Abstract
Background/Objectives: Type 2 diabetes mellitus (T2DM) is a complex metabolic disorder characterized by insulin resistance, impaired insulin secretion, and chronic hyperglycemia. Recent studies have identified microRNAs (miRNAs), a class of small non-coding RNAs that regulate gene expression at the post-transcriptional level, as modulators of pathways involved in T2DM pathophysiology. Dysregulated miRNA expression has been detected in various samples collected from patients with T2DM, implicating these molecules in disease onset and progression. Methods: We systematically searched PubMed, Scopus, and Web of Science for studies published from the earliest available records to 18 August 2025 using the following Boolean search terms: “miRNA AND gliclazide”, “miRNA AND glibenclamide”, “miRNA AND gliquidone”, “miRNA AND glimepiride”, “mirRNA AND metformin”, “miRNA AND pioglitazone”, “miRNA AND rosiglitazone”, “miRNA AND sitagliptin”, “miRNA AND vildagliptin”, “miRNA AND alogliptin”, “miRNA and saxagliptin”, “miRNA AND linagliptin”, “miRNA AND liraglutide”, “miRNA and dulaglutide”, “miRNA AND semaglutide”, “miRNA AND tirzepatide”, “miRNA AND lixisenatide”, “miRNA AND empagliflozin”, “miRNA AND dapagliflozin”, miRNA AND insulin glargine”, “miRNA AND insulin detemir”, “miRNA AND insulin degludec”, “miRNA AND insulin aspart”, “miRNA AND insulin glulisine”, and “miRNA AND insulin lispro”. Additionally, gray literature was searched in ClinicalTrials.gov, the EU Clinical Trials Register (EudraCT), and the ISRCTN Registry to identify unpublished studies. Studies were eligible for inclusion if they were clinical interventional studies assessing the impact of currently available antidiabetic treatments on miRNA expression. Only articles published in English were considered. The risk of bias was evaluated using the RoB2 (Risk of Bias 2) and ROBINS-I (Risk Of Bias In Non-randomized Studies—of Interventions) tools. Study characteristics and major findings were tabulated. Results: A total of 1263 manuscripts was identified initially. After removing duplicates, 726 articles remained for further screening. Ultimately, 17 manuscripts reporting interventional clinical trials on the effects of antidiabetic treatment on miRNA were included, encompassing a total of 1093 patients. Key findings included treatment-associated changes in miRNA expression and their potential utility for the prediction of clinical outcomes. Conclusions: Current evidence supports the hypothesis that antidiabetic treatments modulate miRNA expression, with some findings showing predictive value for metabolic outcomes. However, the available data remain limited and of low grade of certainty, and further large-scale clinical studies are needed to provide deeper insights into these associations.
Keywords:
metformin; thiazolidindiones; DPP-4 inhibitors; GLP-1RAs; insulin; SGLT-2 inhibitors; miRNA 1. Introduction
Diabetes mellitus comprises a group of distinct chronic metabolic diseases with diverse—and in many cases not fully understood—etiologies, specific pathophysiological mechanisms, and diverse treatment approaches. Based on the differences in etiology and clinical characteristics, diabetes is currently classified into type 1 diabetes, type 2 diabetes, and gestational diabetes mellitus, along with other specific forms caused by genetic mutations, endocrine disorders, exocrine pancreatic disease, or medication use [1,2].
Diabetic complications may arise acutely or develop gradually, contributing to both short-term and long-term morbidity and mortality. Globally, in both developed and developing countries, chronic diabetic complications are the leading cause of blindness, renal failure, and non-traumatic lower limb amputations. Diabetes is acknowledged as a major risk factor for cardiovascular disease and stroke [3,4]. Maternal diabetes during pregnancy can result in adverse outcomes for the child [5,6]. In addition, diabetes is increasingly recognized as a risk factor for cognitive decline, functional disability, affective disorders, obstructive sleep apnea and liver disease [7].
The defining clinical characteristic of all types of diabetes is disturbed carbohydrate metabolism, leading to chronic hyperglycemia, whose severity is directly associated with the prevalence and progression of diabetic complications [1,8,9]. The mechanisms underlying hyperglycemia vary among the different types of diabetes and may involve impaired insulin secretion from the pancreas, reduced glucose utilization in tissues, increased hepatic gluconeogenesis and glycogenolysis, hormonal alterations during pregnancy, and other contributing factors [10,11,12]. Regardless of the specific underlying metabolic disturbance, the clinical diagnosis of diabetes is based on the laboratory evidence of elevated blood glucose levels and increased glycated hemoglobin (HbA1c) [1].
Today, type 2 diabetes mellitus (T2DM) is the most prevalent form of the disease, accounting for nearly 90% of patients worldwide [13]. Of further concern, according to the International Diabetes Federation, nearly 11% of the global adult population is diagnosed with diabetes [14]. The figure is still rising, particularly for type 2 diabetes [15].
Furthermore, type 2 diabetes mellitus is increasingly recognized as a heterogeneous group of disorders driven by diverse additional pathophysiological mechanisms. These additional contributing factors include age, obesity, and disordered eating, alterations in gut microbiota, impaired incretin secretion, and aberrant expression of sodium–glucose cotransporters, among others. Based on differences in the etiology of type 2 diabetes, some authors have proposed further subclassification of type 2 diabetes phenotypes at the time of diagnosis, including, but not limited to, mild obesity related phenotypes, mild age-related phenotypes, severe insulin-insufficient phenotypes, and severe insulin-resistant phenotypes [16]. In clinical practice, these phenotypes are further complicated by the presence of diabetic complications, which can substantially influence treatment strategies [17].
However, effective treatments are available, including patient education, empowerment, nutritional therapy, physical activity, and pharmacological interventions. The ultimate goals are normalization of blood glucose and the reduction in the risk of chronic diabetic complications, including cardiovascular and cerebrovascular disease. Contemporary therapies rely on a “treatment-to-target” approach, making diabetes management a highly individualized process. Given the wide spectrum of available pharmacotherapeutic agents, specific national and international guidelines provide recommendations for a structured treatment approach tailored to different patient phenotypes [18,19].
Nevertheless, long-term treatment outcomes remain unpredictable for individual patients and depend on factors such as genetics and epigenetic modifications, post-translational and other specific pathophysiologic mechanisms involved, and treatment availability and adherence [12,20,21,22,23]. Therefore, it is not surprising that many patients with type 2 diabetes mellitus receive suboptimal treatment, thereby increasing their risks of progression of diabetic complications, disability, and reduced life expectancy. The overall societal burden and costs of suboptimal diabetes treatment should not be overlooked. Current antidiabetic treatments are often resource-intensive and expensive, raising concerns about cost-effectiveness, equitable access, and the potential to exacerbate healthcare disparities [24,25,26]. Consequently, there is a pressing need to advance predictive and precision medicine approaches to optimize current treatment strategies, improve patient outcomes, and allocate healthcare resources more efficiently [27].
