Personalized Immunotherapy for T Cell Lymphomas: From Immune Escape to Precision Therapeutics
Abstract
1. Introduction
2. Immunobiology of Peripheral T Cell Lymphomas (PTCLs)
2.1. Tumor-Intrinsic Immune Surveillance Escape Mechanisms
2.1.1. Loss of Antigen Presentation
2.1.2. Downregulation of Adhesion Molecules
2.1.3. Overexpression of “Don’t Eat Me” Signals
2.1.4. Deregulated Apoptotic Pathways
2.2. Tumor Microenvironment (TME)-Mediated Immunosuppression
2.2.1. Cytokine and Chemokine Milieu of PTCLs
2.2.2. Immunosuppressive Cell Populations
2.2.3. Angiogenesis and Vascular Remodeling
2.2.4. Immune Checkpoint Pathways
3. Immunotherapy and Immune-Modulating Agents in TCL
3.1. Checkpoint Inhibitors in T Cell Lymphoma
3.1.1. PD-1/PD-L1 Blockade in PTCL
3.1.2. Immune Checkpoint Expression
3.1.3. Tumor Mutational Burden
3.1.4. Microsatellite Instability (MSI) and Deficient Mismatch Repair (dMMR)
3.1.5. Chr 9p Structural Variants
3.1.6. PD-1/PD-L1 Blockade Combination Regimens in PTCL
| Trial and Phase | Line of Therapy | No of Patients (TCL Cohort) | Regimen | ORR | CR | Median PFS | OS | Notes | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Checkpoint inhibitor monotherapy studies—mixed PTCL | |||||||||
| AVAIL-T NCT03046953 | Median 3 prior lines Range 1–7 | Total: 34 AITL: 11 PTCL NOS: 17 ENKTL: 4 ALCL: 1 Transformed MF: 1 | Avelumab monotherapy | 17.6% | 0% | 2.9 months | 8.9 months | N/A | [65] |
| Phase I NCT01592370 | 5 | Nivolumab monotherapy | 40% | 0% | 14 weeks | N/A | [67] | ||
| Phase II NCT03075553 | Median 2 prior line Range 1–6 | 12 AITL: 6 PTCL NOS: 3 ALCL ALK-: 1 EATL: 1 HSTCL: 1 | Nivolumab monotherapy | 33% | 16.7% | 2.7 months | 6.7 months | Median DOR: 3.6 months Hyperprogression in 4 patients (3 AITL and 1 HSTCL) | [114] |
| GXPLORE-002 Phase II NCT03502629 | Median 2 prior line | 102 (89 in full analysis set) PTCL NOS: 41 ENKTL: 23 ALCL ALK-: 12 ALCL ALK+: 7 EATL: 3 MF: 3 Others: 12 | Geptanolimab monotherapy | 40.4% | 14.6% | 2.7 months | 14.6 months | DOR: 11.4 months ORR by disease subtype ENKTL: 63.2% ALCL ALK-: 53.8% ALCL ALK+: 42.9% PTCL NOS: 17.9% PD-L1 ≥50% enriched (ORR 53.3% vs. 25%) | [68] |
| Phase II NCT02535247 | Median 2 prior lines Range 1–9 | 18 (13 evaluable) PTCL NOS: 7 TFH: 4 Transformed MF: 3 | Pembrolizumab monotherapy | 33% | 27% | 3.2 months | 10.6 months | Halted early after futility analysis | [66] |
| Pembrolizumab maintenance post ASCT Phase II NCT02362997 | N/A | 21 PTCL NOS: 11 AITL: 4 ENKTL: 3 ALCL ALK-: 2 MEITL: 1 | Pembrolizumab monotherapy Post ASCT maintenance study | N/A | N/A | 18-month PFS: 83.6% | 18-month OS: 94.4% | N/A | [70] |
| Duvelisib maintenance post ASCT Phase II NCT04331119 | N/A | 12 (5 PTCL) ALCL: 3 PTCL NOS: 1 AITL: 3 | Duvelisib monotherapy Post ASCT maintenance study | N/A | N/A | Note reported | Not reported | N/A | [69] |
| Checkpoint inhibitor monotherapy studies—ENKTL | |||||||||
| ORIENT-4 Phase II NCT03228836 | Median 3 prior lines | 28 | Sintilimab monotherapy | 75% | 21.4% | Not reported | NR 2yr OS: 78.6% | Median DOR: 4.1 months Pseudoprogression in 17.9% | [75] |
| Avelumab Phase II NCT 03439501 | Median ≥2 prior lines | 21 | Avelumab monotherapy | 38% | 24% | 2.7 months | NR | Response by PDL1 expression: High PD-L1: 73% Low PD-L1: 0% | [77] |
| GEMSTONE-201 Phase II NCT03595657 | Median 1 prior lines | 80 | Sugemalimab monotherapy | 44.9% | 35.9% | Not reported | NR 18-month OS: 57.9% | Median DOR: NR | [115] |
| Pembrolizumab (Retrospective case series) | Median 2 prior lines Range 1–5 | 7 | Pembrolizumab monotherapy | 100% | 71.4% | Not reported | Not reported | Non-trial study | [71] |
| Pembrolizumab (Retrospective case series) | Median 4 prior lines Range 3–10 | 7 | Pembrolizumab monotherapy | 57% | 28.6% | 4.8 months | 5 months | Non-trial study DOR: 4.1 months | [74] |
| Pembrolizumab (Retrospective case series) | 14 | Pembrolizumab monotherapy | 44% | 35.7% | Not reported?? | N/A | N/A | [74] | |
| Checkpoint inhibitor combination studies—Mixed PTCL | |||||||||
| Pembrolizumab + Romidepsin Phase I/II NCT03278782 | Median 2.5 prior lines | 20 (14 evaluable for efficacy) PTCL NOS: 7 TFH: 15 AITL: 2 Transformed MF: 3 ALCL: 3 ENKTL: 2 | Pembrolizumab + Romidepsin | 50% | 35.7% | Not reported | Not reported | Hyperprogression occurred in 2 patients | [116] |
| Camrelizumab + Apatinib Phase II NCT03701022 | Median 3 prior lines Range 1–6 | ENKTL: 7 PTCL NOS: 6 AITL: 5 ALCL ALK-: 2 | Camrelizumab + Apatinib | 30% | 10% | 5.6 months | 16.7 months | DOR: not reached Patients with PD-L1 expression ≥ 50% vs. <50% ORR (66.7% vs. 40%). The two patients with the highest PD-L1 expression showed PFS of 18.2 and 22.3 months, respectively. | [117] |
| PCET (Retrospective case series) | Median 2 prior lines Range 1–6 | ENKTL: 3 | PCET: toripalimab + chidamide + etoposide + thalidomide | 100% | 66.7% | Not reported | Not reported | Non-trial study | [113] |
3.2. Engineered Cellular Therapies (CAR-T, CAR-NK)
3.2.1. CD4
3.2.2. CD5
3.2.3. CD7
3.2.4. TRBC-1
3.2.5. CD30
3.2.6. CD70
3.2.7. CCR4
3.2.8. Other Targets in Preclinical Development
3.3. Bispecific Antibodies in T Cell Lymphoma
3.4. Oncolytic Viruses
3.5. Immunomodulatory Imide Drugs (IMiDs)
3.6. Other Agents
3.6.1. Denileukin Diftitox
3.6.2. Brentuximab Vedotin
3.6.3. Lacutamab
3.6.4. Mogamulizumab
3.6.5. MEDI-570
| Regimen | Phase | Trial | Prior Lines of Therapy | No of Patients (TCL Cohort) | ORR | CR | Median PFS | OS | Reference |
|---|---|---|---|---|---|---|---|---|---|
| CAR-T Cell Therapies | |||||||||
| CD4-specific CAR-T | Phase I | NCT04162340 | N/A | (Preliminary data) Total: 3 SS: 1 Transformed MF: 1 AITL: 1 | 100% (2 CR, 1 PR) | 66% (2 out of 3) | N/A | N/A | [130] |
| Phase I | NCT04712864 | Active, Not Recruiting | |||||||
| Phase I | NCT03829540 | Recruiting | |||||||
| CD5-specific CAR-T | Phase I | NCT03081910 (MAGENTA) | Median: 5 lines (Range 2–18) | Total: 9 MF/SS: 1 CTCL: 1 AITL: 2 PTCL: 4 ATLL: 1 | 44% (2 CRs, 1 PR, 1 mixed radiographic response) | 22% (2 out of 9) | N/A | N/A | [137] |
| Phase I | NCT04767308 | (Range 2–6) | (Preliminary data) Total: 3 AITL: 2 SPTCL: 1 | 100% (1 CR, 2 PR) | 33% (1 out of 3) | N/A | N/A | [136] | |
| Phase I | NCT04594135 | Unknown status | Preclinical and preliminary clinical data of 1 patient [135] | ||||||
| Phase I | NCT06633341 | Recruiting | |||||||
| Phase I | NCT07022964 | Recruiting | |||||||
| CD7-specific CAR-T | Phase I | NCT04928105 | Median: 6 (Range: 2–12) | (Preliminary data) Total: 5 PTCL-NOS: 2 MEITL: 1 HSTCL: 1 NKTCL: 1 | 80% (3 CR, 1 PR) | 60% (3 out of 5) | N/A | N/A | [148] |
| Phase I | NCT05377827 | N/A | (Preliminary data) Total: 5 PTCL:1 T-PLL:2 Gamma-delta TCL: 2 | 80% (2 CR, 2 PR, 1 SD) | 40% (2 out of 5) | N/A | N/A | [268] | |
| Phase I | NCT04004637 | Unknown status | Preliminary data on T-ALL/T-LBL [269] | ||||||
| Phase I | NCT05290155 | Completed, results not published | |||||||
| Phase II | NCT05059912 | Unknown status | |||||||
| Phase I/II | NCT06925464 | Recruiting | |||||||
| Phase I | NCT05979792 | Not yet recruiting | |||||||
| Phase I | NCT04480788 | Unknown status | |||||||
| Phase I | NCT04264078 | Unknown status | |||||||
| Phase I | NCT04934774 | Unknown status | |||||||
| Phase I | NCT04823091 | Recruiting | |||||||
| Phase I | NCT05995028 | Recruiting | |||||||
| N/A | NCT07008872 | Not yet recruiting | |||||||
| N/A | NCT05620680 | Recruiting | |||||||
| TRBC1-specific CAR-T | Phase I/II | NCT03590574 (AUTO4) | Median: 2 (Range 1–5) | Total: 10 AITL: 4 PTCL-NOS: 5 ALCL: 1 | 66.6% (6 of 9) | 44.4% (4 of 9) | Median 4.7 months | Median OS was not reached | [270] |
| Phase I | NCT04828174 | Trial suspended | |||||||
| CD30-specific CAR-T | Phase I | NCT01316146 | N/A | Total: 9 Hodgkin’s lymphoma: 7 ALCL: 2 | 33.3% (3 of 9) | 33.3% (3 of 9) | N/A | N/A | [169] |
| Phase I | ChiCTR-OPN-16009069 | N/A | Total: 9 Hodgkin’s lymphoma: 6 ALCL: 3 | 77.7% (7 CR) | 77.7% | Median 13 months | [170] | ||
| Phase I | NCT04526834 | Active, not recruiting | |||||||
| Phase I | NCT07048353 | Not yet recruiting | |||||||
| Phase I | NCT05208853 | Unknown status | |||||||
| Phase I | NCT02917083 (RELY-30) | Recruiting | |||||||
| Phase I | NCT04653649 | Unknown status | |||||||
| Phase II | NCT04083495 | Recruiting | |||||||
| Phase I | NCT06494371 | Recruiting | |||||||
| Allogeneic CD30 CAR-EBVST cells | Phase I | NCT04288726 | Recruiting | ||||||
| Allogeneic CD30 CAR-EBVST cells with constitutive IL7R (C7R) | Phase I | NCT06176690 | Not yet recruiting | ||||||
| CCR4-expressing CD30-specific CAR-T cells | Phase I | NCT03602157 | Recruiting | ||||||
| CD30-specific CAR-T as consolidation after BEAM and autologous HSCT | Phase I | NCT02663297 | 83% of patients have one line of salvage therapy before autologous HSCT, 17% required second line of therapy | Total: 21 Hodgkin’s lymphoma: 11 ALCL: 4 AITL: 1 PTCL-NOS: 1 Grey zone lymphoma: 1 | At median follow-up of 48.2 months, 5 patients with T cell lymphoma have died | N/A | Median 32.3 months | Not reached | [171] |
| Allogeneic CD70-specific CAR-T cells | Phase I | NCT04502446 (COBALT-LYM) | Median: 2.5 for PTCL, 5 for CTCL | Total: 39 PTCL: 22 (9 ATLL, 8 PTCL-NOS, 4 AITL, 1 ALCL) CTCL: 17 | 46.2% (18 of 39) | 19.4% (6 of 39) | N/A | N/A | [179] |
| Phase I/II | NCT06492304 | Recruiting | |||||||
| CCR4-specific CAR-T cells | Phase I | NCT07055477 | Recruiting | ||||||
| CD37-specific CAR-T cells | Phase I | NCT04136275 | Median 6 (range 3–8) | Total: 5 Double-hit HGBCL: 2 CTCL:1 Hodgkin’s lymphoma: 1 NKTCL: 1 | 80% (3 CR) | 60% (3 of 5) | N/A | N/A | [193] |
| CD56-specific CAR-T | Phase II | NCT05941156 | Recruiting | ||||||
| CAR-NK Cell Therapies | |||||||||
| CD5-specific CAR-NK | Phase I | NCT06909474 | Recruiting | ||||||
| CD70-specific CAR-NK cells | Phase I | NCT06696846 | Not yet recruiting | ||||||
| Oncolytic Virus | |||||||||
| Oncolytic Virus Injection (RT-01) | Phase I | NCT06508463 | Unknown status | ||||||
| Vesicular Stomatitis Virus (VSV) | Phase I | NCT05387226 | Recruiting | ||||||
| Vesicular Stomatitis Virus (VSV) | Phase I | NCT03017820 | Recruiting | ||||||
| Single Agent Immunomodulatory Drugs (IMiDs) | |||||||||
| Lenalidomide Monotherapy | Phase II | NCT01724177 | Median: 2 (Range 1–4) | Total: 26 (All ATLL) | 42% | 19.2% (4 CR and 1 unconfirmed) | 3.8 months | 20.3 months | [228] |
| Lenalidomide Monotherapy | Phase II | NCT00322985 | Median: 1 (range 0–5) | Total: 40 | 26% (10 of 39) | 8% | 4 months | 12 months | [226] |
| Lenalidomide Monotherapy | Phase I | NCT01169298 | Median: 1 (Range 1–3) | Total: 13 ATLL: 9 Other PTCL: 4 | 36% (4 of 11 evaluable) | 0% | 3.4 months | N/A | [271] |
| Lenalidomide Monotherapy | Phase II | NCT00655668 (EXPECT) | Median: 3 (Range 1–11) | Total: 54 AITL: 26 PTCL-NOS: 20 CTCL (MF): 3 sALCL: 3 pcALCL:1 ENKTL: 1 | 22% (12 of 54) | 11% | 2.5 months | N/A | [227] |
| Lenalidomide Monotherapy | Phase II | NCT01036399 | Median: 4 (Range 2–7) | Total: 10 (All PTCL-NOS) | 30% | 30% (3 of 10) | N/A | N/A | [225] |
| Immunomodulatory Drugs (IMiDs) + Chemotherapy | |||||||||
| Chidamide plus prednisone, cyclophosphamide, and thalidomide (CPCT) | Phase II | NCT02879526 | At least 1 prior line | Total: 45 AITL: 20 PTCL-NOS: 17 Other subtypes: 8 | 71.1% (32 of 45) | 28.9% (13 of 45) | 8.5 months | 17.2 months | [232] |
| CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) vs. GDPT (gemcitabine, cisplatin, prednisone, thalidomide) in newly diagnosed PTCL patient | Phase IV | NCT01664975 | 0 (newly diagnosed cohort) | Total: 153 PTCL-NOS: 31 AITL: 37 ALCL: 49 Other subtypes: 36 | 66.3% (GDPT) versus 50.0% (CHOP) | 42.9% (GDPT) vs. 27.6% (CHOP) | 4-year PFS: 63.6% (GDPT) vs. 53.0% (CHOP) | 4-year OS: 66.8% (GDPT) vs. 53.6% (CHOP | [233] |
| Lenalidomide in combination with vorinostat and dexamethasone | Phase I/II | NCT00972842 | Median: 1 (Range 1–2) | Total: 8 AITL: 5 PTCL-NOS:1 ALCL: 1 | 25% (2 of 7 evaluable) | 14.2% (1 of 7) | 2.2 months | 6.7 months | [272] |
| CHOP alone vs. CHOP plus thalidomide | N/A | N/A | N/A | Total: 46 NKTCL: 21 PTCL: 9 ALCL: 7 AITL: 4 Other subtypes: 5 | 79.2% (thalidomide group) vs. 63.6% (CHOP alone) | 50% (thalidomide group) vs. 36.4% (CHOP alone) | 12 months (thalidomide group) vs. 6 months in CHOP alone | Undefined (thalidomide group) vs. 17 months in CHOP alone | [231] |
| Romidepsin, 5-azacitidine, Dexamethasone, plus Lenalidomide | Phase I | NCT04447027 | Median: 2 (Range 1–8) | Total: 26 Nodal TCL: 16 MF: 6 ATLL: 4 | 56% (25 evaluable) | 12% | 1-year PFS: 14.9% | 1-year OS: 63.3% | [273] |
| Lenalidomide plus CHOEP | Phase I/II | NCT02561273 | 0 (newly diagnosed) | Total: 39 PTCL-NOS: 19 AITL: 16 ALCL: 3 | 69% | 49% | 2-year PFS: 55% | 2-year OS: 78% | [274] |
| Romidepsin and Lenalidomide | Phase II | NCT02232516 | 0 (newly diagnosed) | Total: 29 AITL: 16 PTCL-NOS: 10 ATLL: 2 EATL: 1 | 65.2% | 26.1% | 2-year PFS: 31.5% | 2-year OS: 49.5% | [275] |
