Abstract
Background/Objectives: The COVID-19 pandemic increased psychiatric symptoms in patients with pre-pandemic mental health conditions. However, the effects of pandemic on the brain, stress, and mental illness remain largely conjectural. Our objective was to examine how the pandemic affected prefrontal cortical thicknesses (CTs), stress, and PTSD symptoms in people with pre-pandemic trauma histories. Methods: Fifty-one survivors from a pre-pandemic trauma study who had completed a pre-pandemic PTSD Checklist-5 (PCL) to assess PTSD symptoms and a sMRI scan to measure prefrontal CTs were re-recruited after the pandemic. They subsequently completed the COVID Stress Scale (CSS) to assess stress, the Clinician Administered PTSD Scale-5 (CAPS) to diagnose PTSD, and a second sMRI scan. COVID-19 infection was self-reported. Associations between stress and symptom assessments and post-pandemic CTs, differences in CTs in PTSD vs. non-PTSD groups, and changes in pre- to post-pandemic CTs were examined. Results: Pre-pandemic PCL scores were positively associated with CSS scores which, in turn, were higher in the PTSD group. Thicker IFG-opercularis CTs were associated with COVID-19 infection. Post-pandemic rMFG and IFG-orbitalis CTs were positively associated with CAPS scores. rACC CTs were negatively associated with CSS scores. Pre- to post-pandemic rMFG and frontal pole CTs thickened in the PTSD group but thinned in the non-PTSD group, whereas rACC CTs thinned in the PTSD group but thickened in the non-PTSD group. Conclusions: These findings provide novel evidence that the COVID-19 pandemic had diverse effects involving prefrontal cortex structure, stress, and PTSD symptoms in subjects with pre-pandemic trauma history and suggest that treatments are needed to counter these diverse effects.
1. Introduction
The COVID-19 pandemic caused unprecedented mental health challenges, including prolonged and elevated global psychological distress [1,2]. Significant pandemic-related increases in depression, anxiety, and posttraumatic stress disorder (PTSD) in diverse populations, e.g., healthcare workers, young adults, and others, have been attributed to pandemic-related stress [3,4]. High levels of stress, anxiety, and PTSD have been linked to pandemic-related fear and lockdown [5].
Stress affects brain changes that contribute to physical and emotional adaptation to stress and improves survival [6,7]. The prefrontal cortex (PFC) regions serve as integration centers for regulation of emotional behavior, fear extinction, and decision making that is essential for stress adaptation [8]. Prolonged or intense stress can impair PFC function on emotion regulation in healthy individuals and individuals with pre-existing mental health conditions [9]. PFC dysfunction can increase vulnerability to develop abnormal responses to future stressors by exacerbating symptoms like hypervigilance and emotional dysregulation [10,11]. Deficits in emotion regulation are linked to psychiatric disorders, including, e.g., depression, anxiety, and PTSD [12,13,14,15]. From this, it might be expected that individuals with pre-existing conditions, such as previous trauma exposure, may have different responses to pandemic-related stress. Meta-analyses of patients with pre-existing mental illness suggest that these patients had significantly higher stress and psychiatric symptoms during the pandemic than the control groups [16]. A new understanding of brain regions affected by pandemic-related stress in patients who had pre-pandemic traumatic experiences or other pre-existing conditions is desirable and needed to direct clinical interventions and personalized treatment to improve mental health outcome.
Structural changes in PFC have been reported in psychiatric patients. PTSD studies indicate that CT reductions in PFC regions, which are associated with early life trauma and PTSD symptoms, can impair stress responses and emotion regulation [17,18]. Volume reductions in ventral medial PFC (vmPFC), which encompasses the rostral anterior cingulate cortex (rACC) and medial orbitofrontal cortex (mOFC), have been reported in PTSD patients [19,20]. Moreover, greater vmPFC thickness appears to be associated with better recall of fear extinction memory [21]. Recent mega-analyses from our lab suggest that chronic PTSD is associated with reduced cortical volumes in widespread emotion regulation regions, including orbital frontal, rostral middle frontal, and pars-orbitalis inferior frontal regions [22]. A longitudinal imaging study found that trauma survivors had thicker dorsolateral prefrontal (DLPFC), superior frontal (SFG), and inferior frontal (IFG) cortices 1.4 years after trauma that subsequently normalized over 5 years and which, in turn, were associated with PTSD symptoms [23]. PTSD symptoms have also been associated with post-earthquake gray matter volume (GMV) changes in the left lateral OFC [24]. Taken together, the above findings suggest that PFC plays a role in stress and PTSD after trauma exposure. Most brain structural studies of PTSD assess survivors of a single intense traumatic event or trauma occurring in early life. It would be further useful to extend the study to how PFC structure in adults is affected by prolonged stressful living conditions as were common during COVID-19 pandemic.
Early work examined changes in brain structures, which were associated with COVID-19 viral infection and pandemic-related fear [25,26]. Compared to healthy controls, reductions in cortical and subcortical volumes and frontal, temporal, and parietal cortical thicknesses had been observed in COVID-19 patients. These structural changes appear to be associated with COVID-19 symptoms and cognitive deficits [26,27,28,29,30]. Further study in COVID-19 patients reported increased GMV in PFC and other brain regions that correlated with PTSD symptoms [31]. The available literature largely focuses on COVID-19 patients with major neurological symptoms. There is limited work on associations between pandemic-related stress and brain structural changes in the cortical regions involved in stress and emotion regulation, particularly in individuals with pre-existing mental conditions.
The objective of the present study was to examine brain and psychological reactions to the COVID-19 pandemic in subjects who had suffered pre-pandemic trauma. Given that PFC contributes to stress responses, we were particularly interested in how CTs in PFC regions were affected in these subjects. We directly examined associations between COVID-19 viral infection, pandemic-related stress, PTSD symptoms, and PFC cortical thicknesses, as well as PTSD vs. non-PTSD group differences. The findings provide new insights into the impact of pandemic on brain structure, stress, and PTSD in subjects who had pre-pandemic conditions.
2. Materials and Methods
2.1. Participants and Processing
The participants were recruited from a previous trauma study conducted in our lab [32]. Briefly, trauma survivors were recruited from local hospitals immediately after a life-threatening traumatic event. They completed a post-trauma stress symptom assessment using the self-reported PTSD Checklist for DSM-V (PCL) and a brain structural MRI (sMRI) scan one year after the trauma. These survivors were recontacted after the outbreak of the COVID-19 pandemic in March 2020. We excluded survivors who (1) tested COVID-19-positive during the in-person study session, (2) had contraindications for MRI scanning, (3) were diagnosed with severe psychiatric or neurological problems, or (4) were under the influence of alcohol or substances at the time of study. This study was approved by the University Institutional Review Board. All participants provided written informed consent.
The consented subjects completed the online self-report COVID Stress Scales (CSS), a virtual PTSD diagnosis interview conducted by a clinical psychologist, and a sMRI scan after a confirmed negative COVID-19 test. Information about COVID-19 infection and treatment history was tracked by self-report. Pre-pandemic PCL scores and sMRI data were derived from the parent study.
2.2. Pandemic Stress Assessment
The 37-item CSS survey was used to assess stress induced by the COVID-19 pandemic, including fears to danger, contamination, economic consequences, xenophobia, compulsive checking, and traumatic stress [33]. The CSS total score and 6 subscores were calculated from (1) 6 questions about fear of danger; (2) 6 questions about socioeconomic fear; (3) 6 questions about xenophobia; (4) 6 questions about contamination fear; (5) 6 questions about traumatic stress; and (6) 7 questions about compulsive checking. The CSS total score was used to represent a cumulative measure of COVID-19-related stress during the pandemic.
