Real Index of Refraction of Normal and Cancerous Axillary Lymph Nodes in Breast Cancer Patients: Results from an Experimental Study
Abstract
1. Introduction
2. Materials and Methods
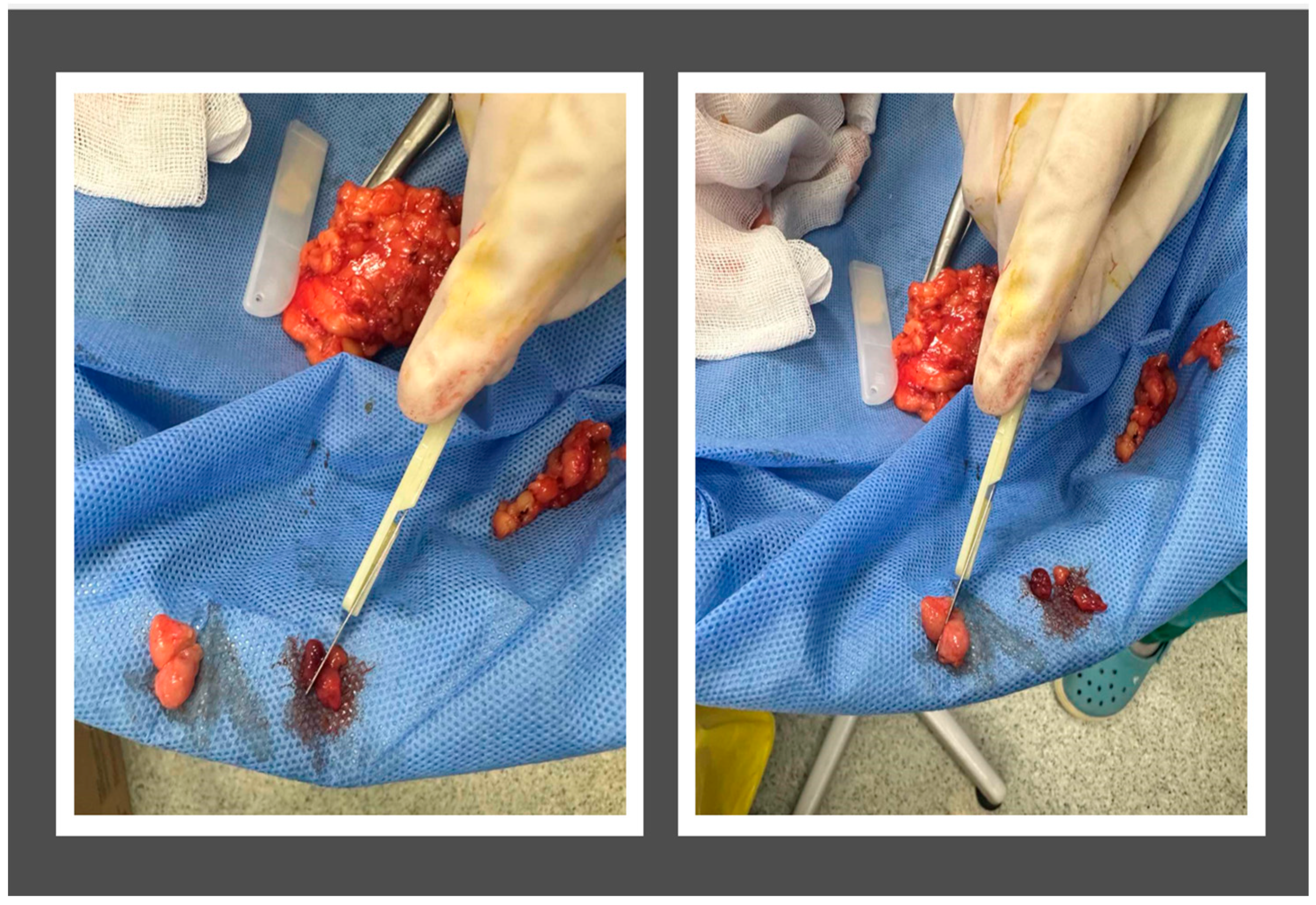
2.1. Subject Population and Sample Preparation
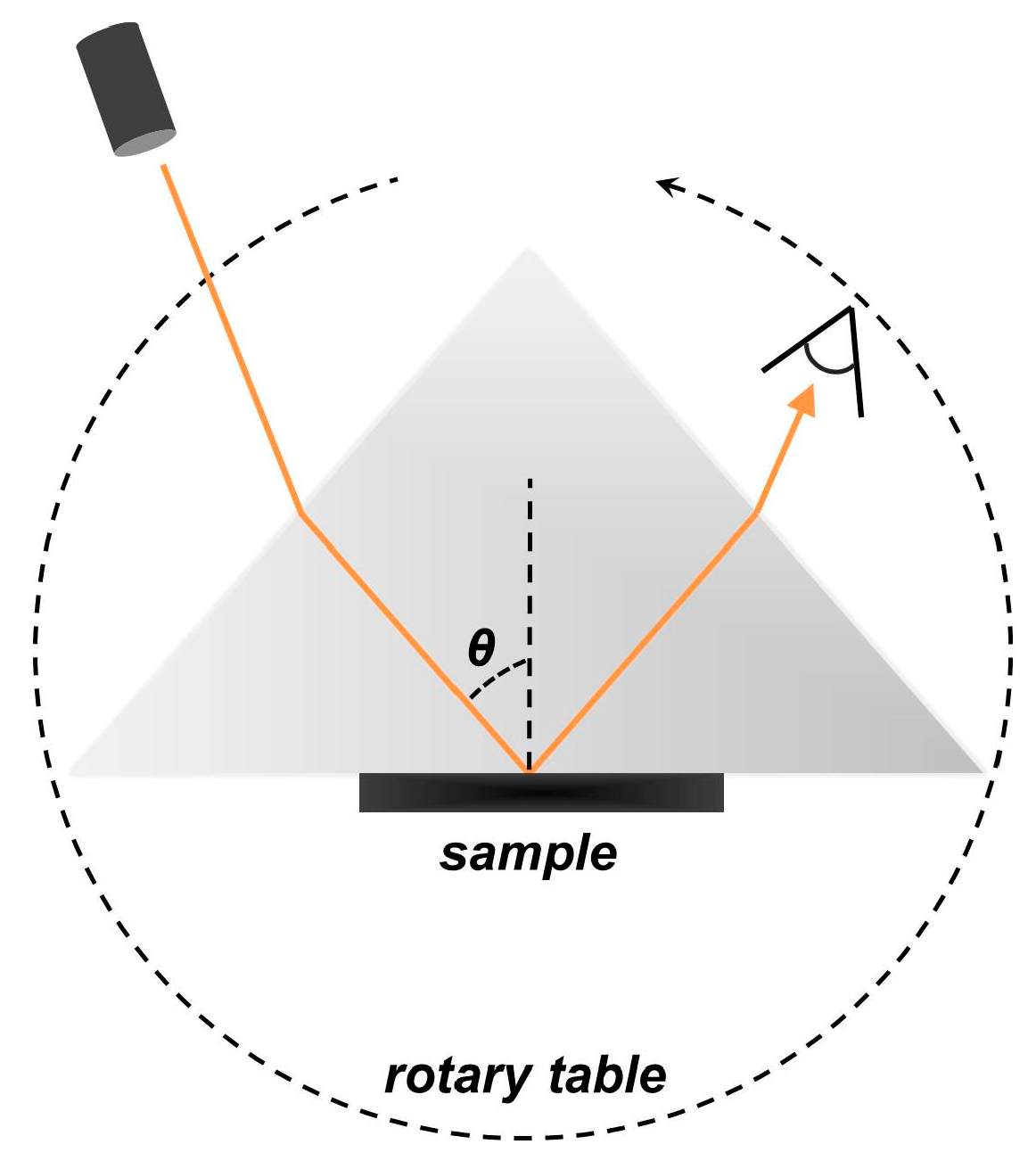
2.2. Experimental Set-Up
2.3. Raw-Data Treatment
2.4. Statistical Analysis
3. Results
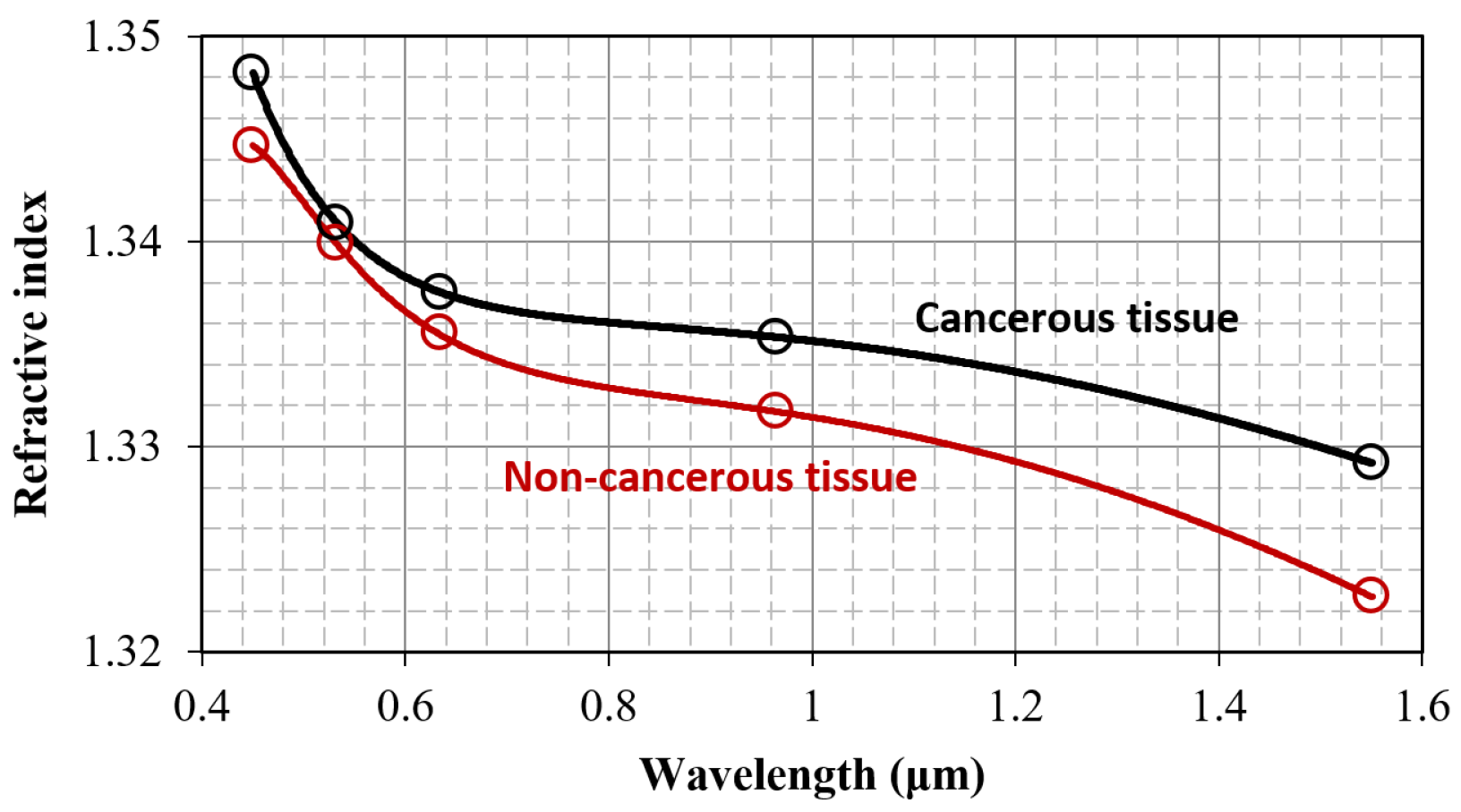
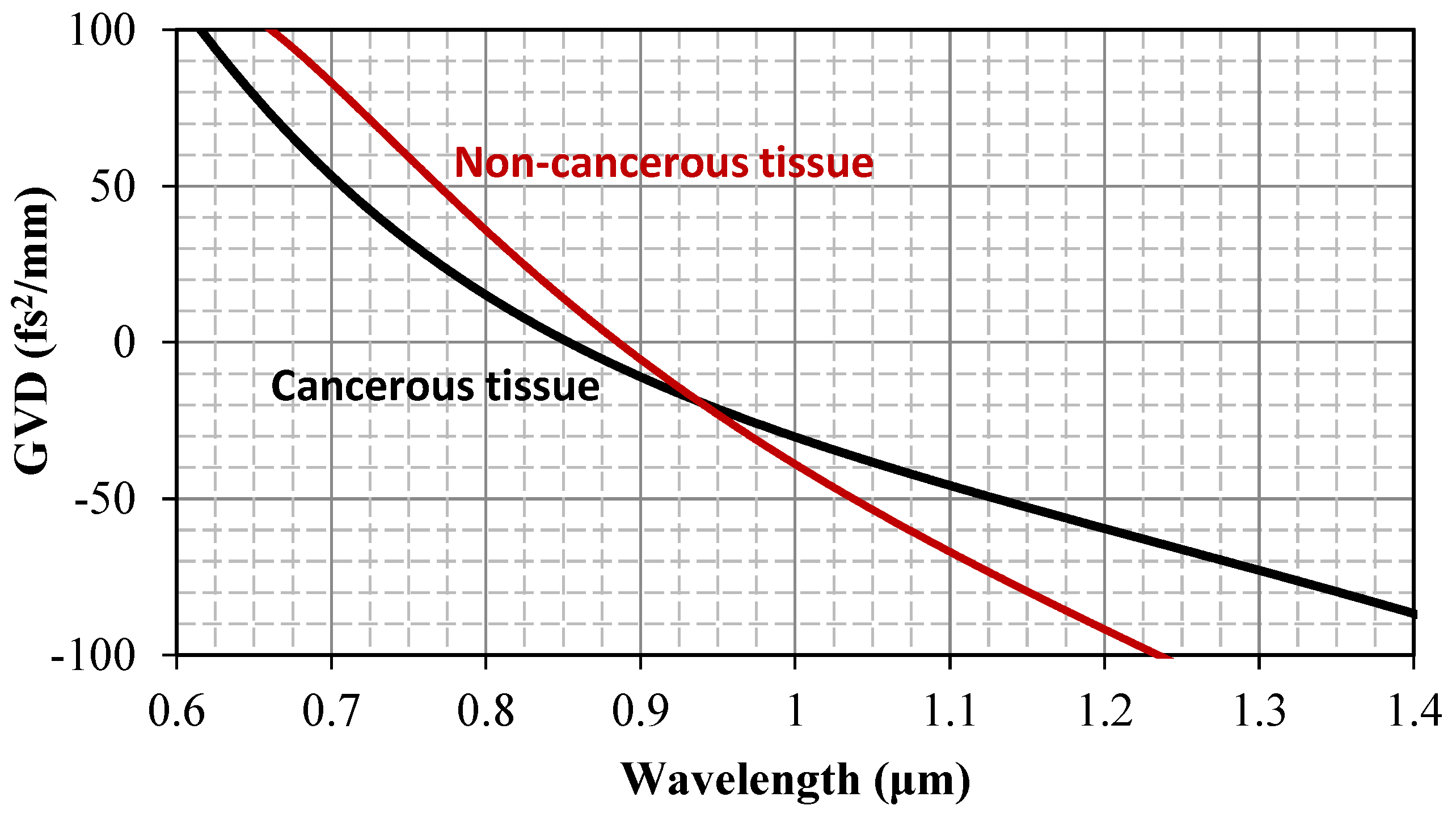
3.1. Refractive Index and Wavelength Dispersion Considerations
3.2. Sensitivity, Specificity, and Receiver Operating Characteristic (ROC) Analysis
3.3. Further Considerations
4. Summary and Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Wolmark, N.; Bauer, M.; Redmond, C.; Gebhardt, M. The accuracy of clinical nodal staging and of limited axillary dissection as a determinant of histologic nodal status in carcinoma of the breast. Surg. Gynecol. Obstet. 1981, 152, 765–772. [Google Scholar] [PubMed]
- Fisher, E.R.; Wang, J.; Bryant, J.; Fisher, B.; Mamounas, E.; Wolmark, N. Pathobiology of preoperative chemotherapy: Findings from the National Surgical Adjuvant Breast and Bowel (NSABP) protocol B-18. Cancer 2002, 95, 681–695. [Google Scholar] [CrossRef]