| Lenalidomide plus Gemcitabine | Phase I/II | NCT05105412 | Terminated | ||||||
| Bendamustine Combined With Chidamide and Lenalidomide | N/A | NCT07072221 | Recruiting | ||||||
| Chidamide Combination with Lenalidomide | Phase II | NCT04329130 | Unknown status | ||||||
| lenalidomide plus CHOP (L-CHOP) versus CHOP alone | Phase II | NCT04922567 | Recruiting | ||||||
| CHOP plus Lenalidomide | Phase II | NCT01553786 | Completed | ||||||
| Immunomodulatory Drugs (IMiDs) + Immunotherapy | |||||||||
| Lenalidomide + Sintilimab (anti-PD1) | Phase II | NCT04231370 | Unknown status | ||||||
| Anti-PD1 + Lenalidomide and azacytidine | Phase II | NCT05182957 | Unknown status | ||||||
| Lenalidomide, anti-PD1, Chidamide and Gemcitabine | Phase IV | NCT04040491 | Unknown status | ||||||
| Lenalidomide, anti-PD1, Chidamide and Etoposide | Phase IV | NCT04038411 | Unknown status | ||||||
| Durvalumab (anti-PDL1) with or without lenalidomide | Phase I/2 | NCT03011814 | Active, not recruiting | ||||||
| Phase II | NCT03054532 | Unknown status | |||||||
| Lenalidomide plus Brentuximab Vedotin | Phase I | NCT03302728 | Completed | ||||||
| Phase II | NCT03409432 | Completed | |||||||
| Lenalidomide and Ipilimumab after Stem cell transplant | Phase II | NCT01919619 | Completed | ||||||
4. Biomarkers for Informing Management of PTCL
4.1. Positron Emission Tomography
4.2. Genomics
4.3. Immunohistochemistry
4.4. Gene Expression
4.5. Integrating Biomarkers
5. Perspectives and Outlook on Immunotherapy in PTCL
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACT | Adoptive cell transfer |
| ADCC | Antibody-directed cellular cytotoxicity |
| AITL | Angioimmunoblastic T cell lymphoma |
| ALCL | Anaplastic large cell lymphoma |
| ALK | Anaplastic lymphoma kinase |
| allo-HSCT | Allogeneic hematopoietic stem cell transplantation |
| AML | Acute myeloid leukemia |
| ATLL | Adult T cell leukemia/lymphoma |
| auSCT | Autologous stem cell transplantation |
| BIA-ALCL | Breast-implant-associated anaplastic large cell lymphoma |
| BV | Brentuximab vedotin |
| BV-CHP | Brentuximab vedotin, cyclophosphamide, doxorubicin and prednisolone |
| c-FLIP | cellular FLICE inhibitory protein |
| CAR | Chimeric Antigen Receptor |
| CHOP | Cyclophosphamide, adriamycin, vincristine, and prednisone |
| CPCT | Chidamide, prednisone, cyclophosphamide, and thalidomide |
| CR | Complete response |
| CRBN | Cereblon |
| CRR | Complete response rate |
| CRS | Cytokine release syndrome |
| CTCL | Cutaneous T cell lymphoma |
| ctDNA | Circulating tumor DNA |
| dMMR | Deficient mismatch repair |
| EATL | Enteropathy-associated T cell lymphoma |
| EBV | Epstein–Barr Virus |
| EFS | Event free survival |
| EGFR | Epidermal Growth Factor Receptor |
| ENKTL | Extranodal NK/T cell lymphoma |
| FDC | Follicular dendritic cell |
| GvHD | Graft-versus-host disease |
| HDACi | Histone deacetylase inhibitor |
| HEV | High endothelial venule |
| HIF | Hypoxia-Inducible Factor |
| HL | Hodgkin lymphoma |
| HSTCL | Hepatosplenic gamma delta T cell lymphoma |
| HTLV-1 | Human T lymphotropic virus 1 |
| ICANS | Immune cell-associated neurotoxicity syndrome |
| ICE | Innate cell engager |
| ICI | Immune checkpoint inhibitor |
| ICOSL | Inducible T cell costimulator ligand |
| IMIDs | Immunomodulatory imide drugs |
| KIR | Killer Immunoglobulin-like Receptor |
| mDC | Myeloid dendritic cell |
| MDSC | Myeloid-derived suppressor cell |
| MEITL | Monomorphic epitheliotropic intestinal T cell lymphoma |
| MHC | Major Histocompatibility Complex |
| MRD | Minimal residual disease |
| MSI | Microsatellite instability |
| nTFHL | Nodal T-follicular helper cell lymphoma |
| nTFHL-AI | Nodal T-follicular helper cell lymphoma, angioimmunoblastic type |
| NHL | Non-Hodgkin lymphoma |
| NK | Natural Killer Cell |
| ORR | Overall response rate |
| PCET | PD-1 antibody + chidamide + etoposide + thalidomide |
| PD | Progressive disease |
| PFS | Progression-free survival |
| PMBCL | Primary mediastinal B cell lymphoma |
| PR | Partial response |
| PTCL | Peripheral T cell lymphomas |
| PTCL-NOS | Peripheral T cell lymphoma, not otherwise specified |
| r/r | relapsed/refractory |
| sALCL | Systemic ALCL |
| SD | Stable disease |
| SIRPα | Signal regulatory protein alpha |
| SS | Sezary Syndrome |
| T-ALL | T cell acute lymphoblastic leukemia |
| T-LBL | T cell lymphoblastic lymphoma |
| TAM | Tumor-associated macrophage |
| TCL | T cell lymphoma |
| TCR | T cell receptor |
| Tfh | T-follicular helper |
| Th1 | T-helper 1 |
| Th17 | T-helper 17 |
| Th2 | T-helper 2 |
| TIL | Tumor-infiltrating lymphocyte |
| TMB | Tumor mutational burden |
| TME | Tumor Microenvironment |
| TRAIL | TNF-related apoptosis-inducing ligand |
| Treg | Regulatory T cell |
| TTNT | Time to next treatment |
| VEGF | Vascular endothelial growth factor |
References
- Kwong, Y.-L.; Zhang, H.; Wang, X.; Tse, E. Epidemiology of mature T-cell and NK-cell neoplasms: East and west. Lancet Reg. Health West. Pac. 2025, 62, 101646. [Google Scholar] [CrossRef]
- Brink, M.; Huisman, F.; Meeuwes, F.O.; van der Poel, M.W.M.; Kersten, M.J.; Wondergem, M.; Böhmer, L.; Woei-A-Jin, F.J.S.H.; Visser, O.; Oostvogels, R.; et al. Treatment strategies and outcome in relapsed peripheral T-cell lymphoma: Results from the Netherlands Cancer Registry. Blood Adv. 2024, 8, 3619–3628. [Google Scholar] [CrossRef]
- Han, J.X.; Koh, M.J.; Boussi, L.; Sorial, M.; McCabe, S.M.; Peng, L.; Singh, S.; Eche-Ugwu, I.J.; Gabler, J.; Turizo, M.J.F.; et al. Global outcomes and prognosis for relapsed/refractory mature T-cell and NK-cell lymphomas: Results from the PETAL consortium. Blood Adv. 2024, 9, 583–602. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, M.; Margolskee, E.; Inghirami, G. Pathogenesis of Peripheral T Cell Lymphoma. Annu. Rev. Pathol. Mech. Dis. 2018, 13, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Fiore, D.; Cappelli, L.V.; Broccoli, A.; Zinzani, P.L.; Chan, W.C.; Inghirami, G. Peripheral T cell lymphomas: From the bench to the clinic. Nat. Rev. Cancer 2020, 20, 323–342. [Google Scholar] [CrossRef] [PubMed]
- Swann, J.B.; Smyth, M.J. Immune surveillance of tumors. J. Clin. Investig. 2007, 117, 1137–1146. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The tumor microenvironment at a glance. J. Cell Sci. 2012, 125, 5591–5596. [Google Scholar] [CrossRef]
- Cornel, A.M.; Mimpen, I.L.; Nierkens, S. MHC Class I Downregulation in Cancer: Underlying Mechanisms and Potential Targets for Cancer Immunotherapy. Cancers 2020, 12, 1760. [Google Scholar] [CrossRef]
- Watatani, Y.; Sato, Y.; Miyoshi, H.; Sakamoto, K.; Nishida, K.; Gion, Y.; Nagata, Y.; Shiraishi, Y.; Chiba, K.; Tanaka, H.; et al. Molecular heterogeneity in peripheral T-cell lymphoma, not otherwise specified revealed by comprehensive genetic profiling. Leukemia 2019, 33, 2867–2883. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Qiu, Y.-R.; Zhang, Q.-L.; Cai, M.-C.; Yu, H.; Zhang, J.-M.; Jiang, L.; Ji, M.-M.; Xu, P.-P.; Wang, L.; et al. Genomic and transcriptomic profiling of peripheral T cell lymphoma reveals distinct molecular and microenvironment subtypes. Cell Rep. Med. 2024, 5, 101416. [Google Scholar] [CrossRef]
- Polprasert, C.; Takeuchi, Y.; Makishima, H.; Wudhikarn, K.; Kakiuchi, N.; Tangnuntachai, N.; Assanasen, T.; Sitthi, W.; Muhamad, H.; Lawasut, P.; et al. Frequent mutations in HLA and related genes in extranodal NK/T cell lymphomas. Leuk. Lymphoma 2021, 62, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Nagata, Y.; Kitanaka, A.; Shiraishi, Y.; Shimamura, T.; Yasunaga, J.; Totoki, Y.; Chiba, K.; Sato-Otsubo, A.; Nagae, G.; et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat. Genet. 2015, 47, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Asano, N.; Miyoshi, H.; Kato, T.; Shimono, J.; Yoshida, N.; Kurita, D.; Sasaki, Y.; Kawamoto, K.; Ohshima, K.; Seto, M. Expression pattern of immunosurveillance-related antigen in adult T cell leukaemia/lymphoma. Histopathology 2018, 72, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Anfossi, N.; André, P.; Guia, S.; Falk, C.S.; Roetynck, S.; Stewart, C.A.; Breso, V.; Frassati, C.; Reviron, D.; Middleton, D.; et al. Human NK Cell Education by Inhibitory Receptors for MHC Class I. Immunity 2006, 25, 331–342. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, Y.; Yue, N.; Xiong, S.; Zhang, S.; Wu, C. A potential prognostic marker for hematologic neoplasms: CD58. Front. Oncol. 2025, 15, 1586842. [Google Scholar] [CrossRef]
- Reina, M.; Espel, E. Role of LFA-1 and ICAM-1 in Cancer. Cancers 2017, 9, 153. [Google Scholar] [CrossRef]
- Younes, S.; Zhao, S.; Bharadwaj, S.; Mosquera, A.P.; Libert, D.; Johnsrud, A.; Majzner, R.G.; Miklos, D.B.; Frank, M.J.; Natkunam, Y. Detection of Aberrant CD58 Expression in a Wide Spectrum of Lymphoma Subtypes: Implications for Treatment Resistance. Mod. Pathol. 2023, 36, 100256. [Google Scholar] [CrossRef]
- Yoshida, N.; Karube, K.; Utsunomiya, A.; Tsukasaki, K.; Imaizumi, Y.; Taira, N.; Uike, N.; Umino, A.; Arita, K.; Suguro, M.; et al. Molecular Characterization of Chronic-type Adult T-cell Leukemia/Lymphoma. Cancer Res. 2014, 74, 6129–6138. [Google Scholar] [CrossRef]
- Tanaka, Y.; Fukudome, K.; Hayashi, M.; Takagi, S.; Yoshie, O. Induction of ICAM-1 and LFA-3 by Tax1 of human T-cell leukemia virus type 1 and mechanism of down-regulation of ICAM-1 or LFA-1 in adult-T-cell-leukemia cell lines. Int. J. Cancer 1995, 60, 554–561. [Google Scholar] [CrossRef]
- Majeti, R.; Chao, M.P.; Alizadeh, A.A.; Pang, W.W.; Jaiswal, S.; Gibbs, K.D.; Van Rooijen, N.; Weissman, I.L. CD47 Is an Adverse Prognostic Factor and Therapeutic Antibody Target on Human Acute Myeloid Leukemia Stem Cells. Cell 2009, 138, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.P.; Alizadeh, A.A.; Tang, C.; Myklebust, J.H.; Varghese, B.; Gill, S.; Jan, M.; Cha, A.C.; Chan, C.K.; Tan, B.T.; et al. Anti-CD47 Antibody Synergizes with Rituximab to Promote Phagocytosis and Eradicate Non-Hodgkin Lymphoma. Cell 2010, 142, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Uger, R.; Johnson, L. Blockade of the CD47-SIRPα axis: A promising approach for cancer immunotherapy. Expert Opin. Biol. Ther. 2020, 20, 5–8. [Google Scholar] [CrossRef]
- Logtenberg, M.E.W.; Scheeren, F.A.; Schumacher, T.N. The CD47-SIRPα Immune Checkpoint. Immunity 2020, 52, 742–752. [Google Scholar] [CrossRef]
- Zhu, M.; Li, N.; Fan, L.; Wu, R.; Cao, L.; Ren, Y.; Lu, C.; Zhang, L.; Cai, Y.; Shi, Y.; et al. Single-cell transcriptomic and spatial analysis reveal the immunosuppressive microenvironment in relapsed/refractory angioimmunoblastic T-cell lymphoma. Blood Cancer J. 2024, 14, 218. [Google Scholar] [CrossRef]
- de Leval, L.; Rickman, D.S.; Thielen, C.; de Reynies, A.; Huang, Y.-L.; Delsol, G.; Lamant, L.; Leroy, K.; Brière, J.; Molina, T.; et al. The gene expression profile of nodal peripheral T-cell lymphoma demonstrates a molecular link between angioimmunoblastic T-cell lymphoma (AITL) and follicular helper T (TFH) cells. Blood 2007, 109, 4952–4963. [Google Scholar] [CrossRef]
- Jain, S.; Scoyk, A.V.; Morgan, E.A.; Matthews, A.; Stevenson, K.; Newton, G.; Powers, F.; Autio, A.; Louissaint, A.; Pontini, G.; et al. Targeted inhibition of CD47-SIRPα requires Fc-FcγR interactions to maximize activity in T-cell lymphomas. Blood 2019, 134, 1430–1440. [Google Scholar] [CrossRef]
- Folkes, A.S.; Feng, M.; Zain, J.M.; Abdulla, F.; Rosen, S.T.; Querfeld, C. Targeting CD47 as a cancer therapeutic strategy: The cutaneous T-cell lymphoma experience. Curr. Opin. Oncol. 2018, 30, 332–337. [Google Scholar] [CrossRef]
- Qin, L.; Li, Y.; Zeng, R.; He, Y.; Chen, X.; Xiao, L.; Zhou, H. A novel anti-CD47 antibody with therapeutic potential for NK/T-cell lymphoma. Hum. Vaccines Immunother. 2024, 20, 2408088. [Google Scholar] [CrossRef]
- Johnson, L.D.S.; Banerjee, S.; Kruglov, O.; Viller, N.N.; Horwitz, S.M.; Lesokhin, A.; Zain, J.; Querfeld, C.; Chen, R.; Okada, C.; et al. Targeting CD47 in Sézary syndrome with SIRPαFc. Blood Adv. 2019, 3, 1145–1153. [Google Scholar] [CrossRef]
- Ansell, S.M.; Maris, M.B.; Lesokhin, A.M.; Chen, R.W.; Flinn, I.W.; Sawas, A.; Minden, M.D.; Villa, D.; Percival, M.-E.M.; Advani, A.S.; et al. Phase I Study of the CD47 Blocker TTI-621 in Patients with Relapsed or Refractory Hematologic Malignancies. Clin. Cancer Res. 2021, 27, 2190–2199. [Google Scholar] [CrossRef]
- Han, Z.; Wu, X.; Yuan, Y.-C.; Qin, H.; Su, C.; Zain, J.; Akilov, O.E.; Rosen, S.T.; Feng, M.; Querfeld, C. Blockade of the Immune Checkpoint CD47 By TTI-621 Potentiates the Response to Anti-PD-L1 in Cutaneous T Cell Lymphoma. Blood 2022, 140, 6376–6377. [Google Scholar] [CrossRef]
- Wilde, L.; Kasner, M. Targeting CD47: Many misses; hopeful for a hit. Blood 2025, 145, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Liang, A.C.T.; Lu, L.; Au, W.Y.; Kwong, Y.-L.; Liang, R.H.S.; Srivastava, G. Frequent Deletion of Fas Gene Sequences Encoding Death and Transmembrane Domains in Nasal Natural Killer/T-Cell Lymphoma. Am. J. Pathol. 2002, 161, 2123–2131. [Google Scholar] [CrossRef] [PubMed]