2.3. PTSD Symptom Assessment and PTSD Diagnosis
A virtual PTSD diagnostic interview was conducted by a clinical psychologist using the Clinician-Administered PTSD Scale for DSM-V (CAPS). CAPS evaluated PTSD symptom severity across four symptom clusters: reexperiencing, avoidance, negative alterations in cognition and mood, and hyperarousal, based on symptom frequency and intensity. A diagnosis of PTSD required at least 1 reexperiencing, 1 avoidance, 2 negative mood, and 2 hyperarousal symptoms by DSM-V criteria [34]. A diagnosis of partial PTSD required at least 1 symptom in each of these symptom clusters. Partial PTSD was included because partial PTSD with impairment of social functioning commonly requires clinical intervention [35]. The CAPS total score was also calculated by summing the ratings of 20 PTSD symptoms. CAPS has high internal consistency (α = 0.88) and interrater reliability (к = 0.91), as well as good test-retest reliability (к = 0.78) [34].
Pre-pandemic stress symptoms 1 year after a pre-pandemic life-threatening traumatic event were reflected by the self-reported PCL scores obtained from the previous study. The PCL survey evaluates the severity of 20 symptoms for reexperiencing, avoidance, negative alterations in cognition and mood, and hyperarousal symptom clusters. These were the same symptoms assessed by CAPS. Each item was rated on a 5-point Likert scale (0 = Not at all, 4 = Extremely) with the total score ranging from 0 to 80. PCL survey has strong internal consistency (α = 0.94) and test–retest reliability (r = 0.82) [36].
2.4. Structural MRI Acquisition, Processing, and Cortical Thickness Measures
All participants were scanned using a 3T General Electric Signa HDx MRI scanner (GE Healthcare, Chicago, IL, USA). A high-resolution T1-weighted sMRI image was obtained using a previously validated 3D FSPGR structural MRI image protocol (TR = 7.836 ms, TE = 2.976 ms, FA = 9°, NEX = 1, field of view = 256 × 256 mm, matrix = 256 × 256, slice thickness = 1 mm, voxel dimensions = 1 × 1 × 1 mm3, 164 contiguous axial slices) [32]. sMRI image quality was checked by a trained MRI team member. The subjects with blurred sMRI images caused by head motion during the scan were excluded from sMRI analyses. The sMRI images were also reviewed by an experienced radiologist to identify clinical abnormalities.
Brain sMRI scans were processed using FreeSurfer software (Version 7) (https://surfer.nmr.mgh.harvard.edu) (accessed on 23 March 2025). Using the automated FreeSurfer processing stream, the surface-based CT map was built from ~150,000 vertex measures from each hemisphere at which CT was defined as the distance from the gray matter pial surface to the gray–white matter border [37]. An experienced MRI team member who was blinded to psychological assessments visually inspected FreeSurfer-created cortical gray matter borders on each MRI slice for all subjects and made necessary corrections.
Based on mega-analyses of PFC cortical changes related to PTSD ([22], see Introduction), PFC regions of interest (ROIs) included the superior-, middle-, and inferior-frontal, orbital, anterior cingulate, and frontal pole regions. The mean CT for each ROI in each hemisphere was defined by FreeSurfer software.
2.5. Statistics/Analysis
2.5.1. PTSD vs. Non-PTSD Group Comparison Analyses
The number of participants having at least one positive COVID-19 test after the pandemic was compared for PTSD vs. non-PTSD groups using a chi-square test to assess if the COVID-19 infection differed between the two groups. The CSS total score and subscores were compared using ANCOVA analyses to assess potential differences in pandemic-related stress across these groups. ANCOVA analyses were also used to evaluate the effects of COVID-19 infection and post-pandemic PTSD diagnosis on post-pandemic CTs, as well as investigate how pre-pandemic PCL scores contributed to these effects.
2.5.2. Correlation Analyses
Partial correlation analyses were used to examine relationships between pre-pandemic PCL scores and pandemic CSS total scores and subscores. Partial correlation analyses were also used to assess relationships between CSS scores, CAPS scores, and prefrontal CTs.
2.5.3. Repeated Measures ANCOVA Analysis
In subjects with both pre-pandemic and post-pandemic sMRI data, post-pandemic CT changes in PFC regions were compared to pre-pandemic measures. PTSD*time interactions and COVID-19 infection*time interactions on CT changes were tested using the repeated measures ANCOVA. The pre- to post-pandemic CT differences in individual subjects and percentages of subjects contributing to the results of the repeated measures analysis were also calculated.
Age, sex, time between pandemic onset to survey and sMRI data collections, and days between two sMRI scans were included as covariant in relevant analyses. Given that depression is commonly comorbid with PTSD [22,38], and that social support is a resilience factor for trauma recovery [39], we assessed pandemic impacts on depression and general anxiety mood using the COIVD-related Impacts on Mood Survey. Similarly, social support during the pandemic was assessed using the Perceived Social Support Scale (see Appendix A). These measures did not differ in PTSD vs. non-PTSD groups and were not significantly correlated to CTs in PFC regions. Thus, we did not include these factors in the analyses presented in this study. Statistical analyses were performed using SPSS version 29 (IBM Corp., Armonk, NY, USA). The data are reported as mean ± SD, with p < 0.05 set as the significant level. Correlation analysis figures were created with SPSS, and Excel charts were used for bar graphs, pre- to post-pandemic line charts, and figure assembly.
3. Results
3.1. Demographics
Fifty-one subjects (37 females, 14 males) who had experienced a life-threatening traumatic event were recruited from our previous study. Five subjects withdrew from sMRI scanning and four were excluded due to blurred sMRI images. Thus, 42 subjects were included in sMRI analyses.
The data regarding age, race, time between pandemic onset (3/01/2020 in US) and the survey, sMRI, days between two sMRI scans, and the numbers of subjects with PTSD diagnosis and COVID-19 infection are summarized in Table 1. Forty-four subjects completed the post-pandemic PTSD diagnosis interview. Using CAPS for DSM-V criteria, 16 subjects were diagnosed with PTSD, whereas 28 were diagnosed as non-PTSD. Forty-nine subjects had COVID-19 test records. Nineteen reported at least one positive COVID-19 test and seven subjects received treatment for COVID-19 infection. No participants were being treated for COVID-19 symptoms at the time of this study. There was no significant difference in the number of subjects who had at least one positive COVID-19 test in the PTSD vs. non-PTSD group (Table 1).

Table 1.
Demographic data.
3.2. Correlation Analysis of Pre-Pandemic PCL Scores and Pandemic CSS Scores
The pre-pandemic PCL scores were significantly positively correlated with the pandemic CSS total scores (r = 0.429, p = 0.009, df = 34, Figure 1, Table 2). Significant positive relationships were also seen between the pre-pandemic PCL scores and CSS subscores for danger, economic, traumatic stress, and compulsive checking (Table 2). These results suggest that subjects with more severe pre-pandemic PTSD symptoms from prior trauma experienced greater pandemic-related stress.
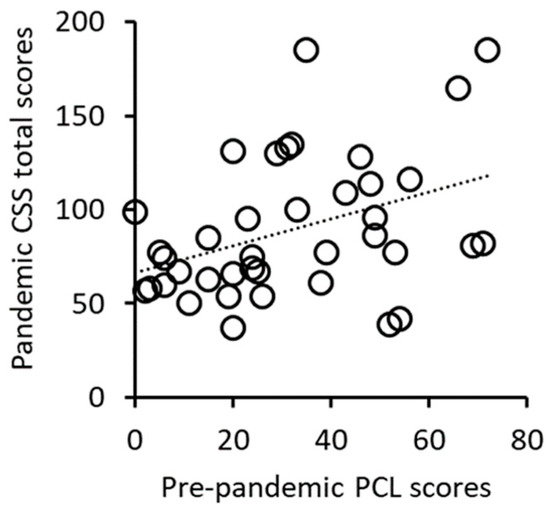
Figure 1.
Positive correlation between pre-pandemic PCL scores and pandemic CSS total scores.

Table 2.
Relationships between pre-pandemic PCL scores and pandemic CSS scores.
