The Actual Role of CPET in Predicting Postoperative Morbidity and Mortality of Patients Undergoing Pneumonectomy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Pre-Admission Exams
2.3. Cardiopulmonary Exercise Testing/Lung Perfusion Scan
2.4. Surgical Procedure and Postoperative Course
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PFT | pulmonary function testing |
| CPET | cardiopulmonary exercise testing |
| 99Tc-MAA | 99Tecnetium (99Tc)-labeled macroaggregate albumin (MAA) |
| VO2max | maximal oxygen consumption |
| (ppoVO2)VO2max | predictive postoperative maximal oxygen consumption |
| FEV1 | expiratory forced volume |
| DLCO | diffusing capacity of the lungs for carbon monoxide |
References
- Schussler, O.; Alifano, M.; Dermine, H.; Strano, S.; Casetta, A.; Sepulveda, S.; Chafik, A.; Coignard, S.; Rabbat, A.; Regnard, J.-F. Postoperative pneumonia after major lung resection. Am. J. Respir. Crit. Care Med. 2006, 173, 1161–1169. [Google Scholar] [PubMed]
- Algar, F.J.; Alvarez, A.; Salvatierra, A.; Baamonde, C.; Aranda, J.L. Predicting pulmonary complications after pneumonectomy for lung cancer. Eur. J. Cardiothorac. Surg. 2003, 23, 201–208. [Google Scholar] [PubMed]
- Mazzella, A.; Pardolesi, A.; Maisonneuve, P.; Petrella, F.; Galetta, D.; Gasparri, R.; Spaggiari, L. Bronchopleural Fistula After pneumonectomy: Risk Factors and Management, Focusing on Open-Window Thoracostomy. Semin. Thorac. Cardiovasc. Surg. 2018, 30, 104–113. [Google Scholar] [PubMed]
- Groth, S.S.; Burt, B.M.; Sugarbaker, D.J. Management of Complications After Pneumonectomy. Thorac. Surg. Clin. 2015, 25, 335–348. [Google Scholar] [CrossRef]
- Janet-Vendroux, A.; Loi, M.; Bobbio, A.; Lococo, F.; Lupo, A.; Ledinot, P.; Magdeleinat, P.; Roche, N.; Damotte, D.; Regnard, J.-F.; et al. Which is the Role of Pneumonectomy in the Era of Parenchymal-Sparing Procedures? Early/Long-Term Survival and Functional Results of a Single-Center Experience. Lung 2015, 193, 965–973. [Google Scholar]
- Mazzella, A.; Bertolaccini, L.; Sedda, G.; Prisciandaro, E.; Loi, M.; Iacono, G.L.; Spaggiari, L. Pneumonectomy and broncho-pleural fistula: Predicting factors and stratification of the risk. Updates Surg. 2022, 74, 1471–1478. [Google Scholar]