- Tuchin, V.V. Tissue Optics: Light Scattering Methods and Instruments for Medical Diagnosis, 3rd ed.; SPIE: Bellingham, WA, USA, 2015. [Google Scholar]
- Tuchin, V.V. Handbook of Optical Biomedical Diagnostics; SPIE: Bellingham, WA, USA, 2016. [Google Scholar]
- Papadoliopoulou, M.; Matiatou, M.; Koutsoumpos, S.; Mulita, F.; Giannios, P.; Margaris, I.; Moutzouris, K.; Arkadopoulos, N.; Michalopoulos, N.V. Optical Imaging in Human Lymph Node Specimens for Detecting Breast Cancer Metastases: A Review. Cancers 2023, 15, 5438. [Google Scholar] [CrossRef]
- Grootendorst, D.J.; Steenbergen, W.; Manohar, S.; Ruers, T.J. Optical techniques for the intraoperative assessment of nodal status. Future Oncol. 2013, 9, 1741–1755. [Google Scholar] [CrossRef]
- Johnson, K.; Chicken, D.W.; Pickard DC, O.; Lee, A.C.; Briggs, G.M.; Falzon, M.; Bigio, I.J.; Keshtgar, M.; Bown, S.G. Elastic Scattering Spectroscopy for Intraoperative Determination of Sentinel Lymph Node Status in the Breast. J. Biomed. Opt. 2004, 9, 1122. [Google Scholar] [CrossRef]
- Nolan, R.M.; Adie, S.G.; Marjanović, M.; Chaney, E.J.; South, F.A.; Monroy, G.L.; Shemonski, N.D.; Erickson-Bhatt, S.J.; Shelton, R.L.; Bower, A.J.; et al. Intraoperative Optical Co-herence Tomography for Assessing Human Lymph Nodes for Metastatic Cancer. BMC Cancer 2016, 16, 144. [Google Scholar] [CrossRef]