Recent studies on genetics and epigenetics in type 2 diabetes mellitus have provided valuable insights into interindividual variability in disease onset, progression, and treatment response [28,29,30]. However, it remains under debate whether this research can be fully translated into clinical practice in humans. Human genetic material has remained relatively stable throughout evolution, and changes may lead to evolutionary shifts. Consequently, the concept of gene therapy for type 2 diabetes raises not only technical but also ethical challenges [31]. Similarly, epigenetic modifications such as DNA methylation, histone modification and chromatin remodeling alter gene expression without changing the original DXA sequence. These modifications have been linked to impaired insulin secretion and increased insulin resistance, impaired glucose metabolism, and inflammation. Importantly, even relatively modest environmental factors like diet, overweight, and physical inactivity can induce epigenetic changes, explaining the link between lifestyle and disease progression [32,33]. However, certain epigenetic changes can also be transmitted to offspring, potentially influencing the disease risk across generations, and thus limiting the therapeutic potential of this approach. In addition, concerns about possible discrimination, patient re-identification, and unexpected findings may arise [34].
By contrast, microRNAs (miRNAs) are small non-coding RNAs that regulate gene expression post-transcriptionally, representing another layer of gene regulation. They target the messenger RNA and may induce its degradation or function loss, thereby modulating protein synthesis and gene expression [35]. Dysregulated miRNAs have been linked to impaired insulin secretion, insulin resistance, and chronic inflammation in type 2 diabetes [36,37]. Several miRNAs, including miR-375, miR-29, miR-34a, and miR-103/107, have been involved in pancreatic β-cell apoptosis, impaired insulin signaling, lipid metabolism abnormalities, and cardiovascular disease [38,39,40,41,42].
The therapeutic impact of miRNAs in type 2 diabetes mellitus could encompass several distinct approaches. Some agents could act as miRNA antagonists (also called antagomirs, or miRNA inhibitors), which inhibit the activity of overexpressed deleterious miRNAs. Others may act as miRNA agonists or mimics (agomirs), restoring the function of beneficial miRNAs and thereby reestablishing physiological regulation of glucose metabolism [43]. In addition, miRNA activity can be modulated by already existing antidiabetic therapies, suggesting that at least a part of the therapeutic effect of currently prescribed antidiabetic drugs may be mediated through alterations in miRNA expression [44].
These directions could imply a novel approach to diabetes care, as novel biomarkers for early diagnosis, risk stratification, and prediction of treatment response are actively investigated, and they may contribute to the development of personalized therapeutic strategies in type 2 diabetes. However, current treatments should not be overlooked, as a growing body of evidence suggests that at least some of these agents possess miRNA modulatory properties, which may partly mediate their clinical effects.
This systematic review aims to evaluate the current evidence regarding the impact of contemporary drug treatments for type 2 diabetes mellitus on miRNA expression, with a particular focus on the potential, yet not fully understood, role of miRNA in modern diabetes pharmacotherapy.
2. Materials and Methods
In preparation for this systematic review, the guidelines specified in the PRISMA2020 statement: An updated guideline for reporting systematic reviews were adhered to [45]. This review is registered in the PROSPERO database (PROSPERO registration number: 2025 CRD420251140020).
2.1. Eligibility Criteria
Studies eligible for inclusion in this review had to be interventional clinical studies on patients with type 2 diabetes that reported the effects of specific currently approved antidiabetic drugs on miRNAs. Only articles published in English were considered.
Manuscripts were excluded if they did not report original research on pharmacological interventional studies on patients with type 2 diabetes. Studies that focused on other types of diabetes were also excluded. Furthermore, conference papers and proceedings, preclinical studies, reviews, editorials, commentaries, letters, notes, errata, retractions, and case reports were not included.
2.2. Search Strategies
We conducted a comprehensive search for interventional clinical studies investigating the effects of antidiabetic drugs on miRNAs.
The search used the following Boolean terms: “miRNA AND gliclazide”, “miRNA AND glibenclamide”, “miRNA AND gliquidone”, “miRNA AND glimepiride”, “miRNA AND metformin”, “miRNA AND pioglitazone”, “miRNA AND rosiglitazone”, “miRNA AND sitagliptin”, “miRNA AND vildagliptin”, “miRNA AND alogliptin”, “miRNA and saxagliptin”, “miRNA AND linagliptin”, “miRNA AND liraglutide”, “miRNA and dulaglutide”, “miRNA AND semaglutide”, “miRNA AND tirzepatide”, “miRNA AND lixisenatide”, “miRNA AND empagliflozin”, “miRNA AND dapagliflozin”, miRNA AND insulin glargine”, “miRNA AND insulin detemir”, “miRNA AND insulin degludec”, “miRNA AND insulin aspart”, “miRNA AND insulin glulisine”, and “miRNA AND insulin lispro”. The search was performed in PubMed, Scopus, and Web of Science. Additionally, gray literature was searched in ClinicalTrials.gov, the EU Clinical Trials Register (EudraCT), and the ISRCTN Registry to identify unpublished studies. Only trials with posted or publicly available results were considered for inclusion.
Manuscripts published from the earliest available records through 18 August 2025 were included, with no filters or limits applied.
2.3. Study Selection and Data Extraction
The eligibility of articles was independently evaluated by two reviewers according to predefined inclusion criteria, specifically the reporting of effects of non-insulin antidiabetic drugs on miRNA. Along with outcome relevance, data on patient characteristics and follow-up length were extracted. Any discrepancies between reviewers were resolved through discussion.
Finally, all titles and abstracts meeting the inclusion criteria were retrieved for full-text review. No automation tools were employed in the selection process.
2.4. Risk of Bias Assessment
The risk of bias was independently determined by two reviewers using the Risk Of Bias 2 (RoB2) tool for randomized clinical trials (RTCs) and the Risk Of Bias In Non-randomized Studies—of Interventions (ROBINS-I) tool for non-randomized studies. A detailed description of these tools is available elsewhere [46,47].
2.5. Data Synthesis
To assess eligibility for data synthesis, the intervention characteristics of each study were first tabulated, including specific details as drug type administered, population characteristics and follow-up duration.
In cases when summary statistics were missing or incomplete, relevant data were systematically extracted from tables, figures, or supplementary materials to ensure comprehensive inclusion in the analysis.
