449. [Google Scholar]
- Collaborative, C. Outcomes and Their State-level Variation in Patients Undergoing Surgery With Perioperative SARS-CoV-2 Infection in the USA: A Prospective Multicenter Study. Ann. Surg. 2022, 275, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Nepogodiev, D.; Bhangu, A.; Glasbey, J.C.; Li, E.; Omar, O.M.; Simoes, J.F.; Abbott, T.E.; Alser, O.; Arnaud, A.P.; Bankhead-Kendall, B.K.; et al. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: An international cohort study. Lancet 2020, 396, 27–38. [Google Scholar] [CrossRef]
- Bohn, M.K.; Hall, A.; Sepiashvili, L.; Jung, B.; Steele, S.; Adeli, K. Pathophysiology of COVID-19: Mechanisms Underlying Disease Severity and Progression. Physiology 2020, 35, 288–301. [Google Scholar] [CrossRef] [PubMed]
- George, P.M.; Barratt, S.L.; Condliffe, R.; Desai, S.R.; Devaraj, A.; Forrest, I.; Gibbons, M.A.; Hart, N.; Jenkins, R.G.; McAuley, D.F.; et al. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax 2020, 75, 1009. [Google Scholar] [CrossRef]
- Patel, B.V.; Arachchillage, D.J.; Ridge, C.A.; Bianchi, P.; Doyle, J.F.; Garfield, B.; Ledot, S.; Morgan, C.; Passariello, M.; Price, S.; et al. Pulmonary Angiopathy in Severe COVID-19: Physiologic, Imaging, and Hematologic Observations. Am. J. Respir. Crit. Care Med. 2020, 202, 690–699. [Google Scholar] [CrossRef]
- Swenson, K.E.; Hardin, C.C. Pathophysiology of Hypoxemia in COVID-19 Lung Disease. Clin. Chest Med. 2023, 44, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Lopez, A.D. Evidence-based health policy-lessons from the Global Burden of Disease Study. Science 1996, 274, 740–743. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.H. Proceed with Caution When Using Real World Data and Real World Evidence. J. Korean Med. Sci. 2019, 34, e28. [Google Scholar] [CrossRef]
- Kyoung, D.S.; Kim, H.S. Understanding and Utilizing Claim Data from the Korean National Health Insurance Service (NHIS) and Health Insurance Review & Assessment (HIRA) Database for Research. J. Lipid Atheroscler. 2022, 11, 103–110. [Google Scholar]
- Park, J.-S.; Lee, C.H. Clinical Study Using Healthcare Claims Database. J. Rheum. Dis. 2021, 28, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Seo, L.; Yoon, D.; Yang, K.; Yi, J.-E.; Kim, Y.; Lee, J.H. Digital Health Profile of South Korea: A Cross Sectional Study. Int. J. Environ. Res. Public. Health 2022, 19, 6329. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, L.C.; Ersbøll, A.K. When the entire population is the sample: Strengths and limitations in register-based epidemiology. Eur. J. Epidemiol. 2014, 29, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Mattingly, A.S.; Rose, L.; Eddington, H.S.; Trickey, A.W.; Cullen, M.R.; Morris, A.M.; Wren, S.M. Trends in US Surgical Procedures and Health Care System Response to Policies Curtailing Elective Surgical Operations During the COVID-19 Pandemic. JAMA Netw. Open 2021, 4, e2138038. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Huang, C.-H.; Luk, H.-N.; Tsai, P.B. Adaptation to the Plastic Barrier Sheet to Facilitate Intubation During the COVID-19 Pandemic. Anesth. Analg. 2020, 131, e97–e99. [Google Scholar] [CrossRef]