- Takakuwa, T.; Dong, Z.; Nakatsuka, S.; Kojya, S.; Harabuchi, Y.; Yang, W.-I.; Nagata, S.; Aozasa, K. Frequent mutations of Fas gene in nasal NK/T cell lymphoma. Oncogene 2002, 21, 4702–4705. [Google Scholar] [CrossRef]
- Hasegawa, H.; Yamada, Y.; Harasawa, H.; Tsuji, T.; Murata, K.; Sugahara, K.; Tsuruda, K.; Ikeda, S.; Imaizumi, Y.; Tomonaga, M.; et al. Sensitivity of adult T-cell leukaemia lymphoma cells to tumour necrosis factor-related apoptosis-inducing ligand. Br. J. Haematol. 2005, 128, 253–265. [Google Scholar] [CrossRef]
- Zheng, Z.; Cheng, S.; Wu, W.; Wang, L.; Zhao, Y.; Shen, Y.; Janin, A.; Zhao, W.-L. c-FLIP is involved in tumor progression of peripheral T-cell lymphoma and targeted by histone deacetylase inhibitors. J. Hematol. Oncol. 2014, 7, 88. [Google Scholar] [CrossRef]
- Spetz, J.; Presser, A.G.; Sarosiek, K.A. T Cells and Regulated Cell Death: Kill or Be Killed. Int. Rev. Cell Mol. Biol. 2018, 342, 27–71. [Google Scholar] [CrossRef]
- Wang, T.; Feldman, A.L.; Wada, D.A.; Lu, Y.; Polk, A.; Briski, R.; Ristow, K.; Habermann, T.M.; Thomas, D.; Ziesmer, S.C.; et al. GATA-3 expression identifies a high-risk subset of PTCL, NOS with distinct molecular and clinical features. Blood 2014, 123, 3007–3015. [Google Scholar] [CrossRef]
- Iqbal, J.; Wright, G.; Wang, C.; Rosenwald, A.; Gascoyne, R.D.; Weisenburger, D.D.; Greiner, T.C.; Smith, L.; Guo, S.; Wilcox, R.A.; et al. Gene expression signatures delineate biological and prognostic subgroups in peripheral T-cell lymphoma. Blood 2014, 123, 2915–2923. [Google Scholar] [CrossRef]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef]
- Dupuis, J.; Boye, K.; Martin, N.; Copie-Bergman, C.; Plonquet, A.; Fabiani, B.; Baglin, A.-C.; Haioun, C.; Delfau-Larue, M.-H.; Gaulard, P. Expression of CXCL13 by neoplastic cells in angioimmunoblastic T-cell lymphoma (AITL): A new diagnostic marker providing evidence that AITL derives from follicular helper T cells. Am. J. Surg. Pathol. 2006, 30, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Gaulard, P.; de Leval, L. The microenvironment in T-cell lymphomas: Emerging themes. Semin. Cancer Biol. 2014, 24, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Weisenburger, D.D.; Greiner, T.C.; Vose, J.M.; McKeithan, T.; Kucuk, C.; Geng, H.; Deffenbacher, K.; Smith, L.; Dybkaer, K.; et al. Molecular signatures to improve diagnosis in peripheral T-cell lymphoma and prognostication in angioimmunoblastic T-cell lymphoma. Blood 2010, 115, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Karube, K.; Ohshima, K.; Tsuchiya, T.; Yamaguchi, T.; Kawano, R.; Suzumiya, J.; Utsunomiya, A.; Harada, M.; Kikuchi, M. Expression of FoxP3, a key molecule in CD4CD25 regulatory T cells, in adult T-cell leukaemia/lymphoma cells. Br. J. Haematol. 2004, 126, 81–84. [Google Scholar] [CrossRef]
- Georgiev, P.; Charbonnier, L.-M.; Chatila, T.A. Regulatory T Cells: The Many Faces of Foxp3. J. Clin. Immunol. 2019, 39, 623–640. [Google Scholar] [CrossRef]
- Amador, C.; Greiner, T.C.; Heavican, T.B.; Smith, L.M.; Galvis, K.T.; Lone, W.; Bouska, A.; D’Amore, F.; Pedersen, M.B.; Pileri, S.; et al. Reproducing the molecular subclassification of peripheral T-cell lymphoma–NOS by immunohistochemistry. Blood 2019, 134, 2159–2170. [Google Scholar] [CrossRef]
- Amador, C.; Weisenburger, D.D.; Gomez, A.; Bouska, A.; Alshomrani, A.; Sharma, S.; Shah, A.R.; Greiner, T.C.; Vega, F.; Rosenwald, A.; et al. Refining Diagnostic Subtypes of Peripheral T-Cell Lymphoma Using a Multiparameter Approach. Mod. Pathol. 2025, 38, 100646. [Google Scholar] [CrossRef]
- Heavican, T.B.; Bouska, A.; Yu, J.; Lone, W.; Amador, C.; Gong, Q.; Zhang, W.; Li, Y.; Dave, B.J.; Nairismägi, M.-L.; et al. Genetic drivers of oncogenic pathways in molecular subgroups of peripheral T-cell lymphoma. Blood 2019, 133, 1664–1676. [Google Scholar] [CrossRef]
- Chiba, S.; Sakata-Yanagimoto, M. Advances in understanding of angioimmunoblastic T-cell lymphoma. Leukemia 2020, 34, 2592–2606. [Google Scholar] [CrossRef]
- Pritchett, J.C.; Yang, Z.-Z.; Kim, H.J.; Villasboas, J.C.; Tang, X.; Jalali, S.; Cerhan, J.R.; Feldman, A.L.; Ansell, S.M. High-dimensional and single-cell transcriptome analysis of the tumor microenvironment in angioimmunoblastic T cell lymphoma (AITL). Leukemia 2022, 36, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, C.; Jiang, J.; He, S.; Liu, Y.; Yan, W.; Xia, Y.; Cui, Q.; Huang, Y.; Lim, J.Q.; et al. Single-Cell Analysis Reveals Malignant Cells Reshape the Cellular Landscape and Foster an Immunosuppressive Microenvironment of Extranodal NK/T-Cell Lymphoma. Adv. Sci. 2023, 10, 2303913. [Google Scholar] [CrossRef]
- Feng, X.; Meng, M.; Li, H.; Gao, Y.; Song, W.; Di, R.; Li, Z.; Zhang, X.; Zhang, M. T-cell dysfunction in natural killer/T-cell lymphoma. Oncoimmunology 2023, 12, 2212532. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-L.; Mourah, S.; Mounier, N.; Leboeuf, C.; Daneshpouy, M.E.; Legrès, L.; Meignin, V.; Oksenhendler, E.; Maignin, C.L.; Calvo, F.; et al. Vascular endothelial growth factor-A is expressed both on lymphoma cells and endothelial cells in angioimmunoblastic T-cell lymphoma and related to lymphoma progression. Lab. Investig. 2004, 84, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Lunning, M.A.; Vose, J.M. Angioimmunoblastic T-cell lymphoma: The many-faced lymphoma. Blood 2017, 129, 1095–1102. [Google Scholar] [CrossRef]
- Martinengo, C.; Poggio, T.; Menotti, M.; Scalzo, M.S.; Mastini, C.; Ambrogio, C.; Pellegrino, E.; Riera, L.; Piva, R.; Ribatti, D.; et al. ALK-Dependent Control of Hypoxia-Inducible Factors Mediates Tumor Growth and Metastasis. Cancer Res. 2014, 74, 6094–6106. [Google Scholar] [CrossRef]
- Bruns, I.; Fox, F.; Reinecke, P.; Kobbe, G.; Kronenwett, R.; Jung, G.; Haas, R. Complete remission in a patient with relapsed angioimmunoblastic T-cell lymphoma following treatment with bevacizumab. Leukemia 2005, 19, 1993–1995. [Google Scholar] [CrossRef]
- Cortes, J.R.; Ambesi-Impiombato, A.; Couronné, L.; Quinn, S.A.; Kim, C.S.; da Silva Almeida, A.C.; West, Z.; Belver, L.; Martin, M.S.; Scourzic, L.; et al. RHOA G17V Induces T Follicular Helper Cell Specification and Promotes Lymphomagenesis. Cancer Cell 2018, 33, 259–273.e7. [Google Scholar] [CrossRef]
- Leca, J.; Lemonnier, F.; Meydan, C.; Foox, J.; Ghamrasni, S.E.; Mboumba, D.-L.; Duncan, G.S.; Fortin, J.; Sakamoto, T.; Tobin, C.; et al. IDH2 and TET2 mutations synergize to modulate T Follicular Helper cell functional interaction with the AITL microenvironment. Cancer Cell 2023, 41, 323–339.e10. [Google Scholar] [CrossRef]
- Miyoshi, H.; Kiyasu, J.; Kato, T.; Yoshida, N.; Shimono, J.; Yokoyama, S.; Taniguchi, H.; Sasaki, Y.; Kurita, D.; Kawamoto, K.; et al. PD-L1 expression on neoplastic or stromal cells is respectively a poor or good prognostic factor for adult T-cell leukemia/lymphoma. Blood 2016, 128, 1374–1381. [Google Scholar] [CrossRef]
- Takeuchi, M.; Miyoshi, H.; Nakashima, K.; Kawamoto, K.; Yamada, K.; Yanagida, E.; Muta, H.; Moritsubo, M.; Umeno, T.; Suzuki, T.; et al. Comprehensive immunohistochemical analysis of immune checkpoint molecules in adult T cell leukemia/lymphoma. Ann. Hematol. 2020, 99, 1093–1098. [Google Scholar] [CrossRef]
- Wartewig, T.; Kurgyis, Z.; Keppler, S.; Pechloff, K.; Hameister, E.; Öllinger, R.; Maresch, R.; Buch, T.; Steiger, K.; Winter, C.; et al. PD-1 is a haploinsufficient suppressor of T cell lymphomagenesis. Nature 2017, 552, 121–125. [Google Scholar] [CrossRef]
- Ratner, L.; Waldmann, T.A.; Janakiram, M.; Brammer, J.E. Rapid Progression of Adult T-Cell Leukemia–Lymphoma after PD-1 Inhibitor Therapy. N. Engl. J. Med. 2018, 378, 1947–1948. [Google Scholar] [CrossRef] [PubMed]
- Rauch, D.A.; Conlon, K.C.; Janakiram, M.; Brammer, J.E.; Harding, J.C.; Ye, B.H.; Zang, X.; Ren, X.; Olson, S.; Cheng, X.; et al. Rapid progression of adult T-cell leukemia/lymphoma as tumor-infiltrating Tregs after PD-1 blockade. Blood 2019, 134, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Ishitsuka, K.; Utsunomiya, A.; Ishida, T. PD-1 Inhibitor Therapy in Adult T-Cell Leukemia–Lymphoma. N. Engl. J. Med. 2018, 379, 695–697. [Google Scholar] [CrossRef]
- Ahearne, M.J.; Gaskell, C.; Jackson, A.E.; Morland, C.; Hopkins, L.; Nawaz, N.; Timmins, M.A.; Fox, C.P.; Collins, G.P.; Davies, A.; et al. AVAIL-T: A Phase 2a Trial of Avelumab, and Anti-PD-L1 Antibody, in Relapsed and Refractory Peripheral T-Cell Lymphoma (PTCL). Blood 2020, 136, 18–19. [Google Scholar] [CrossRef]
- Barta, S.K.; Zain, J.; MacFarlane, A.W.; Smith, S.M.; Ruan, J.; Fung, H.C.; Tan, C.R.; Yang, Y.; Alpaugh, R.K.; Dulaimi, E.; et al. Phase II Study of the PD-1 Inhibitor Pembrolizumab for the Treatment of Relapsed or Refractory Mature T-cell Lymphoma. Clin. Lymphoma Myeloma Leuk. 2019, 19, 356–364.e3. [Google Scholar] [CrossRef]
- Lesokhin, A.M.; Ansell, S.M.; Armand, P.; Scott, E.C.; Halwani, A.; Gutierrez, M.; Millenson, M.M.; Cohen, A.D.; Schuster, S.J.; Lebovic, D.; et al. Nivolumab in Patients with Relapsed or Refractory Hematologic Malignancy: Preliminary Results of a Phase Ib Study. J. Clin. Oncol. 2016, 34, 2698–2704. [Google Scholar] [CrossRef]
- Shi, Y.; Wu, J.; Wang, Z.; Zhang, L.; Wang, Z.; Zhang, M.; Cen, H.; Peng, Z.; Li, Y.; Fan, L.; et al. Efficacy and safety of geptanolimab (GB226) for relapsed or refractory peripheral T cell lymphoma: An open-label phase 2 study (Gxplore-002). J. Hematol. Oncol. 2021, 14, 12. [Google Scholar] [CrossRef]
- Saba, R.; Mehta-Shah, N.; Ghobadi, A.; DiPersio, J.F.; Cashen, A.F.; Wu, N. Phase II Trial of Duvelisib Maintenance after Autologous Stem Cell Transplant in T-Cell and B-Cell Non-Hodgkin Lymphomas: Results of Safety Lead in. Blood 2023, 142, 6245. [Google Scholar] [CrossRef]
- Merrill, M.H.; Dahi, P.B.; Redd, R.A.; McDonough, M.M.; Chen, Y.-B.; DeFilipp, Z.; Herrera, A.F.; Fisher, D.C.; LaCasce, A.S.; Odejide, O.O.; et al. A phase 2 study of pembrolizumab after autologous stem cell transplantation in patients with T-cell non-Hodgkin lymphoma. Blood 2023, 142, 621–628. [Google Scholar] [CrossRef]
- Kwong, Y.-L.; Chan, T.S.Y.; Tan, D.; Kim, S.J.; Poon, L.-M.; Mow, B.; Khong, P.-L.; Loong, F.; Au-Yeung, R.; Iqbal, J.; et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood 2017, 129, 2437–2442. [Google Scholar] [CrossRef]
- Chan, T.; Tse, E. Pembrolizumab in Relapsed/Refractory Extranodal NK/T Cell Lymphoma and Mature T Cell Lymphoma: A Prospective Phase II Study. Blood 2023, 142, 1726. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Y.; Zhang, M.; Yan, J.; Li, L.; Fu, X.; Zhang, X.; Chang, Y.; Sun, Z.; Yu, H.; et al. Activity of pembrolizumab in relapsed/refractory NK/T-cell lymphoma. J. Hematol. Oncol. 2018, 11, 15. [Google Scholar] [CrossRef]
- Kim, S.-J.; Hyeon, J.; Cho, I.; Ko, Y.H.; Kim, W.S. Comparison of Efficacy of Pembrolizumab between Epstein-Barr Virus—Positive and —Negative Relapsed or Refractory Non-Hodgkin Lymphomas. Cancer Res. Treat. 2018, 51, 611–622. [Google Scholar] [CrossRef]
- Tao, R.; Fan, L.; Song, Y.; Hu, Y.; Zhang, W.; Wang, Y.; Xu, W.; Li, J. Sintilimab for relapsed/refractory extranodal NK/T cell lymphoma: A multicenter, single-arm, phase 2 trial (ORIENT-4). Signal Transduct. Target. Ther. 2021, 6, 365. [Google Scholar] [CrossRef]
- Huang, H.; Tao, R.; Zou, L.; Cen, H.; Guo, Y.; Huang, Y.; Qian, W.; Zhang, L.; Zhou, H.; Yang, Y.; et al. Preliminary Results from a Multicenter, Single-Arm, Phase 2 Study of CS1001, an Anti-Programmed Death-Ligand 1 (PD-L1) Human Monoclonal Antibody (mAb), in Patients (pts) with Relapsed or Refractory Extranodal Natural Killer/T Cell Lymphoma (rr-ENKTL). Blood 2019, 134, 2833. [Google Scholar] [CrossRef]
- Kim, S.J.; Lim, J.Q.; Laurensia, Y.; Cho, J.; Yoon, S.E.; Lee, J.Y.; Ryu, K.J.; Ko, Y.H.; Koh, Y.; Cho, D.; et al. Avelumab for the treatment of relapsed or refractory extranodal NK/T-cell lymphoma: An open-label phase 2 study. Blood 2020, 136, 2754–2763. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liu, Y.; Wang, C.; Gan, R. Signaling pathways of EBV-induced oncogenesis. Cancer Cell Int. 2021, 21, 93. [Google Scholar] [CrossRef] [PubMed]
- Green, M.R.; Rodig, S.; Juszczynski, P.; Ouyang, J.; Sinha, P.; O’Donnell, E.; Neuberg, D.; Shipp, M.A. Constitutive AP-1 Activity and EBV Infection Induce PD-L1 in Hodgkin Lymphomas and Posttransplant Lymphoproliferative Disorders: Implications for Targeted Therapy. Clin. Cancer Res. 2012, 18, 1611–1618. [Google Scholar] [CrossRef]
- Wang, J.; Ge, J.; Wang, Y.; Xiong, F.; Guo, J.; Jiang, X.; Zhang, L.; Deng, X.; Gong, Z.; Zhang, S.; et al. EBV miRNAs BART11 and BART17-3p promote immune escape through the enhancer-mediated transcription of PD-L1. Nat. Commun. 2022, 13, 866. [Google Scholar] [CrossRef] [PubMed]