Results from individual studies and syntheses were organized into summary tables to facilitate cross-study comparison. Key variables, including intervention type, outcome measures, and primary findings, were systematically tabulated. Because accurate measurement of miRNAs requires rigorous analytical and reporting standards to ensure reproducibility and clinical relevance, adherence to current guidelines for publication of quantitative real-time PCR experiments was also evaluated and summarized [48]. The results were calculated as the sum of the scores assigned to each MIQE 2.0 category (0 = non-compliant, 0.5 = partially compliant, 1 = fully compliant), divided by the maximum possible score and multiplied by 100%. Finally, the strength of evidence was assessed using the GRADE system [49].
All steps in data synthesis were performed independently by two reviewers, with any discrepancies resolved through discussion.
3. Results
3.1. Study Selection
A total of 1263 articles were identified during the search through prespecified databases (PubMed, n = 544; Scopus, n = 456; Web of Science, n = 263). After removing duplicate manuscripts, 726 articles were screened. Articles written in languages other than English were excluded, as well as editorials, book chapters, errata and retracted articles, letters, perspectives, meeting abstracts and proceedings, study protocols, and reviews. After these exclusions, 523 studies on miRNA and various antidiabetic agents remained. Next, preclinical studies (n = 402), clinical studies not involving patients with type 2 diabetes (n = 70), and non-interventional clinical studies on patients with type 2 diabetes (n = 34) were excluded. No additional eligible studies were found in the gray literature search. Ultimately, 17 interventional studies on patients with type 2 diabetes investigating the effect of various antidiabetic drugs on miRNA were identified for full-text review. All these manuscripts were retrieved for detailed assessment. Figure 1 presents the flow diagram describing the study selection process.
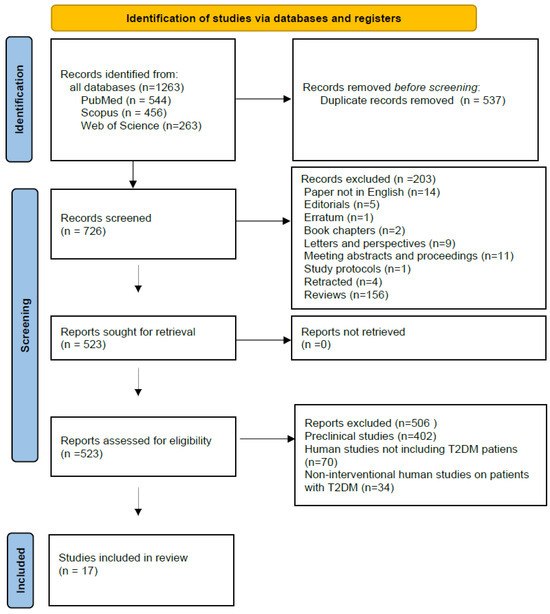
Figure 1.
Flow chart of the sequence of steps in the collection and selection of qualified studies (PROSPERO registration number: 2025 CRD420251140020).
3.2. Study Characteristics
The main finding from the search results is the limited number of relevant evidence-based studies. This may be explained by the relatively recent discoveries and technological advances, considering miRNAs that have only recently enabled such research. In contrast to the abundance of preclinical studies, clinical research in this field remains scarce.
We identified 17 interventional clinical studies investigating the effects of various antidiabetic drugs on miRNA expression in patients with T2DM (Cohen’s κ ≈ 0.91 for inter-rater agreement). Among these, three analyzed the impact of metformin, three evaluated dipeptidyl dipeptidase 4 (DPP-4) inhibitors, three examined thiazolidinediones, six assessed glucagon-like peptide 1 receptor agonists (GLP-1 RAs), three focused on sodium glucose cotransporter 2 (SGLT-2) inhibitors, and one study investigated intensified insulin treatment. In addition, two studies used sulfonylureas as comparators. Notably, some studies included multiple treatment arms investigating different antidiabetic drugs.
The study populations in these studies were diverse. While most of these studies included patients with T2DM without specified comorbidities or complications, some research focused on T2DM patients with arterial hypertension (n = 1), heart failure with preserved ejection fraction (HFpEF) (n = 1), coronary artery disease (CAD) (n = 2), non-alcoholic fatty liver disease (NAFLD) (n = 1), and diabetic kidney disease (n = 1).
Across the 17 included studies, all analyses used real-time quantitative polymerase chain reaction (RT-qPCR)-based platforms, with two studies also employing NanoString for broader profiling. The predominant biological sources were serum or plasma (n = 13), with fewer studies analyzing adipose tissue (n = 2), whole blood (n = 1), urine (n = 1) or extracellular vesicle fractions (n = 1).
Normalization strategies varied considerably among the included studies. Four studies did not report any normalization method. Two studies applied global mean normalization across profiled targets, while spike-in controls with quantile normalization were used in one study. Multiple endogenous reference miRNAs were employed in one study, and single endogenous controls—including miR-191-5p and U6 snRNA—were reported in two studies. NanoString internal normalization was applied in one study, and two studies used exogenous spike-in controls (cel-miR-39). Furthermore, for most studies, hemolysis control data were not provided. Only 1 study explicitly reported hemolysis assessment via visual inspection and evaluation of hemolysis-sensitive miRNAs, while another study applied a ratio-based molecular control. However, hemolysis assessment was not necessary for urine or tissue samples that did not contain blood.
Adherence to MIQE and MIQE 2.0 reporting standards was heterogeneous. The median MIQE 2.0 adherence across the 17 studies was 70% with a range from 35% to 90%. Sixteen of seventeen reports included information on basic methods but lacked substantial details on assay validation, pre-amplification, and reproducibility. Only three studies documented amplification efficiency and dynamic range; two reported intra-assay and inter-assay coefficients of variation. None provided data availability statements or raw Cq values, which are now recommended under MIQE 2.0 to facilitate reproducibility and transparency.
Collectively, these findings indicate that while fundamental analytical procedures are typically described, reporting of validation, normalization justification, and reproducibility metrics remains suboptimal, underscoring the need for stricter adherence to updated MIQE 2.0 guidelines in circulating and tissue miRNA studies.
Last, but not least, a variety of miRNAs were analyzed across studies, with diverse outcome measures reported.
Key characteristics and findings of the included studies are provided in Table 1.

Table 1.
Summary of interventional studies on miRNA in T2DM patients.
3.3. Risk of Bias in Studies
A critical appraisal of the quality of the selected articles was conducted using the Risk Of Bias 2 (RoB2) tool for randomized trials and the ROBINS-I tool for non-randomized studies.