- Doglietto, F.; Vezzoli, M.; Gheza, F.; Lussardi, G.L.; Domenicucci, M.; Vecchiarelli, L.; Zanin, L.; Saraceno, G.; Signorini, L.; Panciani, P.P.; et al. Factors Associated With Surgical Mortality and Complications Among Patients With and Without Coronavirus Disease 2019 (COVID-19) in Italy. JAMA Surg. 2020, 155, 691–702. [Google Scholar] [CrossRef]
- Dorken-Gallastegi, A.; Argandykov, D.; Gebran, A.; Kaafarani, H.M.A. Surgical Implications of Coronavirus Disease-19. Gastroenterol. Clin. N. Am. 2023, 52, 173–183. [Google Scholar] [CrossRef]
- Jonker, P.K.C.; van der Plas, W.Y.; Steinkamp, P.J.; Poelstra, R.; Emous, M.; van der Meij, W.; Thunnissen, F.; Bierman, W.F.W.; Struys, M.M.R.; de Reuver, P.R.; et al. Perioperative SARS-CoV-2 infections increase mortality, pulmonary complications, and thromboembolic events: A Dutch, multicenter, matched-cohort clinical study. Surgery 2021, 169, 264–274. [Google Scholar] [CrossRef]
- Shao, C.C.; McLeod, M.C.; Thogaripally, S.; Mugavero, M.J.; Gleason, L.T.; Dos Santos Marques, I.C.; Chu, D.I.; Gunnells, D.J. Increased Risk of Postoperative Mortality Associated With Prior COVID-19 Infection. Am. J. Prev. Med. 2022, 63, S75–S82. [Google Scholar] [CrossRef]
- Williams, G.W.; Mubashir, T.; Balogh, J.; Rezapour, M.; Hu, J.; Dominique, B.; Gautam, N.K.; Lai, H.; Ahmad, H.S.; Li, X.; et al. Recent COVID-19 infection is associated with increased mortality in the ambulatory surgery population. J. Clin. Anesth. 2023, 89, 111182. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Ahn, E.; Kim, G.H.; Noh, J.-H.; Bang, S.R. Impact of perioperative COVID-19 infection on postoperative complication in cesarean section using Korean National Health insurance data. Sci. Rep. 2024, 14, 16001. [Google Scholar] [CrossRef] [PubMed]
- Khonsari, R.H.; Bernaux, M.; Vie, J.J.; Diallo, A.; Paris, N.; Luong, L.B.; Assouad, J.; Paugam, C.; Simon, T.; Vicaut, E.; et al. Risks of early mortality and pulmonary complications following surgery in patients with COVID-19. Br. J. Surg. 2021, 108, e158–e159. [Google Scholar] [CrossRef]
- Prasad, N.K.; Mayorga-Carlin, M.; Sahoo, S.; Englum, B.R.; Turner, D.J.; Siddiqui, T.; Lake, R.; Sorkin, J.D.; Lal, B.K. Mid-term Surgery Outcomes in Patients With COVID-19. Ann. Surg. 2023, 277, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Diehl, J.L.; Peron, N.; Chocron, R.; Debuc, B.; Guerot, E.; Hauw-Berlemont, C.; Hermann, B.; Augy, J.L.; Younan, R.; Novara, A.; et al. Respiratory mechanics and gas exchanges in the early course of COVID-19 ARDS: A hypothesis-generating study. Ann. Intensive Care 2020, 10, 95. [Google Scholar] [CrossRef]