- Iftimia, N.; Mujat, M.; Ustun, T.E.; Ferguson, R.D.; Danthu, V.; Hammer, D.X. Spectral-Domain Low Coherence Interferometry/Optical Coherence Tomography System for Fine Needle Breast Biopsy Guidance. Rev. Sci. Instrum. 2009, 80, 024302. [Google Scholar] [CrossRef]
- McLaughlin, R.A.; Scolaro, L.; Robbins, P.; Hamza, S.; Saunders, C.; Sampson, D.D. Imaging of Human Lymph Nodes Using Optical Coherence Tomography: Potential for Staging Cancer. Cancer Res. 2010, 70, 2579–2584. [Google Scholar] [CrossRef]
- Podoleanu, A.G. Optical Coherence Tomography. J. Microsc. 2012, 247, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, G.R.; Orr, L.; Christie-Brown, J.; McCarthy, K.; Rose, S.; Thomas, M.; Stone, N. Discrimination between Benign, Primary and Secondary Malignancies in Lymph Nodes from the Head and Neck Utilising Raman Spectroscopy and Multivariate Analysis. Analyst 2013, 138, 3900. [Google Scholar] [CrossRef] [PubMed]
- Haka, A.S.; Volynskaya, Z.; Gardecki, J.A.; Nazemi, J.; Lyons, J.; Hicks, D.G.; Fitzmaurice, M.; Dasari, R.R.; Crowe, J.P.; Feld, M.S. In Vivo Margin Assessment during Partial Mastectomy Breast Surgery Using Raman Spectroscopy. Cancer Res. 2006, 66, 3317–3322. [Google Scholar] [CrossRef] [PubMed]
