Impact of Prolonged Bisphosphonate Therapy on Atypical Femoral Fractures: Insights from a Single-Center Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Declarations
2.2. Research Participants and Data Collection
2.3. Surgical Treatment
2.4. Outcomes
2.5. Statistical Analyses
3. Results
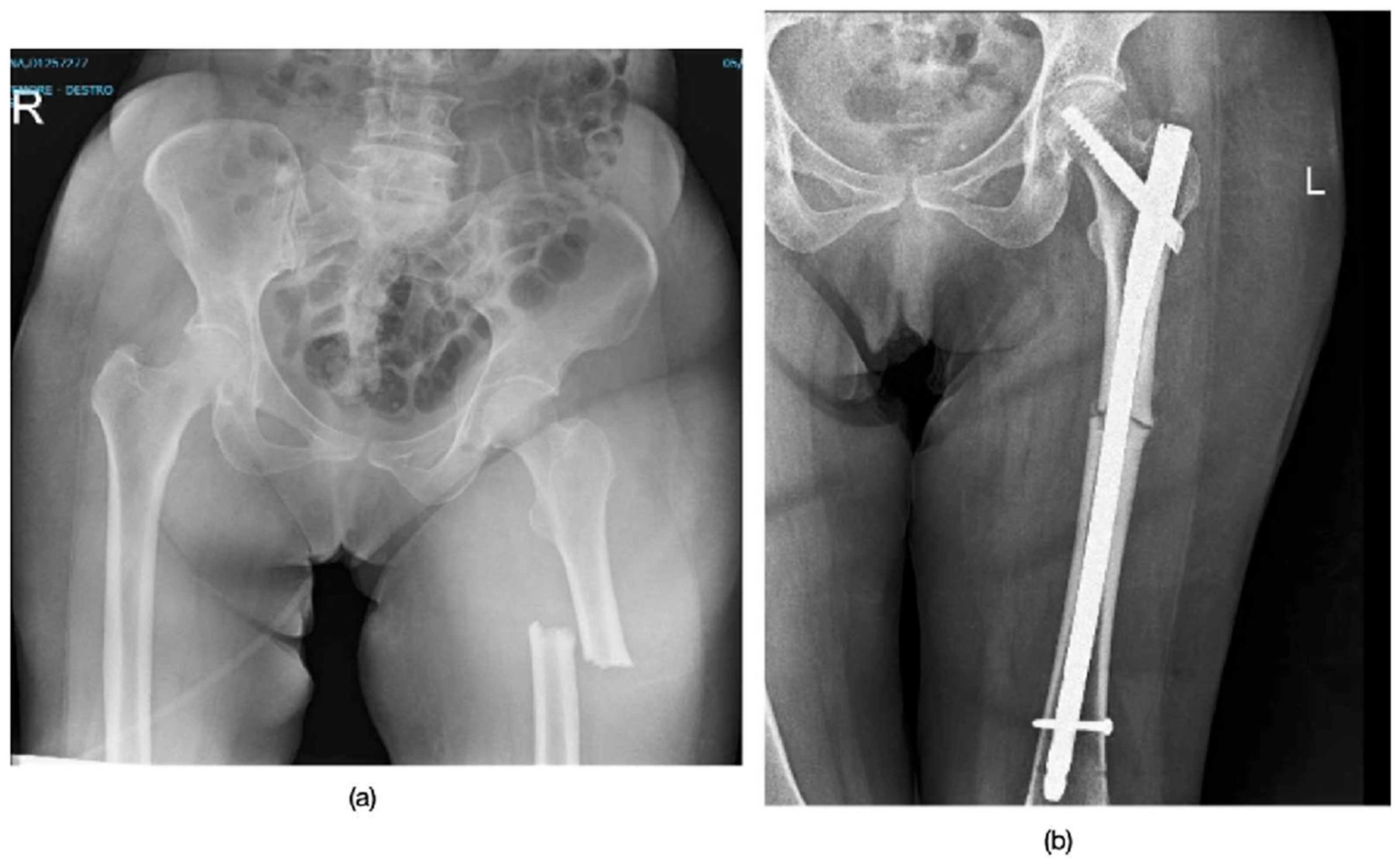
3.1. Patient Demographics and Fracture Characteristics
3.2. Treatment and Healing Process
3.3. Quality of Life and Recovery
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khandelwal, S.; Lane, N.E. Osteoporosis: Review of Etiology, Mechanisms, and Approach to Management in the Aging Population. Endocrinol. Metab. Clin. N. Am. 2023, 52, 259–275. [Google Scholar] [CrossRef]
- Johnston, C.B.; Dagar, M. Osteoporosis in Older Adults. Med. Clin. N. Am. 2020, 104, 873–884. [Google Scholar] [CrossRef]
- Ural, A. Biomechanical mechanisms of atypical femoral fracture. J. Mech. Behav. Biomed. Mater. 2021, 124, 104803. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimpour, A.; Sadighi, M.; Hoveidaei, A.H.; Chehrassan, M.; Minaei, R.; Vahedi, H.; Mortazavi, S.J. Surgical Treatment for Bisphosphonate-related Atypical Femoral Fracture: A Systematic Review. Arch. Bone Jt. Surg. 2021, 9, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Farrukh, A.M.; Reyes, L.C.F.; Capa, G.S.L.; Padilla, T.B.M.; Sunkara, V.; Dhakal, S. Bisphosphonate-induced atypical femoral shaft fracture: A case report. Radiol. Case Rep. 2023, 18, 4048–4051. [Google Scholar] [CrossRef]
- LeBlanc, E.S.; Rosales, A.G.; Genant, H.K.; Dell, R.M.; Friess, D.M.; Boardman, D.L.; Santora, A.C.; Bauer, D.C.; de Papp, A.E.; Black, D.M.; et al. Radiological criteria for atypical features of femur fractures: What we can learn when applied in a clinical study setting. Osteoporos. Int. 2019, 30, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Lins, L.; Carvalho, F.M. SF-36 total score as a single measure of health-related quality of life: Scoping review. SAGE Open Med. 2016, 4, 2050312116671725. [Google Scholar] [CrossRef] [PubMed]
- Toro, G.; Braile, A.; Liguori, S.; Moretti, A.; Landi, G.; Cecere, A.B.; Conza, G.; De Cicco, A.; Tarantino, U.; Iolascon, G. The role of the fracture liaison service in the prevention of atypical femoral fractures. Ther. Adv. Musculoskelet. Dis. 2023, 15, 1759720X231212747. [Google Scholar] [CrossRef]
- Çakmak, S.; Mahiroğulları, M.; Keklikçi, K.; Sarı, E.; Erdik, B.; Rodop, O. Bilateral low-energy sequential femoral shaft fractures in patients on long-term bisphosphonate therapy. Acta Orthop. Traumatol. Turc. 2013, 47, 162–172. [Google Scholar] [CrossRef]
- Capeci, C.M.; Tejwani, N.C. Bilateral low-energy simultaneous or sequential femoral fractures in patients on long-term alendronate therapy. J. Bone Jt. Surg. Am. 2009, 91, 2556–2561. [Google Scholar] [CrossRef]
- Koh, A.; Guerado, E.; Giannoudis, P.V. Atypical femoral fractures related to bisphosphonate treatment: Issues and controversies related to their surgical management. Bone Jt. J. 2017, 99-B, 295–302. [Google Scholar] [CrossRef]