- Upadhya, S.; Rehman, J.; Malik, A.B.; Chen, S. Mechanisms of Lung Injury Induced by SARS-CoV-2 Infection. Physiology 2022, 37, 88–100. [Google Scholar] [CrossRef]
- Camporota, L.; Cronin, J.N.; Busana, M.; Gattinoni, L.; Formenti, F. Pathophysiology of coronavirus-19 disease acute lung injury. Curr. Opin. Crit. Care 2022, 28, 9–16. [Google Scholar] [CrossRef]
- Long, B.; Brady, W.J.; Koyfman, A.; Gottlieb, M. Cardiovascular complications in COVID-19. Am. J. Emerg. Med. 2020, 38, 1504–1507. [Google Scholar] [CrossRef]
- Vahdat, S. A review of pathophysiological mechanism, diagnosis, and treatment of thrombosis risk associated with COVID-19 infection. IJC Heart Vasc. 2022, 41, 101068. [Google Scholar] [CrossRef]
- Duca, Ș.T.; Chetran, A.; Miftode, R.Ș.; Mitu, O.; Costache, A.D.; Nicolae, A.; Iliescu-Halițchi, D.; Halițchi-Iliescu, C.-O.; Mitu, F.; Costache, I.I. Myocardial Ischemia in Patients with COVID-19 Infection: Between Pathophysiological Mechanisms and Electrocardiographic Findings. Life 2022, 12, 1015. [Google Scholar] [CrossRef]
- Mihalj, M.; Mosbahi, S.; Schmidli, J.; Heinisch, P.P.; Reineke, D.; Schoenhoff, F.; Kadner, A.; Schefold, J.C.; Räber, L.; Potapov, E.V.; et al. Providing safe perioperative care in cardiac surgery during the COVID-19 pandemic. Best. Pract. Res. Clin. Anaesthesiol. 2021, 35, 321–332. [Google Scholar] [CrossRef]
- Kaw, R.; Stoller, J.K. Pulmonary Complications After Noncardiac Surgery: A Review of Their Frequency and Prevention Strategies. Clin. Pulm. Med. 2008, 15, 18–23. [Google Scholar] [CrossRef]
- Mi, J.; Zhong, W.; Huang, C.; Zhang, W.; Tan, L.; Ding, L. Gender, age and comorbidities as the main prognostic factors in patients with COVID-19 pneumonia. Am. J. Transl. Res. 2020, 12, 6537–6548. [Google Scholar]
- Aluganti Narasimhulu, C.; Singla, D.K. Mechanisms of COVID-19 pathogenesis in diabetes. Am. J. Physiol.-Heart Circ. Physiol. 2022, 323, H403–H420. [Google Scholar] [CrossRef] [PubMed]
- Dadson, P.; Tetteh, C.D.; Rebelos, E.; Badeau, R.M.; Moczulski, D. Underlying Kidney Diseases and Complications for COVID-19: A Review. Front. Med. 2020, 7, 600144. [Google Scholar] [CrossRef]
- Ambrosino, I.; Barbagelata, E.; Ortona, E.; Ruggieri, A.; Massiah, G.; Giannico, O.V.; Politi, C.; Moretti, A.M. Gender differences in patients with COVID-19: A narrative review. Monaldi Arch. Chest Dis. 2020, 90. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.R.; Kim, G.H.; Cho, S.J.; Yoon, M.J. Risk factors of hypotension during cesarean section with spinal anesthesia in parturients with COVID-19: A retrospective study in comparison with pregnant women without COVID-19. Anesth. Pain. Med. 2024, 19, 326–332. [Google Scholar] [CrossRef]