- Mpakali, A.; Stratikos, E. The Role of Antigen Processing and Presentation in Cancer and the Efficacy of Immune Checkpoint Inhibitor Immunotherapy. Cancers 2021, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, K.M.; Atkins, M.B. Prognostic and predictive markers for the new immunotherapies. Oncology 2014, 28 (Suppl. S3), 39–48. [Google Scholar] [PubMed]
- Goodman, A.M.; Kato, S.; Bazhenova, L.; Patel, S.P.; Frampton, G.M.; Miller, V.; Stephens, P.J.; Daniels, G.A.; Kurzrock, R. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol. Cancer Ther. 2017, 16, 2598–2608. [Google Scholar] [CrossRef]
- Rizvi, H.; Sanchez-Vega, F.; La, K.; Chatila, W.; Jonsson, P.; Halpenny, D.; Plodkowski, A.; Long, N.; Sauter, J.L.; Rekhtman, N.; et al. Molecular Determinants of Response to Anti-Programmed Cell Death (PD)-1 and Anti–Programmed Death-Ligand (PD-L)-Ligand 1 Blockade in Patients With Non–Small-Cell Lung Cancer Profiled With Targeted Next-Generation Sequencing. J. Clin. Oncol. 2018, 36, 633–641. [Google Scholar] [CrossRef]
- Yarchoan, M.; Hopkins, A.; Jaffee, E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017, 377, 2500–2501. [Google Scholar] [CrossRef]
- Wirth, T.C.; Kühnel, F. Neoantigen Targeting—Dawn of a New Era in Cancer Immunotherapy? Front. Immunol. 2017, 8, 1848. [Google Scholar] [CrossRef]
- Galanina, N.; Bejar, R.; Choi, M.; Goodman, A.; Wieduwilt, M.; Mulroney, C.; Kim, L.; Yeerna, H.; Tamayo, P.; Vergilio, J.-A.; et al. Comprehensive Genomic Profiling Reveals Diverse but Actionable Molecular Portfolios across Hematologic Malignancies: Implications for Next Generation Clinical Trials. Cancers 2018, 11, 11. [Google Scholar] [CrossRef]
- Thomas, S.D.; Jeong, A.-R.; Sakowski, P.J.; Sokol, E.S.; Kurzrock, R.; Goodman, A.M. Tumor Mutational Burden and PD-L1 Expression in Hematologic Malignancies. Blood 2020, 136, 15–17. [Google Scholar] [CrossRef]
- Griffin, R.; Wenzl, K.; Sarangi, V.; Rimsza, L.M.; King, R.; Feldman, A.L.; Maurer, M.J.; Nowakowski, G.S.; Link, B.K.; Habermann, T.M.; et al. Tumor Mutational Burden as a Prognostic Factor in Diffuse Large B-Cell Lymphoma. Blood 2023, 142, 1633. [Google Scholar] [CrossRef]
- Cho, J.; Yoon, S.E.; Kim, S.J.; Ko, Y.H.; Kim, W.S. Comparison of tumor mutation burden of 300 various non-Hodgkin lymphomas using panel based massively parallel sequencing. BMC Cancer 2021, 21, 972. [Google Scholar] [CrossRef]
- McGrail, D.J.; Pilié, P.G.; Rashid, N.U.; Voorwerk, L.; Slagter, M.; Kok, M.; Jonasch, E.; Khasraw, M.; Heimberger, A.B.; Lim, B.; et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann. Oncol. 2021, 32, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Thibodeau, S.N.; Bren, G.; Schaid, D. Microsatellite Instability in Cancer of the Proximal Colon. Science 1993, 260, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Li, L.; Jiang, X.; Li, Q. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J. Hematol. Oncol. 2019, 12, 54. [Google Scholar] [CrossRef]
- Chapuy, B.; Stewart, C.; Dunford, A.J.; Kim, J.; Kamburov, A.; Redd, R.A.; Lawrence, M.S.; Roemer, M.G.M.; Li, A.J.; Ziepert, M.; et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat. Med. 2018, 24, 679–690. [Google Scholar] [CrossRef]
- Tian, T.; Li, J.; Xue, T.; Yu, B.; Li, X.; Zhou, X. Microsatellite instability and its associations with the clinicopathologic characteristics of diffuse large B-cell lymphoma. Cancer Med. 2020, 9, 2330–2342. [Google Scholar] [CrossRef]
- Xu-Monette, Z.Y.; Yu, L.; Luo, C.; Li, Y.; Bhagat, G.; Tzankov, A.; Visco, C.; Fan, X.; Fang, X.; Dybkaer, K.; et al. Diffuse Large B-Cell Lymphoma Has a Low Frequency of dMMR and High Frequencies of DNA Mismatch Repair Protein High Expression Associated with Lower T-Cell Infiltration. Blood 2023, 142, 6079. [Google Scholar] [CrossRef]
- Veloza, L.; Fischer, A.; Vallois, D.; Rattina, V.; Lefort, K.; Bisig, B.; Gaulard, P.; Siebert, R.; de Leval, L.; Missiaglia, E. TCL-473 Role of Microsatellite Instability in the Oncogenesis of Primary Intestinal T-Cell Lymphomas. Clin. Lymphoma Myeloma Leuk. 2023, 23, S471. [Google Scholar] [CrossRef]
- Miyashita, K.; Fujii, K.; Taguchi, K.; Shimokawa, M.; Yoshida, M.A.; Abe, Y.; Okamura, J.; Oda, S.; Uike, N. A specific mode of microsatellite instability is a crucial biomarker in adult T-cell leukaemia/lymphoma patients. J. Cancer Res. Clin. Oncol. 2017, 143, 399–408. [Google Scholar] [CrossRef]
- Roemer, M.G.M.; Advani, R.H.; Ligon, A.H.; Natkunam, Y.; Redd, R.A.; Homer, H.; Connelly, C.F.; Sun, H.H.; Daadi, S.E.; Freeman, G.J.; et al. PD-L1 and PD-L2 Genetic Alterations Define Classical Hodgkin Lymphoma and Predict Outcome. J. Clin. Oncol. 2016, 34, 2690–2697. [Google Scholar] [CrossRef]
- Younes, A.; Santoro, A.; Shipp, M.; Zinzani, P.L.; Timmerman, J.M.; Ansell, S.; Armand, P.; Fanale, M.; Ratanatharathorn, V.; Kuruvilla, J.; et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: A multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016, 17, 1283–1294. [Google Scholar] [CrossRef]
- Ansell, S.M.; Lesokhin, A.M.; Borrello, I.; Halwani, A.; Scott, E.C.; Gutierrez, M.; Schuster, S.J.; Millenson, M.M.; Cattry, D.; Freeman, G.J.; et al. PD-1 Blockade with Nivolumab in Relapsed or Refractory Hodgkin’s Lymphoma. N. Engl. J. Med. 2015, 372, 311–319. [Google Scholar] [CrossRef]
- Chen, R.; Zinzani, P.L.; Fanale, M.A.; Armand, P.; Johnson, N.A.; Brice, P.; Radford, J.; Ribrag, V.; Molin, D.; Vassilakopoulos, T.P.; et al. Phase II Study of the Efficacy and Safety of Pembrolizumab for Relapsed/Refractory Classic Hodgkin Lymphoma. J. Clin. Oncol. 2017, 35, 2125–2132. [Google Scholar] [CrossRef] [PubMed]
- Green, M.R.; Monti, S.; Rodig, S.J.; Juszczynski, P.; Currie, T.; O’Donnell, E.; Chapuy, B.; Takeyama, K.; Neuberg, D.; Golub, T.R.; et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood 2010, 116, 3268–3277. [Google Scholar] [CrossRef] [PubMed]
- Armand, P.; Rodig, S.; Melnichenko, V.; Thieblemont, C.; Bouabdallah, K.; Tumyan, G.; Özcan, M.; Portino, S.; Fogliatto, L.; Caballero, M.D.; et al. Pembrolizumab in Relapsed or Refractory Primary Mediastinal Large B-Cell Lymphoma. J. Clin. Oncol. 2019, 37, 3291–3299. [Google Scholar] [CrossRef]
- Kataoka, K.; Miyoshi, H.; Sakata, S.; Dobashi, A.; Couronné, L.; Kogure, Y.; Sato, Y.; Nishida, K.; Gion, Y.; Shiraishi, Y.; et al. Frequent structural variations involving programmed death ligands in Epstein-Barr virus-associated lymphomas. Leukemia 2019, 33, 1687–1699. [Google Scholar] [CrossRef]
- Ng, S.-B.; Chung, T.-H.; Kato, S.; Nakamura, S.; Takahashi, E.; Ko, Y.-H.; Khoury, J.D.; Yin, C.C.; Soong, R.; Jeyasekharan, A.D.; et al. Epstein-Barr virus-associated primary nodal T/NK-cell lymphoma shows a distinct molecular signature and copy number changes. Haematologica 2018, 103, 278–287. [Google Scholar] [CrossRef]
- Beygi, S.; Fernandez-Pol, S.; Duran, G.; Wang, E.B.; Stehr, H.; Zehnder, J.L.; Ramchurren, N.; Fling, S.P.; Cheever, M.A.; Weng, W.-K.; et al. Pembrolizumab in mycosis fungoides with PD-L1 structural variants. Blood Adv. 2021, 5, 771–774. [Google Scholar] [CrossRef]
- Sakihama, S.; Morichika, K.; Saito, R.; Miyara, M.; Miyagi, T.; Hayashi, M.; Uchihara, J.; Tomoyose, T.; Ohshiro, K.; Nakayama, S.; et al. Genetic profile of adult T-cell leukemia/lymphoma in Okinawa: Association with prognosis, ethnicity, and HTLV-1 strains. Cancer Sci. 2021, 112, 1300–1309. [Google Scholar] [CrossRef]
- Kataoka, K.; Iwanaga, M.; Yasunaga, J.; Nagata, Y.; Kitanaka, A.; Kameda, T.; Yoshimitsu, M.; Shiraishi, Y.; Sato-Otsubo, A.; Sanada, M.; et al. Prognostic relevance of integrated genetic profiling in adult T-cell leukemia/lymphoma. Blood 2018, 131, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Duenas, D.E.; Liu, J.; Prakash, R.; Zhou, C.; Koksoy, A.A.; Xu, J.; McAllen, S.A.; Malpica, L.; Luthra, R.; et al. An Integrated Spatial, Codex, and Genomic Analysis Predicts Responsiveness and Survival in the Phase II Combination of Pembrolizumab and Romidepsin in PTCL. Blood 2024, 144, 455. [Google Scholar] [CrossRef]
- Roberts, N.; Lister, J.; Bennani, N.N.; Jain, S.; Battaglia, T.; Ayers, E.C.; Portell, C.A.; Williams, M.E.; Batchala, P.; Pal, I.; et al. Pembrolizumab in Combination with Epigenetic Therapy Is Safe and Active in Heavily Treated Patients with Peripheral T-Cell Lymphoma (PTCL) and Cutaneous T-Cell Lymphoma (CTCL): Preliminary Results from the Embolden Trial. Blood 2022, 140, 9425–9426. [Google Scholar] [CrossRef]
- Du, L.; Zhang, L.; Li, L.; Li, X.; Yan, J.; Wang, X.; Fu, X.; Sun, Z.; Zhang, X.; Li, Z.; et al. Effective Treatment with PD-1 Antibody, Chidamide, Etoposide, and Thalidomide (PCET) for Relapsed/Refractory Natural Killer/T-Cell Lymphoma: A Report of Three Cases. OncoTargets Ther. 2020, 13, 7189–7197. [Google Scholar] [CrossRef]
- Bhabha, F.K.; Weyden, C.V.D.; Casan, J.M.L.; Campbell, B.A.; McCormack, C.; Prince, H.M. Immune pathways, current and potential therapies in Mycosis fungoides and Sezary syndrome. Expert Rev. Clin. Immunol. 2025, 21, 1003–1018. [Google Scholar] [CrossRef]
- Lou, N.; Yang, M.; Xie, Z.; Gao, R.; Zhang, L.; Tang, L.; Yao, J.; Han, X.; Shi, Y. JAK3 A573V and JAK3 M511I mutations in peripheral T-cell lymphoma mediating resistance to anti-PD-1 therapy through the STAT3/PD-L1 pathway. J. Immunother. Cancer 2025, 13, e010783. [Google Scholar] [CrossRef]
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet 2021, 398, 314–324. [Google Scholar] [CrossRef]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T cell immunotherapy for human cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef]
- Uppal, S.S.; Verma, S.; Dhot, P.S. Normal values of CD4 and CD8 lymphocyte subsets in healthy indian adults and the effects of sex, age, ethnicity, and smoking. Cytom. B Clin. Cytom. 2003, 52B, 32–36. [Google Scholar] [CrossRef]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4+T Cells: Differentiation and Functions. J. Immunol. Res. 2012, 2012, 925135. [Google Scholar] [CrossRef]
- Pu, Q.; Qiao, J.; Liu, Y.; Cao, X.; Tan, R.; Yan, D.; Wang, X.; Li, J.; Yue, B. Differential diagnosis and identification of prognostic markers for peripheral T-cell lymphoma subtypes based on flow cytometry immunophenotype profiles. Front. Immunol. 2022, 13, 1008695. [Google Scholar] [CrossRef]
- Pinz, K.; Liu, H.; Golightly, M.; Jares, A.; Lan, F.; Zieve, G.W.; Hagag, N.; Schuster, M.; Firor, A.E.; Jiang, X.; et al. Preclinical targeting of human T-cell malignancies using CD4-specific chimeric antigen receptor (CAR)-engineered T cells. Leukemia 2016, 30, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Shen, J.; Pinz, K.; Wada, M.; Park, J.; Kim, S.; Togano, T.; Tse, W. Targeting T Cell Malignancies Using CD4CAR T-Cells and Implementing a Natural Safety Switch. Stem Cell Rev. Rep. 2019, 15, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Xu, H.; Cinquina, A.; Wu, Z.; Zhang, W.; Sun, L.; Chen, Q.; Tian, L.; Song, L.; Pinz, K.G.; et al. Treatment of aggressive T-cell lymphoma/leukemia with anti-CD4 CAR T cells. Front. Immunol. 2022, 13, 997482. [Google Scholar] [CrossRef]
- Mamonkin, M.; Rouce, R.H.; Tashiro, H.; Brenner, M.K. A T-cell–directed chimeric antigen receptor for the selective treatment of T-cell malignancies. Blood 2015, 126, 983–992. [Google Scholar] [CrossRef]
- Herndler-Brandstetter, D.; Brunner, S.; Weiskopf, D.; van Rijn, R.; Landgraf, K.; Dejaco, C.; Duftner, C.; Schirmer, M.; Kloss, F.; Gassner, R.; et al. Post-thymic regulation of CD5 levels in human memory T cells is inversely associated with the strength of responsiveness to interleukin-15. Hum. Immunol. 2011, 72, 627–631. [Google Scholar] [CrossRef]
- Elghawy, O.; Cao, M.; Xu, J.; Landsburg, D.J.; Svoboda, J.; Nasta, S.D.; Chong, E.A.; Schuster, S.J.; Thomas, C.J.; Carter, J.S.; et al. Prevalence and Prognostication of CD5+ Mature T-Cell Lymphomas. Cancers 2024, 16, 3430. [Google Scholar] [CrossRef]
- Patel, R.P.; Ghilardi, G.; Zhang, Y.; Chiang, Y.-H.; Xie, W.; Guruprasad, P.; Kim, K.H.; Chun, I.; Angelos, M.G.; Pajarillo, R.; et al. CD5 deletion enhances the antitumor activity of adoptive T cell therapies. Sci. Immunol. 2024, 9, eadn6509. [Google Scholar] [CrossRef]
- Wada, M.; Zhang, H.; Fang, L.; Feng, J.; Tse, C.O.; Zhang, W.; Chen, Q.; Sha, S.; Cao, Y.; Chen, K.H.; et al. Characterization of an Anti-CD5 Directed CAR T-Cell against T-Cell Malignancies. Stem Cell Rev. Rep. 2020, 16, 369–384. [Google Scholar] [CrossRef]
- Lin, H.; Cheng, J.; Zhu, L.; Zeng, Y.; Dai, Z.; Zhang, Y.; Zhu, X.; Mu, W. Anti-CD5 CAR-T cells with a tEGFR safety switch exhibit potent toxicity control. Blood Cancer J. 2024, 14, 98. [Google Scholar] [CrossRef] [PubMed]
- Hill, L.C.; Rouce, R.H.; Wu, M.J.; Wang, T.; Ma, R.; Zhang, H.; Mehta, B.; Lapteva, N.; Mei, Z.; Smith, T.S.; et al. Antitumor efficacy and safety of unedited autologous CD5.CAR T cells in relapsed/refractory mature T-cell lymphomas. Blood 2024, 143, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Zhang, M.; Hu, G.; Han, Y.; Mao, X.; Chen, C.; Shen, K.; Dai, Z.; Zhu, X.; Zhou, X.; et al. Case report: Differential diagnosis of highly amplified anti-CD5 CAR T cells and relapsed lymphoma cells in a patient with refractory ALK positive anaplastic large cell lymphoma. Front. Immunol. 2023, 14, 1280007. [Google Scholar] [CrossRef]