3.3. Analyses of Pandemic CSS Scores in Post-Pandemic PTSD vs. Non-PTSD Subjects
The pandemic CSS total scores did not significantly differ in PTSD vs. non-PTSD group; however, the CSS subscores for danger fear and traumatic stress were significantly higher in the PTSD group than non-PTSD group (Table 3). The other CSS subscores did not differ between two groups (Table 3). These results suggest that the post-pandemic PTSD subjects experienced greater pandemic-related stress than non-PTSD subjects with regard to stress related to fear about COVID-19 danger and traumatic stress.

Table 3.
Comparisons of pandemic CSS scores in post-pandemic PTSD vs. non-PTSD groups.
3.4. Correlation Analyses of Post-Pandemic Prefrontal Cortical Thicknesses, PTSD Symptoms, and COVID-19-Related Stress
The post-pandemic CTs in the left rostral middle frontal gyrus (rMFG) and the left pars orbitalis of the inferior frontal gyrus (IFG-orbitalis) were significantly positively associated with CAPS scores (Figure 2 and Figure 3a,b). These results suggest that thicker post-pandemic CTs in these regions were positively related to worse post-pandemic PTSD symptoms.
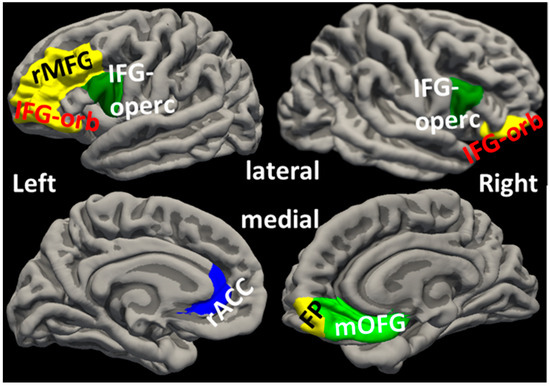
Figure 2.
Locations of cortical regions. FP: frontal pole; IFG-orb: pars orbitalis of inferior frontal gyrus; IFG-operc: pars opercularis of inferior frontal gyrus; mOFG: medial orbital frontal gyrus; rACC: rostral anterior cingulate cortex; rMFG: rostral middle frontal gyrus.
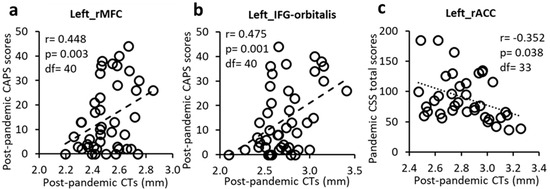
Figure 3.
Post-pandemic CTs positively correlated with post-pandemic CAPS scores in the left rMFG (a) and IFG-orbitalis (b) but negatively correlated with CSS total scores in the left rACC (c).
CT in the left rACC was significantly negatively associated with pandemic CSS total score (Figure 2 and Figure 3c).
Together, these findings suggest that CTs of lateral PFC and rACC regions played roles, respectively, in severity of post-pandemic PTSD symptoms and pandemic stress.
3.5. Effects of COVID-19 Infection and PTSD on Post-Pandemic Prefrontal Cortical Thicknesses
COVID-19 infection significantly impacted IFG-opercularis CTs. Specifically, subjects with a history of a positive COVID-19 test had thicker post-pandemic CTs in the left and right IFG-opercularis than subjects who did not have a positive test history (left: F = 6.106, p = 0.018; right: F = 8.396, p = 0.006, Figure 2 and Figure 4a). These results remained significant when controlled for pre-pandemic PCL scores (left: F = 6.184, p = 0.020; right: F = 7.558, p = 0.011).
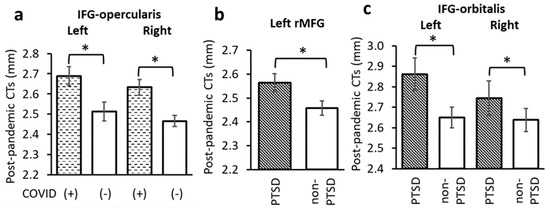
Figure 4.
(a) CTs in the left and right IFG-opercularis were significantly thicker in subjects with a history of COVID-19 infection. CTs in (b) the left rMFG and (c) left and right IFG-orbitalis were significantly thicker in the PTSD than non-PTSD groups. *: p < 0.05 as significant level, controlled for age, sex and time between pandemic onset and sMRI data collection.
The PTSD group had significantly thicker post-pandemic CTs than the non-PTSD group in the left rMFG (F = 4.596, p = 0.040, Figure 2 and Figure 4b). This result remained significant when controlled for pre-pandemic PCL scores (F = 5.456, p = 0.027). Compared to the non-PTSD group, the PTSD group also had significantly thicker post-pandemic CTs in the left and right IFG-orbitalis (left: F = 4.248, p = 0.049; right: F = 4.813, p = 0.037, Figure 2 and Figure 4c).
The effects of pre-pandemic PCL scores and COVID-19*PTSD interaction were not significant for any prefrontal CTs.
Taken together, the above results suggest that COVID-19 infection contributed to thickening CTs in IFG-opercularis. In addition, post-pandemic PTSD diagnosis appeared related to CT thickening in rMFG and IFG-orbitalis.
3.6. Changes in Prefrontal Cortical Thickness from Pre- to Post-Pandemic Time and Associations with PTSD and COVID-19 Infection
An exploratory analysis of CT changes over time from before to after the pandemic was conducted on a subgroup of 9 subjects in the PTSD group and 16 subjects in the non-PTSD group who had undergone pre- and post-pandemic sMRI scans. Repeated measures ANCOVA suggested that post-pandemic CT in the right mOFG was thicker than before the pandemic for all subjects (time effect F = 8.038, p = 0.012, Figure 2 and Figure 5a).
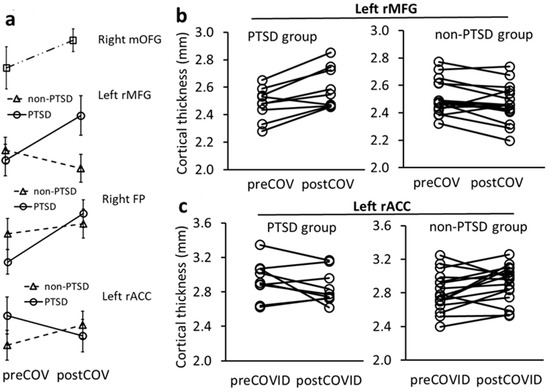
Figure 5.
Repeated measures ANCOVA analysis for CT changes in PFC in PTSD and non-PTSD subjects over pre- to post-pandemic times. (a) Mean (±SD) CT changes over time in the right mOFG across all subjects, and in the left rMFG, right FP and left rACC in PTSD and non-PTSD groups. (b) Over time, CT in the left rMFG increased in 88.9% of subjects in the PTSD group but decreased in 81.2% of non-PTSD subjects. (c) Over time, CT in the left rACC decreased in 55.6% of subjects in the PTSD group but increased in 75% of non-PTSD subjects.
PTSD*time interactions in CT changes in the left rMFG and right frontal pole (FP) were significant and indicated that CTs thickened in the PTSD group and thinned in the non-PTSD group from before to after the pandemic (F = 11.984, p = 0.003; F = 5.179, p = 0.037, respectively, Figure 2 and Figure 5a). These group findings were further supported by individual subject results that 8 out of 9 (88.9%) PTSD subjects had thickening rMFG changes, whereas 13 of 16 (81.2%) non-PTSD subjects had thinning rMFG changes (Figure 5b).
PTSD*time interaction was also significant for CT change in the left rACC and indicated that CT in the left rACC thinned in the PTSD group and thickened in the non-PTSD group (F = 5.568, p = 0.031, Figure 2 and Figure 5a). This finding was supported at the individual subject level by 5 of 9 (55.6%) PTSD subjects with left rACC thinning and 12 of 16 (75%) non-PTSD subjects with left rACC thickening (Figure 5c).