Among the 17 included studies, six randomized controlled trials were evaluated using RoB2 and demonstrated mainly low-to-moderate risk of bias, primarily due to incomplete blinding or relatively short study duration. Of the remaining 11 observational studies, assessed with ROBINS-I, seven exhibited a predominantly moderate risk of bias. The most frequent concerns involved small sample sizes, confounding, selection bias, and the lack of a control group. Serious risk of bias was identified in four studies, particularly those with small sample sizes, no control group, selective outcome reporting, or inadequate adjustment for clinical covariates.
Overall, the body of evidence is at moderate-to serious risk of bias, suggesting findings should be interpreted with caution. The generalizability of the results may also be limited due to potential genetic differences among populations from different countries. Furthermore, the clinical applicability of these findings remains uncertain. Additionally, these studies focused primarily on patients with type 2 diabetes without chronic complications, while other patient populations, such as those with cardiovascular or renal complications, were underrepresented.
The quality assessment outcomes of each included research are presented in Table 2.

Table 2.
Risk of Bias evaluation of interventional studies on miRNA in T2DM patients.
Since no additional studies were identified in the gray literature search, publication bias could not be formally assessed due to the absence of unpublished trials.
3.4. Results of Individual Studies
According to the analyzed studies, currently available standard antidiabetic treatments do affect circulating miRNAs, and the impact varies by drug class. This suggests epigenetic modulations of glucose metabolism and potential other, yet unidentified, mechanisms of action. However, there have been differences between studies in terms of study design, population characteristics, and follow-up periods. In some studies, surrogate clinical outcomes were analyzed [50,53,54,58,59,61,65].
With respect to specific drugs and drug classes, metformin has been associated with a broad downregulation of multiple miRNAs. In contrast, studies on thiazolidinediones, DDP-4 inhibitors, GLP-1Ras, SGLT-2 inhibitors, and insulin have yielded mixed results regarding miRNA upregulation or downregulation [50,51,53,54,55,56,58,59,60,63,64,65,66]. Furthermore, research on the effects of thiazolidinediones, SGLT-2 inhibitors, GLP-1 Ras, and insulin treatment on miRNAs has also investigated associated clinical benefits [50,53,58,59,64,65]. In some studies, the predictive value of baseline levels of specific miRNAs for various clinical outcomes was also investigated [50,52,53,58,59,61,62,64,65].
Patient characteristics like age and comorbidities (CAD, NAFLD, HFpEF) may also influence which miRNAs are responsive; for instance, youth-onset vs. adult-onset T2DM is associated with different predictive markers [62].
The main findings of each research are presented in Table 1.
3.5. Strength of Evidence
The strength of the evidence was formally evaluated using the GRADE system and summarized for each drug and drug class. The details are provided in Table 3.

Table 3.
GRADE assessment of evidence for the effects of antidiabetic drugs on miRNA expression.
For the various drugs, the strength of evidence ranged from low to low-to-moderate. The main limitations of the studies included in this review were the relatively small number of randomized controlled trials (RCTs) with short study durations, a relatively high risk of bias, inconsistent endpoints, and limited clinical correlation.
4. Discussion
The interventional clinical studies included in this review suggest an emerging role of miRNAs as potential predictors of T2DM progression and treatment response. They also provide new insights into the mode of action of currently available treatments. Across diverse drug classes, different miRNAs have been associated with the age of T2DM onset, disease severity, and responsiveness to specific drug classes, highlighting their potential utility in precision and predictive medicine in T2DM. However, substantial heterogeneity in study designs, patient populations, study drugs, as well as in the assays and matrices employed, precluded the conduct of a formal meta-analysis.
4.1. Metformin
Metformin has been the undisputed first-line therapy for T2DM for decades, although it has recently been challenged by newer drug classes like SGLT-2 inhibitors and GLP-1 RAs. Nevertheless, it remains effective, inexpensive, with a good safety profile, and widely used. Its impact likely extends beyond glycemic control, offering benefits in cardiovascular prevention and—albeit to a lesser extent—potentially in oncology, contributing to longevity [67,68,69].
A potential mechanism underlying these effects is metformin’s impact on miRNA expression. Demirsoy et al. demonstrated that several miRNAs, namely let-7e-5p, let-7f-5p, miR21-5p, miR-24-3p, miR-26b-5p, miR-126-5p, miR-129-5p, miR-130b-3p, miR-146a-5p, miR-148a-3p, miR-152-3p, miR-194-5p, miR99a-5p, were downregulated after metformin administration in treatment-naïve T2DM patients. Clinical correlates were not analyzed [51].
However, at least some of these downregulated miRNAs have been suggested to play roles in carbohydrate metabolism and diabetic complications, based on findings from a limited number of animal and human studies. For instance, miRNA let-7e-5p modulates basal GLP-1 secretion [70], miR-26b-5p and miR-148a-3p may contribute to the development of chronic diabetic complications [71,72]. In addition, miR-146a-5p and miR-152-3p are involved in inflammation [73,74]. However, not all miRNAs downregulated by metformin have a clearly defined mechanistic role in diabetes control. Nevertheless, numerous studies link these miRNAs to other effects of metformin, including prevention of muscle atrophy, modulation of cell apoptosis, as well as with improvements in cardiovascular and cancer outcomes [75,76,77,78,79,80,81,82]. Intriguingly, neuropathy, a side effect typically attributed to metformin–induced vitamin B 12 deficiency, may at least in part be explained by the downregulation of miR-130b [83].
Although the clinical studies discussed in this review are encouraging and can be interpreted in the context of previous research, the overall certainty of evidence on the impact of metformin on miRNA expression is low, based on three observational studies that carry a moderate to serious risk of bias due to potential confounding, selective reporting, and missing data.
Nevertheless, further studies with a long follow-up period are needed to establish whether therapeutic modulation of these miRNA shifts translates into predictable clinical outcomes.
4.2. GLP-1 RAs
Evidence regarding another class of antidiabetic drugs, GLP-1Ras, is more heterogeneous. Nevertheless, these drugs have been in clinical use for a couple of decades and have demonstrated beneficial effects beyond glucoregulation. They have been shown to have cardioprotective effects and a positive influence on renal function in patients with T2DM, as well as in other patient groups. In addition, some of these drugs are also approved for obesity treatment [9,69,84].
Formichi et al. reported that baseline expression of miR-21-5p, miR-24-3p, miR-223-3p, and miR-375-5p predicted favorable glycemic outcomes of GLP-1 RA treatment, while miR-15a-5p was associated with weight reduction [58]. Other evidence has confirmed the involvement of miR-24-3p, miR-223-3p, and miR-375-5p in glucose regulation, insulin secretion and insulin resistance [85,86,87]. In contrast, miR-21-5p has been linked to the angiogenesis [88]. The association between miR-15a-5p and obesity has also been supported by previous evidence [89].