- Mazzella, A.; Mohamed, S.; Maisonneuve, P.; Borri, A.; Casiraghi, M.; Bertolaccini, L.; Petrella, F.; Iacono, G.L.; Spaggiari, L. ARDS after Pneumonectomy: How to Prevent It? Development of a Nomogram to Predict the Risk of ARDS after Pneumonectomy for Lung Cancer. Cancers 2022, 14, 6048. [Google Scholar] [CrossRef]
- Deslauriers, J.; Ugalde, P.; Miro, S.; Deslauriers, D.R.; Ferland, S.; Bergeron, S.; Lacasse, Y.; Provencher, S. Long-term physiological consequences of pneumonectomy. Semin. Thorac. Cardiovasc. Surg. 2011, 23, 196–202. [Google Scholar]
- Brunelli, A.; Charloux, A.; Bolliger, C.T.; Rocco, G.; Sculier, J.P.; Varela, G.; Licker, M.; Ferguson, M.K.; Faivre-Finn, C.; Huber, R.M.; et al. European Respiratory Society and European Society of Thoracic Surgeons joint task force on fitness for radical therapy. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur. Respir. J. 2009, 34, 17–41. [Google Scholar]
- American Thoracic Society: American College of Chest Physicians. ATS/ACCP Statement on cardiopulmonary exercise testing. Am. J. Respir. Crit. Care Med. 2003, 167, 211–277. [Google Scholar]
- Brunelli, A.; Kim, A.W.; Berger, K.I.; Addrizzo-Harris, D.J. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed.: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143 (Suppl. S5), e166S–e190S. [Google Scholar] [CrossRef] [PubMed]
- Sawabata, N.; Nagayasu, T.; Kadota, Y.; Goto, T.; Horio, H.; Mori, T.; Yamashita, S.; Iwasaki, A. Risk assessment of lung resection for lung cancer according to pulmonary function: Republication of systematic review and proposals by guideline committee of the Japanese association for chest surgery 2014. Gen. Thorac. Cardiovasc. Surg. 2015, 63, 14–21. [Google Scholar] [CrossRef] [PubMed]
- British Thoracic Society; Society of Cardiothoracic Surgeons of Great Britain and Ireland Working Party. BTS guidelines: Guidelines on the selection of patients with lung cancer for surgery. Thorax 2001, 56, 89–108. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Fernandez, F.G.; Falcoz, P.E.; Kozower, B.D.; Salati, M.; Wright, C.D.; Brunelli, A. The Society of Thoracic Surgeons and the European Society of Thoracic Surgeons general thoracic surgery databases, joint standardization of variable definitions and terminology. Ann. Thorac. Surg. 2015, 99, 368–376. [Google Scholar] [CrossRef]