COVID-19 infection*time interaction did not have a significant effect on pre- to post-pandemic CT change in any PFC regions. Pre-pandemic CTs in the PFC regions did not differ between the PTSD and non-PTSD groups.
Taken together, the above findings suggest that pre- to post-pandemic prefrontal CT changes differed between those pre-pandemic trauma survivors who had PTSD and those who did not. It also appears that different PFC regions, including rMFG, FP, and rACC, underwent different pre- to post-pandemic thickness effects.
4. Discussion
The present study suggests that higher pre-pandemic PTSD symptoms in pre-pandemic trauma survivors were positively associated with more severe COVID-19-related stress during the pandemic. Positive associations were observed between post-pandemic CAPS scores and post-pandemic CTs in the left rMFG and IFG-orbitalis. CTs in the left rMFG and the left and right IFG-orbitalis were also significantly thicker in subjects who were diagnosed with post-pandemic PTSD. A negative association was found between the pandemic CSS score and post-pandemic CT in the left rACC. Post-pandemic CT thickening in the left and right IFG-opercularis was associated with COVID-19 infection. Analysis of pre- to post-pandemic CT changes across all subjects suggested right mOFG CT thickened. PTSD*time interactions indicated significant pre- to post-pandemic CT changes, with the PTSD group showing increased CTs in the left rMFG and right FP and decreased CTs in the left rACC. In contrast, the non-PTSD group had opposite pre- to post-pandemic CT changes in these regions. These results suggest that the COVID-19 pandemic affected stress responses and prefrontal CTs differently between those pre-pandemic trauma survivors who had PTSD and those who did not.
4.1. PFC Involvement in Stress and PTSD
The present results suggest that the PFC regions contributed to the COVID-19 pandemic stress. PFC contains high-order executive processing centers that play key roles in stress, emotion regulation, cognitive control, attention, and working memory [9]. Prefrontal cortical regions are essential for daily adaptations to environmental and physical demands. Stress studies have demonstrated that abnormal levels of stress neurotransmitters and glucocorticoids in response to intense or chronic stress affect PFC function and/or structure [17]. Prolonged or intense stress can induce PFC executive function, emotion regulation, and structural impairment [40]. Patients with mood and anxiety disorders commonly exhibit emotional dysregulation [41]. Subjects in the present study had experienced a life-threatening traumatic event prior to the pandemic. Those who subsequently reported severe PTSD symptoms may have developed PFC dysfunctions that impaired responses to the pandemic. Our results point to a positive association between pre-pandemic PCL scores and pandemic CSS scores. COVID-19-related stress, including fear related to virus danger and traumatic stress, were greater in the post-pandemic PTSD group than non-PTSD group. This suggests that trauma survivors with more severe pre-pandemic PTSD symptoms may have had deficits in stress control, negative attention bias, and emotional dysregulation. Consequently, they may have been more vulnerable to pandemic-related stress and abnormal PFC emotion processing and regulation in response to pandemic threats.
As part of a response to prolonged pandemic stress, PFC likely increased stress management to cope with heightened emotional and cognitive demands [42]. PFC changes occur in response to behavioral and psychological interventions in psychiatric patients [43]. Volume and thickness reductions in DLPFC in PTSD patients are associated with dysfunction in negative emotion regulation [41,44,45]. Alternatively, thicker frontal CTs have been reported to positively associate with PTSD symptom severity [46]. In a trauma longitudinal subway disaster study, trauma survivors who did not have pre-existing trauma experience had thicker DLPFC CTs at 1.4 years after trauma than trauma-free controls, and DLPFC CTs subsequently normalized over 5 years [23]. The present findings suggest that CT in the left rMFG, a part of DLPFC, was thicker in the post-pandemic PTSD group than non-PTSD group. This CT thickening was positively associated with PTSD symptoms. There are notable differences between the present study and a subway disaster study. The subjects in present study had a pre-pandemic life-threatening trauma experience and the pandemic was their further stress, whereas subjects in the subway disaster study did not have a trauma experience before the disaster. This difference may account for different CT effects. In addition, the subway disaster study did not address whether CT normalization in DLPFC occurred in PTSD and non-PTSD survivors. From our results, further work would be useful to longitudinally track if subjects who were diagnosed with post-pandemic PTSD can undergo recovery from PTSD and reversible PFC thickness changes.
rMFG is thought to play key roles in stress and emotion regulation by inhibitory control over, e.g., low-order brain regions and stress perception and appraisal [47]. Increased CTs in the left and right rMFG have been reported to be positively associated with perceived stress [48]. Abnormal rMFG activity has been linked to increased stress perception and depressive symptoms [9]. In the present study, 88.9% of subjects with post-pandemic PTSD had thicker CT in the left rMFG, whereas 81.2% of post-pandemic non-PTSD subjects had thinner left rMFG. These findings suggest that cortical structural alterations in the left rMFG may have contributed to pandemic-related stress that potentially contributed to PTSD.
We observed greater left and right IFG-orbitalis CTs in post-pandemic PTSD than non-PTSD subjects. The left IFG-orbitalis CTs was positively associated with CAPS scores. IFG is thought to play important roles in executive tasks, attention, and memory [49]. IFG is divided in a rostral-caudal direction into the pars orbitalis, pars triangularis, and pars opercularis [50]. IFG-orbitalis is located around Brodmann area 47 that is involved in processing of disgusted, angry, and fearful facial expressions [51]. IFG-orbitalis has been reported to contribute to recognition of emotions and to inhibition of adverse emotion [52,53]. This region may also play a role in integrating emotional recognition with executive prediction and interact with the orbitofrontal cortex in behavioral adaptations [54]. Other work has reported that trauma exposure can significantly impact IFG functions [55]. Previous research suggests that abnormal IFG activation contributed to impaired fear inhibition in trauma survivors with PTSD [56]. In the present study, larger CTs in the left and right IFG-orbitalis were associated with post-pandemic PTSD symptoms. These findings raise the possibility that structural changes in IFG-orbitalis may contribute to brain maladaptations to heightened emotional and cognitive demands from the pandemic.
CT in the left rACC was negatively associated with pandemic-related stress. Analysis of pre- to post-pandemic CT changes suggested CT in the left rACC decreased over time in PTSD subjects but increased over time in non-PTSD subjects, thus, suggesting that left rACC CT underwent different pandemic changes in PTSD vs. non-PTSD subjects. rACC has extensive connections with medial PFC and plays a critical role in emotional regulation and stress control [57]. Previous work reports that thinner rACC is linked to emotion processing disruption in PTSD patients [58]. A study on earthquake survivors reported that thinner left and right rACC CTs in PTSD survivors were negatively correlated with CAPS scores [59]. Taken together, our findings of a negative association between post-pandemic CT in the left rACC and pandemic-related stress, as well as pre- to post-pandemic left rACC thickening in non-PTSD subjects suggest that thicker left rACC CT may be associated with reduced stress and PTSD symptoms. In contrast, rACC CT thinning may be associated with vulnerability to stress.
4.2. PFC Involvement in COVID-19 Viral Infection
About 70% of COVID-19 patients report loss of smell or taste as their initial symptoms [60,61]. In the present study, thicker IFG-opercularis CT was associated with COVID-19 infection. The human gustatory cortex, which perceives and distinguishes different tastes, is located in IFG-opercularis and anterior temporal regions [62]. COVID-19 studies suggest the COVID-19 virus can attack the central nervous system through the olfactory pathway or blood–brain barrier, and, thus, cause loss of taste, smell and other neurological symptoms [63]. A recent study of long COVID-19 symptoms reported decreased odor discrimination that was associated with activation in the right frontal pole and superior frontal gyrus, but did not detect gustatory deficits [64]. Current views on COVID-19-induced taste loss focus on dysfunctions in tongue taste buds as a consequence of viral attack on taste receptors [65]. The present findings suggest that thicker left and right IFG-opercularis CTs were associated with COVID-19 infection and provide a first suggestion that the COVID-19 virus may affect gustatory cortex structure as well as taste receptors.