Al Zamily observed upregulation of miR-146a and miR-222 following liraglutide treatment, both of which were negatively correlated with HbA1c and fasting glucose, alongside downregulation of miR-21 [64]. All of these mRNAs have been previously described as important regulators of blood glucose, both in preclinical and clinical settings [90,91,92].
Similarly, Giglio et al. found liraglutide increased circulating miR-27b, miR-130a, and miR-210, independently of metabolic parameters, while Liu et al. showed reductions in miR-203a-3p and miR-429, potentially contributing to cardiovascular protection [55,63]. The role of miR-27b remains uncertain, as it has not been linked to glucose regulation and diabetic complications in other research [93]. In contrast, the role of miR-130a appears more promising. Evidence suggests beneficial effects of this miRNA on vascular health, aligning with the overall positive vascular effects of liraglutide [94]. In addition, miRNA 203a-3p and miRNA 429 are involved in inflammatory pathways, suggesting a potential role in glucose regulation [95,96]. In contrast, Gaborit et al. and Iacobellis et al. found no significant changes in circulating miRNA with liraglutide [56,66]. These discrepancies may reflect differences in treatment duration, endpoints, or sample types, highlighting the need for larger and more standardized trials.
Although the evidence on GLP-1 RAs is limited and fragmented, it provides fresh insight that expands previous knowledge of the mode of action and clinical impacts of these agents. A substantial limitation is that the impact of weekly GLP-1 RAs with proven cardiovascular benefits on miRNA expression has been investigated in only a single observational study, and neither semaglutide nor tirzepatide has yet been studied.
Conclusively, this review includes six studies on GLP-1 RAs (two of which are randomized controlled trials); however, the mixed directionality of miRNA changes, inconsistent endpoints, and indirect evidence for clinical translation support only a low overall certainty of evidence.
4.3. SGLT2 Inhibitors
SGLT-2 inhibitors are a relatively new class of antidiabetic drugs that exert effects beyond glucose regulation. Similar to GLP-1RAs, they have demonstrated cardioprotective properties and beneficial effects on renal function [9,69].
SGLT2 inhibitors also appear to consistently modulate miRNAs linked to inflammation and vascular function. Pehlivan et al. reported that dapagliflozin reduced miR-21, miR-141, and miR-377, with baseline miR-21 correlating with HbA1c reduction [65]. The clinical effect of these miRNA changes can contribute to improved beta cell function and inhibited hepatic gluconeogenesis, as previously described [92,97]. Although the role of miRNA-141 has been implicated in diabetes control, such a correlation was not confirmed in this study [98,99]. However, evidence from other research suggests that miR-377 exerts a positive effect on the heart and blood vessels [100,101,102,103].
Solini et al. observed upregulation of miR-30e-5p and downregulation of miR-199a-3p following dapagliflozin treatment, although without detectable vascular functional improvements [54]. Pathways mediated by miR-30e-5p are thought to be protective against diabetic nephropathy, a finding supported by animal studies, but not confirmed clinically in this research [102]. Furthermore, miRNA-199a-3p has been implicated in the pathogenesis of chronic diabetic complications [104]. Mone et al. demonstrated that empagliflozin reduced miR-21 and miR-92, both associated with endothelial dysfunction, suggesting potential vascular protective effects beyond glucose lowering [60,105,106].
Taken together, these studies indicate that SGLT2 inhibitors may provide epigenetic benefits relevant to both metabolic and cardiovascular health. The overall level of certainty remains low, although there is an RTC and two observational studies. However, the open-label design of the RCT and the serious risk of bias in the observational studies resulted in a low overall certainty of evidence.
4.4. Thiazolidinediones
Thiazolidinediones are a class of antidiabetic agents with evidence of improving vascular health. However, their clinical use may be limited by fluid retention, weight gain, heart failure, and peripheral bone fractures. They have also been shown to be effective in the treatment of NAFLD [107,108].
Pioglitazone has been shown to consistently influence circulating miRNA expression. Nunez Lopez et al. reported significant downregulation of multiple miRNAs, including miR-7-5p, miR-20a-5p, and miR-374b-5p, in parallel with improvements in HbA1c and insulin resistance [59]. The potential role of miR-7-1-5p as a marker for treatment response in type 2 diabetes remains under investigation, since some research claims that this mRNA is downregulated in patients with T2DM compared to healthy subjects [109]. In contrast, miR-20a-5p has been associated with insulin resistance, whereas circulating exosomal miR-20b-5p is elevated in type 2 diabetes and may impair insulin action in human skeletal muscle, suggesting that downregulation with pioglitazone could be clinically beneficial [110]. Interestingly, downregulation of miR-374b-5p has been linked to heart failure, a finding that was also noticed in this study [111]. Changes in miR-92a-3p appear non-specific, as it is also downregulated in patients treated with metformin [112].
Another miRNA implicated in vascular health is miR-195-5p, which facilitates endothelial dysfunction by inhibiting vascular endothelial growth factor A. Its downregulation by pioglitazone may therefore exert protective effects with clinical relevance [113].
Hong et al. reported increases in circulating miR-24, associated with reduced coronary neointimal hyperplasia and improved endothelial function [50]. These findings suggest that TZDs modulate both metabolic and vascular pathways through miRNA regulation, providing a mechanistic explanation for their vascular effects. Evidence shows that miR-24 appears protective against vascular complications [114].
Intriguingly, Redling et al. identified elevated miRNA-122-5p levels and low miRNA-431-5p and miRNA let-7g-5p levels as predictive markers for thiazolidindione treatment failure in young patients [62].
Collectively, these findings suggest that thiazolidinediones modulate both metabolic and vascular pathways through miRNA regulation, providing also a mechanistic explanation for their vascular effects. Although some miRNA effects appear context-dependent, the evidence highlights the potential of circulating miRNAs as both predictive biomarkers and mediators of TZD action. The overall certainty of evidence for this drug class remains low-to-moderate due to potential confounding, selective reporting, and missing data, while the RCT raised some concerns related to randomization and its open-label design.
4.5. DPP-4 Inhibitors
DPP-4 inhibitors are a class of antidiabetic agents with a favorable safety profile. In addition to improving glycemic control, they have been associated with reductions in albuminuria in patients with diabetes. To date, however, they are not considered to have cardioprotective effects [69,115].