- Butler, H.J.; Ashton, L.; Bird, B.; Cinque, G.; Curtis, K.; Dorney, J.; Esmonde-White, K.A.; Fullwood, N.J.; Gardner, B.; Martin-Hirsch, P.L.; et al. Using Raman Spectroscopy to Characterize Biologi-cal Materials. Nat. Protoc. 2016, 11, 664–687. [Google Scholar] [CrossRef]
- Tian, P.; Zhang, W.; Zhao, H.; Lei, Y.; Cui, L.; Zhang, Y.; Xu, Z. Intraoperative detection of sentinel lymph node metastases in breast carcinoma by Fourier transform infrared spectroscopy. Br. J. Surg. 2015, 102, 1372–1379. [Google Scholar] [CrossRef]
- Bird, B.; Miljkovic, M.; Romeo, M.J.; Smith, J.; Stone, N.; George, M.W.; Diem, M. Infrared micro-spectral imaging: Distinction of tissue types in axillary lymph node histology. BMC Clin. Pathol. 2008, 8, 8. [Google Scholar] [CrossRef]
- Martins, I.S.; Silva, H.F.; Lazareva, E.N.; Chernomyrdin, N.V.; Zaytsev, K.I.; Oliveira, L.M.; Tuchin, V.V. Measurement of tissue optical properties in a wide spectral range: A review [Invited]. Biomed. Opt. Express 2022, 14, 249–298. [Google Scholar] [CrossRef]