COVID-19-related brain changes remain controversial and likely vary due to COVID-19 symptom severity, duration, and other factors [66,67]. Autopsies of COVID-19 patients reveal diverse cellular changes in cerebral cortex and other brain structures, including, e.g., increased synaptic connections, activation of microglia and astrocytes, and neuroinflammation [68]. The impacts of COVID-19 virus on the human brain are complex and its invasion pathways and mechanisms to destroy brain structures are far from being completely understood. The possibilities include immune response and proinflammatory reactions in neurons and glia, cellular infection by binding to cell membrane receptors, and viral RNA replication, transcription, and spread. COVID-19 virus-caused lung and vascular lesions can induce hypoxia and neuron hypoxic injury. In addition, the COVID-19 virus may infect intestinal peripheral neurons and choroidal plexus epithelial cells as an alternative pathway to invade the brain [63].
The current finding, that the left and right IFG-opercularis CT thickening was associated with COVID-19 viral infection, appears inconsistent with findings from the UK Biobank study where post-pandemic CTs in the olfactory cortex, including the orbitofrontal cortex, were thinner in mild COVID-19 patients compared to pre-pandemic baselines [25]. The reasons for this apparent difference remain unclear but may involve study differences. For example, subject ages ranged from 19 to 55 years in the present study and from 51 to 81 years in UK Biobank study. It is well known that age affects cortical thickness [69]; therefore, differences in subject ages and other factors may have contributed to differences in study results.
4.3. Limitations
This study has limitations. First, only individuals who experienced a traumatic event before the pandemic were studied. Second, the findings do not provide direct links between CT changes and COVID-19 symptoms. Third, the data were collected approximately 1.5–2 years after the outbreak of the pandemic and, thus, do not address different potential stages of pandemic-related changes. Fourth, the study focused on pandemic-related stress and PTSD symptoms and did not assess further cognitive functions. Fifth, analyses followed a standard statistical approach with controlling the probability of Type I error for all p-value calculations, while the probability of Type II error was not explicitly considered. Had the pandemic been expected, a more formally designed experiment, including a formal sample size calculation, could have been planned. A potential consequence of the study’s small sample size is an increased probability of Type II error. As a consequence, we may have missed some Type II false negative errors. Thus, from a Type I error perspective, our results are statistically conservative; however, from a Type II error perspective, we may have missed further relevant findings. Finally, the small sample size did not allow for detailed analyses of pre- to post-pandemic CT change dynamics.
5. Conclusions
The present findings provide original evidence that the COVID-19 pandemic had demonstrable effects on prefrontal cortical structure, stress, and PTSD symptoms in subjects who had experienced pre-pandemic trauma. There is conjecture that people who had pre-existing mental health challenges might have been more vulnerable to brain and mental-health impacts caused by the pandemic. The present findings suggest that treatments are needed to counter these diverse effects.
Author Contributions
Conceptualization, funding acquisition, and supervision, H.X. and X.W.; investigation, C.-H.S., S.C. and A.A.; formal analysis, H.X. and Q.S.; visualization, A.A. and S.C.; writing—original draft preparation, S.C. and A.A.; writing—review and editing: C.-H.S., Q.S., H.X. and X.W. All authors contributed to the article and approved the submitted version. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institute of Mental Health R01MH110483, 3R01MH110483 and R21MH126172.
Institutional Review Board Statement
The study involving human subjects was approved by the Institutional Review Board of the University of Toledo (301171-UT, approved on 17 February 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article material. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank the Department of Radiology at the University of Toledo for their clinical and technical support and John Wall for editing the manuscript revision.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
Appendix A.1. Assessments
The COVID-19 pandemic’s impacts on depression and anxiety mood were assessed using the COIVD-related Impacts on Mood Survey (https://www.phenxtoolkit.org/toolkit_content/PDF/UFl_Coing_with_COVID19_Mood.pdf) (assessed on 23 March 2025), which was adapted from Neuro-QOL Item Bank v1.0—Anxiety and Depression—Short Forms. The participants were requested to recall eight depression symptoms and eight general anxiety symptoms before the COVID-19 pandemic and evaluate if each symptom became worse, remained the same, or became better after pandemic, rating from 1 to 5. Lower scores reflected more severe depression and anxiety symptoms after the pandemic.
The social support during the pandemic was assessed using the modified Perceived Social Support Scale (PSS). The PSS questionnaire includes four items about social support from family and friends during the pandemic. A higher score means stronger social support.
Univariate ANCOVA analyses were used to compare the pandemic impacts on mood and social support in post-pandemic PTSD vs. non-PTSD groups. The correlations between these assessments and prefrontal CTs were conducted using partial correlation analysis.
Appendix A.2. Results
The PSS score did not differ in post-pandemic PTSD vs. non-PTSD groups. The pandemic’s impacts on depression and anxiety mood also did not reach significant difference between two groups.

Table A1.
Comparisons of pandemic PSS and impacts on mood changes between post-pandemic PTSD vs. non-PTSD groups [38,39].
Table A1.
Comparisons of pandemic PSS and impacts on mood changes between post-pandemic PTSD vs. non-PTSD groups [38,39].
| PTSD Group | Non-PTSD Group | Comparison (f, p) | |
|---|---|---|---|
| PSS | 18.2 ± 7.3 | 21.1 ± 7.7 | 1.632, 0.210 |
| Depression mood | 17.0 ± 7.5 | 23.6 ± 8.8 | 3.599, 0.067 |
| Anxiety mood | 16.7 ± 9.8 | 22.2 ± 6.8 | 3.888, 0.057 |
There were no significant correlations between post-pandemic prefrontal CTs, PSS scores, and pandemic impacts on depression and anxiety mood.

Table A2.
Correlations between post-pandemic prefrontal CTs, PSS scores, and pandemic impacts on depression and anxiety mood [38,39].
Table A2.
Correlations between post-pandemic prefrontal CTs, PSS scores, and pandemic impacts on depression and anxiety mood [38,39].