- Kwek, E.B.; Goh, S.K.; Koh, J.S.; Png, M.A.; Howe, T.S. An emerging pattern of subtrochanteric stress fractures: A long-term complication of alendronate therapy? Injury 2008, 39, 224–231. [Google Scholar] [CrossRef]
- Shkolnikova, J.; Flynn, J.; Choong, P. Burden of bisphosphonate-associated femoral fractures. ANZ J. Surg. 2013, 83, 175–181. [Google Scholar] [CrossRef]
- Komatsubara, S.; Mori, S.; Mashiba, T.; Ito, M.; Li, J.; Kaji, Y.; Akiyama, T.; Miyamoto, K.; Cao, Y.; Kawanishi, J.; et al. Long-term treatment of incadronate disodium accumulates microdamage but improves the trabecular bone microarchitecture in dog vertebra. J. Bone Miner. Res. 2003, 18, 512–520. [Google Scholar] [CrossRef]
- Mashiba, T.; Saito, M.; Yamagami, Y.; Tanaka, M.; Iwata, K.; Yamamoto, T. Effects of suppressed bone remodeling by minodronic acid and alendronate on bone mass, microdamage accumulation, collagen crosslinks and bone mechanical properties in the lumbar vertebra of ovariectomized cynomolgus monkeys. Bone 2017, 97, 184–191. [Google Scholar] [CrossRef]
- Park, K.T.; Lee, K.B. Sequential subtrochanteric femoral fracture after atypical diaphyseal fracture in a long-term bisphosphonate user: A case report. Acta Chir. Orthop. Traumatol. Cech. 2015, 82, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Weil, Y.A.; Rivkin, G.; Safran, O.; Liebergall, M.; Foldes, A.J. The outcome of surgically treated femur fractures associated with long-term bisphosphonate use. J. Trauma. 2011, 71, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Savaridas, T.; Wallace, R.J.; Salter, D.M.; Simpson, A.H. Do bisphosphonates inhibit direct fracture healing: A laboratory investigation using an animal model. Bone Jt. J. 2013, 95-B, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Phillips, H.K.; Harrison, S.J.; Akrawi, H.; Sidhom, S.A. Retrospective review of patients with atypical bisphosphonate related proximal femoral fractures. Injury 2017, 48, 1159–1164. [Google Scholar] [CrossRef]
- Goh, S.K.; Yang, K.Y.; Koh, J.S.; Wong, M.K.; Chua, S.Y.; Chua, D.T.; Howe, T.S. Subtrochanteric insufficiency fractures in patients on alendronate therapy: A caution. J. Bone Jt. Surg. Br. 2007, 89, 349–353. [Google Scholar] [CrossRef]
- Byun, S.E.; Lee, K.J.; Shin, W.C.; Moon, N.H.; Kim, C.H. The effect of teriparatide on fracture healing after atypical femoral fracture: A systematic review and meta-analysis. Osteoporos. Int. 2023, 34, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Kendler, D.L.; Marin, F.; Zerbini, C.A.F.; Russo, L.A.; Greenspan, S.L.; Zikan, V.; Bagur, A.; Malouf-Sierra, J.; Lakatos, P.; Fahrleitner-Pammer, A.; et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): A multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 2018, 391, 230–240. [Google Scholar] [CrossRef]
- Saleh, A.; Hegde, V.V.; Potty, A.G.; Schneider, R.; Cornell, C.N.; Lane, J.M. Management strategy for symptomatic bisphosphonate-associated incomplete atypical femoral fractures. HSS J. 2012, 8, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Mangano, G.R.A.; Avola, M.; Blatti, C.; Caldaci, A.; Sapienza, M.; Chiaramonte, R.; Vecchio, M.; Pavone, V.; Testa, G. Non- Adherence to Anti-Osteoporosis Medication: Factors Influencing and Strategies to Overcome It. A Narrative Review. J. Clin. Med. 2022, 12, 14. [Google Scholar] [CrossRef] [PubMed]