- Giannios, P.; Toutouzas, K.G.; Matiatou, M.; Stasinos, K.; Konstadoulakis, M.M.; Zografos, G.C.; Moutzouris, K. Visible to near-infrared refractive properties of freshly-excised human-liver tissues: Marking hepatic malignancies. Sci. Rep. 2016, 6, 27910. [Google Scholar] [CrossRef]
- Giannios, P.; Koutsoumpos, S.; Toutouzas, K.G.; Matiatou, M.; Zografos, G.C.; Moutzouris, K. Complex refractive index of normal and malignant human colorectal tissue in the visible and near-infrared. J. Biophotonics 2017, 10, 303–310. [Google Scholar] [CrossRef]
- Matiatou, M.; Giannios, P.; Koutsoumpos, S.; Toutouzas, K.G.; Zografos, G.C.; Moutzouris, K. Data on the refractive index of freshly-excised human tissues in the visible and near-infrared spectral range. Results Phys. 2021, 22, 103833. [Google Scholar] [CrossRef]
- Matiatou, M.; Giannios, P.; Koutsoumpos, S.; Michalopoulos, N.V.; Toutouzas, K.G.; Zografos, G.C.; Moutzouris, K. Complex refractive index of freshly excised human breast tissue as a marker of disease. Lasers Med. Sci. 2022, 37, 2597–2604. [Google Scholar] [CrossRef] [PubMed]
- Bechger, T. Practical Guidelines on the Required Sample Size in a Two Group Multivariate Mean Comparison. Available online: https://www.vvsor.nl/wp-content/uploads/2020/06/KM1997056007.pdf (accessed on 19 January 2025).
