Beyond Standard Parameters: Precision Hemodynamic Monitoring in Patients on Veno-Arterial ECMO
Abstract
1. Introduction
2. Materials and Methods
3. Relevant Sections
3.1. Hemodynamic Principles in V-A ECMO
3.2. Hemodynamic Challenges in V-A ECMO: Differential Hypoxemia and Pulmonary Congestion
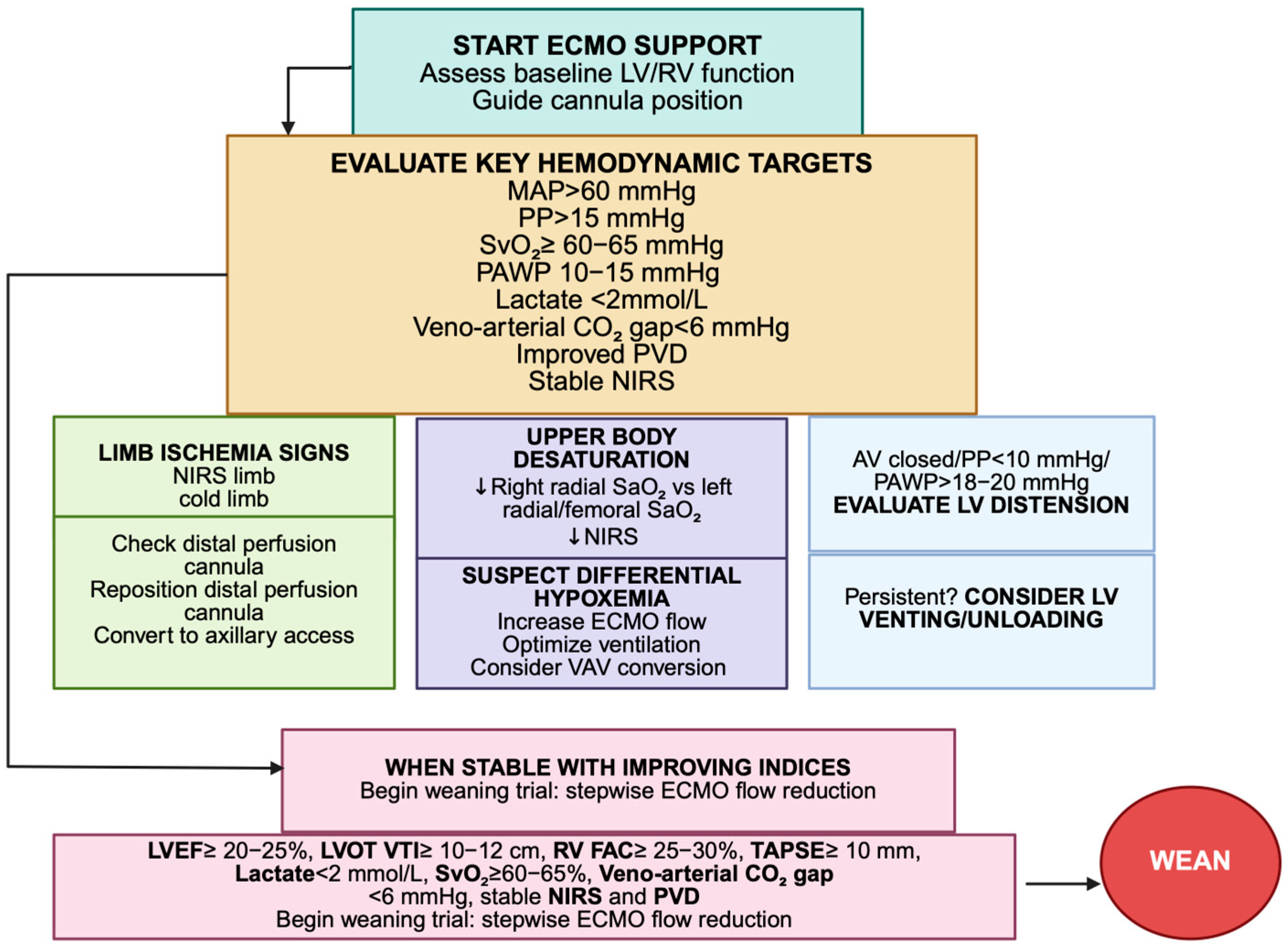
3.2.1. Optimization of Aortic Valve Opening and Pulsatility and Left Ventricular Decompression
- •
- Intra-aortic balloon pump (IABP): Reduces afterload and promotes intermittent AV opening by enhancing diastolic runoff. It is generally preferred when mild to moderate LV loading is present. Advantages include ease of insertion; however, its decompressive effect may be insufficient in cases of severe LV distension [24].
- •
- Microaxial pumps (e.g., Impella devices): Actively unload the LV by draining blood from the LV cavity into the ascending aorta, thereby decreasing end-diastolic volume and wall stress. This method offers powerful unloading and improved LV recovery but carries risks of hemolysis, device migration and vascular injury [25,26].
- •
- Trans-septal or atrial septostomy venting: Allows left-to-right shunting to decompress the left atrium, effectively reducing pulmonary congestion. It is minimally invasive but can result in right-sided volume overload or residual shunt post-decannulation [27].
- •
- Apical LV venting, typically performed via a left mini-thoracotomy, allows for direct insertion of the cannula into the LV apex, which can be connected to the ECMO venous return or to a separate centrifugal pump (the latter configuration may function as a temporary LVAD-like system). This technique provides excellent unloading, but carries bleeding and myocardial injury risks [16,28].
- •
- Right pulmonary vein venting, most often through via median sternotomy, is preferred in patients already undergoing open cardiac surgery. It ensures efficient decompression under direct visualization, but it is inherently more invasive and associated with postoperative bleeding and infection risks [29].
3.2.2. Differential Hypoxemia (Harlequin Syndrome)
- •
- Optimize pulmonary gas exchange: increase FiO2 and PEEP, treat pulmonary edema or atelectasis, perform recruitment maneuvers and adjust ventilator settings to improve oxygenation of native LV output [6].
- •
- Adjust ECMO flow: temporarily increase flow to move the mixing zone proximally, while avoiding complete suppression of LV ejection that may worsen stasis and distension [14].
- •
- •
- Central aortic cannulation: in post-cardiotomy or persistent cases, this ensures uniform antegrade perfusion to both coronary and cerebral arteries, providing definitive correction [13].
3.3. Volume Management and Hemodynamic Monitoring in V-A ECMO
3.4. Perfusion Monitoring in V-A ECMO: Clinical Parameters and Laboratory Indicators
Monitoring and Prevention of Distal Limb Ischemia
3.5. Monitoring Mean Arterial Pressure in V-A ECMO
3.5.1. Arterial Pulse Pressure
3.5.2. ETCO2
3.6. Role of the Pulmonary Catheter in V-A ECMO
3.7. Echocardiographic Monitoring in V-A ECMO
3.8. Monitoring Microcirculation in V-A ECMO
3.9. Arterial Waveform Analysis
3.10. Monitoring of ECMO Blood Flow
3.11. Rhythm Monitoring and Arrhythmia Management
3.12. Integrative Hemodynamic and Echocardiographic Assessment for Weaning from V-A ECMO
3.12.1. Bridge Strategies and Hemodynamic Guidance After ECMO
- •
- •
- Bridge to durable MCS or LVAD implantation should be considered in patients with insufficient myocardial recovery who remain dependent on ECMO flow to maintain perfusion or end-organ function. Key parameters include persistently low LVOT VTI (<10 cm), reduced pulsatility, elevated filling pressures (PAWP > 20 mmHg) or poor right ventricular reserve (FAC < 25%). Early transition to durable support is associated with improved outcomes compared with prolonged ECMO dependence [101,102,103].