| PFC Cortical Thicknesses | PSS Score | Depression Mood | Anxiety Mood | |
|---|---|---|---|---|
| L_caudalanteriorcingulate | Correlation | 0.189 | −0.344 | −0.258 |
| p | 0.277 | 0.054 | 0.141 | |
| df | 33 | 30 | 32 | |
| L_caudalmiddlefrontal | Correlation | 0.078 | 0.138 | 0.017 |
| p | 0.655 | 0.451 | 0.922 | |
| df | 33 | 30 | 32 | |
| L_lateralorbitofrontal | Correlation | 0.081 | 0.049 | −0.116 |
| p | 0.643 | 0.792 | 0.513 | |
| df | 33 | 30 | 32 | |
| L_medialorbitofrontal | Correlation | 0.134 | −0.228 | −0.201 |
| p | 0.444 | 0.21 | 0.255 | |
| df | 33 | 30 | 32 | |
| L_parsopercularis | Correlation | 0.018 | 0.012 | −0.081 |
| p | 0.918 | 0.946 | 0.651 | |
| df | 33 | 30 | 32 | |
| L_parsorbitalis | Correlation | −0.142 | −0.177 | −0.21 |
| p | 0.416 | 0.332 | 0.232 | |
| df | 33 | 30 | 32 | |
| L_parstriangularis | Correlation | −0.109 | 0.004 | −0.169 |
| p | 0.535 | 0.983 | 0.338 | |
| df | 33 | 30 | 32 | |
| L_rostralanteriorcingulate | Correlation | 0.27 | −0.04 | −0.061 |
| p | 0.117 | 0.828 | 0.734 | |
| df | 33 | 30 | 32 | |
| L_rostralmiddlefrontal | Correlation | −0.084 | −0.008 | −0.084 |
| p | 0.632 | 0.964 | 0.638 | |
| df | 33 | 30 | 32 | |
| L_superiorfrontal | Correlation | 0.063 | −0.007 | −0.067 |
| p | 0.721 | 0.971 | 0.705 | |
| df | 33 | 30 | 32 | |
| L_frontalpole | Correlation | −0.201 | −0.111 | −0.088 |
| p | 0.246 | 0.545 | 0.62 | |
| df | 33 | 30 | 32 | |
| R_caudalanteriorcingulate | Correlation | −0.19 | −0.013 | −0.001 |
| p | 0.275 | 0.945 | 0.994 | |
| df | 33 | 30 | 32 | |
| R_caudalmiddlefrontal | Correlation | 0.232 | 0.204 | 0.079 |
| p | 0.179 | 0.264 | 0.659 | |
| df | 33 | 30 | 32 | |
| R_lateralorbitofrontal | Correlation | 0.099 | 0.066 | −0.02 |
| p | 0.57 | 0.721 | 0.908 | |
| df | 33 | 30 | 32 | |
| R_medialorbitofrontal | Correlation | −0.085 | 0.195 | −0.01 |
| p | 0.627 | 0.285 | 0.955 | |
| df | 33 | 30 | 32 | |
| R_parsopercularis | Correlation | 0.073 | −0.16 | −0.168 |
| p | 0.679 | 0.383 | 0.343 | |
| df | 33 | 30 | 32 | |
| R_parsorbitalis | Correlation | −0.284 | 0.059 | −0.103 |
| p | 0.099 | 0.749 | 0.563 | |
| df | 33 | 30 | 32 | |
| R_parstriangularis | Correlation | −0.008 | −0.121 | −0.161 |
| p | 0.965 | 0.509 | 0.363 | |
| df | 33 | 30 | 32 | |
| R_rostralanteriorcingulate | Correlation | −0.037 | −0.02 | −0.143 |
| p | 0.831 | 0.912 | 0.419 | |
| df | 33 | 30 | 32 | |
| R_rostralmiddlefrontal | Correlation | −0.083 | −0.02 | −0.158 |
| p | 0.634 | 0.915 | 0.373 | |
| df | 33 | 30 | 32 | |
| R_superiorfrontal | Correlation | −0.001 | 0.086 | −0.037 |
| p | 0.995 | 0.64 | 0.834 | |
| df | 33 | 30 | 32 | |
| R_frontalpole | Correlation | 0.001 | −0.001 | 0.026 |
| p | 0.994 | 0.997 | 0.882 | |
| df | 33 | 30 | 32 |
References
- Holmes, E.A.; O’Connor, R.C.; Perry, V.H.; Tracey, I.; Wessely, S.; Arseneault, L.; Ballard, C.; Christensen, H.; Cohen Silver, R.; Everall, I.; et al. Multidisciplinary research priorities for the COVID-19 pandemic: A call for action for mental health science. Lancet Psychiatry 2020, 7, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Vahia, I.V.; Jeste, D.V.; Reynolds, C.F., III. Older Adults and the Mental Health Effects of COVID-19. JAMA 2020, 324, 2253–2254. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Scherer, N.; Felix, L.; Kuper, H. Prevalence of depression, anxiety and post-traumatic stress disorder in health care workers during the COVID-19 pandemic: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0246454. [Google Scholar] [CrossRef]
- Liu, C.H.; Zhang, E.; Wong, G.T.F.; Hyun, S.; Hahm, H.C. Factors associated with depression, anxiety, and PTSD symptomatology during the COVID-19 pandemic: Clinical implications for U.S. young adult mental health. Psychiatry Res. 2020, 290, 113172. [Google Scholar] [CrossRef]
- Calegaro, V.C.; Ramos-Lima, L.F.; Hoffmann, M.S.; Zoratto, G.; Kerber, N.; Costa, F.C.D.; Picinin, V.D.; Köchler, J.; Rodrigues, L.; Maciel, L.; et al. Closed doors: Predictors of stress, anxiety, depression, and PTSD during the onset of COVID-19 pandemic in Brazil. J. Affect. Disord. 2022, 310, 441–451. [Google Scholar] [CrossRef]
- Diniz, C.R.A.F.; Crestani, A.P. The times they are a-changin’: A proposal on how brain flexibility goes beyond the obvious to include the concepts of “upward” and “downward” to neuroplasticity. Mol. Psychiatry 2023, 28, 977–992. [Google Scholar] [CrossRef]
- Huether, G.; Doering, S.; Rüger, U.; Rüther, E.; Schüssler, G. The stress-reaction process and the adaptive modification and reorganization of neuronal networks. Psychiatry Res. 1999, 87, 83–95. [Google Scholar] [CrossRef]
- Cerqueira, J.J.; Almeida, O.F.X.; Sousa, N. The stressed prefrontal cortex. Left? Right! Brain Behav. Immun. 2008, 22, 630–638. [Google Scholar] [CrossRef]
- Girotti, M.; Adler, S.M.; Bulin, S.E.; Fucich, E.A.; Paredes, D.; Morilak, D.A. Prefrontal cortex executive processes affected by stress in health and disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 85, 161–179. [Google Scholar] [CrossRef]
- Sherin, J.E.; Nemeroff, C.B. Post-traumatic stress disorder: The neurobiological impact of psychological trauma. Dialogues Clin. Neurosci. 2011, 13, 263–278. [Google Scholar] [CrossRef]
- Esagoff, A.I.; Stevens, D.A.; Kosyakova, N.; Woodard, K.; Jung, D.; Richey, L.N.; Daneshvari, N.O.; Luna, L.P.; Bray, M.J.C.; Bryant, B.R.; et al. Neuroimaging Correlates of Post-Traumatic Stress Disorder in Traumatic Brain Injury: A Systematic Review of the Literature. J. Neurotrauma 2023, 40, 1029–1044. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.F.; Miskowiak, K.K.; Hyphantis, T.N.; Kohler, C.A.; Alves, G.S.; Bortolato, B.; Sales, P.M.G.; Machado-Vieira, R.; Berk, M.; McIntyre, R.S. Cognitive Dysfunction in Depression—Pathophysiology and Novel Targets. CNS Neurol. Disord. Drug Targets 2014, 13, 1819–1835. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, F.; Lapp, L.K.; Peretti, C.-S. Current research on cognitive aspects of anxiety disorders. Curr. Opin. Psychiatry 2011, 24, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Polak, A.R.; Witteveen, A.B.; Reitsma, J.B.; Olff, M. The role of executive function in posttraumatic stress disorder: A systematic review. J. Affect. Disord. 2012, 141, 11–21. [Google Scholar] [CrossRef]
- Morey, R.A.; Dunsmoor, J.E.; Haswell, C.C.; Brown, V.M.; Vora, A.; Weiner, J.; Stjepanovic, D.; Wagner, H.R.; Brancu, M.; Marx, C.E.; et al. Fear learning circuitry is biased toward generalization of fear associations in posttraumatic stress disorder. Transl. Psychiatry 2015, 5, e700. [Google Scholar] [CrossRef]