- Feng, J.; Xu, H.; Cinquina, A.; Wu, Z.; Chen, Q.; Zhang, P.; Wang, X.; Shan, H.; Xu, L.; Zhang, Q.; et al. Treatment of Aggressive T Cell Lymphoblastic Lymphoma/leukemia Using Anti-CD5 CAR T Cells. Stem Cell Rev. Rep. 2021, 17, 652–661. [Google Scholar] [CrossRef]
- Pan, J.; Tan, Y.; Shan, L.; Seery, S.; Deng, B.; Ling, Z.; Xu, J.; Duan, J.; Wang, Z.; Wang, K.; et al. Allogeneic CD5-specific CAR-T therapy for relapsed/refractory T-ALL: A phase 1 trial. Nat. Med. 2025, 31, 126–136. [Google Scholar] [CrossRef]
- Haynes, B.F.; Eisenbarth, G.S.; Fauci, A.S. Human lymphocyte antigens: Production of a monoclonal antibody that defines functional thymus-derived lymphocyte subsets. Proc. Natl. Acad. Sci. USA 1979, 76, 5829–5833. [Google Scholar] [CrossRef]
- Png, Y.T.; Vinanica, N.; Kamiya, T.; Shimasaki, N.; Coustan-Smith, E.; Campana, D. Blockade of CD7 expression in T cells for effective chimeric antigen receptor targeting of T-cell malignancies. Blood Adv. 2017, 1, 2348–2360. [Google Scholar] [CrossRef]
- Karube, K.; Aoki, R.; Nomura, Y.; Yamamoto, K.; Shimizu, K.; Yoshida, S.; Komatani, H.; Sugita, Y.; Ohshima, K. Usefulness of flow cytometry for differential diagnosis of precursor and peripheral T-cell and NK-cell lymphomas: Analysis of 490 cases. Pathol. Int. 2008, 58, 89–97. [Google Scholar] [CrossRef]
- Gomes-Silva, D.; Srinivasan, M.; Sharma, S.; Lee, C.M.; Wagner, D.L.; Davis, T.H.; Rouce, R.H.; Bao, G.; Brenner, M.K.; Mamonkin, M. CD7-edited T cells expressing a CD7-specific CAR for the therapy of T-cell malignancies. Blood 2017, 130, 285–296. [Google Scholar] [CrossRef]
- Watanabe, N.; Mo, F.; Zheng, R.; Ma, R.; Bray, V.C.; van Leeuwen, D.G.; Sritabal-Ramirez, J.; Hu, H.; Wang, S.; Mehta, B.; et al. Feasibility and preclinical efficacy of CD7-unedited CD7 CAR T cells for T cell malignancies. Mol. Ther. 2023, 31, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Liu, Y.; Yang, J.; Zhang, X.; Yang, X.; Wang, H.; Wang, L.; Wang, Q.; Jin, D.; Li, J.; et al. Naturally selected CD7 CAR-T therapy without genetic manipulations for T-ALL/LBL: First-in-human phase 1 clinical trial. Blood 2022, 140, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, X.; Li, J.; Qiu, L.; Wang, L.; Lu, P. CD7-Targeted CAR-T Cell Therapy Shows Promising Efficacy and Safety in Treating Refractory/Relapsed Peripheral T-Cell Lymphoma: Phase I Clinical Trial. Blood 2024, 144, 2075. [Google Scholar] [CrossRef]
- Tan, Y.; Shan, L.; Zhao, L.; Deng, B.; Ling, Z.; Zhang, Y.; Peng, S.; Xu, J.; Duan, J.; Wang, Z.; et al. Long-term follow-up of donor-derived CD7 CAR T-cell therapy in patients with T-cell acute lymphoblastic leukemia. J. Hematol. Oncol. 2023, 16, 34. [Google Scholar] [CrossRef]
- Went, P.; Agostinelli, C.; Gallamini, A.; Piccaluga, P.P.; Ascani, S.; Sabattini, E.; Bacci, F.; Falini, B.; Motta, T.; Paulli, M.; et al. Marker Expression in Peripheral T-Cell Lymphoma: A Proposed Clinical-Pathologic Prognostic Score. J. Clin. Oncol. 2006, 24, 2472–2479. [Google Scholar] [CrossRef]
- Maciocia, P.M.; Wawrzyniecka, P.A.; Philip, B.; Ricciardelli, I.; Akarca, A.U.; Onuoha, S.C.; Legut, M.; Cole, D.K.; Sewell, A.K.; Gritti, G.; et al. Targeting the T cell receptor β-chain constant region for immunotherapy of T cell malignancies. Nat. Med. 2017, 23, 1416–1423. [Google Scholar] [CrossRef]
- Berg, H.; Otteson, G.E.; Corley, H.; Shi, M.; Horna, P.; Jevremovic, D.; Olteanu, H. Flow cytometric evaluation of TRBC1 expression in tissue specimens and body fluids is a novel and specific method for assessment of T-cell clonality and diagnosis of T-cell neoplasms. Cytom. B Clin. Cytom. 2021, 100, 361–369. [Google Scholar] [CrossRef]
- Horna, P.; Weybright, M.J.; Ferrari, M.; Jungherz, D.; Peng, Y.; Akbar, Z.; Ilca, F.T.; Otteson, G.E.; Seheult, J.N.; Ortmann, J.; et al. Dual T-cell constant β chain (TRBC)1 and TRBC2 staining for the identification of T-cell neoplasms by flow cytometry. Blood Cancer J. 2024, 14, 34. [Google Scholar] [CrossRef]
- Cwynarski, K.; Iacoboni, G.; Tholouli, E.; Menne, T.F.; Irvine, D.A.; Balasubramaniam, N.; Wood, L.; Shang, J.; Zhang, Y.; Basilico, S.; et al. First in Human Study of AUTO4, a TRBC1-Targeting CAR T-Cell Therapy in Relapsed/Refractory TRBC1-Positive Peripheral T-Cell Lymphoma. Blood 2022, 140, 10316–10317. [Google Scholar] [CrossRef]
- Nichakawade, T.D.; Ge, J.; Mog, B.J.; Lee, B.S.; Pearlman, A.H.; Hwang, M.S.; DiNapoli, S.R.; Wyhs, N.; Marcou, N.; Glavaris, S.; et al. TRBC1-targeting antibody–drug conjugates for the treatment of T cell cancers. Nature 2024, 628, 416–423. [Google Scholar] [CrossRef]
- Baguet, C.; Larghero, J.; Mebarki, M. Early predictive factors of failure in autologous CAR T-cell manufacturing and/or efficacy in hematologic malignancies. Blood Adv. 2023, 8, 337–342. [Google Scholar] [CrossRef]
- Ferrari, M.; Baldan, V.; Ghongane, P.; Nicholson, A.; Bughda, R.; Akbar, Z.; Wawrzyniecka, P.; Maciocia, P.; Cordoba, S.; Thomas, S.; et al. Abstract 2183: Targeting TRBC1 and 2 for the treatment of T cell lymphomas. Cancer Res. 2020, 80, 2183. [Google Scholar] [CrossRef]
- Stein, H.; Mason, D.Y.; Gerdes, J.; O’Connor, N.; Wainscoat, J.; Pallesen, G.; Gatter, K.; Falini, B.; Delsol, G.; Lemke, H.; et al. The Expression of the Hodgkin’s Disease Associated Antigen Ki-1 in Reactive and Neoplastic Lymphoid Tissue: Evidence That Reed-Sternberg Cells and Histiocytic Malignancies Are Derived from Activated Lymphoid Cells. Blood 1985, 66, 848–858. [Google Scholar] [CrossRef]
- van der Weyden, C.A.; Pileri, S.A.; Feldman, A.L.; Whisstock, J.; Prince, H.M. Understanding CD30 biology and therapeutic targeting: A historical perspective providing insight into future directions. Blood Cancer J. 2017, 7, e603. [Google Scholar] [CrossRef] [PubMed]
- Bossard, C.; Dobay, M.P.; Parrens, M.; Lamant, L.; Missiaglia, E.; Haioun, C.; Martin, A.; Fabiani, B.; Delarue, R.; Tournilhac, O.; et al. Immunohistochemistry as a valuable tool to assess CD30 expression in peripheral T-cell lymphomas: High correlation with mRNA levels. Blood 2014, 124, 2983–2986. [Google Scholar] [CrossRef] [PubMed]
- Sabattini, E.; Pizzi, M.; Tabanelli, V.; Baldin, P.; Sacchetti, C.S.; Agostinelli, C.; Zinzani, P.L.; Pileri, S.A. CD30 expression in peripheral T-cell lymphomas. Haematologica 2013, 98, e81–e82. [Google Scholar] [CrossRef]
- Prince, H.M.; Kim, Y.H.; Horwitz, S.M.; Dummer, R.; Scarisbrick, J.; Quaglino, P.; Zinzani, P.L.; Wolter, P.; Sanches, J.A.; Ortiz-Romero, P.L.; et al. Brentuximab vedotin or physician’s choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): An international, open-label, randomised, phase 3, multicentre trial. Lancet 2017, 390, 555–566. [Google Scholar] [CrossRef]
- Horwitz, S.; O’Connor, O.A.; Pro, B.; Illidge, T.; Fanale, M.; Advani, R.; Bartlett, N.L.; Christensen, J.H.; Morschhauser, F.; Domingo-Domenech, E.; et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): A global, double-blind, randomised, phase 3 trial. Lancet 2019, 393, 229–240. [Google Scholar] [CrossRef]
- Bradley, A.M.; Devine, M.; DeRemer, D. Brentuximab vedotin: An anti-CD30 antibody–drug conjugate. Am. J. Health Syst. Pharm. 2013, 70, 589–597. [Google Scholar] [CrossRef]
- Kim, Y.H.; Tavallaee, M.; Sundram, U.; Salva, K.A.; Wood, G.S.; Li, S.; Rozati, S.; Nagpal, S.; Krathen, M.; Reddy, S.; et al. Phase II Investigator-Initiated Study of Brentuximab Vedotin in Mycosis Fungoides and Sézary Syndrome with Variable CD30 Expression Level: A Multi-Institution Collaborative Project. J. Clin. Oncol. 2015, 33, 3750–3758. [Google Scholar] [CrossRef]
- Melenhorst, J.J.; Chen, G.M.; Wang, M.; Porter, D.L.; Chen, C.; Collins, M.A.; Gao, P.; Bandyopadhyay, S.; Sun, H.; Zhao, Z.; et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature 2022, 602, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Voorhees, T.J.; Ghosh, N.; Grover, N.; Block, J.; Cheng, C.; Morrison, K.; Ivanova, A.; Dotti, G.; Serody, J.; Savoldo, B.; et al. Long-term remission in multiply relapsed enteropathy-associated T-cell lymphoma following CD30 CAR T-cell therapy. Blood Adv. 2020, 4, 5925–5928. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.A.; Ballard, B.; Zhang, H.; Dakhova, O.; Gee, A.P.; Mei, Z.; Bilgi, M.; Wu, M.-F.; Liu, H.; Grilley, B.; et al. Clinical and immunological responses after CD30-specific chimeric antigen receptor–redirected lymphocytes. J. Clin. Investig. 2017, 127, 3462–3471. [Google Scholar] [CrossRef]
- Wang, D.; Zeng, C.; Xu, B.; Xu, J.-H.; Wang, J.; Jiang, L.-J.; Wang, Q.-X.; Li, C.-R.; Wang, N.; Huang, L.; et al. Anti-CD30 chimeric antigen receptor T cell therapy for relapsed/refractory CD30+ lymphoma patients. Blood Cancer J. 2020, 10, 8. [Google Scholar] [CrossRef]
- Grover, N.S.; Hucks, G.; Riches, M.L.; Ivanova, A.; Moore, D.T.; Shea, T.C.; Seegars, M.B.; Armistead, P.M.; Kasow, K.A.; Beaven, A.W.; et al. Anti-CD30 CAR T cells as consolidation after autologous haematopoietic stem-cell transplantation in patients with high-risk CD30+ lymphoma: A phase 1 study. Lancet Haematol. 2024, 11, e358–e367. [Google Scholar] [CrossRef]
- O’Neill, R.E.; Du, W.; Mohammadpour, H.; Alqassim, E.; Qiu, J.; Chen, G.; McCarthy, P.L.; Lee, K.P.; Cao, X. T Cell–Derived CD70 Delivers an Immune Checkpoint Function in Inflammatory T Cell Responses. J. Immunol. 2017, 199, 3700–3710. [Google Scholar] [CrossRef]
- Tesselaar, K.; Xiao, Y.; Arens, R.; van Schijndel, G.M.; Schuurhuis, D.H.; Mebius, R.E.; Borst, J.; van Lier, R.A. Expression of the Murine CD27 Ligand CD70 In Vitro and In Vivo. J. Immunol. 2003, 170, 33–40. [Google Scholar] [CrossRef]
- Flieswasser, T.; den Eynde, A.V.; Audenaerde, J.V.; Waele, J.D.; Lardon, F.; Riether, C.; de Haard, H.; Smits, E.; Pauwels, P.; Jacobs, J. The CD70-CD27 axis in oncology: The new kids on the block. J. Exp. Clin. Cancer Res. 2022, 41, 12. [Google Scholar] [CrossRef]
- Masamoto, I.; Yoshimitsu, M.; Kuroki, A.; Horai, S.; Ezinne, C.C.; Kozako, T.; Hachiman, M.; Kamada, Y.; Baba, M.; Arima, N. Clinical significance of CD70 expression on T cells in human T-lymphotropic virus type-1 carriers and adult T cell leukemia/ lymphoma patients. Leuk. Lymphoma 2016, 57, 685–691. [Google Scholar] [CrossRef]
- Marques-Piubelli, M.L.; Soto, L.S.; Iyer, S.P.; Sagert, J.; Pham, M.T.; Tipton, K.; Lu, W.; Khan, K.B.; Zorrilla, L.G.H.; Chapman, J.R.; et al. CD70 Expression in Mature T-Cell Lymphomas. Blood 2021, 138, 4493. [Google Scholar] [CrossRef]
- Wu, C.-H.; Wang, L.; Yang, C.-Y.; Wen, K.W.; Hinds, B.R.; Gill, R.; McCormick, F.; Moasser, M.; Pincus, L.; Ai, W.Z. Targeting CD70 in cutaneous T-cell lymphoma using an antibody-drug conjugate in patient-derived xenograft models. Blood Adv. 2021, 6, 2290–2302. [Google Scholar] [CrossRef]
- Leupin, N.; Zinzani, P.L.; Morschhauser, F.; Dalle, S.; Maerevoet, M.; Michot, J.; Ribrag, V.; Offner, F.; Beylot-Barry, M.; Moins-Teisserenc, H.; et al. Cusatuzumab for treatment of CD70-positive relapsed or refractory cutaneous T-cell lymphoma. Cancer 2022, 128, 1004–1014. [Google Scholar] [CrossRef]
- Iyer, S.P.; Sica, R.A.; Ho, P.J.; Prica, A.; Zain, J.; Foss, F.M.; Hu, B.; Beitinjaneh, A.; Weng, W.-K.; Kim, Y.H.; et al. Safety and activity of CTX130, a CD70-targeted allogeneic CRISPR-Cas9-engineered CAR T-cell therapy, in patients with relapsed or refractory T-cell malignancies (COBALT-LYM): A single-arm, open-label, phase 1, dose-escalation study. Lancet Oncol. 2024, 26, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.; He, X.; Mo, Z.; Zhao, M.; Liang, X.; Hu, K.; Wang, K.; Yue, Y.; Mo, G.; et al. CD70-targeted iPSC-derived CAR-NK cells display potent function against tumors and alloreactive T cells. Cell Rep. Med. 2025, 6, 101889. [Google Scholar] [CrossRef] [PubMed]
- Ferenczi, K.; Fuhlbrigge, R.C.; Kupper, T.S.; Pinkus, J.L.; Pinkus, G.S. Increased CCR4 Expression in Cutaneous T Cell Lymphoma. J. Investig. Dermatol. 2002, 119, 1405–1410. [Google Scholar] [CrossRef]
- Ishida, T.; Utsunomiya, A.; Iida, S.; Inagaki, H.; Takatsuka, Y.; Kusumoto, S.; Takeuchi, G.; Shimizu, S.; Ito, M.; Komatsu, H.; et al. Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma: Its close association with skin involvement and unfavorable outcome. Clin. Cancer Res. 2003, 9, 3625–3634. [Google Scholar]
- Ishida, T.; Inagaki, H.; Utsunomiya, A.; Takatsuka, Y.; Komatsu, H.; Iida, S.; Takeuchi, G.; Eimoto, T.; Nakamura, S.; Ueda, R. CXC Chemokine Receptor 3 and CC Chemokine Receptor 4 Expression in T-Cell and NK-Cell Lymphomas with Special Reference to Clinicopathological Significance for Peripheral T-Cell Lymphoma, Unspecified. Clin. Cancer Res. 2004, 10, 5494–5500. [Google Scholar] [CrossRef]
- Asano, N.; Suzuki, R.; Ohshima, K.; Kagami, Y.; Ishida, F.; Yoshino, T.; Ogawa, H.; Morishima, Y.; Nakamura, S. Linkage of expression of chemokine receptors (CXCR3 and CCR4) and cytotoxic molecules in peripheral T cell lymphoma, not otherwise specified and ALK-negative anaplastic large cell lymphoma. Int. J. Hematol. 2010, 91, 426–435. [Google Scholar] [CrossRef]