| SEX | BMI | Fracture Type | Side Fracture | Time of Surgery | ASA Score | |
|---|---|---|---|---|---|---|
| Patient 1 | F | 20 | Subtrochanteric | Left | 48 h | 2 |
| Patient 2 | F | 23 | Mid-third diaphysis | Right | 48 h | 2 |
| Patient 3 | F | 31 | Subtrochanteric | Left | 24 h | 2 |
| Patient 4 | F | 22 | Mid-third diaphysis | Right | 48 h | 3 |
| Patient 5 | F | 26 | Mid-third diaphysis | Right | 72 h | 2 |
| Patient 6 | F | 18.5 | Mid-third diaphysis | Right | 48 h | 3 |
| Post-Surgery | 6 Months | 12 Months | |
|---|---|---|---|
| Patient 1 | 0–40° | 0–110° | 0–130° |
| Patient 2 | 0–30° | 0–109° | 0–120° |
| Patient 3 | 0–40° | 0–120° | 0–140° |
| Patient 4 | 0–40° | 0–110° | 0–140° |
| Patient 5 | 0–20° | 0–100° | 0–110° |
| Patient 6 | 0–40° | 0–110° | 0–130° |
| Post-Surgery | 6 Months | 12 Months | |
|---|---|---|---|
| Patient 1 | 0–30° | 0–80° | 0–120° |
| Patient 2 | 0–20° | 0–80° | 0–110° |
| Patient 3 | 0–40° | 0–90° | 0–140° |
| Patient 4 | 0–30° | 0–80° | 0–110° |
| Patient 5 | 0–20° | 0–90° | 0–110° |
| Patient 6 | 0–30° | 0–85° | 0–110° |
| Mean Age | Involved Side | Localization | Time of Use of Bisphosphonates | Surgical Treatment | Pseudoarthrosis |
|---|---|---|---|---|---|
| 70.3 ± 6.5 y | 4 Right, 2 Left | 2 subtrochanteric 4 mid-diaphyseal | 7.3 ± 1.5 y | long intramedullary nail | 0% |
| Radiographic Healing Time | Quality of Life | ROM Average Recovery Time | Residual Pain at 12 Months |
|---|---|---|---|
| 5 ± 1.5 months | SF36 of 77 ± 15.4 pre (before surgery) SF36 of 57 ± 12.5 post (a year after surgery) | 4.5 ± 1.2 months | VAS of 2 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caldaci, A.; Panebianco, P.; Condorelli, S.; Zappalà, N.; Costarella, L.; Sapienza, M.; Testa, G.; Pavone, V. Impact of Prolonged Bisphosphonate Therapy on Atypical Femoral Fractures: Insights from a Single-Center Experience. J. Pers. Med. 2025, 15, 565. https://doi.org/10.3390/jpm15120565
Caldaci A, Panebianco P, Condorelli S, Zappalà N, Costarella L, Sapienza M, Testa G, Pavone V. Impact of Prolonged Bisphosphonate Therapy on Atypical Femoral Fractures: Insights from a Single-Center Experience. Journal of Personalized Medicine. 2025; 15(12):565. https://doi.org/10.3390/jpm15120565
Chicago/Turabian StyleCaldaci, Alessia, Pierpaolo Panebianco, Sveva Condorelli, Noemy Zappalà, Luciano Costarella, Marco Sapienza, Gianluca Testa, and Vito Pavone. 2025. "Impact of Prolonged Bisphosphonate Therapy on Atypical Femoral Fractures: Insights from a Single-Center Experience" Journal of Personalized Medicine 15, no. 12: 565. https://doi.org/10.3390/jpm15120565
APA StyleCaldaci, A., Panebianco, P., Condorelli, S., Zappalà, N., Costarella, L., Sapienza, M., Testa, G., & Pavone, V. (2025). Impact of Prolonged Bisphosphonate Therapy on Atypical Femoral Fractures: Insights from a Single-Center Experience. Journal of Personalized Medicine, 15(12), 565. https://doi.org/10.3390/jpm15120565