- Neelam, K.; Duddu, V.; Anyim, N.; Neelam, J.; Lewis, S. Pandemics and pre-existing mental illness: A systematic review and meta-analysis. Brain Behav. Immun. Health 2021, 10, 100177. [Google Scholar] [CrossRef]
- Arnsten, A.F.T. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009, 10, 410–422. [Google Scholar] [CrossRef]
- Kredlow, M.A.; Fenster, R.J.; Laurent, E.S.; Ressler, K.J.; Phelps, E.A. Prefrontal cortex, amygdala, and threat processing: Implications for PTSD. Neuropsychopharmacology 2022, 47, 247–259. [Google Scholar] [CrossRef]
- Baldaçara, L.; Zugman, A.; Araújo, C.; Cogo-Moreira, H.; Lacerda, A.L.T.; Schoedl, A.; Pupo, M.; Mello, M.F.; Andreoli, S.B.; de Jesus Mari, J.; et al. Reduction of anterior cingulate in adults with urban violence- related PTSD. J. Affect. Disord. 2014, 168, 13–20. [Google Scholar] [CrossRef]
- Morey, R.A.; Haswell, C.C.; Hooper, S.R.; De Bellis, M.D. Amygdala, Hippocampus, and Ventral Medial Prefrontal Cortex Volumes Differ in Maltreated Youth with and without Chronic Posttraumatic Stress Disorder. Neuropsychopharmacology 2016, 41, 791–801. [Google Scholar] [CrossRef]
- Milad, M.R.; Quinn, B.T.; Pitman, R.K.; Orr, S.P.; Fischl, B.; Rauch, S.L. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc. Natl. Acad. Sci. USA 2005, 102, 10706–10711. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xie, H.; Chen, T.; Cotton, A.S.; Salminen, L.E.; Logue, M.W.; Clarke-Rubright, E.K.; Wall, J.; Dennis, E.L.; O’Leary, B.M.; et al. Cortical volume abnormalities in posttraumatic stress disorder: An ENIGMA-psychiatric genomics consortium PTSD workgroup mega-analysis. Mol. Psychiatry 2021, 26, 4331–4343. [Google Scholar] [CrossRef] [PubMed]
- Lyoo, I.K.; Kim, J.E.; Yoon, S.J.; Hwang, J.; Bae, S.; Kim, D.J. The Neurobiological Role of the Dorsolateral Prefrontal Cortex in Recovery From Trauma: Longitudinal Brain Imaging Study Among Survivors of the South Korean Subway Disaster. Arch. Gen. Psychiatry 2011, 68, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, A.; Sugiura, M.; Taki, Y.; Kotozaki, Y.; Nouchi, R.; Takeuchi, H.; Araki, T.; Hanawa, S.; Nakagawa, S.; Miyauchi, C.M.; et al. Brain structural changes as vulnerability factors and acquired signs of post-earthquake stress. Mol. Psychiatry 2013, 18, 618–623. [Google Scholar] [CrossRef]
- Douaud, G.; Lee, S.; Alfaro-Almagro, F.; Arthofer, C.; Wang, C.; McCarthy, P.; Lange, F.; Andersson, J.L.R.; Griffanti, L.; Duff, E.; et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 2022, 604, 697–707. [Google Scholar] [CrossRef]
- Kamaşak Arpaçay, B.; Ulcay, T.; Nisari, M.; Görgülü, Ö.; Akca, V.; Alpaslan, M.; Yetis, A.; Hizmali, L.; Karahocagil, M.; Aycan, K. Effects of COVID-19 on brain and cerebellum: A voxel based morphometrical analysis. Bratisl. Lek. Listy 2023, 124, 442–448. [Google Scholar] [CrossRef]
- Bendella, Z.; Widmann, C.N.; Layer, J.P.; Layer, Y.L.; Haase, R.; Sauer, M.; Bieler, L.; Lehnen, N.C.; Paech, D.; Heneka, M.T.; et al. Brain Volume Changes after COVID-19 Compared to Healthy Controls by Artificial Intelligence-Based MRI Volumetry. Diagnostics 2023, 13, 1716. [Google Scholar] [CrossRef]
- Lu, Y.; Li, X.; Geng, D.; Mei, N.; Wu, P.-Y.; Huang, C.-C.; Jia, T.; Zhao, Y.; Wang, D.; Xiao, A.; et al. Cerebral Micro-Structural Changes in COVID-19 Patients—An MRI-based 3-month Follow-up Study. EClinicalMedicine 2020, 25, 1–12. [Google Scholar] [CrossRef]
- Sanabria-Diaz, G.; Etter, M.M.; Melie-Garcia, L.; Lieb, J.M.; Psychogios, M.-N.; Hutter, G.; Granziera, C. Brain cortical alterations in COVID-19 patients with neurological symptoms. Front. Neurosci. 2022, 16, 992165. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, W.; Huang, S.; Huang, C.; Li, C.; Chen, Y.; Huang, Y.; Yang, L.; Li, C.; Zhang, H.; et al. Gray Matter Thickness and Subcortical Nuclear Volume in Men After SARS-CoV-2 Omicron Infection. JAMA Netw. Open 2023, 6, e2345626. [Google Scholar] [CrossRef]
- Besteher, B.; Machnik, M.; Troll, M.; Toepffer, A.; Zerekidze, A.; Rocktäschel, T.; Heller, C.; Kikinis, Z.; Brodoehl, S.; Finke, K.; et al. Larger gray matter volumes in neuropsychiatric long-COVID syndrome. Psychiatry Res. 2022, 317, 114836. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Shih, C.-H.; Aldoohan, S.D.; Wall, J.T.; Wang, X. Hypothalamus volume mediates the association between adverse childhood experience and PTSD development after adulthood trauma. Transl. Psychiatry 2023, 13, 274. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.; Landry, C.A.; Paluszek, M.M.; Fergus, T.A.; McKay, D.; Asmundson, G.J.G. Development and initial validation of the COVID Stress Scales. J. Anxiety Disord. 2020, 72, 102232. [Google Scholar] [CrossRef] [PubMed]
- Weathers, F.W.; Bovin, M.J.; Lee, D.J.; Sloan, D.M.; Schnurr, P.P.; Kaloupek, D.G.; Keane, T.M.; Marx, B.P. The Clinician-Administered PTSD Scale for DSM–5 (CAPS-5): Development and initial psychometric evaluation in military veterans. Psychol. Assess. 2018, 30, 383–395. [Google Scholar] [CrossRef]
- Mylle, J.; Maes, M. Partial posttraumatic stress disorder revisited. J. Affect. Disord. 2004, 78, 37–48. [Google Scholar] [CrossRef]
- Blevins, C.A.; Weathers, F.W.; Davis, M.T.; Witte, T.K.; Domino, J.L. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): Development and Initial Psychometric Evaluation. J. Trauma. Stress. 2015, 28, 489–498. [Google Scholar] [CrossRef]
- Fischl, B.; Dale, A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. USA 2000, 97, 11050–11055. [Google Scholar] [CrossRef]
- Spinhoven, P.; Penninx, B.W.; van Hemert, A.M.; de Rooij, M.; Elzinga, B.M. Comorbidity of PTSD in anxiety and depressive disorders: Prevalence and shared risk factors. Child. Abus. Negl. 2014, 38, 1320–1330. [Google Scholar] [CrossRef]
- Lubomirsky, B.; Wang, X.; Xie, H.; Smirnoff, J.B.; Biehn, T.L.; Contractor, A.A.; Elhai, J.D.; Sutu, C.; Brickman, K.R.; Liberzon, I.; et al. Preliminary Study on the Relationship Between Visitation in the Emergency Department and Posttraumatic Mental Health. Soc. Work. Ment. Health 2014, 12, 69–80. [Google Scholar] [CrossRef]
- McEwen, B.S.; Morrison, J.H. The Brain on Stress: Vulnerability and Plasticity of the Prefrontal Cortex over the Life Course. Neuron 2013, 79, 16–29. [Google Scholar] [CrossRef]
- New, A.S.; Fan, J.; Murrough, J.W.; Liu, X.; Liebman, R.E.; Guise, K.G.; Tang, C.Y.; Charney, D.S. A functional magnetic resonance imaging study of deliberate emotion regulation in resilience and posttraumatic stress disorder. Biol. Psychiatry 2009, 66, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Kaul, D.; Schwab, S.G.; Mechawar, N.; Matosin, N. How stress physically re-shapes the brain: Impact on brain cell shapes, numbers and connections in psychiatric disorders. Neurosci. Biobehav. Rev. 2021, 124, 193–215. [Google Scholar] [CrossRef] [PubMed]