- •
- Bridge to transplantation represents an option for patients with irreversible myocardial failure but preserved end-organ function who are suitable candidates for heart transplantation. The decision to proceed toward transplant candidacy requires evidence of stable systemic perfusion, recovery of multi-organ function and the absence of fixed pulmonary hypertension which markedly increases the risk of early graft failure. Optimal pre-transplant hemodynamics are characterized by a mean pulmonary artery pressure (mPAP) < 25–30 mmHg, pulmonary vascular resistance (PVR) < 4 Wood units and a transpulmonary gradient (TPG) < 15 mmHg, ensuring that the donor’s right ventricle can adequately adapt to the recipient’s pulmonary circulation. Maintaining controlled afterload, adequate oxygenation and optimized ECMO flow during the bridging phase helps preserve a low pulmonary vasculature tone. In selected cases, transition to a temporary durable device (such as a paracorporeal LVAD or BiVAD) may be required to achieve longer-term stabilization and ensure transplant eligibility [104,105,106].
- •
- For patients unsuitable for immediate recovery or transplantation, a bridge to decision approach allows ongoing stabilization while assessing neurological, renal and hepatic recovery to determine long-term candidacy for advanced therapies [107].
3.12.2. Follow-Up and Long-Term Surveillance
3.13. Artificial Intelligence and Machine Learning in Hemodynamic Monitoring
4. Discussion
5. Conclusions
6. Future Directions
7. Limitations of the Literature
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ECMO | Extracorporeal membrane oxygenation |
| V-A ECMO | Veno-arterial ECMO |
| LV | Left ventricle |
| RV | Right ventricle |
| TAPSE | Tricuspid annular plane systolic excursion |
| FAC | Fractional area change |
| PVD | Perfused vessel density |
| PAPi | Pulmonary artery pulsatility index |
| DO2 | Oxygen delivery |
| VO2 | Oxygen consumption |
References
- Rao, P.; Khalpey, Z.; Smith, R.; Burkhoff, D.; Kociol, R.D. Venoarterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock and Cardiac Arrest. Circ. Heart Fail. 2018, 11, e004905. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Xu, L. Hemodynamic Management During Veno-Arterial Extracorporeal Membrane Oxygenation in Patients with Cardiogenic Shock: A Review. Intensive Care Res. 2023, 3, 131–139. [Google Scholar] [CrossRef]
- Lüsebrink, E.; Stremmel, C.; Stark, K.; Joskowiak, D.; Czermak, T.; Born, F.; Kupka, D.; Scherer, C.; Orban, M.; Petzold, T.; et al. Update on Weaning from Veno-Arterial Extracorporeal Membrane Oxygenation. J. Clin. Med. 2020, 9, 992. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bharadwaj, M.S.; Bora, V. Venoarterial ECMO Hemodynamics. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Rozencwajg, S.; Wu, E.L.; Heinsar, S.; Stevens, M.; Chinchilla, J.; Fraser, J.F.; Pauls, J.P. A mock circulation loop to evaluate differential hypoxemia during peripheral venoarterial extracorporeal membrane oxygenation. Perfusion 2024, 39, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Torre, D.E.; Pirri, C. Harlequin Syndrome in Venoarterial ECMO and ECPELLA: When ECMO and Native or Impella Circulations Collide—A Comprehensive Review. Rev. Cardiovasc. Med. 2025, 26, 39992. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Truby, L.; Mundy, L.; Kalesan, B.; Kirtane, A.; Colombo, P.C.; Takeda, K.; Fukuhara, S.; Naka, Y.; Takayama, H. Contemporary Outcomes of Venoarterial Extracorporeal Membrane Oxygenation for Refractory Cardiogenic Shock at a Large Tertiary Care Center. ASAIO J. 2015, 61, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Ricarte Bratti, J.P.; Cavayas, Y.A.; Noly, P.E.; Serri, K.; Lamarche, Y. Modalities of Left Ventricle Decompression during VA-ECMO Therapy. Membranes 2021, 11, 209. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cevasco, M.; Takayama, H.; Ando, M.; Garan, A.R.; Naka, Y.; Takeda, K. Left ventricular distension and venting strategies for patients on venoarterial extracorporeal membrane oxygenation. J. Thorac. Dis. 2019, 11, 1676–1683. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Torre, D.E.; Pirri, C. Alternative Arterial Access in Veno-Arterial ECMO: The Role of the Axillary Artery. J. Clin. Med. 2025, 14, 5413. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fresiello, L.; Hermens, J.A.J.; Pladet, L.; Meuwese, C.L.; Donker, D.W. The physiology of venoarterial extracorporeal membrane oxygenation—A comprehensive clinical perspective. Perfusion 2024, 39 (Suppl. S1), 5S–12S. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mariscalco, G.; Salsano, A.; Fiore, A.; Dalén, M.; Ruggieri, V.G.; Saeed, D.; Jónsson, K.; Gatti, G.; Zipfel, S.; Dell’Aquila, A.M.; et al. Peripheral versus central extracorporeal membrane oxygenation for postcardiotomy shock: Multicenter registry, systematic review, and meta-analysis. J. Thorac. Cardiovasc. Surg. 2020, 160, 1207–1216.e44. [Google Scholar] [CrossRef] [PubMed]
- Hou, X. How to mange differential hypoxemia during veno-arterial extracorporeal membrane oxygenation. Perfusion 2024, 39, 443–444. [Google Scholar] [CrossRef] [PubMed]