- Geller, S.; Hollmann, T.J.; Horwitz, S.M.; Myskowski, P.L.; Pulitzer, M. C-C chemokine receptor 4 expression in CD8+ cutaneous T-cell lymphomas and lymphoproliferative disorders, and its implications for diagnosis and treatment. Histopathology 2020, 76, 222–232. [Google Scholar] [CrossRef]
- Shono, Y.; Suga, H.; Kamijo, H.; Fujii, H.; Oka, T.; Miyagaki, T.; Shishido-Takahashi, N.; Sugaya, M.; Sato, S. Expression of CCR3 and CCR4 Suggests a Poor Prognosis in Mycosis Fungoides and Sézary Syndrome. Acta Derm. Venereol. 2019, 99, 809–812. [Google Scholar] [CrossRef]
- Duvic, M.; Pinter-Brown, L.C.; Foss, F.M.; Sokol, L.; Jorgensen, J.L.; Challagundla, P.; Dwyer, K.M.; Zhang, X.; Kurman, M.R.; Ballerini, R.; et al. Phase 1/2 study of mogamulizumab, a defucosylated anti-CCR4 antibody, in previously treated patients with cutaneous T-cell lymphoma. Blood 2015, 125, 1883–1889. [Google Scholar] [CrossRef]
- Kim, Y.H.; Bagot, M.; Pinter-Brown, L.; Rook, A.H.; Porcu, P.; Horwitz, S.M.; Whittaker, S.; Tokura, Y.; Vermeer, M.; Zinzani, P.L.; et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): An international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018, 19, 1192–1204. [Google Scholar] [CrossRef]
- Ishida, T.; Jo, T.; Takemoto, S.; Suzushima, H.; Uozumi, K.; Yamamoto, K.; Uike, N.; Saburi, Y.; Nosaka, K.; Utsunomiya, A.; et al. Dose-intensified chemotherapy alone or in combination with mogamulizumab in newly diagnosed aggressive adult T-cell leukaemia-lymphoma: A randomized phase II study. Br. J. Haematol. 2015, 169, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Joh, T.; Uike, N.; Yamamoto, K.; Utsunomiya, A.; Yoshida, S.; Saburi, Y.; Miyamoto, T.; Takemoto, S.; Suzushima, H.; et al. Defucosylated Anti-CCR4 Monoclonal Antibody (KW-0761) for Relapsed Adult T-Cell Leukemia-Lymphoma: A Multicenter Phase II Study. J. Clin. Oncol. 2012, 30, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Ogura, M.; Ishida, T.; Hatake, K.; Taniwaki, M.; Ando, K.; Tobinai, K.; Fujimoto, K.; Yamamoto, K.; Miyamoto, T.; Uike, N.; et al. Multicenter Phase II Study of Mogamulizumab (KW-0761), a Defucosylated Anti-CC Chemokine Receptor 4 Antibody, in Patients With Relapsed Peripheral T-Cell Lymphoma and Cutaneous T-Cell Lymphoma. J. Clin. Oncol. 2014, 32, 1157–1163. [Google Scholar] [CrossRef]
- Perera, L.P.; Zhang, M.; Nakagawa, M.; Petrus, M.N.; Maeda, M.; Kadin, M.E.; Waldmann, T.A.; Perera, P. Chimeric antigen receptor modified T cells that target chemokine receptor CCR4 as a therapeutic modality for T-cell malignancies. Am. J. Hematol. 2017, 92, 892–901. [Google Scholar] [CrossRef]
- Watanabe, K.; Gomez, A.M.; Kuramitsu, S.; Siurala, M.; Da, T.; Agarwal, S.; Song, D.; Scholler, J.; Rotolo, A.; Posey, A.D.; et al. Identifying highly active anti-CCR4-CAR T cells for the treatment of T-cell lymphoma. Blood Adv. 2023, 7, 3416–3430. [Google Scholar] [CrossRef]
- Ni, X.; Jorgensen, J.L.; Goswami, M.; Challagundla, P.; Decker, W.K.; Kim, Y.H.; Duvic, M.A. Reduction of Regulatory T Cells by Mogamulizumab, a Defucosylated Anti-CC Chemokine Receptor 4 Antibody, in Patients with Aggressive/Refractory Mycosis Fungoides and Sézary Syndrome. Clin. Cancer Res. 2015, 21, 274–285. [Google Scholar] [CrossRef]
- Chen, K.H.; Wada, M.; Firor, A.E.; Pinz, K.G.; Jares, A.; Liu, H.; Salman, H.; Golightly, M.; Lan, F.; Jiang, X.; et al. Novel anti-CD3 chimeric antigen receptor targeting of aggressive T cell malignancies. Oncotarget 2016, 7, 56219–56232. [Google Scholar] [CrossRef]
- Kobayashi, E.; Kamihara, Y.; Arai, M.; Wada, A.; Kikuchi, S.; Hatano, R.; Iwao, N.; Susukida, T.; Ozawa, T.; Adachi, Y.; et al. Development of a Novel CD26-Targeted Chimeric Antigen Receptor T-Cell Therapy for CD26-Expressing T-Cell Malignancies. Cells 2023, 12, 2059. [Google Scholar] [CrossRef]
- Zi, Z.; Zhao, H.; Wang, H.; Ma, X.; Wei, F. B7-H3 Chimeric Antigen Receptor Redirected T Cells Target Anaplastic Lymphoma Kinase-Positive Anaplastic Large Cell Lymphoma. Cancers 2020, 12, 3815. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Wang, X.; Cheng, L.; Qin, L.; Jiang, Z.; Zhao, R.; Li, Y.; Shi, J.; Wu, Q.; Long, Y.; et al. The Chemokine Receptor CCR8 Is a Target of Chimeric Antigen T Cells for Treating T Cell Malignancies. Front. Immunol. 2022, 13, 808347. [Google Scholar] [CrossRef]
- Frigault, M.J.; Graham, C.E.; Berger, T.R.; Ritchey, J.; Horick, N.K.; El-Jawahri, A.; Scarfò, I.; Schmidts, A.; Haradhvala, N.J.; Wehrli, M.; et al. Phase 1 study of CAR-37 T cells in patients with relapsed or refractory CD37+ lymphoid malignancies. Blood 2024, 144, 1153–1167. [Google Scholar] [CrossRef]
- Acker, H.H.V.; Capsomidis, A.; Smits, E.L.; Tendeloo, V.F.V. CD56 in the Immune System: More Than a Marker for Cytotoxicity? Front. Immunol. 2017, 8, 892. [Google Scholar] [CrossRef]
- Dalle, I.A.; Dulery, R.; Moukalled, N.; Ricard, L.; Stocker, N.; El-Cheikh, J.; Mohty, M.; Bazarbachi, A. Bi- and Tri-specific antibodies in non-Hodgkin lymphoma: Current data and perspectives. Blood Cancer J. 2024, 14, 23. [Google Scholar] [CrossRef]
- Paul, S.; Pearlman, A.H.; Douglass, J.; Mog, B.J.; Hsiue, E.H.-C.; Hwang, M.S.; DiNapoli, S.R.; Konig, M.F.; Brown, P.A.; Wright, K.M.; et al. TCR β chain–directed bispecific antibodies for the treatment of T cell cancers. Sci. Transl. Med. 2021, 13, eabd3595. [Google Scholar] [CrossRef]
- Kerbauy, L.N.; Marin, N.D.; Kaplan, M.; Banerjee, P.P.; Berrien-Elliott, M.M.; Becker-Hapak, M.; Basar, R.; Foster, M.; Melo, L.G.; Neal, C.C.; et al. Combining AFM13, a Bispecific CD30/CD16 Antibody, with Cytokine-Activated Blood and Cord Blood–Derived NK Cells Facilitates CAR-like Responses Against CD30+ Malignancies. Clin. Cancer Res. 2021, 27, 3744–3756. [Google Scholar] [CrossRef]
- Rothe, A.; Sasse, S.; Topp, M.S.; Eichenauer, D.A.; Hummel, H.; Reiners, K.S.; Dietlein, M.; Kuhnert, G.; Kessler, J.; Buerkle, C.; et al. A phase 1 study of the bispecific anti-CD30/CD16A antibody construct AFM13 in patients with relapsed or refractory Hodgkin lymphoma. Blood 2015, 125, 4024–4031. [Google Scholar] [CrossRef]
- Sawas, A.; Chen, P.-H.; Lipschitz, M.; Rodig, S.; Vlad, G. Title: Clinical and Biological Evaluation of the Novel CD30/CD16A Tetravalent Bispecific Antibody (AFM13) in Relapsed or Refractory CD30-Positive Lymphoma with Cutaneous Presentation: A Biomarker Phase Ib/IIa Study (NCT03192202). Blood 2020, 136, 25–26. [Google Scholar] [CrossRef]
- Bartlett, N.L.; Herrera, A.F.; Domingo-Domenech, E.; Mehta, A.; Forero-Torres, A.; Garcia-Sanz, R.; Armand, P.; Devata, S.; Izquierdo, A.R.; Lossos, I.S.; et al. A phase 1b study of AFM13 in combination with pembrolizumab in patients with relapsed or refractory Hodgkin lymphoma. Blood 2020, 136, 2401–2409. [Google Scholar] [CrossRef]
- Kim, W.S.; Shortt, J.; Zinzani, P.L.; Mikhailova, N.; Radeski, D.; Ribrag, V.; Domenech, E.D.; Sawas, A.; Alexis, K.; Emig, M.; et al. A Phase II Study of Acimtamig (AFM13) in Patients with CD30-Positive, Relapsed, or Refractory Peripheral T-cell Lymphomas. Clin. Cancer Res. 2025, 31, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Nieto, Y.; Banerjee, P.; Kaur, I.; Basar, R.; Li, Y.; Daher, M.; Rafei, H.; Kerbauy, L.N.; Kaplan, M.; Marin, D.; et al. Allogeneic NK cells with a bispecific innate cell engager in refractory relapsed lymphoma: A phase 1 trial. Nat. Med. 2025, 31, 1987–1993. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.P. Genmab Axes 3 Programs to Focus on Plumped-Up Pivotal Pipeline. 2024. Available online: https://www.fiercebiotech.com/biotech/genmab-axes-3-programs-focus-plumped-pivotal-pipeline (accessed on 25 September 2025).
- Chikada, T.; Asano, H.; Terashima, M.; Tsuchiya, T.; Kabumoto, Y.; Okita, G.; Takahashi, T.; Nakakubo, H.; Mieno, R.; Ito, Y.; et al. Bispecific T Cell Engager with Trbc-Targeting Antibody Against Effector T Cells for the Treatment of T Cell Malignancy. Blood 2024, 144, 2060. [Google Scholar] [CrossRef]
- Wang, J.; Qi, J.; Li, W.; Zhai, Z.; Li, P.; Zou, W.; Ding, M.; Yang, X.; Wang, R.; Guo, W.; et al. GNC-038, a tetra-specific antibody, in patients with R/R non-Hodgkin lymphoma or acute lymphoblastic leukemia: A phase 1 study design and rationale. J. Clin. Oncol. 2023, 41, TPS2668. [Google Scholar] [CrossRef]
- Tateshita, N.; Watanabe, N.; Shimbo, T.; Morimoto, A.; Takeda, K.; Tanihiro, T. Abstract 7324: ONO-4685: A novel PD-1/CD3 bispecific T-cell engager (TCE) for the treatment of T-cell malignancies. Cancer Res. 2025, 85, 7324. [Google Scholar] [CrossRef]
- Park, J.A.; Santich, B.H.; Xu, H.; Lum, L.G.; Cheung, N.-K.V. Potent ex vivo armed T cells using recombinant bispecific antibodies for adoptive immunotherapy with reduced cytokine release. J. Immunother. Cancer 2021, 9, e002222. [Google Scholar] [CrossRef]
- Fukuhara, H.; Ino, Y.; Todo, T. Oncolytic virus therapy: A new era of cancer treatment at dawn. Cancer Sci. 2016, 107, 1373–1379. [Google Scholar] [CrossRef]
- Macedo, N.; Miller, D.M.; Haq, R.; Kaufman, H.L. Clinical landscape of oncolytic virus research in 2020. J. Immunother. Cancer 2020, 8, e001486. [Google Scholar] [CrossRef]
- Pasin, F.; Calveri, M.M.; Pizzarelli, G.; Calabrese, A.; Andreoli, M.; Bongiovanni, I.; Cattaneo, C.; Rignanese, G. Oncolytic effect of SARS-CoV2 in a patient with NK lymphoma. Acta Biomed. 2020, 91, 2531–6745. [Google Scholar] [CrossRef]
- Snowden, C.; Ng, S.; Choi, J. Partial remission of advanced untreated Sézary syndrome after COVID-19. JAAD Case Rep. 2022, 21, 165–168. [Google Scholar] [CrossRef]
- Ohadi, L.; Hosseinzadeh, F.; Dadkhahfar, S.; Nasiri, S. Oncolytic effect of SARS-CoV-2 in a patient with mycosis fungoides: A case report. Clin. Case Rep. 2022, 10, e05682. [Google Scholar] [CrossRef]
- Heinzerling, L.; Künzi, V.; Oberholzer, P.A.; Kündig, T.; Naim, H.; Dummer, R. Oncolytic measles virus in cutaneous T-cell lymphomas mounts antitumor immune responses in vivo and targets interferon-resistant tumor cells. Blood 2005, 106, 2287–2294. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.; Peng, K.W.W.; Witzig, T.E.; Broski, S.M.; Villasboas, J.C.; Paludo, J.; Patnaik, M.M.; Rajkumar, V.; Dispenzieri, A.; Leung, N.; et al. Clinical Activity of Single Dose Systemic Oncolytic VSV Virotherapy in Patients with Relapsed Refractory T-Cell Lymphoma. Blood Adv. 2022, 6, 3268–3279. [Google Scholar] [CrossRef] [PubMed]
- Angelova, A.; Barf, M.; Just, A.; Leuchs, B.; Rommelaere, J.; Ungerechts, G. H-1 Parvovirus-Induced Oncolysis and Tumor Microenvironment Immune Modulation in a Novel Heterotypic Spheroid Model of Cutaneous T-Cell Lymphoma. Cancers 2024, 16, 2711. [Google Scholar] [CrossRef]
- Chesney, J.; Puzanov, I.; Collichio, F.; Singh, P.; Milhem, M.M.; Glaspy, J.; Hamid, O.; Ross, M.; Friedlander, P.; Garbe, C.; et al. Randomized, Open-Label Phase II Study Evaluating the Efficacy and Safety of Talimogene Laherparepvec in Combination With Ipilimumab Versus Ipilimumab Alone in Patients With Advanced, Unresectable Melanoma. J. Clin. Oncol. 2017, 36, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Quach, H.; Ritchie, D.; Stewart, A.K.; Neeson, P.; Harrison, S.; Smyth, M.J.; Prince, H.M. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia 2010, 24, 22–32. [Google Scholar] [CrossRef]
- Colley, A.; Brauns, T.; Sluder, A.E.; Poznansky, M.C.; Gemechu, Y. Immunomodulatory drugs: A promising clinical ally for cancer immunotherapy. Trends Mol. Med. 2024, 30, 765–780. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Pellegrini, C.; Broccoli, A.; Stefoni, V.; Gandolfi, L.; Quirini, F.; Argnani, L.; Berti, E.; Derenzini, E.; Pileri, S.; et al. Lenalidomide monotherapy for relapsed/refractory peripheral T-cell lymphoma not otherwise specified. Leuk. Lymphoma 2011, 52, 1585–1588. [Google Scholar] [CrossRef]
- Toumishey, E.; Prasad, A.; Dueck, G.; Chua, N.; Finch, D.; Johnston, J.; van der Jagt, R.; Stewart, D.; White, D.; Belch, A.; et al. Final report of a phase 2 clinical trial of lenalidomide monotherapy for patients with T-cell lymphoma. Cancer 2015, 121, 716–723. [Google Scholar] [CrossRef]
- Morschhauser, F.; Fitoussi, O.; Haioun, C.; Thieblemont, C.; Quach, H.; Delarue, R.; Glaisner, S.; Gabarre, J.; Bosly, A.; Lister, J.; et al. A phase 2, multicentre, single-arm, open-label study to evaluate the safety and efficacy of single-agent lenalidomide (Revlimid®) in subjects with relapsed or refractory peripheral T-cell non-Hodgkin lymphoma: The EXPECT trial. Eur. J. Cancer. 2013, 49, 2869–2876. [Google Scholar] [CrossRef]
- Ishida, T.; Fujiwara, H.; Nosaka, K.; Taira, N.; Abe, Y.; Imaizumi, Y.; Moriuchi, Y.; Jo, T.; Ishizawa, K.; Tobinai, K.; et al. Multicenter Phase II Study of Lenalidomide in Relapsed or Recurrent Adult T-Cell Leukemia/Lymphoma: ATLL-002. J. Clin. Oncol. 2016, 34, 4086–4093. [Google Scholar] [CrossRef]