- Bonanad, C.; García-Blas, S.; Tarazona-Santabalbina, F.; Sanchis, J.; Bertomeu-González, V.; Fácila, L.; Ariza, A.; Núñez, J.; Cordero, A. The Effect of Age on Mortality in Patients with COVID-19: A Meta-Analysis with 611,583 Subjects. J. Am. Med. Dir. Assoc. 2020, 21, 915–918. [Google Scholar] [CrossRef]
- Brown, W.A.; Moore, E.M.; Watters, D.A. Mortality of patients with COVID-19 who undergo an elective or emergency surgical procedure: A systematic review and meta-analysis. ANZ J. Surg. 2021, 91, 33–41. [Google Scholar] [CrossRef]
- Vranis, N.M.; Bekisz, J.M.; Daar, D.A.; Chiu, E.S.; Wilson, S.C. Clinical Outcomes of 2019 COVID-19 Positive Patients Who Underwent Surgery: A New York City Experience. J. Surg. Res. 2021, 261, 113–122. [Google Scholar] [CrossRef]
- Gansevoort, R.T.; Hilbrands, L.B. CKD is a key risk factor for COVID-19 mortality. Nat. Rev. Nephrol. 2020, 16, 705–706. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Zhang, J.; Zhu, Y.; Liu, L.; Liu, Y.; He, Q. Mortality in chronic kidney disease patients with COVID-19: A systematic review and meta-analysis. Int. Urol. Nephrol. 2021, 53, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- Ferrandis, R.; Llau, J.V.; Afshari, A.; Douketis, J.D.; Gómez-Luque, A.; Samama, C.M. Management of perioperative thromboprophylaxis for surgery following COVID-19: An expert-panel survey. Br. J. Anaesth. 2021, 127, e143–e145. [Google Scholar] [CrossRef]
- Dexter, F.; Parra, M.C.; Brown, J.R.; Loftus, R.W. Perioperative COVID-19 Defense: An Evidence-Based Approach for Optimization of Infection Control and Operating Room Management. Anesth. Analg. 2020, 131, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Landoni, G.; Pisano, A.; Lomivorotov, V.; Alvaro, G.; Hajjar, L.; Paternoster, G.; Nigro Neto, C.; Latronico, N.; Fominskiy, E.; Pasin, L.; et al. Randomized Evidence for Reduction of Perioperative Mortality: An Updated Consensus Process. J. Cardiothorac. Vasc. Anesth. 2017, 31, 719–730. [Google Scholar] [CrossRef]
- Urbach, D.R.; Baxter, N.N. Reducing variation in surgical care. BMJ 2005, 330, 1401–1402. [Google Scholar] [CrossRef]
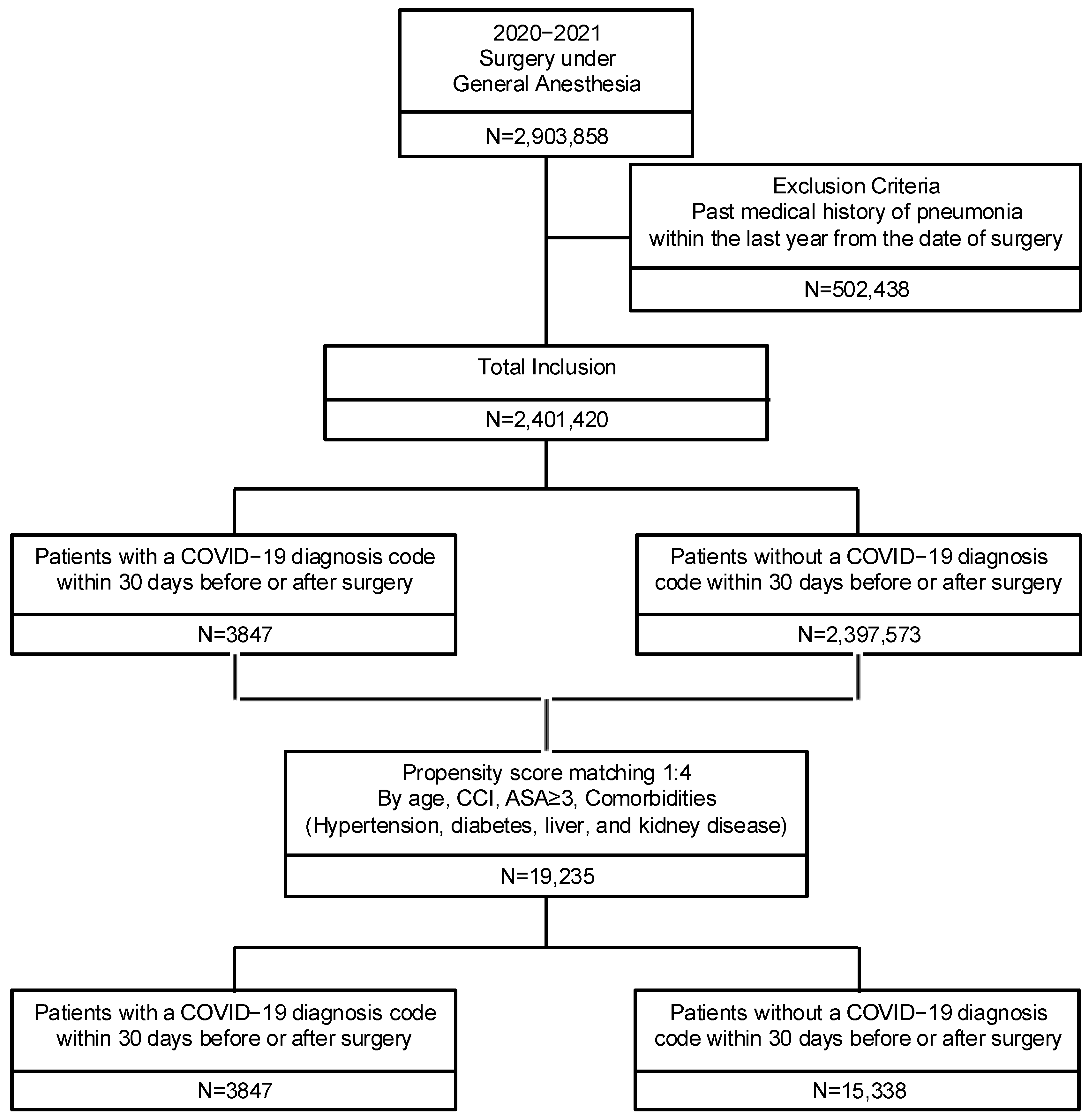

| Variable | Prematching | Postmatching | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (N = 2,401,420) | COVID (+) (N = 3847) | COVID (−) (N = 2,397,573) | p-Value | SMD | Total (N = 19,235) | COVID (+) (N = 3847) | COVID (−) (N = 15,388) | p-Value | SMD | |
| Year | ||||||||||
| 2020 | 1,200,631 (50) | 759 (19.73) | 1,199,872 (50.05) | <0.0001 | 8308 (43.19) | 759 (19.73) | 7549 (49.06) | <0.0001 | ||
| 2021 | 1,200,789 (50) | 3088 (80.27) | 1,197,701 (49.95) | 10,927 (56.81) | 3088 (80.27) | 7839 (50.94) | ||||
| Sex | ||||||||||