- Villani, F.; De Maria, P.; Busia, A. Exercise testing as a predictor of surgical risk after pneumonectomy for bronchogenic carcinoma. Respir. Med. 2003, 97, 1296–1298. [Google Scholar] [CrossRef]
- Bolliger, C.T.; Wyser, C.; Roser, H.; Solèr, M.; Perruchoud, A.P. Lung scanning and exercise testing for the prediction of postoperative performance in lung resection candidates at increased risk for complications. Chest 1995, 108, 341–348. [Google Scholar] [CrossRef]
- Kallianos, A.; Rapti, A.; Tsimpoukis, S.; Charpidou, A.; Dannos, I.; Kainis, E.; Syrigos, K. Cardiopulmonary exercise testing (CPET) as preoperative test before lung resection. In Vivo 2014, 28, 1013–1020. [Google Scholar]
- Gooseman, M.R.; Brunelli, A. Cardio-Pulmonary Exercise Testing Prior to Major Surgery. Ann. Surg. Oncol. 2020, 27, 3583–3584. [Google Scholar] [CrossRef]
- Jiang, G.; Zhang, L.; Zhu, Y.; Chen, C.; Zhou, X.; Liu, J.; Zhang, P.; Wang, H.; Xie, B.; Wang, H.; et al. Clinical consensus on preoperative pulmonary function assessment in patients undergoing pulmonary resection (first edition). Curr. Chall. Thorac. Surg. 2019, 1, 7. [Google Scholar] [CrossRef]
- Loewen, G.M.; Watson, D.; Kohman, L.; Herndon, J.E.; Shennib, H.; Kernstine, K.; Olak, J.; Mador, M.J.; Harpole, D.; Sugarbaker, D.; et al. Cancer and Leukemia Group B. Preoperative exercise Vo2 measurement for lung resection candidates: Results of Cancer and Leukemia Group B Protocol 9238. J. Thorac. Oncol. 2007, 2, 619–625. [Google Scholar] [PubMed]
- Arbee-Kalidas, N.; Moutlana, H.J.; Moodley, Y.; Kebalepile, M.M.; Chakane, P.M. The association between cardiopulmonary exercise testing and postoperative outcomes in patients with lung cancer undergoing lung resection surgery: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0295430. [Google Scholar]
- Benzo, R.; Kelley, G.A.; Recchi, L.; Hofman, A.; Sciurba, F. Complications of lung resection and exercise capacity: A meta-analysis. Respir. Med. 2007, 101, 1790–1797. [Google Scholar] [PubMed]