Data on the effects of DPP-4 inhibitors on miRNA expression are mixed. Catanzaro et al. found that miR-126-3p and miR-223 were associated with favorable responses to sitagliptin, while miR-378 was linked to poor responses in elderly patients [52]. Interestingly, in other research high miR-126-3p levels were associated with cardiovascular events in a general population [116]. In diabetic nephropathy, miR-223 is downregulated, and miR-223-3p has been shown to mediate the diabetic kidney disease progression by targeting IL6ST/STAT3 pathway, indicating protective mechanisms against diabetic kidney disease [117,118]. Furthermore, overexpression of miR-378 is associated with low adiponectin levels, suggesting a potential mechanism for increased insulin resistance that may reduce the treatment response [119].
In contrast to Catanzaro, Cho et al. observed no significant urinary exosomal miRNA differences between patients on DPP-4 inhibitors and those on sulfonylureas, except for overexpression of miR-23a-3p in type 2 diabetic patients compared with healthy volunteers [57]. These discrepancies may reflect differences in biological sample types (serum vs. urine), populations studied, or treatment durations.
Similarly, Tain et al. reported that miR-210 overexpression negatively correlated with metabolic control [61]. Intriguingly, miR-210 may have deleterious effects on endothelial progenitor cells in newly diagnosed type 2 diabetes [120], compounding the harmful impact of hyperglycemia and endothelial function [121]. However, other studies found no difference in miR-210 expression between treatment groups [122].
In summary, while DPP-4 inhibitors show benefits on glycemic control and albuminuria, their effects on miRNA profiles are variable and widely not understood. Three studies have been identified (one of which is an RCT); however, the lack of blinding and selective reporting lowered the overall certainty of evidence.
4.6. Insulin Therapy
Insulin remains an important antidiabetic agent. Among patients with T2DM, it is indicated for those with reduced insulin secretion to improve glucose control and decrease the risk of acute hyperglycemic diabetic complications [123].
Insulin itself can reshape the miRNA landscape. Nunez Lopez et al. demonstrated that baseline miR-145-5p and miR-29c-3p predicted glycemic response to short-term intensive insulin therapy [53]. miR-29c-3p has been associated with hyperglycemia, renal fibrosis, and apoptosis. In vitro, the lncRNA TUG1/miR-29c-3p/SIRT1 axis regulates endoplasmic reticulum stress-mediated injury in renal epithelial cells in a diabetic nephropathy model [124]. Similarly, overexpression of miR-145 has been linked to diabetic complications [125]. Furthermore, responders to insulin therapy in the mentioned study exhibited longitudinal changes in miR-138-5p, miR-192-5p, and miR-320b, which correlated with improved β-cell function and insulin sensitivity [50]. This suggests that miRNAs not only predict insulin response but also serve as dynamic markers of treatment-driven pathophysiological adaptation. miR-138-5p has been reported to decrease insulin secretion, with obesity-induced adipocytes promoting diabetes through exosomal miR-138-5p-mediated regulation of β-cell function [126]. miR-192-5p is involved in diabetic retinopathy, as its upregulation inhibits the ELAVL1/PI3Kδ axis and attenuates microvascular endothelial cell proliferation, migration, and angiogenesis [127]. Other investigated miRNAs also highlight cardiovascular and microvascular risks. Downregulation of miR-195-5p inhibits endothelial-mesenchymal transition and myocardial fibrosis in diabetic cardiomyopathy by targeting Smad7 and the TGF-β1/Smads/Snail pathway [128]. Low serum miR-320b has been identified as a novel indicator of carotid atherosclerosis [129]. Additionally, let-7a-5p has been implicated in diabetic retinopathy through miRNA-mRNA regulatory networks [130].
In conclusion, insulin therapy improves glycemic control and modulates multiple miRNAs, which may serve as predictive biomarkers and mechanistic mediators of metabolic, microvascular, and cardiovascular adaptations in T2DM. However, with only a single observational study available, the overall certainty of evidence remains low due to a serious risk of bias, primarily arising from post hoc classification of responders and non-responders and potential confounding, despite the use of rigorous laboratory and statistical methods.
4.7. Other Therapies
Additional therapies have also been shown to influence circulating miRNAs. Tian et al. and Cho et al. used sulfonylureas as comparators in their studies [57,61]. Although some changes in miRNA expression were observed, these differences were not significant compared with the comparator groups. Overall, while additional antidiabetic therapies may modulate circulating miRNAs, current evidence suggests that these effects are modest and not consistently different from those observed with standard comparator treatments such as DPP-4 inhibitors. Therefore, the overall certainty of evidence remains low.
4.8. Overview of miRNAs Affected by Currently Approved Diabetes Drugs
A brief overview summarizing the specific miRNAs affected by diabetes pharmacotherapy and their biological effects is presented in Table 4.

Table 4.
Predominant miRNAs, canonical functions and clinical links for analysed drug classes.
5. Future Directions
The findings presented in this review highlight miRNAs as both promising biomarkers and potential therapeutic targets in T2DM management. Nonetheless, several critical steps are required to translate these early insights into clinical practice.
Robust, multi-center, randomized, and longitudinal studies with standardized methodologies are essential to confirm the predictive and prognostic value of specific miRNAs across diverse populations.
Furthermore, large interventional studies are needed to clarify the mechanistic role of individual miRNAs in mediating drug effects. While associations between miRNA expression and glycemic or cardiovascular outcomes have been described, causal links remain uncertain. Preclinical models and integrative approaches combining transcriptomics, proteomics, and metabolomics could help delineate whether miRNA modulation actively drives therapeutic benefits or merely reflects treatment response.
Finally, miRNAs may represent therapeutic targets. Antagomirs or miRNA mimics directed against disease-relevant pathways could provide novel adjunctive treatments for T2DM, particularly in individuals with suboptimal responses to existing therapies. Future clinical trials should explore whether modifying miRNA expression enhances treatment efficacy or reduces long-term complications.
6. Strengths and Limitations
The evidence summarized in this review offers notable strengths. The inclusion of interventional clinical studies provides valuable insights into causal relationships between glucose-lowering therapies and miRNA modulation, moving beyond purely observational associations. By analyzing a wide range of therapeutic classes—including metformin, GLP-1 receptor agonists, SGLT2 inhibitors, TZDs, DPP-4 inhibitors, and insulin—the reviewed literature highlights both shared and drug-specific miRNA signatures. This breadth allows comparison across different mechanisms of action and suggests that miRNA regulation may serve as a common pathway through which drug classes exert metabolic and other clinical benefits. Additionally, several studies explored longitudinal changes in miRNA expression, strengthening the case for their utility as dynamic biomarkers that reflect treatment response rather than static indicators of disease.