| Male | 1,013,593 (42.21) | 1780 (46.27) | 1,011,813 (42.2) | <0.0001 | 0.1463 | 8898 (46.26) | 1780 (46.27) | 7118 (46.26) | 0.9885 | 0.00026 |
| Female | 1,387,827 (57.79) | 2067 (53.73) | 1,385,760 (57.8) | 10,337 (53.74) | 2067 (53.73) | 8270 (53.74) | ||||
| Age | ||||||||||
| <18 years | 154,092 (6.42) | 240 (6.24) | 153,852 (6.42) | <0.0001 | 0.3812 | 1201 (6.24) | 240 (6.24) | 961 (6.25) | 0.9999 | 0.00022 |
| 18–69 years | 1,830,632 (76.23) | 2737 (71.15) | 1,827,895 (76.24) | 13,685 (71.15) | 2737 (71.15) | 10,948 (71.15) | ||||
| ≥70 years | 416,696 (17.35) | 870 (22.62) | 415,826 (17.34) | 4349 (22.61) | 870 (22.62) | 3479 (22.61) | ||||
| Hospital type | ||||||||||
| Tertiary Hospital | 840,699 (35.01) | 1978 (51.42) | 838,721 (34.98) | <0.0001 | 8032 (41.76) | 1978 (51.42) | 6054 (39.34) | <0.0001 | ||
| General Hospital | 787,954 (32.81) | 1125 (29.24) | 786,829 (32.82) | 6245 (32.47) | 1125 (29.24) | 5120 (33.27) | ||||
| Hospital | 515,418 (21.46) | 507 (13.18) | 514,911 (21.48) | 3428 (17.82) | 507 (13.18) | 2921 (18.98) | ||||
| Clinic | 257,349 (10.72) | 237 (6.16) | 257,112 (10.72) | 1530 (7.95) | 237 (6.16) | 1293 (8.4) | ||||
| Hospitalization Pathway | ||||||||||
| Emergency | 289,143 (12.04) | 518 (13.47) | 242,904 (10.13) | <0.0001 | 1379 (7.17) | 518 (13.47) | 1893 (12.3) | 0.0008 | ||
| Outpatient | 1,869,146 (77.84) | 3102 (80.63) | 288,625 (12.04) | 2411 (12.53) | 3102 (80.63) | 12,343 (80.21) | ||||
| Others | 243,131 (10.12) | 227 (5.9) | 1,866,044 (77.83) | 15,445 (80.3) | 227 (5.9) | 1152 (7.49) | ||||
| Emergency surgery | 31,493 (1.31) | 44 (1.14) | 31,449 (1.31) | 0.3602 | 274 (1.42) | 44 (1.14) | 230 (1.49) | 0.1004 | ||
| Night surgery | 61,790 (2.57) | 127 (3.3) | 61,663 (2.57) | 0.0043 | 542 (2.82) | 127 (3.3) | 415 (2.7) | 0.0428 | ||
| ASA 3 or higher | 237,558 (9.89) | 562 (14.61) | 236,996 (9.88) | <0.0001 | 2628 (13.66) | 562 (14.61) | 2066 (13.43) | 0.0561 | ||
| Comorbidities | ||||||||||
| History of Hypertension | 776,749 (32.35) | 1494 (38.84) | 775,255 (32.33) | <0.0001 | 7742 (40.25) | 1494 (38.84) | 6248 (40.6) | 0.0455 | ||
| History of Diabetes | 466,756 (19.44) | 972 (25.27) | 465,784 (19.43) | <0.0001 | 5347 (27.8) | 972 (25.27) | 4375 (28.43) | <0.0001 | ||
| History of liver disease | 680,836 (28.35) | 1461 (37.98) | 679,375 (28.34) | <0.0001 | 7136 (37.1) | 1461 (37.98) | 5675 (36.88) | 0.2072 | ||
| History of kidney disease | 87,212 (3.63) | 231 (6) | 86,981 (3.63) | <0.0001 | 1228 (6.38) | 231 (6) | 997 (6.48) | 0.2817 | ||
| History of CVA | 8327 (0.35) | 13 (0.34) | 8314 (0.35) | 0.9257 | 103 (0.54) | 13 (0.34) | 90 (0.58) | 0.0605 | ||
| Charson Comorbidity Index | ||||||||||
| Continuous | 1.91 (1.51–2.38) | 1.95 (1.59–2.59) | 1.91 (1.51–2.38) | 0.0471 | 0.010393 | 1.86 (1.59–2.52) | 1.9513 (1.59–2.59) | 1.863855 (1.60–2.52) | 1 | 0.00033 |