- Dogan, A.; Ngu, L.S.P.; Ng, S.H.; Cervi, P.L. Pathology and clinical features of angioimmunoblastic T-cell lymphoma after successful treatment with thalidomide. Leukemia 2005, 19, 873–875. [Google Scholar] [CrossRef] [PubMed]
- Gottardi, M.; Danesin, C.; Canal, F.; Tos, A.P.D.; Stefani, P.M.; Calistri, E.; Salvadori, U.; Gherlinzoni, F. Complete remission induced by thalidomide in a case of angioimmunoblastic T-cell lymphoma refractory to autologous stem cell transplantation. Leuk. Lymphoma 2008, 49, 1836–1838. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhao, C.; Gu, K.; Jiao, Y.; Hao, J.; Sun, G. Thalidomide plus chemotherapy exhibit enhanced efficacy in the clinical treatment of T-cell non-Hodgkin’s lymphoma: A prospective study of 46 cases. Mol. Clin. Oncol. 2014, 2, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wang, L.; Wang, X.; Cui, G.; Zhou, J.; Xing, T.; Du, K.; Xu, J.; Wang, L.; Liang, R.; et al. Chidamide plus prednisone, cyclophosphamide, and thalidomide for relapsed or refractory peripheral T-cell lymphoma: A multicenter phase II trial. Chin. Med. J. 2024, 137, 1576–1582. [Google Scholar] [CrossRef]
- Sun, Y.; Li, L.; Li, X.; Zhang, L.; Wang, X.; Fu, X.; Sun, Z.; Zhang, X.; Li, Z.; Wu, J.; et al. Outcomes of GDPT (gemcitabine, cisplatin, prednisone, thalidomide) versus CHOP in newly diagnosed peripheral T-cell lymphoma patients. Ther. Adv. Med. Oncol. 2020, 12, 1758835920923829. [Google Scholar] [CrossRef]
- Querfeld, C.; Chen, L.; Wu, X.; Han, Z.; Su, C.; Banez, M.; Quach, J.; Barnhizer, T.; Crisan, L.; Rosen, S.T.; et al. Preliminary Analysis Demonstrates Durvalumab (Anti-PD-L1) & Lenalidomide Is Superior to Single-Agent Durvalumab (anti-PD-L1) in Refractory/Advanced Cutaneous T Cell Lymphoma in a Randomized Phase 2 Trial. Blood 2023, 142, 3077. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Schjesvold, F.; Oriol, A.; Karlin, L.; Cavo, M.; Rifkin, R.M.; Yimer, H.A.; LeBlanc, R.; Takezako, N.; McCroskey, R.D.; et al. Pembrolizumab plus lenalidomide and dexamethasone for patients with treatment-naive multiple myeloma (KEYNOTE-185): A randomised, open-label, phase 3 trial. Lancet Haematol. 2019, 6, e448–e458. [Google Scholar] [CrossRef]
- Mateos, M.-V.; Blacklock, H.; Schjesvold, F.; Oriol, A.; Simpson, D.; George, A.; Goldschmidt, H.; Larocca, A.; Chanan-Khan, A.; Sherbenou, D.; et al. Pembrolizumab plus pomalidomide and dexamethasone for patients with relapsed or refractory multiple myeloma (KEYNOTE-183): A randomised, open-label, phase 3 trial. Lancet Haematol. 2019, 6, e459–e469. [Google Scholar] [CrossRef]
- Leonard, J.P.; Trneny, M.; Izutsu, K.; Fowler, N.H.; Hong, X.; Zhu, J.; Zhang, H.; Offner, F.; Scheliga, A.; Nowakowski, G.S.; et al. AUGMENT: A Phase III Study of Lenalidomide Plus Rituximab Versus Placebo Plus Rituximab in Relapsed or Refractory Indolent Lymphoma. J. Clin. Oncol. 2019, 37, 1188–1199. [Google Scholar] [CrossRef]
- van der Weyden, C.; Bressel, M.; Khot, A.; Prince, H.M.; Dickinson, M. Assessment of the Tolerability and Optimal Dosing of the Combination of Brentuximab Vedotin and Lenalidomide in Patients with Relapsed or Refractory T-cell Lymphoma: Results of a Single-centre Phase 1 Dose-escalation Study. EJHaem 2025, 6, e70033. [Google Scholar] [CrossRef] [PubMed]
- Reneau, J.C.; Huang, Y.; Hanel, W.; Brammer, J.E.; Chung, C.; William, B. Final Results of a Phase II Study of Brentuximab Vedotin and Lenalidomide in Relapsed and Refractory T-Cell Lymphomas. Blood 2024, 144, 4438. [Google Scholar] [CrossRef]
- Prince, H.M.; Duvic, M.; Martin, A.; Sterry, W.; Assaf, C.; Sun, Y.; Straus, D.; Acosta, M.; Negro-Vilar, A. Phase III Placebo-Controlled Trial of Denileukin Diftitox for Patients with Cutaneous T-Cell Lymphoma. J. Clin. Oncol. 2010, 28, 1870–1877. [Google Scholar] [CrossRef] [PubMed]
- Foss, F.M.; Kim, Y.H.; Prince, H.M.; Akilov, O.E.; Querfeld, C.; Seminario-Vidal, L.; Fisher, D.C.; Kuzel, T.M.; Yannakou, C.K.; Geskin, L.J.; et al. Efficacy and Safety of Denileukin Diftitox-Cxdl, an Improved Purity Formulation of Denileukin Diftitox, in Patients with Relapsed or Refractory Cutaneous T-Cell Lymphoma. J. Clin. Oncol. 2025, 43, 1198–1209. [Google Scholar] [CrossRef]
- Foss, F.M.; Sjak-Shie, N.; Goy, A.; Jacobsen, E.; Advani, R.; Smith, M.R.; Komrokji, R.; Pendergrass, K.; Bolejack, V. A multicenter phase II trial to determine the safety and efficacy of combination therapy with denileukin diftitox and cyclophosphamide, doxorubicin, vincristine and prednisone in untreated peripheral T-cell lymphoma: The CONCEPT study. Leuk. Lymphoma 2013, 54, 1373–1379. [Google Scholar] [CrossRef]
- Younes, A.; Bartlett, N.L.; Leonard, J.P.; Kennedy, D.A.; Lynch, C.M.; Sievers, E.L.; Forero-Torres, A. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N. Engl. J. Med. 2010, 363, 1812–1821. [Google Scholar] [CrossRef]
- Pro, B.; Advani, R.; Brice, P.; Bartlett, N.L.; Rosenblatt, J.D.; Illidge, T.; Matous, J.; Ramchandren, R.; Fanale, M.; Connors, J.M.; et al. Brentuximab Vedotin (SGN-35) in Patients with Relapsed or Refractory Systemic Anaplastic Large-Cell Lymphoma: Results of a Phase II Study. J. Clin. Oncol. 2012, 30, 2190–2196. [Google Scholar] [CrossRef]
- Duvic, M.; Tetzlaff, M.T.; Gangar, P.; Clos, A.L.; Sui, D.; Talpur, R. Results of a Phase II Trial of Brentuximab Vedotin for CD30+ Cutaneous T-Cell Lymphoma and Lymphomatoid Papulosis. J. Clin. Oncol. 2015, 33, 3759–3765. [Google Scholar] [CrossRef]
- Pro, B.; Advani, R.; Brice, P.; Bartlett, N.L.; Rosenblatt, J.D.; Illidge, T.; Matous, J.; Ramchandren, R.; Fanale, M.; Connors, J.M.; et al. Five-year results of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma. Blood 2017, 130, 2709–2717. [Google Scholar] [CrossRef]
- Horwitz, S.M.; Advani, R.H.; Bartlett, N.L.; Jacobsen, E.D.; Sharman, J.P.; O’Connor, O.A.; Siddiqi, T.; Kennedy, D.A.; Oki, Y. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood 2014, 123, 3095–3100. [Google Scholar] [CrossRef]
- Horwitz, S.; O’Connor, O.A.; Pro, B.; Trümper, L.; Iyer, S.; Advani, R.; Bartlett, N.L.; Christensen, J.H.; Morschhauser, F.; Domingo-Domenech, E.; et al. The ECHELON-2 Trial: 5-year results of a randomized, phase III study of brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma. Ann. Oncol. 2022, 33, 288–298. [Google Scholar] [CrossRef]
- Horwitz, S.M.; Scarisbrick, J.J.; Dummer, R.; Whittaker, S.; Duvic, M.; Kim, Y.H.; Quaglino, P.; Zinzani, P.L.; Bechter, O.; Eradat, H.; et al. Randomized phase 3 ALCANZA study of brentuximab vedotin vs physician’s choice in cutaneous T-cell lymphoma: Final data. Blood Adv. 2021, 5, 5098–5106. [Google Scholar] [CrossRef]
- Marie-Cardine, A.; Viaud, N.; Thonnart, N.; Joly, R.; Chanteux, S.; Gauthier, L.; Bonnafous, C.; Rossi, B.; Bléry, M.; Paturel, C.; et al. IPH4102, a Humanized KIR3DL2 Antibody with Potent Activity against Cutaneous T-cell Lymphoma. Cancer Res. 2014, 74, 6060–6070. [Google Scholar] [CrossRef]
- Ortonne, N.; Gouvello, S.L.; Tabak, R.; Marie-Cardine, A.; Setiao, J.; Berrehar, F.; Nghe-Tang, A.; Martin, N.; Bagot, M.; Bensussan, A. CD158k/KIR3DL2 and NKp46 are frequently expressed in transformed mycosis fungoides. Exp. Dermatol. 2012, 21, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Thonnart, N.; Caudron, A.; Legaz, I.; Bagot, M.; Bensussan, A.; Marie-Cardine, A. KIR3DL2 is a coinhibitory receptor on Sézary syndrome malignant T cells that promotes resistance to activation-induced cell death. Blood 2014, 124, 3330–3332. [Google Scholar] [CrossRef] [PubMed]
- Battistella, M.; Janin, A.; Jean-Louis, F.; Collomb, C.; Leboeuf, C.; Sicard, H.; Bonnafous, C.; Dujardin, A.; Ram-Wolff, C.; Kadin, M.E.; et al. KIR3DL2 (CD158k) is a potential therapeutic target in primary cutaneous anaplastic large-cell lymphoma. Br. J. Dermatol. 2016, 175, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Battistella, M.; Leboeuf, C.; Ram-Wolff, C.; Hurabielle, C.; Bonnafous, C.; Sicard, H.; Bensussan, A.; Bagot, M.; Janin, A. KIR3DL2 expression in cutaneous T-cell lymphomas: Expanding the spectrum for KIR3DL2 targeting. Blood 2017, 130, 2900–2902. [Google Scholar] [CrossRef]
- Ghazi, B.; Thonnart, N.; Bagot, M.; Bensussan, A.; Marie-Cardine, A. KIR3DL2/CpG ODN Interaction Mediates Sézary Syndrome Malignant T Cell Apoptosis. J. Investig. Dermatol. 2015, 135, 229–237. [Google Scholar] [CrossRef]
- Bagot, M.; Porcu, P.; Ram-Wolff, C.; Khodadoust, M.; Basem, W.; Battistella, M.; Marie-Cardine, A.; Vermeer, M.; Mathieu, S.; Whittaker, S.; et al. Phase I Study of IPH4102, Anti-KIR3DL2 Mab, in Relapsed/Refractory Cutaneous T-Cell Lymphomas (CTCL): Dose-escalation Safety, Biomarker and Clinical Activity Results. Hematol. Oncol. 2017, 35, 48–49. [Google Scholar] [CrossRef]
- Bagot, M.; Kim, Y.; Zinzani, P.L.; Dalle, S.; Beylot-Barry, M.; Ortiz-Romero, P.L.; Cambalia, A.; Dereure, O.; Mortier, L.; Jacobsen, E.; et al. Tre-O2-07 Lacutamab in patients (pts) with advanced mycosis fungoides (MF) according to KIR3DL2 expression: Early results from the TELLOMAK phase 2 trial. Eur. J. Cancer. 2021, 156, S20–S21. [Google Scholar] [CrossRef]
- Porcu, P.; Bagot, M.; Kim, Y.H.; Ram-Wolff, C.; Geskin, L.J.; Ortiz-Romero, P.L.; Kim, E.J.; Mehta-Shah, N.; Dereure, O.; Ingen-Housz-Oro, S.; et al. Lacutamab in patients with relapsed and refractory Sézary syndrome: Long term follow-up from the TELLOMAK phase 2 trial. J. Clin. Oncol. 2025, 43, 2522. [Google Scholar] [CrossRef]
- Decroos, A.; Cheminant, M.; Bruneau, J.; Carras, S.; Parinet, V.; Pelletier, L.; Lacroix, L.; Martin, N.; Giustiniani, J.; Lhermitte, L.; et al. KIR3DL2 may represent a novel therapeutic target in aggressive systemic peripheral T-cell lymphoma. Haematologica 2023, 108, 2830–2836. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.P.; Ayyappan, S.R.; Greenwell, I.B.; Kim, W.S.; Lee, S.T.; Kim, J.S.; Lee, W.-S.; Jeon, Y.; Johnson, W.; Dave, U.P.; et al. Strategies to Develop Anti-KIR Mab Lacutamab in Patients with Peripheral T-Cell Lymphoma: Preliminary Monotherapy Clinical Data and Pre-Clinical Combinability Data. Blood 2023, 142, 3072. [Google Scholar] [CrossRef]
- Iyer, S.P.; Greenwell, I.B.; Shea, L.K.; Lee, J.-H.; Muller, M. 647TiP A multi-center phase Ib trial evaluating the safety and efficacy of lacutamab in patients with relapsed/refractory peripheral T-cell lymphoma that express KIR3DL2. Ann. Oncol. 2022, 33, S837. [Google Scholar] [CrossRef]
- Bratulic, A. Innate Pulls Back on Lacutumab Monotherapy for PTCL After Efficacy Loss. 2024. Available online: https://firstwordpharma.com/story/5839110 (accessed on 20 September 2025).
- Cheminant, M.; Carras, S.; Bruneau, J.; Lemonnier, F.; Bachy, E.; Herbaux, C.; Feugier, P.; Daguindau, N.; Gastinne, T.; Guillermin, Y.; et al. Kilt: A randomized non-comparative phase II LYSA study of lacutamab with gemox versus gemox in relapsed/refractory peripheral t-cell lymphoma. Hematol. Oncol. 2023, 41, 838–839. [Google Scholar] [CrossRef]
- Cowan, R.A.; Scarisbrick, J.J.; Zinzani, P.L.; Nicolay, J.P.; Sokol, L.; Pinter-Brown, L.; Quaglino, P.; Iversen, L.; Dummer, R.; Musiek, A.; et al. Efficacy and safety of mogamulizumab by patient baseline blood tumour burden: A post hoc analysis of the MAVORIC trial. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 2225–2238. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Karlin, L.; Radford, J.; Caballero, D.; Fields, P.; Chamuleau, M.E.D.; d’Amore, F.; Haioun, C.; Thieblemont, C.; González-Barca, E.; et al. European phase II study of mogamulizumab, an anti-CCR4 monoclonal antibody, in relapsed/refractory peripheral T-cell lymphoma. Haematologica 2016, 101, e407–e410. [Google Scholar] [CrossRef]
- Ishitsuka, K.; Yasukawa, T.; Tsuji, Y. Safety and effectiveness of mogamulizumab in relapsed or refractory CC chemokine receptor 4-positive peripheral T-cell lymphoma and relapsed or refractory cutaneous T-cell lymphoma: A post-marketing surveillance in Japan. Hematol. Oncol. 2024, 42, e3292. [Google Scholar] [CrossRef]
- Cordover, E.; Pradhan, K.; Townsend, L.; Goldfinger, M.; Mantzaris, I.; Shah, N.; Shastri, A.; Kornblum, N.; Cooper, D.; Gupta, R.; et al. Efficacy and manageable toxicity of mogamulizumab in a real-world North American cohort of adult T-cell leukemia/lymphoma (ATLL). J. Clin. Oncol. 2025, 43, e18603. [Google Scholar] [CrossRef]
- Yonekura, K.; Kusumoto, S.; Choi, I.; Nakano, N.; Ito, A.; Suehiro, Y.; Imaizumi, Y.; Yoshimitsu, M.; Nosaka, K.; Ohtsuka, E.; et al. Mogamulizumab for adult T-cell leukemia-lymphoma: A multicenter prospective observational study. Blood Adv. 2020, 4, 5133–5145. [Google Scholar] [CrossRef]
- Satake, A.; Azuma, Y.; Tsubokura, Y.; Yoshimura, H.; Hotta, M.; Nakanishi, T.; Fujita, S.; Nakaya, A.; Ito, T.; Ishii, K.; et al. Clinical Impact of Mogamulizumab Against Aggressive ATL: A Single-Center Retrospective Analysis. Blood 2017, 130, 4092. Available online: https://ashpublications.org/blood/article/130/Supplement%201/4092/73007/Clinical-Impact-of-Mogamulizumab-Against (accessed on 11 November 2025).