- Pineda-Vázquez, D.; García-Valenzuela, A. Functional refractive-index sensor by internal reflection of diffuse light. Sens. Rev. 2022, 43, 12–21. [Google Scholar] [CrossRef]
- Acevedo-Barrera, A.; García-Valenzuela, A. Probing bio-tissue films by optical internal reflectivity: Modeling and measurements. Appl. Opt. 2021, 60, 8588–8599. [Google Scholar] [CrossRef] [PubMed]
- Morales-Luna, G.; Contreras-Tello, H.; García-Valenzuela, A.; Barrera, R.G. Experimental test of reflectivity formulas for turbid colloids: Beyond the Fresnel reflection amplitudes. J. Phys. Chem. B 2016, 120, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Tello, H.; García-Valenzuela, A. Refractive index measurement of turbid media by transmission of backscattered light near the critical angle. Appl. Opt. 2014, 53, 4768–4778. [Google Scholar] [CrossRef]
- Gutiérrez-Reyes, E.; García-Valenzuela, A.; Barrera, R.G. Extension of Fresnel’s formulas for turbid colloidal suspensions: A rigorous treatment. J. Phys. Chem. B 2014, 118, 6015–6031. [Google Scholar] [CrossRef]
- Contreras-Tello, H.; Márquez-Islas, R.; Vázquez-Estrada, O.; Sánchez-Pérez, C.; García-Valenzuela, A. Understanding the performance of Abbe-type refractometers with optically absorbing fluids. Meas. Sci. Technol. 2014, 25, 075201. [Google Scholar] [CrossRef]
- García-Valenzuela, A.; Contreras-Tello, H. Optical model enabling the use of Abbe-type refractometers on turbid suspensions. Opt. Lett. 2013, 38, 775–777. [Google Scholar] [CrossRef]
- Koutsoumpos, S.; Giannios, P.; Stavrakas, I.; Moutzouris, K. The derivative method of critical-angle refractometry for attenuating media. J. Opt. 2020, 22, 075601. [Google Scholar] [CrossRef]
- Photiou, C.; Bousi, E.; Zouvani, I.; Pitris, C. Using speckle to measure tissue dispersion in optical coherence tomography. Biomed. Opt. Express 2017, 8, 2528–2535. [Google Scholar] [CrossRef]
- Liu, B.; Macdonald, E.A.; Stamper, D.L.; Brezinski, M.E. Group velocity dispersion effects with water and lipid in 1.3 microm optical coherence tomography system. Phys. Med. Biol. 2004, 49, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Photiou, C.; Pitris, C. Comparison of tissue dispersion measurement techniques based on optical coherence tomography. J. Biomed. Opt. 2019, 24, 046003. [Google Scholar] [CrossRef] [PubMed]
- De Boniface, J.; Filtenborg Tvedskov, T.; Rydén, L.; Szulkin, R.; Reimer, T.; Kühn, T.; Kontos, M.; Gentilini, O.D.; Bagge, R.O.; Sund, M.; et al. Omitting Axillary Dissection in Breast Cancer with Sentinel-Node Metastases. N. Engl. J. Med. 2024, 390, 1163–1175. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Wang, Y.; Ma, L.; Zheng, R.; Ding, J.; Gong, Y.; Yao, H.; Wang, J.; Zha, X. Omission of sentinel lymph node biopsy in patients with clinically axillary lymph node-negative early breast cancer (OMSLNB): Protocol for a prospective, non-inferiority, single-arm, phase II clinical trial in China. BMJ Open 2024, 14, e087700. [Google Scholar] [CrossRef]
- De Wild, S.R.; van Roozendaal, L.M.; de Wilt, J.H.W.; van Dalen, T.; van der Hage, J.A.; van Duijnhoven, F.H.; Simons, J.M.; Schipper, R.-J.; de Munck, L.; van Kuijk, S.M.J.; et al. De-escalation of axillary treatment in the event of a positive sentinel lymph node biopsy in cT1-2 N0 breast cancer treated with mastectomy: Nationwide registry study (BOOG 2013-07). Br. J. Surg. 2024, 111, znae077. [Google Scholar] [CrossRef]