| 0 | 814,029 (33.9) | 855 (22.23) | 813,174 (33.92) | <0.0001 | 4275 (22.23) | 855 (22.23) | 3420 (22.23) | 1 | ||
| 1 | 512,184 (21.33) | 665 (17.29) | 511,519 (21.33) | 3325 (17.29) | 665 (17.29) | 2660 (17.29) | ||||
| 2 | 374,303 (15.59) | 558 (14.5) | 373,745 (15.59) | 2790 (14.5) | 558 (14.5) | 2232 (14.5) | ||||
| 3+ | 700,904 (29.19) | 1769 (45.98) | 699,135 (29.16) | 8845 (45.98) | 1769 (45.98) | 7076 (45.98) | ||||
| Elixhauser’s comorbidities | ||||||||||
| Weight (Continuous) | 4.68 (2.29–7.69) | 5.64 (3.53–9.16) | 4.68 (2.28–7.69) | <0.0001 | 5.03 (3.20–8.25) | 5.64 (3.52–9.16) | 4.88 (3.12–7.99) | <0.0001 | ||
| Variable | Prematching | Postmatching | ||||||
|---|---|---|---|---|---|---|---|---|
| Total (N = 2,401,420) | COVID (+) (N = 3847) | COVID (−) (N = 2,397,573) | p-Value | Total (N = 19,235) | COVID (+) (N = 3847) | COVID (−) (N = 15,388) | p-Value | |
| Overall mortality | 92,734 (3.86) | 280 (7.28) | 92,454 (3.86) | <0.0001 * | 1193 (6.20) | 280 (7.28) | 913 (5.93) | 0.002 * |
| 30 d mortality | 11,709 (0.49) | 33 (0.86) | 11,709 (0.49) | 0.001 * | 141 (0.73) | 33 (0.86) | 108 (0.70) | 0.3104 |
| Time to death (days) | 305.2 (167.9) | 220.9 (155.9) | 305.5 (168.0) | <0.0001 * | 290.2 (157.5) | 220.9 (155.9) | 311.4 (158.2) | <0.0001 * |
| Postoperative complication | ||||||||
| Pneumonia | 73,402 (3.06) | 481 (12.5) | 72,921 (3.04) | <0.0001 * | 1097 (5.7) | 481 (12.5) | 616 (4) | <0.0001 * |
| ARDS | 1774 (0.07) | 33 (0.86) | 1741 (0.07) | <0.0001 * | 41 (0.21) | 33 (0.86) | 8 (0.05) | <0.0001 * |
| PTE | 17,452 (0.73) | 67 (1.74) | 17,385 (0.73) | <0.0001 * | 194 (1.01) | 67 (1.74) | 127 (0.83) | <0.0001 * |
| Thromboembolic event | 18,333 (0.76) | 40 (1.04) | 18,293 (0.76) | 0.0487 * | 197 (1.02) | 40 (1.04) | 157 (1.02) | 0.9145 |
| Mechanical ventilation | 1177 (0.05) | 5 (0.13) | 1172 (0.05) | 0.0232 * | 12 (0.06) | 5 (0.13) | 7 (0.05) | 0.0605 |
| Cardiac arrest | 8300 (0.35) | 14 (0.36) | 8286 (0.35) | 0.8466 | 89 (0.46) | 14 (0.36) | 75 (0.49) | 0.3128 |
| MI | 23,132 (0.96) | 40 (1.04) | 23,092 (0.96) | 0.6268 | 265 (1.38) | 40 (1.04) | 225 (1.46) | 0.0444 |
| Surgical site infection | 66,537 (2.77) | 258 (6.71) | 66,279 (2.76) | <0.0001 * | 766 (3.98) | 258 (6.71) | 508 (3.3) | <0.0001 * |
| Sepsis | 37,158 (1.55) | 123 (3.2) | 37,035 (1.54) | <0.0001 * | 492 (2.56) | 123 (3.2) | 369 (2.4) | 0.005 * |
| ARF | 33,711 (1.4) | 72 (1.87) | 33,639 (1.4) | 0.0136 * | 386 (2.01) | 72 (1.87) | 314 (2.04) | 0.5039 |
| Hepatic failure | 8392 (0.35) | 32 (0.83) | 8360 (0.35) | <0.0001 * | 124 (0.64) | 32 (0.83) | 92 (0.6) | 0.1049 |
| Hospitalization | ||||||||
| Hospitalization | 2,241,214 (93.38) | 3739 (90.30) | 2,237,475 (93.32) | 18,388 (95.60) | 3739 (90.30) | 14,649 (95.20) | ||