- Falk, L.; Sallisalmi, M.; Lindholm, J.A.; Lindfors, M.; Frenckner, B.; Broomé, M.; Broman, L.M. Differential hypoxemia during venoarterial extracorporeal membrane oxygenation. Perfusion 2019, 34 (Suppl. S1), 22–29. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Kim, M.C.; Jeong, I.S. Left ventricle unloading during veno-arterial extracorporeal membrane oxygenation: Review with updated evidence. Acute Crit. Care 2024, 39, 473–487. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lorusso, R.; Meani, P.; Raffa, G.M.; Kowalewski, M. Extracorporeal membrane oxygenation and left ventricular unloading: What is the evidence? JTCVS Tech. 2022, 13, 101–114. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meani, P.; Delnoij, T.; Raffa, G.M.; Morici, N.; Viola, G.; Sacco, A.; Oliva, F.; Heuts, S.; Sels, J.W.; Driessen, R.; et al. Protracted aortic valve closure during peripheral veno-arterial extracorporeal life support: Is intra-aortic balloon pump an effective solution? Perfusion 2019, 34, 35–41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grandin, E.W.; Nunez, J.I.; Willar, B.; Kennedy, K.; Rycus, P.; Tonna, J.E.; Kapur, N.K.; Shaefi, S.; Garan, A.R. Mechanical Left Ventricular Unloading in Patients Undergoing Venoarterial Extracorporeal Membrane Oxygenation. J. Am. Coll. Cardiol. 2022, 79, 1239–1250. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hermens, J.A.J.; van Til, J.A.; Meuwese, C.L.; van Dijk, D.; Donker, D.W. Clinical decision making for VA ECMO weaning in patients with cardiogenic shock A formative qualitative study. Perfusion 2024, 39 (Suppl. S1), 39S–48S. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.R.; Sekhar, P.; Grover, J.; Tian, D.H.; Downey, C.; Maudlin, B.; Dissanayake, C.; Dennis, M. Predictors of successful weaning from veno-arterial extracorporeal membrane oxygenation (V-A ECMO): A systematic review and meta-analysis. PLoS ONE 2025, 20, e0310289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, H.; Wang, T.; Wang, J.; Liu, G.; Yan, S.; Teng, Y.; Wang, J.; Ji, B. Different strategies in left ventricle unloading during venoarterial extracorporeal membrane oxygenation: A network meta-analysis. Int. J. Cardiol. Heart Vasc. 2024, 54, 101506. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lüsebrink, E.; Binzenhöfer, L.; Kellnar, A.; Müller, C.; Scherer, C.; Schrage, B.; Joskowiak, D.; Petzold, T.; Braun, D.; Brunner, S.; et al. Venting during venoarterial extracorporeal membrane oxygenation. Clin. Res. Cardiol. 2023, 112, 464–505. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jorde, U.P.; Saeed, O.; Uehara, M.; Goldstein, D.J.; Castagna, F. Left Ventricular Venting Based on Acute Shock ECMO Phenotype. J. Am. Coll. Cardiol. 2022, 80, e153–e154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zeng, P.; Yang, C.; Chen, J.; Fan, Z.; Cai, W.; Huang, Y.; Xiang, Z.; Yang, J.; Zhang, J.; Yang, J. Comparison of the Efficacy of ECMO with or Without IABP in Patients with Cardiogenic Shock: A Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 917610. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gandhi, K.D.; Moras, E.C.; Niroula, S.; Lopez, P.D.; Aggarwal, D.; Bhatia, K.; Balboul, Y.; Daibes, J.; Correa, A.; Dominguez, A.C.; et al. Left Ventricular Unloading with Impella Versus IABP in Patients with VA-ECMO: A Systematic Review and Meta-Analysis. Am. J. Cardiol. 2023, 208, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Meani, P.; Lorusso, R.; Pappalardo, F. ECPella: Concept, Physiology and Clinical Applications. J. Cardiothorac. Vasc. Anesth. 2022, 36, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Broomé, M.; Donker, D. Atrial Septostomy for Left Ventricular Unloading. JACC Cardiovasc. Interv. 2021, 14, 2708–2710. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.; Senanayake, E.; Thomson, B. Simple left ventricular apical cannulation for temporary mechanical circulatory support. J. Card. Surg. 2022, 37, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Russo, J.J.; Aleksova, N.; Pitcher, I.; Couture, E.; Parlow, S.; Faraz, M.; Visintini, S.; Simard, T.; Di Santo, P.; Mathew, R.; et al. Left Ventricular Unloading During Extracorporeal Membrane Oxygenation in Patients with Cardiogenic Shock. J. Am. Coll. Cardiol. 2019, 73, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Badulak, J.; Abrams, D.; Luks, A.M.; Zakhary, B.; Conrad, S.A.; Bartlett, R.; MacLaren, G.; Vercaemst, L.; Lorusso, R.; Broman, L.M.; et al. Correction: Position paper on the physiology and nomenclature of dual circulation during venoarterial ECMO in adults. Intensive Care Med. 2025, 51, 661–662, Erratum in Intensive Care Med. 2024, 50, 1994–2004. https://doi.org/10.1007/s00134-024-07645-8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cha, H.J.; Kim, J.W.; Kang, D.H.; Moon, S.H.; Kim, S.H.; Jung, J.J.; Yang, J.H.; Byun, J.H. Conversion to Veno-arteriovenous Extracorporeal Membrane Oxygenation for Differential Hypoxia. J. Chest Surg. 2023, 56, 274–281. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsuyoshi, T.; Shimizu, K.; Kaneko, H.; Kohsen, D.; Suzuki, H. Reconfiguration from veno-arterial to veno-arterio-venous extracorporeal membrane oxygenation for massive pulmonary embolism. J. Artif. Organs 2022, 25, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Bailey, M.; Kelly, J.; Hodgson, C.; Cooper, D.J.; Scheinkestel, C.; Pellegrino, V.; Bellomo, R.; Pilcher, D. Impact of fluid balance on outcome of adult patients treated with extracorporeal membrane oxygenation. Intensive Care Med. 2014, 40, 1256–1266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jendoubi, A.; de Roux, Q.; Ribot, S.; Vanden Bulcke, A.; Miard, C.; Tiquet, B.; Ghaleh, B.; Tissier, R.; Kohlhauer, M.; Mongardon, N. Fluid management in adult patients undergoing venoarterial extracorporeal membrane oxygenation: A scoping review. J. Crit. Care 2025, 86, 155007. [Google Scholar] [CrossRef] [PubMed]
- Selewski, D.T.; Wille, K.M. Continuous renal replacement therapy in patients treated with extracorporeal membrane oxygenation. Semin. Dial. 2021, 34, 537–549. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coudroy, R.; Jamet, A.; Frat, J.P.; Veinstein, A.; Chatellier, D.; Goudet, V.; Cabasson, S.; Thille, A.W.; Robert, R. Incidence and impact of skin mottling over the knee and its duration on outcome in critically ill patients. Intensive Care Med. 2015, 41, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Hariri, G.; Luxey, X.; Wenger, S.; Dureau, P.; Hariri, S.; Charfeddine, A.; Lebreton, G.; Djavidi, N.; Lancelot, A.; Duceau, B.; et al. Capillary refill time assessment after fluid challenge in patients on venoarterial extracorporeal membrane oxygenation: A retrospective study. J. Crit. Care 2024, 82, 154770. [Google Scholar] [CrossRef] [PubMed]
