Hepatic Arterial Infusion Chemotherapy in the Treatment of Unresectable Hepatocellular Carcinoma with and Without Extrahepatic Spread: A Propensity Score Matching Study
Abstract
1. Introduction
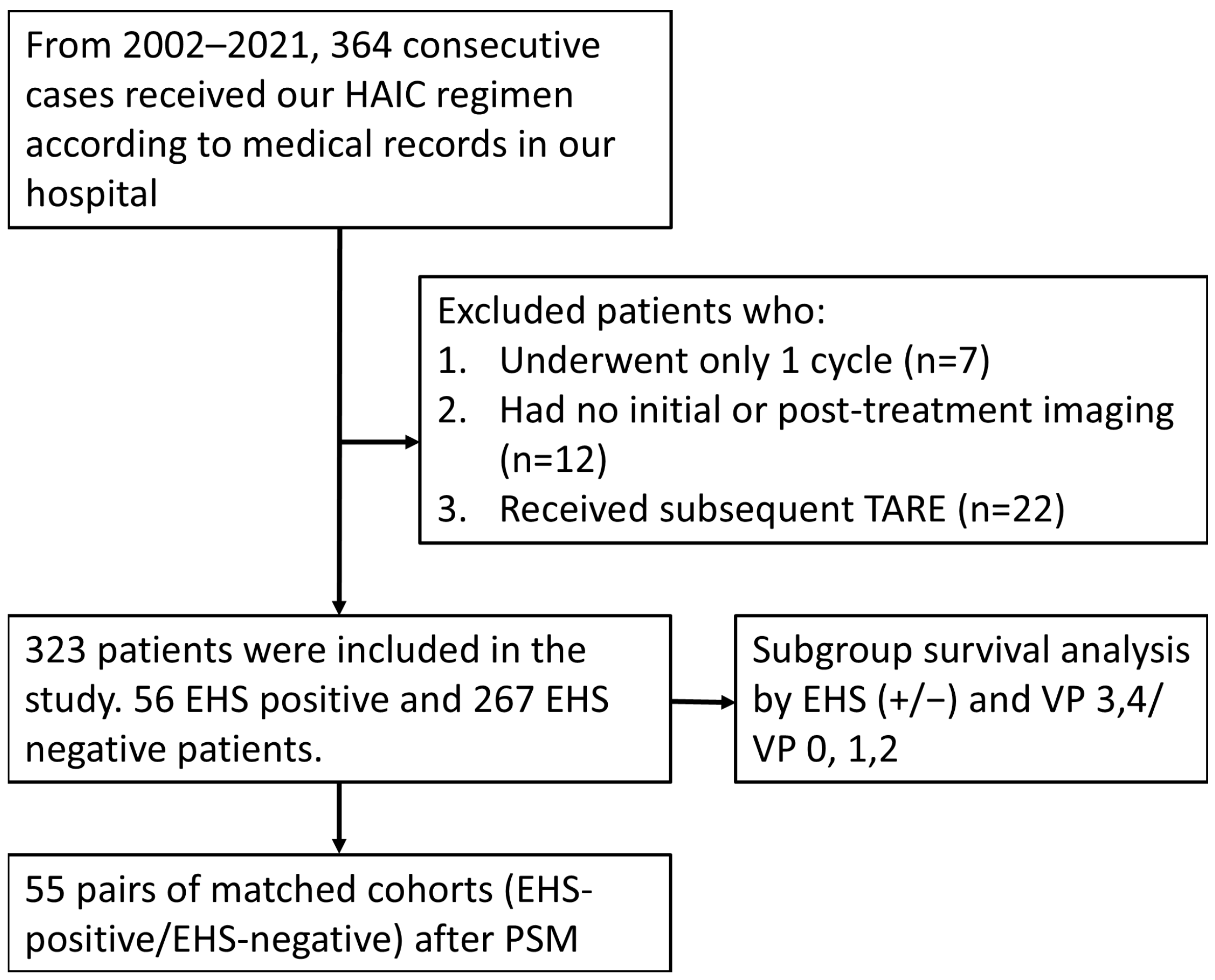
2. Method
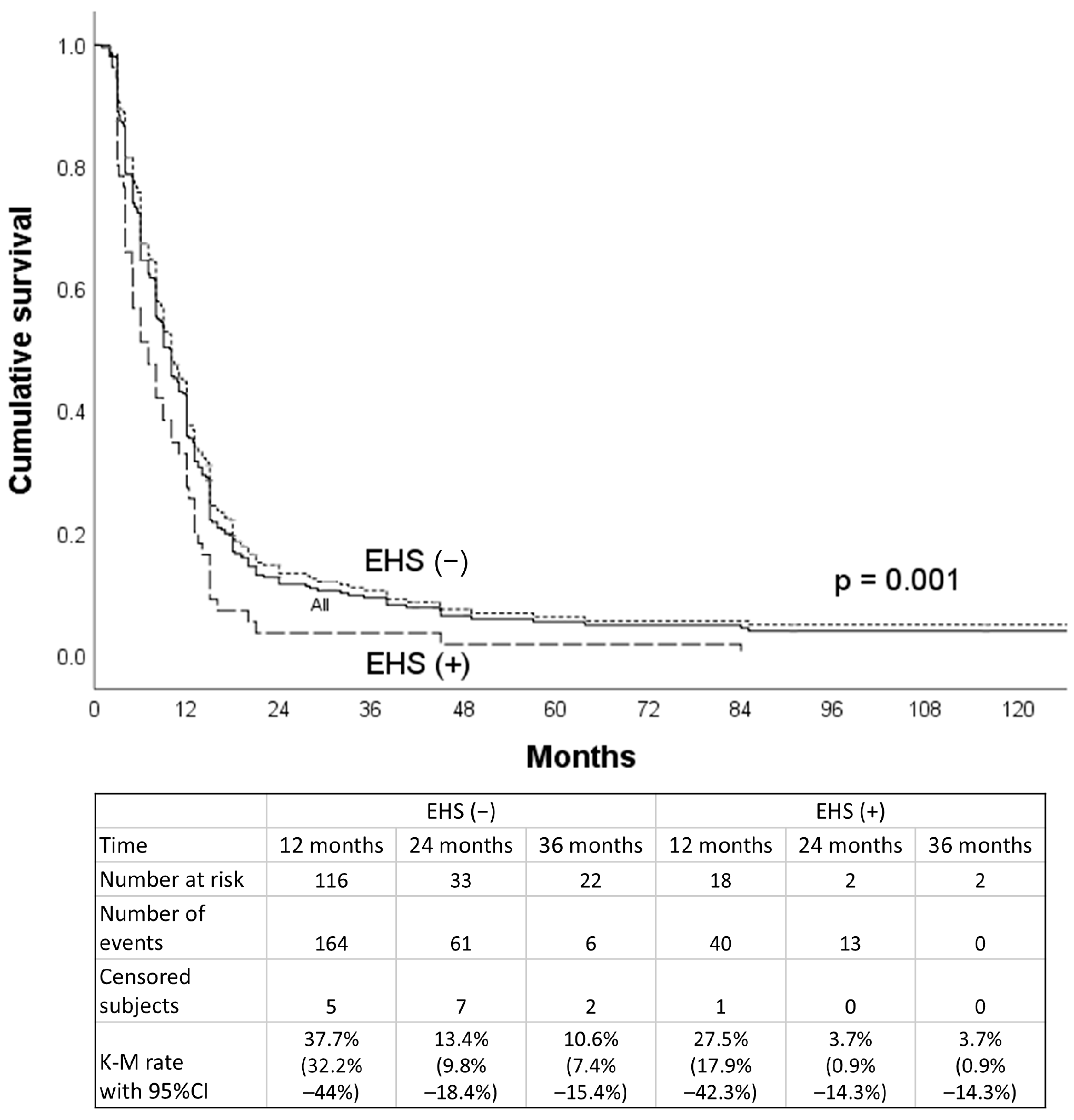
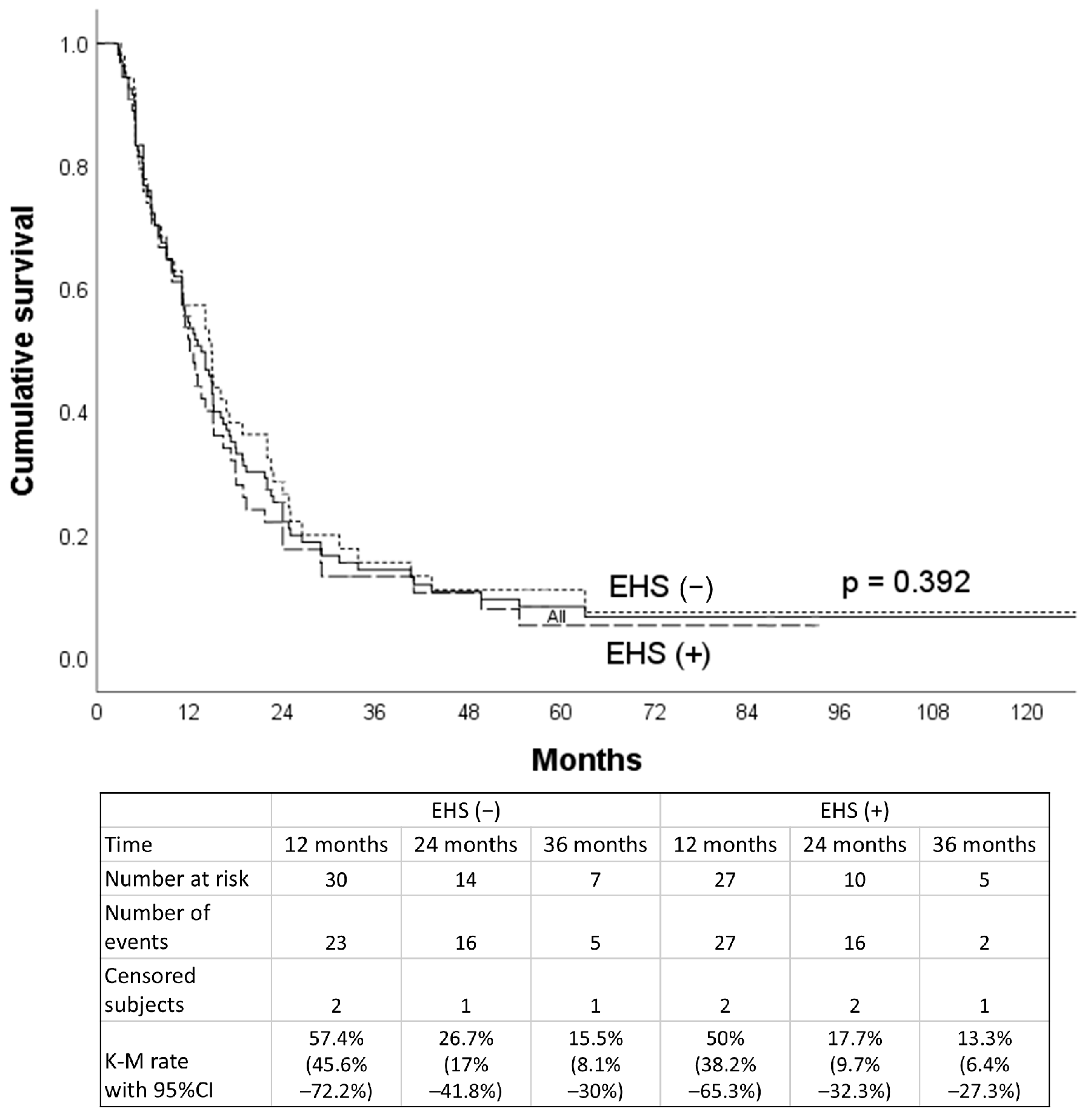
3. Result
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reig, M.; Sanduzzi-Zamparelli, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Díaz, A.; Llarch, N.; Iserte, G. BCLC strategy for prognosis prediction and treatment recommendations: The 2025 update. J. Hepatol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.-L.; Kang, Y.-K.; Chen, Z.; Tsao, C.-J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.-S. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Rimassa, L.; Santoro, A. Sorafenib therapy in advanced hepatocellular carcinoma: The SHARP trial. Expert Rev. Anticancer Ther. 2009, 9, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.; Psarelli, E.-E.; Berhane, S.; Khan, H.; Johnson, P. Impact of viral status on survival in patients receiving sorafenib for advanced hepatocellular cancer: A meta-analysis of randomized phase III trials. J. Clin. Oncol. 2017, 35, 622–628. [Google Scholar] [CrossRef]
- Nakano, M.; Tanaka, M.; Kuromatsu, R.; Nagamatsu, H.; Tajiri, N.; Satani, M.; Niizeki, T.; Aino, H.; Okamura, S.; Iwamoto, H. Sorafenib for the treatment of advanced hepatocellular carcinoma with extrahepatic metastasis: A prospective multicenter cohort study. Cancer Med. 2015, 4, 1836–1843. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, B.-W.; Li, W.; Xie, X.-H.; Hu, H.-T.; Lu, M.-D.; Xie, X.-Y. Sorafenib versus hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma: A systematic review and meta-analysis. Jpn. J. Clin. Oncol. 2019, 49, 845–855. [Google Scholar] [CrossRef]