| Hospital length of stay | 21.8 (14.7–52.3) | 32.3 (10.0–63.7) | 21.7 (14.7–52.2) | <0.0001 * | 24.4 (13.2–55.9) | 32.3 (10.00–63.7) | 22.4 (14.0–53.6) | <0.0001 * |
| Hospitalization costs | 11,867 (5933–24,562) | 7473 (3345–17,618) | 11,857 (5931–24,544) | <0.0001 * | 13,499 (6887–27,580) | 17,619 (7473–33,452) | 12,452 (6737–25,773) | <0.0001 * |
| ICU Admission | ||||||||
| ICU admission | 213,008 (8.87) | 569 (14.79) | 212,439 (8.86) | <0.0001 * | 2310 (12.01) | 569 (14.79) | 1741 (11.31) | <0.0001 * |
| ICU length of stay | 72.2 (17.8–245.6) | 156.0 (17.7–1487.6) | 72.0 (17.8–236.0) | 0.9038 | 89.9 (17.8–746.1) | 156.0 (17.7–1487.6) | 69.3 (17.8–190.4) | 0.9415 |
| ICU costs | 76,450 (19,885–238,958) | 103,490 (20,067–1,003,210) | 76,322 (19,885–234,360) | 0.8133 | 88,293 (19,998–523,600) | 103,490 (20,067–136,210) | 73,246 (19,976–154,000) | 0.928 |
| ECMO implementation | 18,360 (0.76) | 29 (0.75) | 18,331 (0.76) | 0.9391 | 158 (0.82) | 29 (0.75) | 129 (0.84) | 0.6036 |
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Crude OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | |
| COVID Group | ||||
| COVID (–) | 1 | 1 | ||
| COVID (+) | 1.224 (0.828–1.811) | 0.311 | 1.189 (0.788–1.794) | 0.4087 |
| Year | ||||
| 2020 | 1 | |||
| 2021 | 1.057 (0.756–1.479) | 0.7457 | ||
| Sex | ||||
| Male | 1 | 1 | ||
| Female | 0.548 (0.39–0.77) | 0.0005 | ||
| Age | ||||
| <70 years | 1 | 1 | ||
| ≥70 years | 4.974 (3.551–6.969) | <0.0001 | 2.407 (1.68–3.449) | <0.0001 * |
| Hospital type | ||||
| Tertiary care hospital | 1 | 1 | ||
| General hospital | 1.45 (1.036–2.028) | <0.0001 | 1.46 (1.037–2.055) | 0.0008 * |
| Hospital | 0.072 (0.018–0.292) | 0.0143 | 0.167 (0.04–0.692) | 0.0667 |
| Clinic | 0.08 (0.011–0.578) | 0.0858 | 0.242 (0.033–1.775) | 0.3595 |
| Emergency surgery | 7.985 (4.537–14.055) | <0.0001 | 5.468 (3.026–9.881) | <0.0001 * |
| ASA 3 or higher | 10.821 (7.677–15.254) | <0.0001 | 4.803 (3.297–6.997) | <0.0001 * |
| Comorbidities | ||||
| History of hypertension | 3.531 (2.459–5.072) | <0.0001 | ||
| History of diabetes | 2.302 (1.651–3.209) | <0.0001 | ||
| History of liver disease | 1.829 (1.313–2.548) | 0.0004 | 1.429 (1.019–2.005) | 0.0386 * |
| History of kidney disease | 5.969 (4.12–8.649) | <0.0001 | 2.555 (1.724–3.786) | <0.0001 * |
| History of CVA | 2.706 (0.661–11.077) | 0.1663 | ||
| Charson Comorbidity Index | ||||
| 0 | 1 | |||
| 1 | 1.674 (0.733–3.822) | 0.3033 | ||
| 2 | 2.46 (1.115–5.429) | 0.5035 | ||
| 3+ | 4.976 (2.596–9.536) | <0.0001 | ||
| Elixhauser’s comorbidities | ||||
| Weight(Continuous) | 1.26 (1.201–1.321) | <0.0001 | ||
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Crude OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | |
| COVID Group | ||||
| COVID (–) | 1 | 1 | ||
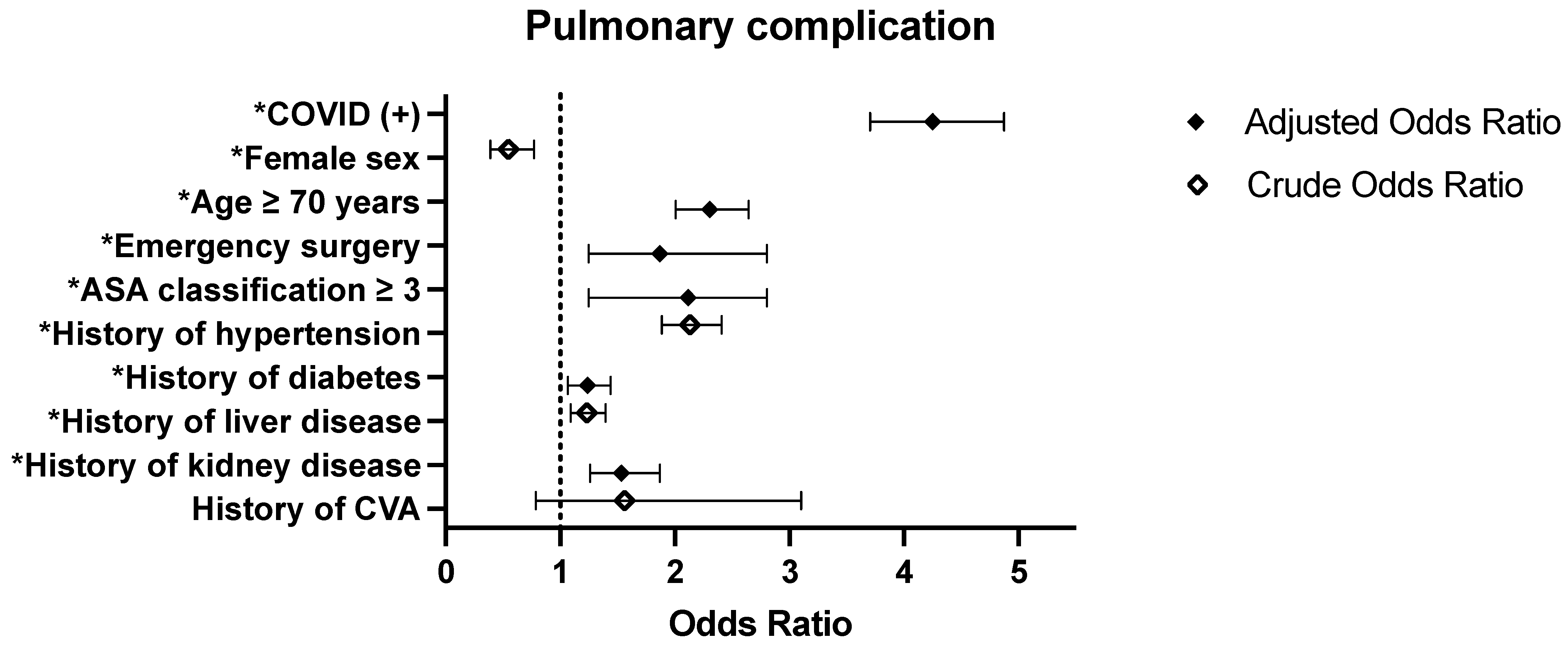
| COVID (+) | 3.503 (3.095–3.966) | <0.0001 | 4.25 (3.704–4.875) | <0.0001 * |