- Cruz, G.; Pedroza Gómez, S.; Arango, A.; Guevara, P.A.; González, C.; Aguirre, J.; Valencia-Orozco, A.; Suguimoto, A.J. Capillary Refill Time and Serum Lactate as Predictors of Mortality and Postoperative Extracorporeal Membrane Oxygenation Requirement in Congenital Heart Surgery. Children 2023, 10, 875. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mungan, İ.; Kazancı, D.; Bektaş, Ş.; Ademoglu, D.; Turan, S. Does lactate clearance prognosticates outcomes in ECMO therapy: A retrospective observational study. BMC Anesthesiol. 2018, 18, 152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kurniawati, E.; Weerwind, P.; Maessen, J. Understanding lactate and its clearance during extracorporeal membrane oxygenation for supporting refractory cardiogenic shock patients. BMC Cardiovasc. Disord. 2023, 23, 305. [Google Scholar] [CrossRef] [PubMed]
- Gallet, R.; Lellouche, N.; Mitchell-Heggs, L.; Bouhemad, B.; Bensaid, A.; Dubois-Randé, J.L.; Gueret, P.; Lim, P. Prognosis value of central venous oxygen saturation in acute decompensated heart failure. Arch. Cardiovasc. Dis. 2012, 105, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Ladakis, C.; Myrianthefs, P.; Karabinis, A.; Karatzas, G.; Dosios, T.; Fildissis, G.; Gogas, J.; Baltopoulos, G. Central venous and mixed venous oxygen saturation in critically ill patients. Respiration 2001, 68, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, Y.; Zhang, Y.; Wu, W.; He, P. Risk factors for refractory septic shock treated with VA ECMO. Ann. Transl. Med. 2019, 7, 476. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wagner, K.; Risnes, I.; Abdelnoor, M.; Karlsen, H.M.; Svennevig, J.L. Is it possible to predict outcome in cardiac ECMO? Analysis of preoperative risk factors. Perfusion 2007, 22, 225–229. [Google Scholar] [CrossRef] [PubMed]
- John, K.J.; Belani, D.J.; Kapur, N.K.; Lussier, L.; Chweich, H. Comparison of Mixed Venous Oxygen Saturation and Premembranous Venous Oxygen Saturation in Patients on Peripheral Venous-Arterial Extracorporeal Membrane Oxygenation. ASAIO J. 2024, 70, e130–e132. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.P.; Liu, Y.N.; Zeng, Y.T.; Zheng, Y.R.; Chen, Q. Early changes in cardiac troponin T and NT-proBNP levels in neonates receiving ECMO support: A single-center experience. BMC Cardiovasc. Disord. 2024, 24, 233. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Falkensammer, C.B.; Heinle, J.S.; Chang, A.C. Serial plasma BNP levels in assessing inadequate left ventricular decompression on ECMO. Pediatr. Cardiol. 2008, 29, 808–811. [Google Scholar] [CrossRef] [PubMed]
- Volleman, C.; Dubelaar, D.P.C.; Phelp, P.G.; Ibelings, R.; Tuip-de Boer, A.M.; Polet, C.A.; van den Bergh, W.M.; Vlaar, A.P.J.; van den Brom, C.E. Cell-free hemoglobin is associated with microcirculatory perfusion disturbances and acute kidney injury in rats on extracorporeal membrane oxygenation. BMC Anesthesiol. 2025, 25, 411. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bezak, B.; Artemiou, P.; Ondrusek, M.; Hulman, M. Comparison of PCO2gap, SvO2 and plasmatic lactate in patients on venoarterial extracorporeal circulation support. Bratisl. Lek. Listy. 2024, 125, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Coelho, R.; Tavares, J.; Marinheiro, C.; Costa, C.; Ferreira, S.; Gregório, T. The effectiveness of NIRS technology to the early diagnosis of lower limb ischemia in patients on peripheral VA ECMO: A systematic review and meta-analysis. Intensive Crit. Care Nurs. 2025, 89, 104039. [Google Scholar] [CrossRef] [PubMed]
- Hunt, M.F.; Clark, K.T.; Whitman, G.; Choi, C.W.; Geocadin, R.G.; Cho, S.M. The Use of Cerebral NIRS Monitoring to Identify Acute Brain Injury in Patients with VA-ECMO. J. Intensive Care Med. 2021, 36, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Schewe, J.C.; Schumacher, S.; Erdfelder, F.; Ehrentraut, S.F.; Weißbrich, C.; Lehmann, F.; Kögl, F.; Muders, T.; Thudium, M.; Putensen, C.; et al. Monitoring of Cerebral Oxygen Saturation in Interhospital Transport of Patients Receiving Extracorporeal Membrane Oxygenation. ASAIO J. 2023, 69, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Lu, A.; Pan, C.; Zhang, B.; Wa, Y.L.; Qu, W.; Bai, M. Limb Ischemia Complications of Veno-Arterial Extracorporeal Membrane Oxygenation. Front Med. 2022, 9, 938634. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nejim, B.; Snow, R.; Chau, M.; Sakya, S.; Castello-Ramirez, M.; Flohr, T.R.; Brehm, C.; Aziz, F. Acute Limb Ischemia in Patients on Veno-Arterial Extracorporeal Membrane Oxygenation (VA-ECMO) Support: A Ten-Year Single-Center Experience. Ann. Vasc. Surg. 2025, 111, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Simons, J.; Mees, B.; MacLaren, G.; Fraser, J.F.; Zaaqoq, A.M.; Cho, S.-M.; Patel, B.M.; Brodie, D.; Bělohlávek, J.; Belliato, M.; et al. Evolution of distal limb perfusion management in adult peripheral venoarterial extracorporeal membrane oxygenation with femoral artery cannulation. Perfusion 2024, 39 (Suppl. S1), 23S–38S. [Google Scholar] [CrossRef]