- Prathibha, S.; White, M.; Kolbow, M.; Hui, J.Y.C.; Brauer, D.; Ankeny, J.; Jensen, E.H.; LaRocca, C.J.; Marmor, S.; Tuttle, T.M. Omission of axillary lymph node dissection for breast cancer patients with three or more positive sentinel lymph nodes. Breast Cancer Res. Treat. 2024, 205, 127–133. [Google Scholar] [CrossRef]
- Alamoodi, M.; Patani, N.; Mokbel, K.; Wazir, U.; Mokbel, K. Reevaluating Axillary Lymph Node Dissection in Total Mastectomy for Low Axillary Burden Breast Cancer: Insights from a Meta-Analysis including the SINODAR-ONE Trial. Cancers 2024, 16, 742. [Google Scholar] [CrossRef]
- Solouma, N.; Hamdy, O. Ex Vivo Optical Properties Estimation for Reliable Tissue Characterization. Photonics 2023, 10, 891. [Google Scholar] [CrossRef]
- Maliszewski, K.A.; Urbańska, M.A.; Kolenderski, P.; Vetrova, V.; Kolenderska, S.M. Extracting Group Velocity Dispersion values using quantum-mimic Optical Coherence Tomography and Machine Learning. Sci. Rep. 2023, 13, 6596. [Google Scholar] [CrossRef]
- Qiu, S.; Zeng, J.; Hou, Y.; Chen, L.; Ge, J.; Wen, L.; Liu, C.; Zhang, Y.; Zhu, R.; Gao, M. Detection of lymph node metastasis with near-infrared upconversion luminescent nanoprobes. Nanoscale 2018, 10, 21772–21781. [Google Scholar] [CrossRef]
- Wunderbaldinger, P. Optical imaging of lymph nodes. Eur. J. Radiol. 2006, 58, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Zysk, A.M.; Adie, S.G.; Armstrong, J.J.; Leigh, M.S.; Paduch, A.; Sampson, D.D.; Nguyen, F.T.; Boppart, S.A. Needle-based refractive index measurement using low-coherence interferometry. Opt. Lett. 2007, 32, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Setchfield, K.; Gorman, A.; Simpson, A.H.R.W.; Somekh, M.G.; Wright, A.J. Relevance and utility of the in-vivo and ex-vivo optical properties of the skin reported in the literature: A review [Invited]. Biomed. Opt. Express 2023, 14, 3555–3583. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, P.J.; Cheng, Y.; Shen, M.; Gregori, G.; Wang, R.K. Unleashing the power of optical attenuation coefficients to facilitate segmentation strategies in OCT imaging of age-related macular degeneration: Perspective. Biomed. Opt. Express 2023, 14, 4947–4963. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, M.R.; Tuchin, V.V.; Oliveira, L.M. Analysis of the experimental absorption spectrum of the rabbit lung and identification of its components. J. Biophotonics 2024, e202300494. [Google Scholar] [CrossRef]
- Pinheiro, M.; Carvalho, M.; Oliveira, L. Tutorial on the Use of the Photon Diffusion Approximation for Fast Calculation of Tissue Optical Properties. J. Biophotonics 2025, e202400257. [Google Scholar] [CrossRef]
- Selifonov, A.A.; Rusanova, T.Y.; Selifonova, E.I.; Tuchin, V.V. Study of the Diffusion of Tetracycline in the Dentin of the Human Tooth Ex Vivo. J. Biomed. Photonics Eng. 2022, 8, 030303. [Google Scholar] [CrossRef]

| Age (Years); Median(IQR) | 66 (25) | |
|---|---|---|
| Gender | Female | 34 (100%) |
| Male | 0 (0) | |