- Liu, M.; Shi, J.; Mou, T.; Wang, Y.; Wu, Z.; Shen, A. Systematic review of hepatic arterial infusion chemotherapy versus sorafenib in patients with hepatocellular carcinoma with portal vein tumor thrombosis. J. Gastroenterol. Hepatol. 2020, 35, 1277–1287. [Google Scholar] [CrossRef]
- Zheng, K.; Zhu, X.; Fu, S.; Cao, G.; Li, W.-Q.; Xu, L.; Chen, H.; Wu, D.; Yang, R.; Wang, K. Sorafenib plus hepatic arterial infusion chemotherapy versus sorafenib for hepatocellular carcinoma with major portal vein tumor thrombosis: A randomized trial. Radiology 2022, 303, 455–464. [Google Scholar] [CrossRef]
- He, M.; Li, Q.; Zou, R.; Shen, J.; Fang, W.; Tan, G.; Zhou, Y.; Wu, X.; Xu, L.; Wei, W. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: A randomized clinical trial. JAMA Oncol. 2019, 5, 953–960. [Google Scholar] [CrossRef]
- Iwamoto, H.; Niizeki, T.; Nagamatsu, H.; Ueshima, K.; Tani, J.; Kuzuya, T.; Kasai, K.; Kooka, Y.; Hiraoka, A.; Sugimoto, R. The clinical impact of hepatic arterial infusion chemotherapy new-FP for hepatocellular carcinoma with preserved liver function. Cancers 2022, 14, 4873. [Google Scholar] [CrossRef]
- Li, M.-F.; Liang, H.-L.; Chiang, C.-L.; Tsai, W.-L.; Chen, W.-C.; Tsai, C.-C.; Chen, I.-S. New regimen of combining hepatic arterial infusion chemotherapy and lipiodol embolization in treating hepatocellular carcinoma with main portal vein invasion. J. Pers. Med. 2022, 13, 88. [Google Scholar] [CrossRef]