- Hanley, S.C.; Melikian, R.; Mackey, W.C.; Salehi, P.; Iafrati, M.D.; Suarez, L. Distal perfusion cannulae reduce extracorporeal membrane oxygenation-related limb ischemia. Int. Angiol. 2021, 40, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Liu, K.; Zheng, J.L.; Li, X.; Zhu, D.M.; Zhang, Y.; Zhang, Y.J.; Wang, C.S.; Shi, T.T.; Luo, Z.; et al. Hemodynamic monitoring in patients with venoarterial extracorporeal membrane oxygenation. Ann. Transl. Med. 2020, 8, 792. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Merdji, H.; Levy, B.; Jung, C.; Ince, C.; Siegemund, M.; Meziani, F. Microcirculatory dysfunction in cardiogenic shock. Ann. Intensive Care 2023, 13, 38. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rilinger, J.; Riefler, A.M.; Bemtgen, X.; Jäckel, M.; Zotzmann, V.; Biever, P.M.; Duerschmied, D.; Benk, C.; Trummer, G.; Kaier, K.; et al. Impact of pulse pressure on clinical outcome in extracorporeal cardiopulmonary resuscitation (eCPR) patients. Clin. Res. Cardiol. 2021, 110, 1473–1483. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shou, B.L.; Wilcox, C.; Florissi, I.; Kalra, A.; Caturegli, G.; Zhang, L.Q.; Bush, E.; Kim, B.; Keller, S.P.; Whitman, G.J.R.; et al. Early Low Pulse Pressure in VA-ECMO Is Associated with Acute Brain Injury. Neurocrit. Care 2023, 38, 612–621. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mourad, M.; Eliet, J.; Zeroual, N.; Saour, M.; Sentenac, P.; Manna, F.; Molinari, N.; Gandet, T.; Colson, P.H.; Gaudard, P. Pulse pressure and end-tidal carbon dioxide for monitoring low native cardiac output during veno-arterial ECLS: A prospective observational study. Crit. Care 2020, 24, 569. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eliet, J.; Gaudard, P.; Zeroual, N.; Rouvière, P.; Albat, B.; Mourad, M.; Colson, P.H. Effect of Impella During Veno-Arterial Extracorporeal Membrane Oxygenation on Pulmonary Artery Flow as Assessed by End-Tidal Carbon Dioxide. ASAIO J. 2018, 64, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Dauwe, D.F.; Saugel, B.; De Backer, D. Which cardiovascular monitoring on veno-arterial extracorporeal membrane oxygenation. Curr. Opin. Crit. Care 2025, 31, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.; Umbarkar, S. Pulmonary artery catheter-Dilemma is still on? Ann. Card. Anaesth. 2021, 24, 1–3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ortoleva, J.P.; Alfadhel, A.; Dalia, A.A. Invasive Hemodynamic and Physiologic Considerations in Patients Undergoing Extracorporeal Membrane Oxygenation. J. Cardiothorac. Vasc. Anesth. 2021, 35, 2549–2551. [Google Scholar] [CrossRef] [PubMed]
- Haller, M.; Zöllner, C.; Manert, W.; Briegel, J.; Kilger, E.; Polasek, J.; Hummel, T.; Forst, H.; Peter, K. Thermodilution cardiac output may be incorrect in patients on venovenous extracorporeal lung assist. Am. J. Respir. Crit. Care Med. 1995, 152 Pt 1, 1812–1817. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.N.V.; El-Sabbagh, A.; Nishimura, R.A. Comparing Pulmonary Arterial Wedge Pressure and Left Ventricular End Diastolic Pressure for Assessment of Left-Sided Filling Pressures. JAMA Cardiol. 2018, 3, 453–454. [Google Scholar] [CrossRef]
- Russ, M.; Steiner, E.; Boemke, W.; Busch, T.; Melzer-Gartzke, C.; Taher, M.; Badulak, J.; Weber-Carstens, S.; Swenson, E.R.; Francis, R.C.E.; et al. Extracorporeal Membrane Oxygenation Blood Flow and Blood Recirculation Compromise Thermodilution-Based Measurements of Cardiac Output. ASAIO J. 2022, 68, 721–729. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Linden, K.; Schmandt, M.; Muders, T.; Theuerkauf, N.; Schewe, J.C.; Herberg, U.; Putensen, C.; Ehrentraut, S.F.; Kreyer, S. Estimation of Cardiac Output Under Veno-Venous Extracorporeal Membrane Oxygenation: Comparing Thermodilution Methods to 3D Echocardiography. ASAIO J. 2025, 71, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; Schmidt, M.; Hekimian, G.; Combes, A.; Saura, O. Clinical validation of pulmonary artery catheter use for continuous thermodilution cardiac output monitoring in VA-ECMO patients. Intensive Care Med. 2025, 51, 785–787. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, P.M. Role of echocardiography in ECMO. Qatar Med. J. 2017, 2017, 17. [Google Scholar] [CrossRef] [PubMed Central]
- Hussey, P.T.; von Mering, G.; Nanda, N.C.; Ahmed, M.I.; Addis, D.R. Echocardiography for extracorporeal membrane oxygenation. Echocardiography 2022, 39, 339–370. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tavazzi, G.; Colombo, C.N.J.; Klersy, C.; Dammassa, V.; Civardi, L.; Degani, A.; Biglia, A.; Via, G.; Camporotondo, R.; Pellegrini, C.; et al. Echocardiographic parameters for weaning from extracorporeal membrane oxygenation-the role of longitudinal function and cardiac time intervals. Eur. Heart J. Cardiovasc. Imaging 2025, 26, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.; Kostibas, M.P. Echocardiographic and Point-of-Care Ultrasonography (POCUS) Guidance in the Management of the ECMO Patient. J. Clin. Med. 2024, 13, 2630. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Estep, J.D.; Nicoara, A.; Cavalcante, J.; Chang, S.M.; Cole, S.P.; Cowger, J.; Daneshmand, M.A.; Hoit, B.D.; Kapur, N.K.; Kruse, E.; et al. Recommendations for Multimodality Imaging of Patients with Left Ventricular Assist Devices and Temporary Mechanical Support: Updated Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2024, 37, 820–871. [Google Scholar] [CrossRef] [PubMed]
- Douflé, G.; Roscoe, A.; Billia, F.; Fan, E. Echocardiography for adult patients supported with extracorporeal membrane oxygenation. Crit. Care 2015, 19, 326, Erratum in Crit. Care 2016, 20, 34. https://doi.org/10.1186/s13054-016-1214-8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ince, C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit. Care 2015, 19 (Suppl. S3), S8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Backer, D.; Creteur, J.; Dubois, M.J.; Sakr, Y.; Vincent, J.L. Microvascular alterations in patients with acute severe heart failure and cardiogenic shock. Am. Heart J. 2004, 147, 91–99. [Google Scholar] [CrossRef] [PubMed]