| Year | ||||
| 2020 | 1 | 1 | ||
| 2021 | 0.991 (0.877–1.119) | 0.8807 | 0.697 (0.609–0.798) | <0.0001 * |
| Sex | ||||
| Male | 1 | 1 | ||
| Female | 0.548 (0.39–0.77) | 0.0005 | ||
| Age | ||||
| <70 years | 1 | 1 | ||
| ≥70 years | 3.327 (2.942–3.762) | <0.0001 | 2.303 (2.006–2.644) | <0.0001 * |
| Hospital type | ||||
| Tertiary care hospital | 1 | 1 | ||
| General hospital | 1.113 (0.976–1.27) | <0.0001 | 1.243 (1.08–1.43) | 0.0011 * |
| Hospital | 0.551 (0.451–0.674) | 0.004 | 0.958 (0.774–1.187) | 0.5446 |
| Clinic | 0.384 (0.276–0.535) | <0.0001 | 0.868 (0.614–1.227) | 0.2457 |
| Emergency surgery | 2.104 (1.441–3.074) | 0.0001 | 1.869 (1.246–2.803) | 0.0025 * |
| ASA 3 or higher | 3.601 (3.157–4.108) | <0.0001 | 2.115 (1.246–2.803) | <0.0001 * |
| Comorbidities | ||||
| History of hypertension | 2.132 (1.886–2.409) | <0.0001 | ||
| History of diabetes | 2.018 (1.784–2.283) | <0.0001 | 1.238 (1.066–1.437) | 0.0052 * |
| History of liver disease | 1.233 (1.09–1.394) | 0.0008 | ||
| History of kidney disease | 2.878 (2.416–3.429) | <0.0001 | 1.533 (1.259–1.868) | <0.0001 * |
| History of CVA | 1.56 (0.785–3.101) | 0.204 | ||
| Charson Comorbidity Index | ||||
| 0 | 1 | 1 | ||
| 1 | 1.381 (1.082–1.762) | 0.0261 | 1.231 (0.959–1.581) | 0.9933 |
| 2 | 1.85 (1.457–2.349) | 0.0627 | 1.344 (1.045–1.728) | 0.2247 |
| 3+ | 2.715 (2.243–3.286) | <0.0001 | 1.384 (1.103–1.737) | 0.0505 |
| Elixhauser’s comorbidities | ||||
| Weight (Continuous) | 1.182 (1.16–1.205) | <0.0001 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.J.; Ahn, E.; Oh, E.J.; Bang, S.R. Perioperative Coronavirus Disease 2019 Infection and Its Impact on Postoperative Outcomes: Pulmonary Complications and Mortality Based on Korean National Health Insurance Data. J. Pers. Med. 2025, 15, 157. https://doi.org/10.3390/jpm15040157
Kim HJ, Ahn E, Oh EJ, Bang SR. Perioperative Coronavirus Disease 2019 Infection and Its Impact on Postoperative Outcomes: Pulmonary Complications and Mortality Based on Korean National Health Insurance Data. Journal of Personalized Medicine. 2025; 15(4):157. https://doi.org/10.3390/jpm15040157
Chicago/Turabian StyleKim, Hyo Jin, EunJin Ahn, Eun Jung Oh, and Si Ra Bang. 2025. "Perioperative Coronavirus Disease 2019 Infection and Its Impact on Postoperative Outcomes: Pulmonary Complications and Mortality Based on Korean National Health Insurance Data" Journal of Personalized Medicine 15, no. 4: 157. https://doi.org/10.3390/jpm15040157
APA StyleKim, H. J., Ahn, E., Oh, E. J., & Bang, S. R. (2025). Perioperative Coronavirus Disease 2019 Infection and Its Impact on Postoperative Outcomes: Pulmonary Complications and Mortality Based on Korean National Health Insurance Data. Journal of Personalized Medicine, 15(4), 157. https://doi.org/10.3390/jpm15040157