- Nakashima, T.; Okuda, K.; Kojiro, M.; Jimi, A.; Yamaguchi, R.; Sakamoto, K.; Ikari, T. Pathology of hepatocellular carcinoma in Japan: 232 consecutive cases autopsied in ten years. Cancer 1983, 51, 863–877. [Google Scholar] [CrossRef]
- Kudo, M. Surveillance, diagnosis, and treatment outcomes of hepatocellular carcinoma in Japan: 2021 update. Liver Cancer 2021, 10, 167–180. [Google Scholar] [CrossRef]
- Kim, B.; Won, J.H.; Kim, J.; Kwon, Y.; Cho, H.J.; Huh, J.; Kim, J.K. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma: Radiologic and clinical factors predictive of survival. Am. J. Roentgenol. 2021, 216, 1566–1573. [Google Scholar] [CrossRef]
- Sung, P.S.; Yang, K.; Bae, S.H.; Oh, J.S.; Chun, H.J.; Nam, H.C.; Jang, J.W.; Choi, J.Y.; Yoon, S.K. Reduction of intrahepatic tumour by hepatic arterial infusion chemotherapy prolongs survival in hepatocellular carcinoma. Anticancer Res. 2019, 39, 3909–3916. [Google Scholar] [CrossRef]
- Lyu, N.; Kong, Y.; Pan, T.; Mu, L.; Li, S.; Liu, Y.; Deng, H.; Li, J.; Shi, M.; Xu, L. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin in hepatocellular cancer with extrahepatic spread. J. Vasc. Interv. Radiol. 2019, 30, 349–357.e2. [Google Scholar] [CrossRef]
- Liang, R.-B.; Zhao, Y.; He, M.-K.; Wen, D.-S.; Bu, X.-Y.; Huang, Y.-X.; Lai, Z.-C.; Xu, Y.-J.; Kan, A.; Wei, W. Hepatic arterial infusion chemotherapy of oxaliplatin, fluorouracil, and leucovorin with or without sorafenib as initial treatment for advanced hepatocellular carcinoma. Front. Oncol. 2021, 11, 619461. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.; Zhang, N.; Deng, M.; Lin, Y.; Huang, G.; Fu, Y.; Zheng, Z.; Wei, W.; Zhong, C.; Zhao, H. Patients with hepatocellular carcinoma extrahepatic metastases can benefit from hepatic arterial infusion chemotherapy combined with lenvatinib plus programmed death-1 inhibitors. Int. J. Surg. 2024, 110, 4062–4073. [Google Scholar] [CrossRef]