- Brunelli, A.; Belardinelli, R.; Refai, M.; Socci, L.; Pompili, C.; Sabbatini, A. Peak oxygen consumption during cardiopulmonary exercise test improves risk stratification in candidates to major lung resection. Chest 2009, 135, 1260–1267. [Google Scholar] [CrossRef]
- Brunelli, A.; Pompili, C.; Refai, M.; Xiumè, F.; Salati, M.; Sabbatini, A. Predicted versus observed peak oxygen consumption after major pulmonary resection. Ann. Thorac. Surg. 2012, 94, 222–225. [Google Scholar]
- Lim, E.; Beckles, M.; Warburton, C.; Baldwin, D. Cardiopulmonary exercise testing for the selection of patients undergoing surgery for lung cancer: Friend or foe? Thorax 2010, 65, 847–849. [Google Scholar]
- Jones, G.D.; Caso, R.; Tan, K.S.; Dycoco, J.; Adusumilli, P.S.; Bains, M.S.; Downey, R.J.; Huang, J.; Isbell, J.M.; Molena, D.; et al. Propensity-matched Analysis Demonstrates Long-term Risk of Respiratory and Cardiac Mortality After Pneumonectomy Compared with Lobectomy for Lung Cancer. Ann. Surg. 2022, 275, 793–799. [Google Scholar]
- Gooseman, M.R.; Falcoz, P.E.; Decaluwe, H.; Szanto, Z.; Brunelli, A. Morbidity and mortality of lung resection candidates defined by the American College of Chest Physicians as ‘moderate risk’: An analysis from the European Society of Thoracic Surgeons database. Eur. J. Cardiothorac. Surg. 2021, 60, 91–97. [Google Scholar]
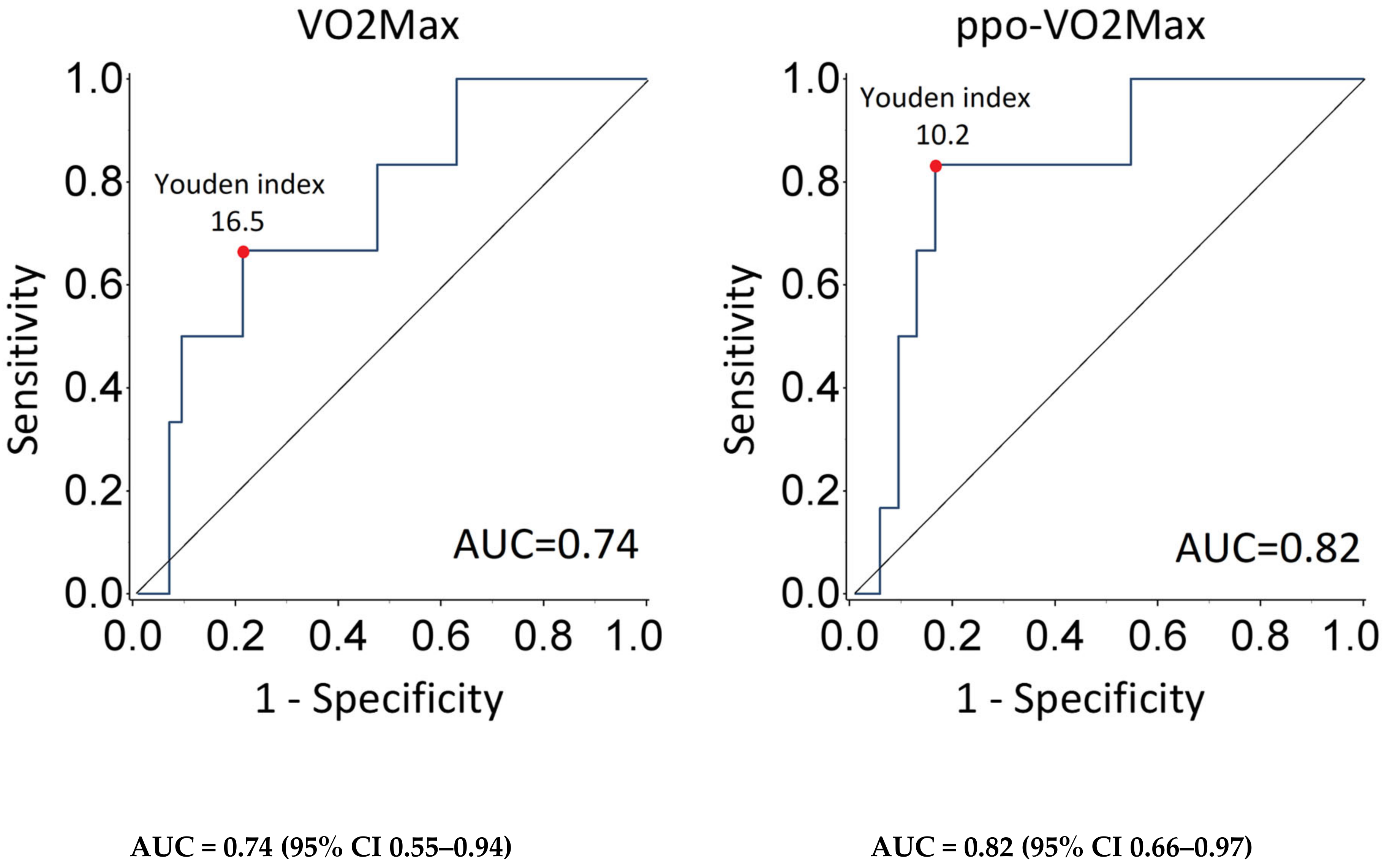
| Patients | VO2max | ppo-VO2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | Mean ± SD | p-Value | <15 | ≥15 | p-Value | Median (IQR) | p-Value | <10 | ≥10 | p-Value | ||
| All | 90 (100.0) | 19.5 ± 4.3 | 11 (100.0) | 79 (100.0) | 12.2 (10.4–14.1) | 17 (100.0) | 73 (100.0) | |||||
| Age | ||||||||||||
| <60 | 19 (21.1) | 20.7 ± 4.6 | 2 (18.2) | 17 (21.5) | 12.5 (11.7–15.1) | 3 (17.6) | 16 (21.9) | |||||
| 60–64 | 17 (18.9) | 20.1 ± 3.6 | 2 (18.2) | 15 (19.0) | 13.0 (11.1–14.9) | 2 (11.8) | 15 (20.5) | |||||
| 65–69 | 25 (27.8) | 19.4 ± 5.3 | 4 (36.4) | 21 (26.6) | 12.2 (10.2–13.1) | 5 (29.4) | 20 (27.4) | |||||
| 70+ | 29 (32.2) | 18.6 ± 3.6 | 0.36 | 3 (27.3) | 26 (32.9) | 0.84 | 11.7 (10.1–13.7) | 0.38 | 7 (41.2) | 22 (30.1) | 0.56 | |
| Sex | ||||||||||||
| Male | 61 (67.8) | 20.1 ± 4.4 | 6 (54.5) | 55 (69.6) | 12.6 (10.6–14.6) | 11 (64.7) | 50 (68.5) | |||||