- Phillips, A.A.; Fields, P.A.; Hermine, O.; Ramos, J.C.; Beltran, B.E.; Pereira, J.; Wandroo, F.; Feldman, T.; Taylor, G.P.; Sawas, A.; et al. Mogamulizumab versus investigator’s choice of chemotherapy regimen in relapsed/refractory adult T-cell leukemia/lymphoma. Haematologica 2019, 104, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Yoshimitsu, M.; Kusumoto, S.; Shimokawa, M.; Utsunomiya, A.; Suehiro, Y.; Hidaka, T.; Nosaka, K.; Sasaki, H.; Rai, S.; et al. A phase 2 trial of CHOP with anti-CCR4 antibody mogamulizumab for elderly patients with CCR4-positive adult T-cell leukemia/lymphoma. J. Clin. Oncol. 2023, 41, 7504. [Google Scholar] [CrossRef]
- Edner, N.M.; Carlesso, G.; Rush, J.S.; Walker, L.S.K. Targeting co-stimulatory molecules in autoimmune disease. Nat. Rev. Drug Discov. 2020, 19, 860–883. [Google Scholar] [CrossRef]
- Chavez, J.C.; Foss, F.M.; William, B.M.; Brammer, J.E.; Smith, S.M.; Prica, A.; Zain, J.M.; Tuscano, J.M.; Shah, H.; Mehta-Shah, N.; et al. Targeting the Inducible T-cell Costimulator (ICOS) in Patients with Relapsed/Refractory T-follicular Helper Phenotype Peripheral T-cell and Angioimmunoblastic T-cell Lymphoma. Clin. Cancer Res. 2023, 29, 1869–1878. [Google Scholar] [CrossRef]
- Wei, C.; Wang, W.; Zhang, Y.; Zhao, D.; Zhang, W.; Zhou, D. Mutation profiling, tumour burden assessment, outcome prediction and disease monitoring by circulating tumour DNA in peripheral T-cell lymphoma. Br. J. Haematol. 2023, 202, 86–95. [Google Scholar] [CrossRef]
- Zhang, M.; Fu, X.; Meng, H.; Wang, M.; Wang, Y.; Zhang, L.; Li, L.; Li, X.; Wang, X.; Sun, Z.; et al. A Single-Arm, Open-Label, Pilot Trial of Autologous CD7-CAR-T Cells for CD7 Positive Relapsed and Refractory T-Lymphoblastic Leukemia/Lymphoma. Blood 2021, 138, 3829. [Google Scholar] [CrossRef]
- Palomero, T.; Couronné, L.; Khiabanian, H.; Kim, M.-Y.; Ambesi-Impiombato, A.; Perez-Garcia, A.; Carpenter, Z.; Abate, F.; Allegretta, M.; Haydu, J.E.; et al. Recurrent mutations in epigenetic regulators, RHOA and FYN kinase in peripheral T cell lymphomas. Nat. Genet. 2014, 46, 166–170. [Google Scholar] [CrossRef]
- Zhang, C.; Mou, B.; Xu, J.; Wang, J.; Liu, Q.; Yang, Y.; Tang, W.; Zhong, X.; Xu, C. Angioimmunoblastic T-cell lymphoma: Novel recurrent mutations and prognostic biomarkers by cell-free DNA profiling. Br. J. Haematol. 2023, 203, 807–819. [Google Scholar] [CrossRef]
- Sibon, D.; Bisig, B.; Bonnet, C.; Poullot, E.; Bachy, E.; Cavalieri, D.; Fataccioli, V.; Bregnard, C.; Drieux, F.; Bruneau, J.; et al. ALK-negative anaplastic large cell lymphoma with DUSP22 rearrangement has distinctive disease characteristics with better progression-free survival: A LYSA study. Haematologica 2022, 108, 1590–1603. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Ishida, T.; Masaki, A.; Murase, T.; Yonekura, K.; Tashiro, Y.; Tokunaga, M.; Utsunomiya, A.; Ito, A.; Kusumoto, S.; et al. CCR4 mutations associated with superior outcome of adult T-cell leukemia/lymphoma under mogamulizumab treatment. Blood 2018, 132, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Fujii, K.; Sakamoto, Y.; Masaki, A.; Murase, T.; Tashiro, Y.; Yonekura, K.; Utsunomiya, A.; Ito, A.; Kusumoto, S.; Iida, S.; et al. Immunohistochemistry for CCR4 C-terminus predicts CCR4 mutations and mogamulizumab efficacy in adult T-cell leukemia/lymphoma. J. Pathol. Clin. Res. 2021, 7, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Qiu, C.; Li, W.-S.; Wang, B.; Han, X.-L.; Lin, S.-W.; Fu, X.-H.; Hou, J.; Huang, Z.-F. Spatial Transcriptomics Analysis Reveals that CCL17 and CCL22 are Robust Indicators of a Suppressive Immune Environment in Angioimmunoblastic T Cell Lymphoma (AITL). Front. Biosci. Landmark 2022, 27, 270. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Arias, C.G.; Cunningham, D.; Peckitt, C.; Thomas, K.; Du, Y.; Hujairi, N.; To, Y.M.; Chen, H.C.; Patel, S.; et al. The role of PET/CT in peripheral T-cell lymphoma: Results from the PET/CT substudy of the UK NCRI phase 2 CHEMO-T trial. Br. J. Haematol. 2025, 207, 407–416. [Google Scholar] [CrossRef]
- Pellegrini, C.; Argnani, L.; Broccoli, A.; Stefoni, V.; Derenzini, E.; Gandolfi, L.; Casadei, B.; Maglie, R.; Pileri, S.; Zinzani, P.L. Prognostic Value of Interim Positron Emission Tomography in Patients with Peripheral T-Cell Lymphoma. Oncologist 2014, 19, 746–750. [Google Scholar] [CrossRef]
- Song, G.-Y.; Jung, S.-H.; Ahn, S.-Y.; Kim, M.; Ahn, J.-S.; Lee, J.-J.; Kim, H.-J.; Moon, J.B.; Yoo, S.W.; Kwon, S.Y.; et al. Prognostic Significance Of Sequential 18f-fdg Pet/Ct During Frontline Treatment of Peripheral T Cell Lymphomas. Korean J. Intern. Med. 2024, 39, 327–337. [Google Scholar] [CrossRef]
- Krutzek, F.; Kopka, K.; Stadlbauer, S. Development of Radiotracers for Imaging of the PD-1/PD-L1 Axis. Pharmaceuticals 2022, 15, 747. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, Y.J.; Yoon, S.E.; Ryu, K.J.; Park, B.; Park, D.; Cho, D.; Kim, H.-Y.; Cho, J.; Ko, Y.H.; et al. Circulating Tumor DNA-Based Genotyping and Monitoring for Predicting Disease Relapses of Patients with Peripheral T-Cell Lymphomas. Cancer Res. Treat. 2022, 55, 291–303. [Google Scholar] [CrossRef]
- Yannakou, C.K.; Wu, S.; Rajah, K.; Abeyakoon, C.; Nguyen-Ngo, C.; Yap, Y.Z.; Sheldon, J.; Blombery, P.; Prince, H.M. Circulating Tumour DNA Is a Biomarker of Response in Angioimmunoblastic T-Cell Lymphoma. Int. J. Mol. Sci. 2025, 26, 6719. [Google Scholar] [CrossRef]
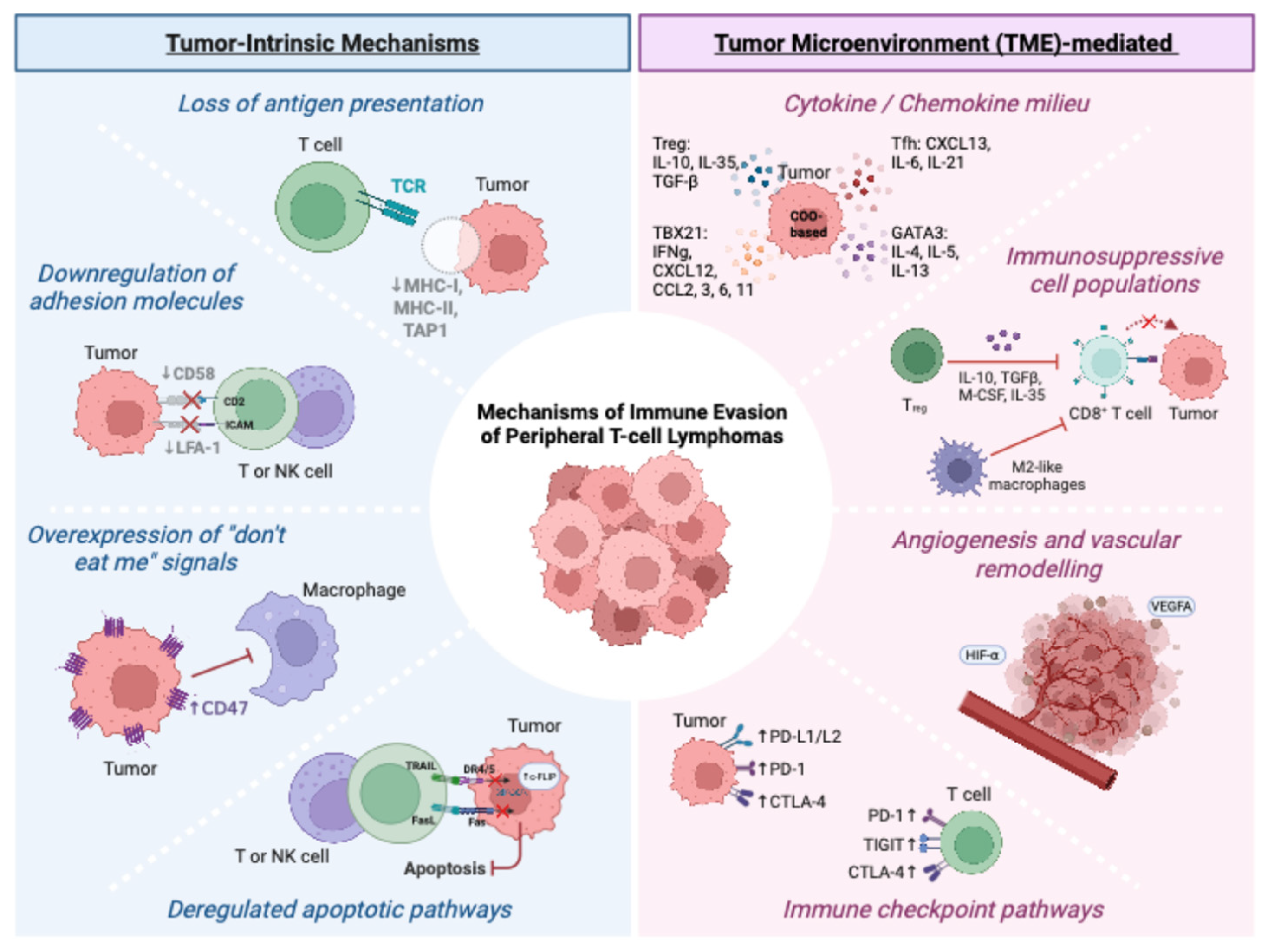
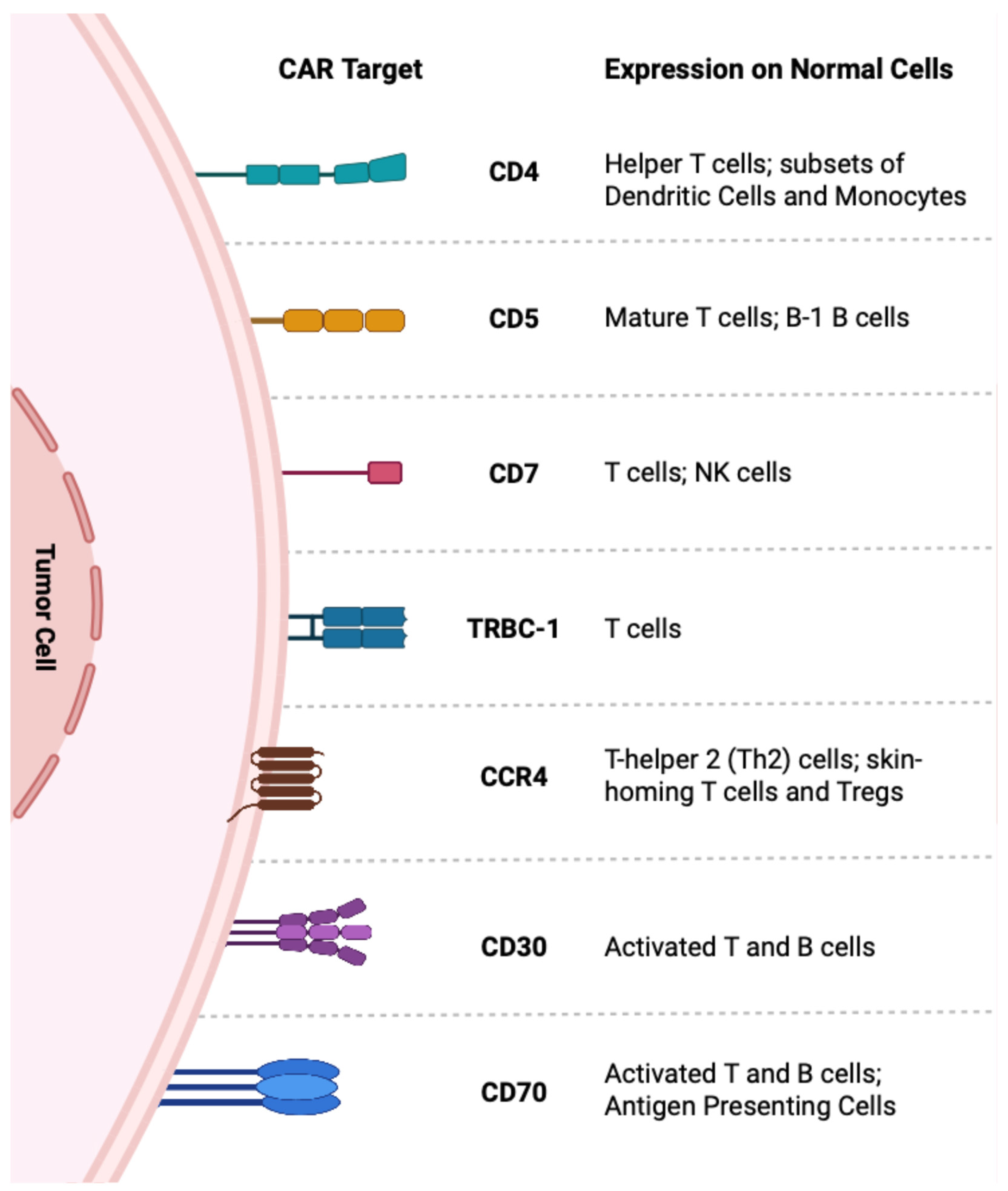
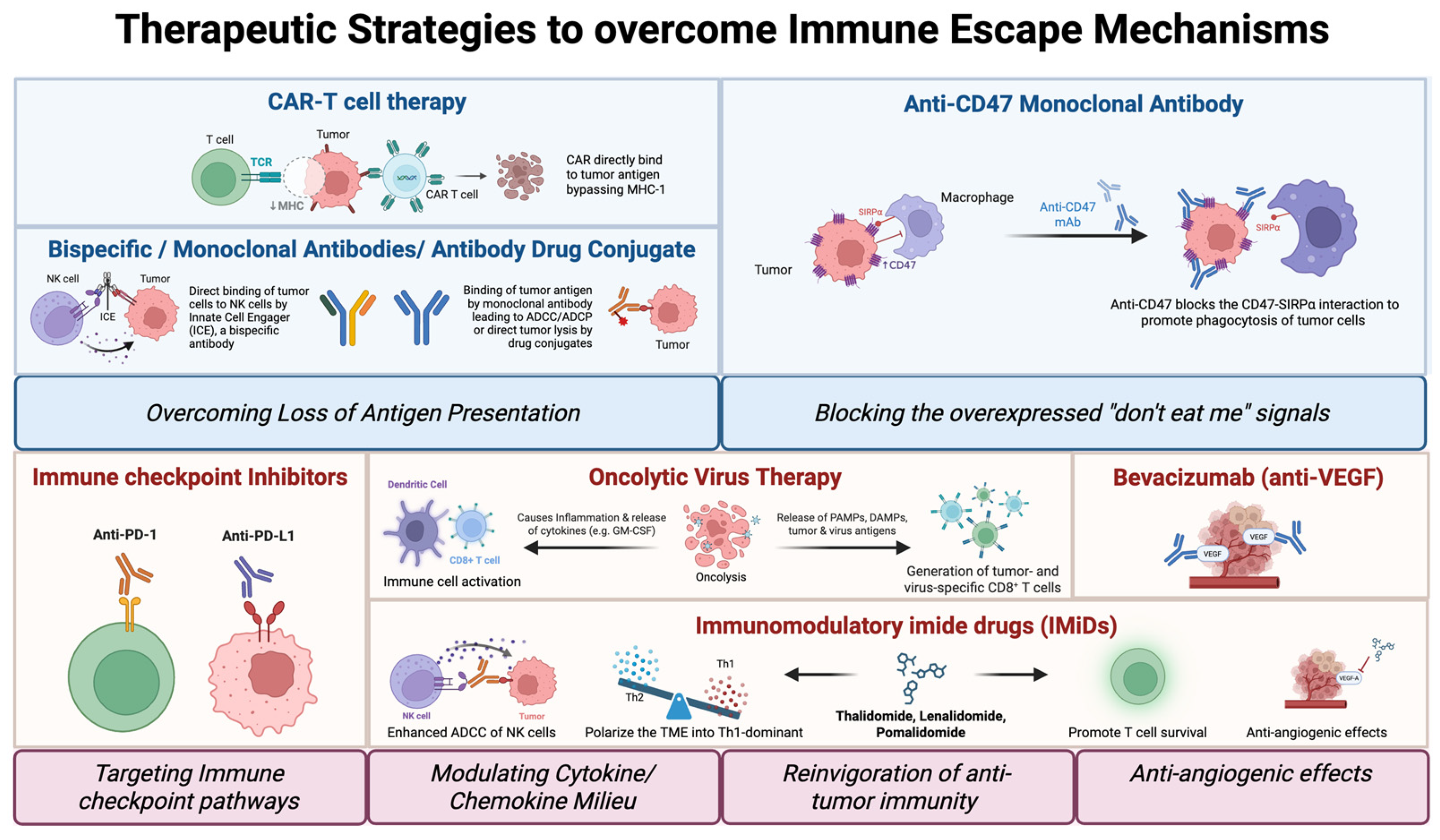
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casan, J.M.L.; Ong, X.J.; van der Weyden, C.; Yannakou, C.K.; Zhu, J.; D’Souza, C.; Neeson, P.; Prince, H.M. Personalized Immunotherapy for T Cell Lymphomas: From Immune Escape to Precision Therapeutics. J. Pers. Med. 2025, 15, 560. https://doi.org/10.3390/jpm15110560
Casan JML, Ong XJ, van der Weyden C, Yannakou CK, Zhu J, D’Souza C, Neeson P, Prince HM. Personalized Immunotherapy for T Cell Lymphomas: From Immune Escape to Precision Therapeutics. Journal of Personalized Medicine. 2025; 15(11):560. https://doi.org/10.3390/jpm15110560
Chicago/Turabian StyleCasan, Joshua M. L., Xiao Jing Ong, Carrie van der Weyden, Costas K. Yannakou, Joe Zhu, Criselle D’Souza, Paul Neeson, and H. Miles Prince. 2025. "Personalized Immunotherapy for T Cell Lymphomas: From Immune Escape to Precision Therapeutics" Journal of Personalized Medicine 15, no. 11: 560. https://doi.org/10.3390/jpm15110560
APA StyleCasan, J. M. L., Ong, X. J., van der Weyden, C., Yannakou, C. K., Zhu, J., D’Souza, C., Neeson, P., & Prince, H. M. (2025). Personalized Immunotherapy for T Cell Lymphomas: From Immune Escape to Precision Therapeutics. Journal of Personalized Medicine, 15(11), 560. https://doi.org/10.3390/jpm15110560