- Chen, S.; Wang, N.; Xiao, Y.; Jiang, X.; Shi, F.; Cai, H.; Tang, S.; Guo, W.; Zhuang, W. Efficiency and Safety of HAIC Combined with Lenvatinib and PD-1 Inhibitor for Advanced Hepatocellular Carcinoma with Lung Metastasis: A Multicenter Propensity Score Matching Analysis. ImmunoTargets Ther. 2025, 14, 953–965. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Lai, E.C.; Li, N.; Guo, W.-X.; Xue, J.; Lau, W.-Y.; Wu, M.-C.; Cheng, S.-Q. A new classification for hepatocellular carcinoma with portal vein tumor thrombus. J. Hepato-Biliary-Pancreat. Sci. 2011, 18, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.-L.; Huang, J.-S.; Lin, Y.-H.; Lai, K.-H.; Yang, C.-F.; Pan, H.-B. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma by placing a temporary catheter via the subclavian route. Acta Radiol. 2007, 48, 734–740. [Google Scholar] [CrossRef]
- Lencioni, R.; Montal, R.; Torres, F.; Park, J.-W.; Decaens, T.; Raoul, J.-L.; Kudo, M.; Chang, C.; Ríos, J.; Boige, V. Objective response by mRECIST as a predictor and potential surrogate end-point of overall survival in advanced HCC. J. Hepatol. 2017, 66, 1166–1172. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; De Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Quirk, M.; Kim, Y.H.; Saab, S.; Lee, E.W. Management of hepatocellular carcinoma with portal vein thrombosis. World J. Gastroenterol. WJG 2015, 21, 3462. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. Changing the treatment paradigm for hepatocellular carcinoma using atezolizumab plus bevacizumab combination therapy. Cancers 2021, 13, 5475. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.-J.; Gao, S.; Zhu, X.; Guo, J.-H.; Kou, F.-X.; Liu, S.-X.; Zhang, X.; Wang, X.-D.; Cao, G.; Chen, H. Combination Therapy of Chemoembolization and Hepatic Arterial Infusion Chemotherapy in Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis Compared with Chemoembolization Alone: A Propensity Score-Matched Analysis. BioMed Res. Int. 2021, 2021, 6670367. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Jian, Y.; Wang, M.; Liu, F.; Zhang, Q.; Peng, Z.; Cheng, N.; Zhang, W. Hepatic artery intervention combined with immune-targeted therapy is superior to sequential therapy in BCLC-C hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2023, 149, 5405–5416. [Google Scholar] [CrossRef]




| EHS (Yes/No) | Before PSM n = 323 | After PSM n = 110 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | p-Value | Yes | No | p-Value | SMD | |||
| Gender (M/F) | 49/7 | 218/49 | 0.338 | 48/7 | 87.3 ± 4.5% | 51/4 | 92.7 ± 3.5% | 0.527 | 0.183 |
| Age (≥/<60) | 26/30 | 147/120 | 0.243 | 25/30 | 45.5 ± 6.7% | 23/32 | 41.8 ± 6.7% | 0.848 | 0.073 |
| HBV or HCV/Others | 47/9 | 232/35 | 0.526 | 47/8 | 85.5 ± 4.8% | 50/5 | 30.9 ± 3.9% | 0.556 | 0.168 |
| Tumor size (≥/<8) cm | 41/15 | 121/146 | <0.001 | 41/14 | 74.5 ± 5.9% | 41/14 | 74.5 ± 5.9% | >0.999 | <0.001 |
| Tumor number (≥/<10) | 14/42 | 41/226 | 0.116 | 14/41 | 25.5 ± 5.9% | 12/43 | 21.8 ± 5.6% | 0.823 | 0.085 |
| VP3,4/VP 0,1,2 | 32/24 | 127/140 | 0.24 | 32/23 | 58.2 ± 6.7% | 33/22 | 60.0 ± 6.6% | >0.999 | 0.037 |
| Child–Pugh A/B | 49/7 | 230/37 | >0.999 | 48/7 | 87.3 ± 4.5% | 48/7 | 87.3 ± 4.5% | >0.999 | <0.001 |