Despite these promising findings, important limitations temper the conclusions. Most studies were limited by small sample sizes, reducing statistical power and generalizability. Considerable heterogeneity in patient populations, disease duration, and comorbidities further complicates comparisons across studies. Methodological variability—including differences in biological sample types (plasma, serum, urine) may introduce significant bias and may explain inconsistent results. Moreover, the relatively short duration of most studies makes it difficult to determine whether observed miRNA changes translate into long-term clinical benefits. Another limitation is the lack of mechanistic validation; while many miRNAs have been associated with treatment response, their direct role in mediating drug effects remains unclear. In addition, possible language bias needs to be addressed, as four additional interventional studies in other languages were identified (three in Chinese and one in Ukrainian) [131,132,133,134]. Although the overall results were statistically significant, these studies were relatively small, recruiting a total of 137 participants with T2DM, of whom 66 received the study drug. The study drugs included metformin, dapagliflozin, and insulin, while various miRNAs were analyzed (miR-423-5p, hsa-miR-29a-3p, hsa-miR-133a-5p, hsa-miR-21-5p, hsa-miR-30c-5p, hsa-miR-1-3p, miR-let-7b-5p, miR-182-5p, miR-200c-3p, and miR-126) in different patient populations, including patients with T2DM with heart failure, and chronic kidney disease. Only a single trial was a randomized clinical trial. Compared with the English-language studies, these non-English studies had a lower proportion of randomized controlled trials and smaller sample sizes. The interventions and outcomes assessed in the English studies were largely similar, and no contradictory findings were observed.
7. Conclusions
Current evidence for personalized pharmacotherapy in type 2 diabetes remains limited and heterogeneous. Although patterns are emerging—such as the vascular-protective signature of SGLT2 inhibitors, the metabolic and cardiovascular effects of TZDs, and predictive roles for GLP-1RA and insulin responses—most available studies are small and exploratory, and vary considerably in study design, outcomes, and patient populations.
Progress toward clinical translation will require standardized profiling methods, longer follow-up periods, and larger patient cohorts.
Taken together, while current evidence demonstrates a low to low-to-moderate level of certainty, it nonetheless provides a rationale for further research. Robust, large-scale, and well-controlled clinical studies with standardized methodology are required before miRNAs can be integrated into routine precision medicine for T2DM.
In summary, miRNAs represent an emerging field with potential to reshape precision medicine in diabetes care. Realizing this promise will depend on coordinated translational efforts that integrate mechanistic studies, biomarker validation, and well-designed interventional trials.
Author Contributions
Conceptualization, V.A. and J.M.R.; methodology, V.A.; validation, V.A. and J.M.R.; formal analysis, V.A. and J.M.R.; investigation, V.A.; writing—original draft preparation, V.A.; writing—review and editing, V.A. and J.M.R.; visualization, V.A. and J.M.R.; supervision, V.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- ElSayed, N.A.; McCoy, R.G.; Aleppo, G.; Balapattabi, K.; Beverly, E.A.; Briggs Early, K.; Bruemmer, D.; Ebekozien, O.; Echouffo-Tcheugui, J.B.; Ekhlaspour, L.; et al. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48, S27–S49. [Google Scholar]
- Burns, C.; Francis, N. Type 2 Diabetes-Etiology, Epidemiology, Pathogenesis, Treatment. In Metabolic Syndrome; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–20. [Google Scholar]
- Gregg, E.W.; Buckley, J.; Ali, M.K.; Davies, J.; Flood, D.; Mehta, R.; Griffiths, B.; Lim, L.-L.; Manne-Goehler, J.; Pearson-Stuttard, J.; et al. Improving Health Outcomes of People with Diabetes: Target Setting for the WHO Global Diabetes Compact. Lancet 2023, 401, 1302–1312. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Khurana, R.N.; Nguyen, Q.D.; Kelly, S.P.; Lum, F.; Hall, R.; Abbass, I.M.; Abolian, A.M.; Stoilov, I.; To, T.M.; et al. Risk of Blindness among Patients with Diabetes and Newly Diagnosed Diabetic Retinopathy. Diabetes Care 2021, 44, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.; Immanuel, J.; Hague, W.M.; Teede, H.; Nolan, C.J.; Peek, M.J.; Flack, J.R.; McLean, M.; Wong, V.; Hibbert, E.; et al. Treatment of Gestational Diabetes Mellitus Diagnosed Early in Pregnancy. N. Engl. J. Med. 2023, 388, 2132–2144. [Google Scholar] [CrossRef]
- Malaza, N.; Masete, M.; Adam, S.; Dias, S.; Nyawo, T.; Pheiffer, C. A Systematic Review to Compare Adverse Pregnancy Outcomes in Women with Pregestational Diabetes and Gestational Diabetes. Int. J. Environ. Res. Public. Health 2022, 19, 10846. [Google Scholar] [CrossRef]
- Tomic, D.; Shaw, J.E.; Magliano, D.J. The Burden and Risks of Emerging Complications of Diabetes Mellitus. Nat. Rev. Endocrinol. 2022, 18, 525–539. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 3. Prevention or Delay of Diabetes and Associated Comorbidities: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48, S50–S58. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48, S207–S238. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.T.; Greenway, F.; Tucker, T.R.; Alexander, M.; Jackson, L.K.; Hepford, S.A.; Loveridge, B.; Lakey, J.R. A Receptor Story: Insulin Resistance Pathophysiology and Physiologic Insulin Resensitization’s Role as a Treatment Modality. Int. J. Mol. Sci. 2023, 24, 10927. [Google Scholar] [CrossRef]
- Barroso, E.; Jurado-Aguilar, J.; Wahli, W.; Palomer, X.; Vázquez-Carrera, M. Increased Hepatic Gluconeogenesis and Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2024, 35, 1062–1077. [Google Scholar] [CrossRef]
- Lima, J.E.; Moreira, N.C.; Sakamoto-Hojo, E.T. Mechanisms Underlying the Pathophysiology of Type 2 Diabetes: From Risk Factors to Oxidative Stress, Metabolic Dysfunction, and Hyperglycemia. Mutat. Res. Toxicol. Environ. Mutagen. 2022, 874, 503437. [Google Scholar] [CrossRef]
- Ahmad, E.; Lim, S.; Lamptey, R.; Webb, D.R.; Davies, M.J. Type 2 Diabetes. Lancet 2022, 400, 1803–1820. [Google Scholar] [CrossRef] [PubMed]
- Genitsaridi, I.; Salpea, P.; Salim, A.; Sajjadi, S.F.; Tomic, D.; James, S.; Thirunavukkarasu, S.; Issaka, A.; Chen, L.; Basit, A.; et al. IDF Diabetes Atlas: Global, Regional and National Diabetes Prevalence Estimates for 2024 and Projections for 2050. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=5327047 (accessed on 24 August 2025).