| Female | 29 (32.2) | 18.3 ± 4.0 | 0.06 | 5 (45.5) | 24 (30.4) | 0.32 | 11.6 (10.2–12.9) | 0.08 | 6 (35.3) | 23 (31.5) | 0.78 | |
| BMI | ||||||||||||
| Normal weight | 55 (61.1) | 20.8 ± 4.6 | 3 (27.3) | 52 (65.8) | 12.9 (11.2–14.9) | 8 (47.1) | 47 (64.4) | |||||
| Over weight | 30 (33.3) | 17.6 ± 3.2 | 6 (54.5) | 24 (30.4) | 11.5 (9.2–12.8) | 8 (47.1) | 22 (30.1) | |||||
| Obese | 5 (5.6) | 17.3 ± 2.9 | 0.002 | 2 (18.2) | 3 (3.8) | 0.008 | 12.2 (10.4–13.4) | 0.038 | 1 (5.9) | 4 (5.5) | 0.23 | |
| ASA score | ||||||||||||
| 1 | 2 (2.2) | 29.3 ± 4.2 | 0 (0.0) | 2 (2.5) | 18.5 (15.1–21.9) | 0 (0.0) | 2 (2.7) | |||||
| 2 | 55 (61.1) | 20.7 ± 4.0 | 5 (45.5) | 50 (63.3) | 13.0 (11.5–14.8) | 5 (29.4) | 50 (68.5) | |||||
| 3 | 33 (36.7) | 17.1 ± 3.1 | <0.0001 | 6 (54.5) | 27 (34.2) | 0.17 | 11.0 (9.7–12.5) | 0.0002 | 12 (70.6) | 21 (28.8) | 0.002 | |
| CCI | ||||||||||||
| Mild | 6 (6.7) | 24.2 ± 5.8 | 1 (9.1) | 5 (6.3) | 17.0 (11.8–20.9) | 1 (5.9) | 5 (6.8) | |||||
| Moderate | 35 (38.9) | 20.3 ± 4.1 | 3 (27.3) | 32 (40.5) | 12.4 (11.5–14.5) | 5 (29.4) | 30 (41.1) | |||||
| Severe | 49 (54.4) | 18.5 ± 3.9 | 0.004 | 7 (63.6) | 42 (53.2) | 0.61 | 11.7 (10.2–13.6) | 0.039 | 11 (64.7) | 38 (52.1) | 0.38 | |
| Comorbidity | ||||||||||||
| No | 39 (43.3) | 21.6 ± 4.0 | 1 (9.1) | 38 (48.1) | 13.3 (11.5–14.9) | 4 (23.5) | 35 (47.9) | |||||
| Yes | 51 (56.7) | 18.0 ± 3.9 | <0.0001 | 10 (90.9) | 41 (51.9) | 0.02 | 11.7 (9.9–13.1) | 0.005 | 13 (76.5) | 38 (52.1) | 0.10 | |
| Cardiovascular | ||||||||||||
| No | 60 (66.7) | 20.7 ± 4.1 | 4 (36.4) | 56 (70.9) | 12.9 (11.1–14.8) | 8 (47.1) | 52 (71.2) | |||||
| Yes | 30 (33.3) | 17.2 ± 3.8 | 0.0002 | 7 (63.6) | 23 (29.1) | 0.04 | 11.1 (9.9–12.6) | 0.001 | 9 (52.9) | 21 (28.8) | 0.09 | |
| Pulmonary | ||||||||||||
| No | 81 (90.0) | 19.8 ± 4.3 | 9 (81.8) | 72 (91.1) | 12.3 (10.6–14.2) | 13 (76.5) | 68 (93.2) | |||||
| Yes | 9 (10.0) | 17.1 ± 4.0 | 0.07 | 2 (18.2) | 7 (8.9) | 0.30 | 10.2 (9.2–12.5) | 0.09 | 4 (23.5) | 5 (6.8) | 0.06 | |
| Previous malignancy | ||||||||||||
| No | 74 (82.2) | 20.0 ± 4.2 | 7 (63.6) | 67 (84.8) | 12.3 (11.0–14.2) | 13 (76.5) | 61 (83.6) | |||||
| Yes | 16 (17.8) | 17.6 ± 4.6 | 0.047 | 4 (36.4) | 12 (15.2) | 0.10 | 10.6 (9.8–14.0) | 0.30 | 4 (23.5) | 12 (16.4) | 0.49 | |
| Smoking | ||||||||||||