- Crocker, L.D.; Heller, W.; Warren, S.L.; O’Hare, A.J.; Infantolino, Z.P.; Miller, G.A. Relationships among cognition, emotion, and motivation: Implications for intervention and neuroplasticity in psychopathology. Front. Hum. Neurosci. 2013, 7, 261. [Google Scholar] [CrossRef] [PubMed]
- Lanius, R.A.; Williamson, P.C.; Boksman, K.; Densmore, M.; Gupta, M.; Neufeld, R.W.; Gati, J.S.; Menon, R.S. Brain activation during script-driven imagery induced dissociative responses in PTSD: A functional magnetic resonance imaging investigation. Biol. Psychiatry 2002, 52, 305–311. [Google Scholar] [CrossRef]
- Lee, H.; Oh, S.; Ha, E.; Joo, Y.; Suh, C.; Kim, Y.; Jeong, H.; Lyoo, I.K.; Yoon, S.; Hong, H. Cerebral cortical thinning in brain regions involved in emotional regulation relates to persistent symptoms in subjects with posttraumatic stress disorder. Psychiatry Res. 2023, 327, 115345. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Zhao, Y.; Li, Z.; Kemp, G.J.; Wu, M.; Gong, Q. Cortical thickness abnormalities in patients with post-traumatic stress disorder: A vertex-based meta-analysis. Neurosci. Biobehav. Rev. 2022, 134, 104519. [Google Scholar] [CrossRef]
- Raschle, N.M.; Fehlbaum, L.V.; Menks, W.M.; Martinelli, A.; Prätzlich, M.; Bernhard, A.; Ackermann, K.; Freitag, C.; De Brito, S.; Fairchild, G.; et al. Atypical dorsolateral prefrontal activity in female adolescents with conduct disorder during effortful emotion regulation. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2019, 4, 984–994. [Google Scholar] [CrossRef]
- Michalski, L.J.; Demers, C.H.; Baranger, D.A.A.; Barch, D.M.; Harms, M.P.; Burgess, G.C.; Bogdan, R. Perceived stress is associated with increased rostral middle frontal gyrus cortical thickness: A family-based and discordant-sibling investigation. Genes Brain Behav. 2017, 16, 781–789. [Google Scholar] [CrossRef]
- Tops, M.; Boksem, M.A. A potential role of the inferior frontal gyrus and anterior insula in cognitive control, brain rhythms, and event-related potentials. Front. Psychol. 2011, 2, 330. [Google Scholar] [CrossRef]
- Greenlee, J.D.W.; Oya, H.; Kawasaki, H.; Volkov, I.O.; Severson, M.A., III; Howard, M.A., III; Brugge, J.F. Functional connections within the human inferior frontal gyrus. J. Comp. Neurol. 2007, 503, 550–559. [Google Scholar] [CrossRef]
- Sprengelmeyer, R.; Rausch, M.; Eysel, U.T.; Przuntek, H. Neural structures associated with recognition of facial expressions of basic emotions. Proc. Biol. Sci. 1998, 265, 1927–1931. [Google Scholar] [CrossRef] [PubMed]
- Dapretto, M.; Bookheimer, S.Y. Form and Content: Dissociating Syntax and Semantics in Sentence Comprehension. Neuron 1999, 24, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Hampshire, A.; Chamberlain, S.R.; Monti, M.M.; Duncan, J.; Owen, A.M. The role of the right inferior frontal gyrus: Inhibition and attentional control. Neuroimage 2010, 50, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T.; Hornak, J.; Wade, D.; McGrath, J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. J. Neurol. Neurosurg. Psychiatry 1994, 57, 1518–1524. [Google Scholar] [CrossRef]
- Quidé, Y.; O’Reilly, N.; Watkeys, O.J.; Carr, V.J.; Green, M.J. Effects of childhood trauma on left inferior frontal gyrus function during response inhibition across psychotic disorders. Psychol. Med. 2018, 48, 1454–1463. [Google Scholar] [CrossRef]
- van Rooij, S.J.H.; Rademaker, A.R.; Kennis, M.; Vink, M.; Kahn, R.S.; Geuze, E. Impaired right inferior frontal gyrus response to contextual cues in male veterans with PTSD during response inhibition. J. Psychiatry Neurosci. 2014, 39, 330–338. [Google Scholar] [CrossRef]
- Bush, G.; Luu, P.; Posner, M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000, 4, 215–222. [Google Scholar] [CrossRef]
- Mueller, S.G.; Ng, P.; Neylan, T.; Mackin, S.; Wolkowitz, O.; Mellon, S.; Yan, X.; Flory, J.; Yehuda, R.; Marmar, C.R.; et al. Evidence for disrupted gray matter structural connectivity in posttraumatic stress disorder. Psychiatry Res. Neuroimaging 2015, 234, 194–201. [Google Scholar] [CrossRef]
- Qi, S.; Mu, Y.; Liu, K.; Zhang, J.; Huan, Y.; Tan, Q.; Shi, M.; Wang, Q.; Chen, Y.; Wang, H.; et al. Cortical inhibition deficits in recent onset PTSD after a single prolonged trauma exposure. Neuroimage Clin. 2013, 3, 226–233. [Google Scholar] [CrossRef]
- Kaye, R.; Chang, C.W.D.; Kazahaya, K.; Brereton, J.; Denneny, J.C., III. COVID-19 Anosmia Reporting Tool: Initial Findings. Otolaryngol.–Head. Neck Surg. 2020, 163, 132–134. [Google Scholar] [CrossRef]
- Santos, R.E.A.; da Silva, M.G.; do Monte Silva, M.C.B.; Barbosa, D.A.M.; Gomes, A.L.d.V.; Galindo, L.C.M.; da Silva Aragão, R.; Ferraz-Pereira, K.N. Onset and duration of symptoms of loss of smell/taste in patients with COVID-19: A systematic review. Am. J. Otolaryngol. 2021, 42, 102889. [Google Scholar] [CrossRef] [PubMed]
- Obiefuna, S.; Donohoe, C. Neuroanatomy, Nucleus Gustatory; StatPearls: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554522/ (accessed on 17 January 2025).
- Sun, Z.; Shi, C.; Jin, L. Mechanisms by Which SARS-CoV-2 Invades and Damages the Central Nervous System: Apart from the Immune Response and Inflammatory Storm, What Else Do We Know? Viruses 2024, 16, 663. [Google Scholar] [CrossRef] [PubMed]
- Masala, C.; Porcu, M.; Orofino, G.; Defazio, G.; Pinna, I.; Solla, P.; Ercoli, T.; Suri, J.S.; Spinato, G.; Saba, L. Neuroimaging evaluations of olfactory, gustatory, and neurological deficits in patients with long-term sequelae of COVID-19. Brain Imaging Behav. 2024, 18, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M. Taste Dysfunction and Long COVID-19. Front. Cell. Infect. Microbiol. 2021, 11, 716563. [Google Scholar] [CrossRef]
- Alhazmi, F.H.; Alsharif, W.M.; Alshoabi, S.A.; Gameraddin, M.; Aloufi, K.M.; Abdulaal, O.M.; Qurashi, A.A. Identifying cerebral microstructural changes in patients with COVID-19 using MRI: A systematic review. Brain Circ. 2023, 9, 6–15. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, W.; Huang, S.; Huang, Y.; Chen, Y.; Zhang, H.; Guo, H.; Liu, J. Two-year follow-up of brain structural changes in patients who recovered from COVID-19: A prospective study. Psychiatry Res. 2023, 319, 114969. [Google Scholar] [CrossRef]
- Mukerji, S.S.; Solomon, I.H. What can we learn from brain autopsies in COVID-19? Neurosci. Lett. 2021, 742, 135528. [Google Scholar] [CrossRef]
- Sele, S.; Liem, F.; Mérillat, S.; Jäncke, L. Age-related decline in the brain: A longitudinal study on inter-individual variability of cortical thickness, area, volume, and cognition. Neuroimage 2021, 240, 118370. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).