| Neoadjuvant treatment | No | 21 (62%) |
| Yes | 13 (38%) | |
| Menopause | No | 7 (21%) |
| Yes | 27 (79%) | |
| Cancer histology | NST ǂ | 27 (79%) |
| Apocrine | 3 (9%) | |
| Lobular | 3 (9%) | |
| Metaplastic | 1 (3%) | |
| Not available | 1 (3%) | |
| Lymphovascular invasion (LVI) | No | 18 (53%) |
| Yes | 11 (32%) | |
| Not available | 5 (15%) | |
| Tumor grade | 1 | 0 (0) |
| 2 | 11 (32%) | |
| 3 | 18 (53%) | |
| Not available | 5 (15%) | |
| Wavelength | Refractive Index | ||||||
|---|---|---|---|---|---|---|---|
| Cancerous Tissue | Non-Cancerous Tissue | ||||||
| n | Median | IQR | n | Median | IQR | p-Values | |
| 450 nm | 39 | 1.3482 | 0.0174 | 46 | 1.3447 | 0.0076 | 0.035 * |
| 532 nm | 41 | 1.3409 | 0.0105 | 47 | 1.3399 | 0.0080 | 0.60 |
| 632.8 nm | 41 | 1.3376 | 0.0114 | 48 | 1.3355 | 0.0072 | 0.05 * |
| 964 nm | 40 | 1.3353 | 0.0196 | 45 | 1.3295 | 0.0086 | 0.02 * |
| 1551 nm | 40 | 1.3292 | 0.0166 | 46 | 1.3227 | 0.0097 | 0.014 * |
| Dispersion Coefficients | Cancerous Tissue | Non-Cancerous Tissue | ||
|---|---|---|---|---|
| Mean | Median | Mean | Median | |
| 1.81824385 | 1.81197661 | 1.83761605 | 1.82203329 | |
| −0.01758911 | −0.01597675 | −0.02768208 | −0.02479342 | |
| −0.01284216 | −0.01854048 | −0.03426113 | −0.03630494 | |
| 0.00318697 | 0.00543981 | 0.01146329 | 0.01300119 | |
| 0.00000094 | −0.00026687 | −0.0009907 | 0.00121783 | |
| Metric | Wavelength | ||||
|---|---|---|---|---|---|
| 450 nm | 532 nm | 632.8 nm | 964 nm | 1551 nm | |
| Optimal refractive index threshold | 1.351 | 1.348 | 1.333 | 1.329 | 1.327 |
| Sensitivity | 44% | 29% | 90% | 78% | 63% |
| Specificity | 83% | 83% | 33% | 49% | 70% |
| AUC | 63% | 53% | 62% | 65% | 65% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadoliopoulou, M.; Koutsoumpos, S.; Margaris, I.; Matiatou, M.; Giannios, P.; Arkadopoulos, N.; Moutzouris, K.; Michalopoulos, N.V. Real Index of Refraction of Normal and Cancerous Axillary Lymph Nodes in Breast Cancer Patients: Results from an Experimental Study. J. Pers. Med. 2025, 15, 71. https://doi.org/10.3390/jpm15020071
Papadoliopoulou M, Koutsoumpos S, Margaris I, Matiatou M, Giannios P, Arkadopoulos N, Moutzouris K, Michalopoulos NV. Real Index of Refraction of Normal and Cancerous Axillary Lymph Nodes in Breast Cancer Patients: Results from an Experimental Study. Journal of Personalized Medicine. 2025; 15(2):71. https://doi.org/10.3390/jpm15020071
Chicago/Turabian StylePapadoliopoulou, Maria, Spyridon Koutsoumpos, Ioannis Margaris, Maria Matiatou, Panagiotis Giannios, Nikolaos Arkadopoulos, Konstantinos Moutzouris, and Nikolaos V. Michalopoulos. 2025. "Real Index of Refraction of Normal and Cancerous Axillary Lymph Nodes in Breast Cancer Patients: Results from an Experimental Study" Journal of Personalized Medicine 15, no. 2: 71. https://doi.org/10.3390/jpm15020071
APA StylePapadoliopoulou, M., Koutsoumpos, S., Margaris, I., Matiatou, M., Giannios, P., Arkadopoulos, N., Moutzouris, K., & Michalopoulos, N. V. (2025). Real Index of Refraction of Normal and Cancerous Axillary Lymph Nodes in Breast Cancer Patients: Results from an Experimental Study. Journal of Personalized Medicine, 15(2), 71. https://doi.org/10.3390/jpm15020071