| No | 27 (30.0) | 20.1 ± 4.5 | 3 (27.3) | 24 (30.4) | 12.5 (10.8–16.0) | 3 (17.6) | 24 (32.9) | |||||
| Current | 24 (26.7) | 20.4 ± 4.3 | 2 (18.2) | 22 (27.8) | 12.3 (11.0–13.7) | 3 (17.6) | 21 (28.8) | |||||
| Ex | 37 (41.1) | 18.4 ± 4.1 | 0.15 | 6 (54.5) | 31 (39.2) | 0.72 | 11.7 (9.7–13.6) | 0.16 | 11 (64.7) | 26 (35.6) | 0.13 | |
| COPD | ||||||||||||
| No | 73 (81.1) | 19.7 ± 4.4 | 8 (72.7) | 65 (82.3) | 12.2 (10.6–13.9) | 10 (58.8) | 63 (86.3) | |||||
| Yes | 17 (18.9) | 18.9 ± 4.2 | 0.50 | 3 (27.3) | 14 (17.7) | 0.43 | 12.2 (9.2–14.2) | 0.46 | 7 (41.2) | 10 (13.7) | 0.02 | |
| Diabetes | ||||||||||||
| No | 82 (91.1) | 19.6 ± 4.3 | 10 (90.9) | 72 (91.1) | 12.2 (10.4–14.2) | 16 (94.1) | 66 (90.4) | |||||
| Yes | 8 (8.9) | 18.5 ± 4.7 | 0.50 | 1 (9.1) | 7 (8.9) | 1.00 | 12.5 (11.4–13.0) | 0.76 | 1 (5.9) | 7 (9.6) | 1.00 | |
| Cardiac (Hypertension) | ||||||||||||
| No | 55 (61.1) | 20.5 ± 4.3 | 4 (36.4) | 51 (64.6) | 12.5 (11.0–14.9) | 6 (35.3) | 49 (67.1) | |||||
| Yes | 35 (38.9) | 18.0 ± 4.0 | 0.006 | 7 (63.6) | 28 (35.4) | 0.10 | 11.7 (9.2–13.1) | 0.014 | 11 (64.7) | 24 (32.9) | 0.03 | |
| Patients | VO2max | ppo-VO2 | Mortality | ||||
|---|---|---|---|---|---|---|---|
| <15 | ≥15 | <10 | ≥10 | 30 days | 90 days | ||
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| All patients | 90 (100) | 12 (12.2) | 78 (87.8) | 17 (18.9) | 73 (81.1) | 2 (2.2) | 6 (6.7) |
| ASA score | |||||||
| I | 2 (2.2) | 0 (0.0) | 2 (100.) | 0 (0.0) | 2 (100.) | 0 (0.0) | 0 (0.0) |
| II | 55 (61.1) | 5 (9.1) | 50 (90.1) | 5 (9.1) | 50 (90.1) | 1 (1.8) | 3 (5.5) |
| III | 33 (36.7) | 6 (18.2) | 27 (81.8) | 12 (36.4) | 21 (63.6) | 1 (3.0) | 3 (9.1) |
| p-value | 0.17 | 0.002 | 0.67 | 0.45 | |||
| Patients | VO2max | ppo-VO2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | Mean ± SD | p-Value | <15 | ≥15 | p-Value | Median (IQR) | p-Value | <10 | ≥10 | p-Value | ||
| All | 90 (100.0) | 19.5 ± 4.3 | 11 | 79 | 12.2 (10.4–14.1) | 17 (100) | 73 (100) | |||||
| ARDS | 6 (6.7) | 16.6 ± 3.1 | 0.09 | 2 (18.2) | 4 (5.1) | 0.16 | 9.5 (8.8–9.9) | 0.007 | 5 (29.4) | 1 (1.4) | 0.0007 | |
| Fistula | 9 (10.0) | 22.1 ± 5.3 | 0.06 | 0 (0.0) | 9 (11.4) | 0.59 | 13.8 (12.5–14.9) | 0.09 | 0 (0.0) | 9 (12.3) | 0.20 | |