- den Uil, C.A.; Lagrand, W.K.; van der Ent, M.; Jewbali, L.S.; Cheng, J.M.; Spronk, P.E.; Simoons, M.L. Impaired microcirculation predicts poor outcome of patients with acute myocardial infarction complicated by cardiogenic shock. Eur. Heart J. 2010, 31, 3032–3039. [Google Scholar] [CrossRef] [PubMed]
- Massey, M.J.; Hou, P.C.; Filbin, M.; Wang, H.; Ngo, L.; Huang, D.T.; Aird, W.C.; Novack, V.; Trzeciak, S.; Yealy, D.M.; et al. Microcirculatory perfusion disturbances in septic shock: Results from the ProCESS trial. Crit. Care 2018, 22, 308. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- den Uil, C.A.; Maat, A.P.; Lagrand, W.K.; van der Ent, M.; Jewbali, L.S.; van Thiel, R.J.; Spronk, P.E.; Simoons, M.L. Mechanical circulatory support devices improve tissue perfusion in patients with end-stage heart failure or cardiogenic shock. J. Heart Lung Transplant. 2009, 28, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Chommeloux, J.; Montero, S.; Franchineau, G.; Bréchot, N.; Hékimian, G.; Lebreton, G.; Le Guennec, L.; Bourcier, S.; Nieszkowska, A.; Leprince, P.; et al. Microcirculation Evolution in Patients on Venoarterial Extracorporeal Membrane Oxygenation for Refractory Cardiogenic Shock. Crit. Care Med. 2020, 48, e9–e17. [Google Scholar] [CrossRef] [PubMed]
- Kara, A.; Akin, S.; Dos Reis Miranda, D.; Struijs, A.; Caliskan, K.; van Thiel, R.J.; Dubois, E.A.; de Wilde, W.; Zijlstra, F.; Gommers, D.; et al. Microcirculatory assessment of patients under VA-ECMO. Crit. Care 2016, 20, 344. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akin, S.; Dos Reis Miranda, D.; Caliskan, K.; Soliman, O.I.; Guven, G.; Struijs, A.; van Thiel, R.J.; Jewbali, L.S.; Lima, A.; Gommers, D.; et al. Functional evaluation of sublingual microcirculation indicates successful weaning from VA-ECMO in cardiogenic shock. Crit. Care 2017, 21, 265. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Slagt, C.; Malagon, I.; Groeneveld, A.B. Systematic review of uncalibrated arterial pressure waveform analysis to determine cardiac output and stroke volume variation. Br. J. Anaesth. 2014, 112, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Tran, N.P.; Alexander, B.S.; Laning, K.; Chen, G.; Kain, Z.N.; Cannesson, M. The impact of phenylephrine, ephedrine, and increased preload on third-generation Vigileo-FloTrac and esophageal doppler cardiac output measurements. Anesth. Analg. 2011, 113, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Hamzaoui, O.; Monnet, X.; Richard, C.; Osman, D.; Chemla, D.; Teboul, J.L. Effects of changes in vascular tone on the agreement between pulse contour and transpulmonary thermodilution cardiac output measurements within an up to 6-hour calibration-free period. Crit. Care Med. 2008, 36, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Guglin, M.; Zucker, M.J.; Bazan, V.M.; Bozkurt, B.; El Banayosy, A.; Estep, J.D.; Gurley, J.; Nelson, K.; Malyala, R.; Panjrath, G.S.; et al. Venoarterial ECMO for Adults: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019, 73, 698–716. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.S. Conceptualizing Liberation From Venoarterial Extracorporeal Membrane Oxygenation. Circ. Heart Fail. 2022, 15, e009183. [Google Scholar] [CrossRef] [PubMed]
- Cheypesh, A.; Yu, X.; Li, J. Measurement of systemic oxygen consumption in patients during extracorporeal membrane oxygenation--description of a new method and the first clinical observations. Perfusion 2014, 29, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Makdisi, G.; Makdisi, T.; Wang, I.W. Use of distal perfusion in peripheral extracorporeal membrane oxygenation. Ann. Transl. Med. 2017, 5, 103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bonicolini, E.; Martucci, G.; Simons, J.; Raffa, G.M.; Spina, C.; Lo Coco, V.; Arcadipane, A.; Pilato, M.; Lorusso, R. Limb ischemia in peripheral veno-arterial extracorporeal membrane oxygenation: A narrative review of incidence, prevention, monitoring, and treatment. Crit. Care 2019, 23, 266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Charbonneau, F.; Chahinian, K.; Bebawi, E.; Lavigueur, O.; Lévesque, É.; Lamarche, Y.; Serri, K.; Albert, M.; Noly, P.E.; Cournoyer, A.; et al. Parameters associated with successful weaning of veno-arterial extracorporeal membrane oxygenation: A systematic review. Crit. Care 2022, 26, 375. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ortuno, S.; Delmas, C.; Diehl, J.L.; Bailleul, C.; Lancelot, A.; Naili, M.; Cholley, B.; Pirracchio, R.; Aissaoui, N. Weaning from veno-arterial extra-corporeal membrane oxygenation: Which strategy to use? Ann. Cardiothorac. Surg. 2019, 8, E1–E8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Puerto, E.; Tavazzi, G.; Gambaro, A.; Cirillo, C.; Pecoraro, A.; Martín-Asenjo, R.; Delgado, J.; Bueno, H.; Price, S. Interaction between VA-ECMO and the right ventricle. Hellenic J. Cardiol. 2022, 68, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Kawakami, S.; Murata, S.; Nishimura, K.; Tahara, Y.; Hosoda, H.; Nakashima, T.; Kataoka, Y.; Asaumi, Y.; Noguchi, T.; et al. Predicting Parameters for Successful Weaning from Veno-Arterial Extracorporeal Membrane Oxygenation in Cardiogenic Shock. ESC Heart Fail. 2021, 8, 471–480. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, T.Y.; Chang, C.H.; Hsu, J.Y.; Sheu, J.J.; Kuo, H.C.; Hsu, M.H.; Cheng, M.C.; Hsieh, K.S.; Lin, Y.J. Comparison of the predictive ability of lactate and central venous blood gas in pediatric venoarterial mode extracorporeal membrane oxygenation outcome. Pediatr. Neonatol. 2022, 63, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, Y.; Chen, X.; Song, Y.; Zhu, L.; Gong, X.; Zhang, H.; Xu, Z. Application of Near-Infrared Spectroscopy to Monitor Perfusion During Extracorporeal Membrane Oxygenation After Pediatric Heart Surgery. Front Med. 2021, 8, 762731. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duong, V.D.; Aludaat, C.; Kouadri, G.; Scherrer, V.; Clavier, T.; Demailly, Z.; Compère, V.; Rey, N.; Selim, J.; Besnier, E. Association Between Pulmonary Artery Pulsatility Index and Radial Artery Pulse Pressure and Successful Separation from Peripheral Veno-Arterial Extracorporeal Membrane Oxygenation: A French Single-Center Retrospective Study From 2017 to 2021. J. Cardiothorac. Vasc. Anesth. 2025, 39, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.J.; Wang, C.H.; Chan, W.S.; Huang, C.H.; Lai, C.H.; Wang, M.J.; Chen, Y.S.; Ince, C.; Lin, T.Y.; Yeh, Y.C. Microcirculatory Response to Changes in Venoarterial Extracorporeal Membrane Oxygenation Pump Flow: A Prospective Observational Study. Front Med. 2021, 8, 649263. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Møller, J.E.; Thiele, H.; Morrow, D.; Kjærgaard, J.; Hassager, C. Mechanical circulatory support: When, how, and for whom. Eur. Heart J. 2025, 46, 1480–1492. [Google Scholar] [CrossRef] [PubMed]
- Loyaga-Rendon, R.Y.; Acharya, D.; Kirklin, J.K. LVAD Implantation or Heart Transplantation for ECMO-Supported Patients. J. Am. Coll. Cardiol. 2020, 76, 2575–2576. [Google Scholar] [CrossRef] [PubMed]
- Toda, K.; Fujita, T.; Seguchi, O.; Yanase, M.; Nakatani, T. Role of percutaneous veno-arterial extracorporeal membrane oxygenation as bridge to left ventricular assist device. J. Artif. Organs. 2018, 21, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.; Singer Englar, T.; Cole, R.; Catarino, P.; Chang, D.; Czer, L.; Emerson, D.; Geft, D.; Kobashigawa, J.; Megna, D.; et al. Extracorporeal membrane oxygenation as a bridge to durable mechanical circulatory support or heart transplantation. Int. J. Artif. Organs. 2022, 45, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, J.R.; Pahwa, S.V.; Slaughter, M.S. Mechanical circulatory support for bridge to transplant therapy: Data on use and patient outcomes. Indian. J. Thorac. Cardiovasc. Surg. 2023, 39 (Suppl. S1), 3–7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Velleca, A.; Shullo, M.A.; Dhital, K.; Azeka, E.; Colvin, M.; DePasquale, E.; Farrero, M.; García-Guereta, L.; Jamero, G.; Khush, K.; et al. The International Society for Heart and Lung Transplantation (ISHLT) guidelines for the care of heart transplant recipients. J. Heart Lung Transplant. 2023, 42, e1–e141. [Google Scholar] [CrossRef] [PubMed]
- Rousse, N.; Juthier, F.; Pinçon, C.; Hysi, I.; Banfi, C.; Robin, E.; Fayad, G.; Jegou, B.; Prat, A.; Vincentelli, A. ECMO as a bridge to decision: Recovery, VAD, or heart transplantation? Int. J. Cardiol. 2015, 187, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.T.; Lerakis, S.; Delgado, V.; Addetia, K.; Burkhoff, D.; Muraru, D.; Pinney, S.; Friedberg, M.K. Multimodality Imaging of Right Heart Function: JACC Scientific Statement. J. Am. Coll. Cardiol. 2023, 81, 1954–1973. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tao, S.; Zhu, Y.; Gu, Q.; Ni, P.; Zhang, W.; Wu, C.; Zhao, R.; Hu, W.; Diao, M. AI-powered model for predicting mortality risk in VA-ECMO patients: A multicenter cohort study. Sci. Rep. 2025, 15, 10362. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deng, B.; Zhao, Z.; Ruan, T.; Zhou, R.; Liu, C.; Li, Q.; Cheng, W.; Wang, J.; Wang, F.; Xie, H.; et al. Development and external validation of a machine learning model for brain injury in pediatric patients on extracorporeal membrane oxygenation. Crit. Care 2025, 29, 17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, W.; Wu, J.; Zhang, Z.; Gao, Z.; Chen, X.; Zhang, Y.; Lin, Z.; Tang, Z.; Yu, W.; Fan, S.; et al. Artificial intelligence-assisted echocardiographic monitoring in pediatric patients on extracorporeal membrane oxygenation. Front. Cardiovasc. Med. 2024, 11, 1418741. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raissi-Dehkordi, N.; Raissi-Dehkordi, N.; Xu, B. Contemporary applications of artificial intelligence and machine learning in echocardiography. NPJ Cardiovasc. Health 2025, 2, 30. [Google Scholar] [CrossRef]
- Tsangaris, A.; Alexy, T.; Kalra, R.; Kosmopoulos, M.; Elliott, A.; Bartos, J.A.; Yannopoulos, D. Overview of Veno-Arterial Extracorporeal Membrane Oxygenation (VA-ECMO) Support for the Management of Cardiogenic Shock. Front. Cardiovasc. Med. 2021, 8, 686558. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hockstein, M.A.; Singam, N.S.; Papolos, A.I.; Kenigsberg, B.B. The Role of Echocardiography in Extracorporeal Membrane Oxygenation. Curr. Cardiol. Rep. 2023, 25, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Ezad, S.M.; Ryan, M.; Donker, D.W.; Pappalardo, F.; Barrett, N.; Camporota, L.; Price, S.; Kapur, N.K.; Perera, D. Unloading the Left Ventricle in Venoarterial ECMO: In Whom, When, and How? Circulation 2023, 147, 1237–1250. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, X.; Chen, B.; Hu, C. A tip for assessing blood flow in distal perfusion catheter during veno-arterial extracorporeal membrane oxygenation. Crit. Care 2025, 29, 13. [Google Scholar] [CrossRef] [PubMed]
- Cikes, M.; Solomon, S.D. Beyond ejection fraction: An integrative approach for assessment of cardiac structure and function in heart failure. Eur. Heart J. 2016, 37, 1642–1650. [Google Scholar] [CrossRef] [PubMed]
- Silva-Jr, J.M.; Menezes, P.F.L.; Lobo, S.M.; de Carvalho, F.H.S.; de Oliveira, M.A.N.; Cardoso Filho, F.N.F.; Fernando, B.N.; Carmona, M.J.C.; Teich, V.D.; Malbouisson, L.M.S. Impact of perioperative hemodynamic optimization therapies in surgical patients: Economic study and meta-analysis. BMC Anesthesiol. 2020, 20, 71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saxena, A.; Garan, A.R.; Kapur, N.K.; O’Neill, W.W.; Lindenfeld, J.; Pinney, S.P.; Uriel, N.; Burkhoff, D.; Kern, M. Value of Hemodynamic Monitoring in Patients with Cardiogenic Shock Undergoing Mechanical Circulatory Support. Circulation 2020, 141, 1184–1197. [Google Scholar] [CrossRef] [PubMed]