| AFP (≥/<400) ng/mL | 37/19 | 127/170 | 0.013 | 36/19 | 65.5 ± 6.4% | 36/19 | 65.5 ± 6.4% | >0.999 | <0.001 |
| Median OS (m) | Extrahepatic Spread | p-Value | |
|---|---|---|---|
| Yes | No | ||
| VP3/4 subgroup | 13.0 (9.2–16.8) | 15.0 (12.2–17.8) | 0.407 |
| VP ≤ 2 subgroup | 11.4 (10.6–12.2) | 19.4 (16.8–22.0) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-T.; Liang, H.-L.; Chiang, C.-L.; Tsai, W.-L.; Chen, Y.-C. Hepatic Arterial Infusion Chemotherapy in the Treatment of Unresectable Hepatocellular Carcinoma with and Without Extrahepatic Spread: A Propensity Score Matching Study. J. Pers. Med. 2025, 15, 561. https://doi.org/10.3390/jpm15110561
Chen C-T, Liang H-L, Chiang C-L, Tsai W-L, Chen Y-C. Hepatic Arterial Infusion Chemotherapy in the Treatment of Unresectable Hepatocellular Carcinoma with and Without Extrahepatic Spread: A Propensity Score Matching Study. Journal of Personalized Medicine. 2025; 15(11):561. https://doi.org/10.3390/jpm15110561
Chicago/Turabian StyleChen, Chao-Ting, Huei-Lung Liang, Chia-Ling Chiang, Wei-Lun Tsai, and Yu-Chia Chen. 2025. "Hepatic Arterial Infusion Chemotherapy in the Treatment of Unresectable Hepatocellular Carcinoma with and Without Extrahepatic Spread: A Propensity Score Matching Study" Journal of Personalized Medicine 15, no. 11: 561. https://doi.org/10.3390/jpm15110561
APA StyleChen, C.-T., Liang, H.-L., Chiang, C.-L., Tsai, W.-L., & Chen, Y.-C. (2025). Hepatic Arterial Infusion Chemotherapy in the Treatment of Unresectable Hepatocellular Carcinoma with and Without Extrahepatic Spread: A Propensity Score Matching Study. Journal of Personalized Medicine, 15(11), 561. https://doi.org/10.3390/jpm15110561