| Any complications | 42 (46.7) | 19.4 ± 4.7 | 0.74 | 7 (63.6) | 35 (44.3) | 0.34 | 12.2 (9.9–13.8) | 0.40 | 12 (70.6) | 30 (41.1) | 0.03 | |
| Pulmonary | 11 (12.2) | 17.3 ± 4.6 | 0.07 | 3 (27.3) | 8 (10.1) | 0.13 | 9.9 (8.7–12.5) | 0.008 | 7 (41.2) | 4 (5.5) | 0.0005 | |
| Cardiac | 18 (20.0) | 18.2 ± 4.7 | 0.15 | 3 (27.3) | 15 (19.0) | 0.69 | 10.7 (9.2–12.6) | 0.008 | 8 (47.1) | 10 (13.7) | 0.005 | |
| Other | 32 (35.6) | 19.4 ± 4.3 | 0.85 | 4 (36.4) | 28 (35.4) | 1.00 | 12.3 (10.5–14.3) | 0.81 | 7 (41.2) | 25 (34.3) | 0.59 | |
| Mortality 30 days | 2 (2.2) | 17.5 ± 5.3 | 0.49 | 1 (9.1) | 1 (1.3) | 0.23 | 9.3 (8.7–9.9) | 0.31 | 2 (11.8) | 0 (0.0) | 0.03 | |
| Mortality 90 days | 6 (6.7) | 16.5 ± 3.1 | 0.07 | 3 (27.3) | 3 (3.8) | 0.02 | 9.5 (9.0–10.2) | 0.055 | 4 (23.5) | 2 (2.7) | 0.01 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzella, A.; Orlandi, R.; Maisonneuve, P.; Uslenghi, C.; Chiari, M.; Casiraghi, M.; Bertolaccini, L.; Caffarena, G.; Spaggiari, L. The Actual Role of CPET in Predicting Postoperative Morbidity and Mortality of Patients Undergoing Pneumonectomy. J. Pers. Med. 2025, 15, 136. https://doi.org/10.3390/jpm15040136
Mazzella A, Orlandi R, Maisonneuve P, Uslenghi C, Chiari M, Casiraghi M, Bertolaccini L, Caffarena G, Spaggiari L. The Actual Role of CPET in Predicting Postoperative Morbidity and Mortality of Patients Undergoing Pneumonectomy. Journal of Personalized Medicine. 2025; 15(4):136. https://doi.org/10.3390/jpm15040136
Chicago/Turabian StyleMazzella, Antonio, Riccardo Orlandi, Patrick Maisonneuve, Clarissa Uslenghi, Matteo Chiari, Monica Casiraghi, Luca Bertolaccini, Giovanni Caffarena, and Lorenzo Spaggiari. 2025. "The Actual Role of CPET in Predicting Postoperative Morbidity and Mortality of Patients Undergoing Pneumonectomy" Journal of Personalized Medicine 15, no. 4: 136. https://doi.org/10.3390/jpm15040136
APA StyleMazzella, A., Orlandi, R., Maisonneuve, P., Uslenghi, C., Chiari, M., Casiraghi, M., Bertolaccini, L., Caffarena, G., & Spaggiari, L. (2025). The Actual Role of CPET in Predicting Postoperative Morbidity and Mortality of Patients Undergoing Pneumonectomy. Journal of Personalized Medicine, 15(4), 136. https://doi.org/10.3390/jpm15040136