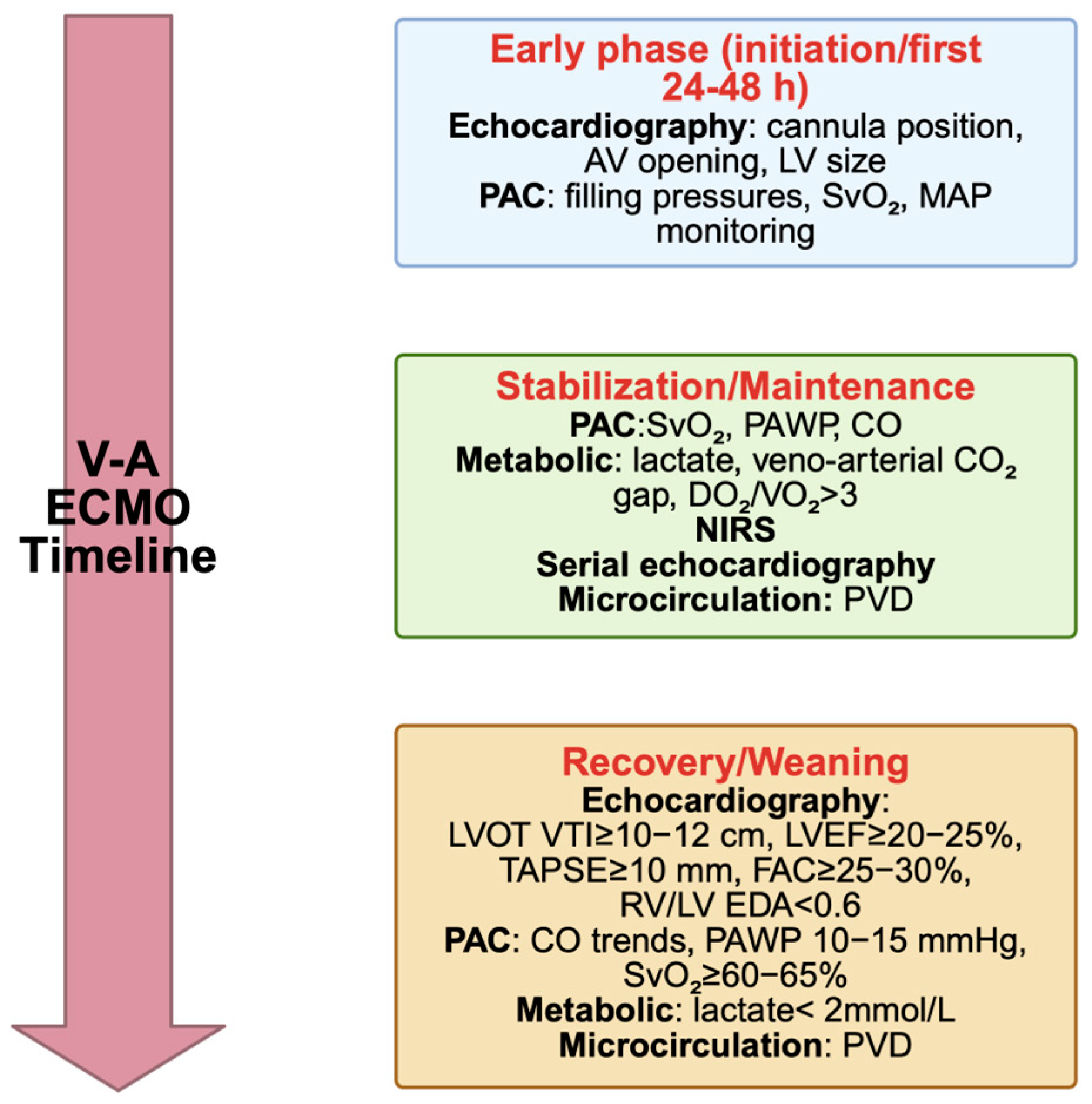

| Phase | Purpose of Echocardiography | Preferred Modality | Main Parameters | Limitations/Notes |
|---|---|---|---|---|
| Pre-implantation | Assess LV/RV function, exclude contraindications | TTE or TEE | LV and RV size and function, valvular competence, presence of thrombus | Limited by acoustic window in ventilated patients; TEE preferred in postoperative cases |
| During cannulation | Guide vascular access and confirm cannula positioning | TEE (preferred), TTE | Visualize guidewire and cannular tips in SVC/RA/aorta | Real-time ultrasound guidance to avoid vessel injury |
| During support | Monitor ventricular loading, LV distension, valve function, pericardial effusion | TTE or TEE | LVOT VTI, AV opening, MR, TAPSE, FAC, pericardial effusion | TTE limited by ventilation, dressings; TEE semi-invasive |
| Weaning trial | Assess myocardial recovery and readiness for decannulation | TEE or TTE | LVEF ≥ 20–25% LVOT VTI ≥ 10 cm Mitral S’ ≥ 6 cm/s RV performance adequate | Perform under low ECMO flow (<1.5 L/min). Requires stable anticoagulation |
| Post-decannulation | Detect vascular complications, residual dysfunction | TTE | Thrombus | Recommended follow-up after cannula removal |
| Domain | Parameter | Proposed Threshold Weaning | Notes |
|---|---|---|---|
| Left ventricular function | LVOT VTI | ≥10–12 cm | Reflects forward stroke volume; trend more relevant than single measure |
| LVEF | ≥20–25% | Low sensitivity alone; best if combined with LVOT VTI | |
| Right ventricular function | TAPSE | ≥10 mm | Associated with preserved RV systolic performance |
| FAC | ≥25–30% | Complementary to TAPSE; assesses global RV systolic function | |
| TDI S’ velocity | >6–10 cm/s | Reflects longitudinal RV systolic function | |
| RV/LV end diastolic area ratio | <0.6 | Marker of balanced ventricular loading | |
| Biventricular interaction | IV septal position and motion | Midline or mildly rightward; absence of paradoxical septal shift | Reflects interdependence; leftward shift indicates RV overload masking LV recovery |
| Coupled trends in LVOT VTI and PAPi | LVOT VTI ≥ 10 cm with PAPi > 1.5 | Suggests synchronized RV-LV recovery | |
| Invasive monitoring (PAC) | SvO2 | ≥60–65% | Reflects global oxygen balance; declining SvO2 suggests inadequate CO |
| PAWP | 10–15 mmHg | Avoid congestion while maintaining adequate preload | |
| Native CO (continuous thermodilution) | Rising trend during flow reduction | Suggest ventricular recovery and readiness for ECMO weaning | |
| Metabolic indices | Arterial lactate | <2 mmol/L, declining trajectory | Indicates adequate systemic perfusion and tissue oxygenation |
| Veno-arterial CO2 gap | <6 mmHg | Reflects adequate tissue perfusion and oxygen utilization | |
| Cerebral NIRS | Stable or improving values | Proxy of cerebral oxygenation; should be integrated with systemic data | |
| Microcirculation | Perfused vessel density (PVD)—sublingual | Improving/sustained values | Emerging tool; promising for predicting weaning success |
| Clinical Phenotype | Monitoring Priorities | Key Parameter/Tools |
|---|---|---|
| Predominant LV failure | Prevention of LV distension and optimization of unloading and weaning | Echo (LVEF, LV dimensions, AV opening, MR, LVOT VTI); PAC (PAWP, CO); markers of congestion; DO2/VO2 balance |
| Predominant RV failure | Assessment of RV systolic performance and pulmonary afterload | Echo (TAPSE, FAC, TDI S’, RV/LV EDA ratio); PAC (PAP, SvO2, PAPi); metabolic indices; response to vasodilators |
| Biventricular failure | Assessment of LV and RV performance | LVOT VTI, AV opening, RV and LV size, TAPSE, FAC, septal shift, pulmonary pressures, pulmonary congestion |
| Sepsis-related cardiogenic shock | Ensuring tissue-level perfusion despite normalized systemic hemodynamics | Lactate clearance, veno-arterial CO2 gap, NIRS, sublingual PVD (if available); DO2/VO2 ratio |
| Post-cardiotomy ECMO | Early recognition of myocardial stunning vs. irreversible dysfunction; bleeding and tamponade surveillance | Echo (pericardial effusion, biventricular function); PAC (filling pressures, CO); coagulation and lactate trends |
| Peripheral cannulation | Prevention of limb ischemia and monitoring of distal perfusion | Clinical exam; NIRS limb monitoring; DPC flow measurement (ultrasound or flowmeter); Doppler ultrasound |
| Harlequin syndrome | Detection of differential hypoxemia and distribution of oxygenated blood | Right radial arterial oximetry; echo (TEE to locate mixing zone); comparison with femoral PaO2/SaO2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torre, D.E.; Pirri, C. Beyond Standard Parameters: Precision Hemodynamic Monitoring in Patients on Veno-Arterial ECMO. J. Pers. Med. 2025, 15, 541. https://doi.org/10.3390/jpm15110541
Torre DE, Pirri C. Beyond Standard Parameters: Precision Hemodynamic Monitoring in Patients on Veno-Arterial ECMO. Journal of Personalized Medicine. 2025; 15(11):541. https://doi.org/10.3390/jpm15110541
Chicago/Turabian StyleTorre, Debora Emanuela, and Carmelo Pirri. 2025. "Beyond Standard Parameters: Precision Hemodynamic Monitoring in Patients on Veno-Arterial ECMO" Journal of Personalized Medicine 15, no. 11: 541. https://doi.org/10.3390/jpm15110541
APA StyleTorre, D. E., & Pirri, C. (2025). Beyond Standard Parameters: Precision Hemodynamic Monitoring in Patients on Veno-Arterial ECMO. Journal of Personalized Medicine, 15(11), 541. https://doi.org/10.3390/jpm15110541