- Voeltz, D.; Tönnies, T.; Brinks, R.; Hoyer, A. Future Prevalence of Type 2 Diabetes—A Comparative Analysis of Chronic Disease Projection Methods. PLoS ONE 2022, 17, e0264739. [Google Scholar] [CrossRef]
- Deutsch, A.J.; Ahlqvist, E.; Udler, M.S. Phenotypic and Genetic Classification of Diabetes. Diabetologia 2022, 65, 1758–1769. [Google Scholar] [CrossRef]
- Gouda, P.; Zheng, S.; Peters, T.; Fudim, M.; Randhawa, V.K.; Ezekowitz, J.; Mavrakanas, T.A.; Giannetti, N.; Tsoukas, M.; Lopes, R.; et al. Clinical Phenotypes in Patients with Type 2 Diabetes Mellitus: Characteristics, Cardiovascular Outcomes and Treatment Strategies. Curr. Heart Fail. Rep. 2021, 18, 253–263. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 6. Glycemic Goals and Hypoglycemia: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48, S128–S145. [Google Scholar] [CrossRef]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycaemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2022, 65, 1925–1966. [Google Scholar] [CrossRef]
- Mizukami, H.; Kudoh, K. Diversity of Pathophysiology in Type 2 Diabetes Shown by Islet Pathology. J. Diabetes Investig. 2022, 13, 6–13. [Google Scholar] [CrossRef]
- Sanches, J.M.; Zhao, L.N.; Salehi, A.; Wollheim, C.B.; Kaldis, P. Pathophysiology of Type 2 Diabetes and the Impact of Altered Metabolic Interorgan Crosstalk. FEBS J. 2023, 290, 620–648. [Google Scholar] [CrossRef] [PubMed]
- Piragine, E.; Petri, D.; Martelli, A.; Calderone, V.; Lucenteforte, E. Adherence to Oral Antidiabetic Drugs in Patients with Type 2 Diabetes: Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 1981. [Google Scholar] [CrossRef]
- Altabas, V.; Orlović, Z.; Baretić, M. Addressing the Shortage of GLP-1 RA and Dual GIP/GLP-1 RA-Based Therapies—A Systematic Review. Diabetology 2025, 6, 52. [Google Scholar] [CrossRef]
- Perez, A.; Redondo-Anton, J.; Romera, I.; Lizan, L.; Rubio-de Santos, M.; Diaz-Cerezo, S.; Orozco-Beltran, D. Disease and Economic Burden of Poor Metabolic and Weight Control in Type 2 Diabetes in Spain: A Systematic Literature Review. Diabetes Ther. 2024, 15, 325–341. [Google Scholar] [CrossRef]
- Chew, B.-H.; Mohd-Yusof, B.-N.; Lai, P.S.M.; Khunti, K. Overcoming Therapeutic Inertia as the Achilles’ Heel for Improving Suboptimal Diabetes Care: An Integrative Review. Endocrinol. Metab. 2023, 38, 34–42. [Google Scholar] [CrossRef]
- Fralick, M.; Jenkins, A.J.; Khunti, K.; Mbanya, J.C.; Mohan, V.; Schmidt, M.I. Global Accessibility of Therapeutics for Diabetes Mellitus. Nat. Rev. Endocrinol. 2022, 18, 199–204. [Google Scholar] [CrossRef]
- Tobias, D.K.; Merino, J.; Ahmad, A.; Aiken, C.; Benham, J.L.; Bodhini, D.; Clark, A.L.; Colclough, K.; Corcoy, R.; Cromer, S.J.; et al. Second International Consensus Report on Gaps and Opportunities for the Clinical Translation of Precision Diabetes Medicine. Nat. Med. 2023, 29, 2438–2457. [Google Scholar] [CrossRef]
- Laakso, M.; Fernandes Silva, L. Genetics of Type 2 Diabetes: Past, Present, and Future. Nutrients 2022, 14, 3201. [Google Scholar] [CrossRef] [PubMed]
- DeForest, N.; Majithia, A.R. Genetics of Type 2 Diabetes: Implications from Large-Scale Studies. Curr. Diab. Rep. 2022, 22, 227–235. [Google Scholar] [CrossRef]
- Yang, Y.; Luan, Y.; Feng, Q.; Chen, X.; Qin, B.; Ren, K.-D.; Luan, Y. Epigenetics and beyond: Targeting Histone Methylation to Treat Type 2 Diabetes Mellitus. Front. Pharmacol. 2022, 12, 807413. [Google Scholar] [CrossRef] [PubMed]
- Ansah, E.O. Ethical Challenges and Controversies in the Practice and Advancement of Gene Therapy. Adv. Cell Gene Ther. 2022, 2022, 1015996. [Google Scholar] [CrossRef]
- Capparelli, R.; Iannelli, D. Role of Epigenetics in Type 2 Diabetes and Obesity. Biomedicines 2021, 9, 977. [Google Scholar] [CrossRef]
- Ling, C.; Bacos, K.; Rönn, T. Epigenetics of Type 2 Diabetes Mellitus and Weight Change—A Tool for Precision Medicine? Nat. Rev. Endocrinol. 2022, 18, 433–448. [Google Scholar] [CrossRef]
- Santaló, J.; Berdasco, M. Ethical Implications of Epigenetics in the Era of Personalized Medicine. Clin. Epigenet. 2022, 14, 44. [Google Scholar] [CrossRef]
- Diener, C.; Keller, A.; Meese, E. Emerging Concepts of miRNA Therapeutics: From Cells to Clinic. Trends Genet. 2022, 38, 613–626. [Google Scholar] [CrossRef]
- Mendonca, A.; Thandapani, P.; Nagarajan, P.; Venkatesh, S.; Sundaresan, S. Role of microRNAs in Regulation of Insulin Secretion and Insulin Signaling Involved in Type 2 Diabetes Mellitus. J. Biosci. 2022, 47, 58. [Google Scholar] [CrossRef]
- Auddino, S.; Aiello, E.; Grieco, G.E.; Dotta, F.; Sebastiani, G. A Three-Layer Perspective on miRNA Regulation in β Cell Inflammation. Trends Endocrinol. Metab. 2025, 36, 623–637. [Google Scholar] [CrossRef]
- Jiménez-Lucena, R.; Rangel-Zúñiga, O.A.; Alcalá-Díaz, J.F.; López-Moreno, J.; Roncero-Ramos, I.; Molina-Abril, H.; Yubero-Serrano, E.M.; Caballero-Villarraso, J.; Delgado-Lista, J.; Castaño, J.P.; et al. Circulating miRNAs as Predictive Biomarkers of Type 2 Diabetes Mellitus Development in Coronary Heart Disease Patients from the CORDIOPREV Study. Mol. Ther. Nucleic Acids 2018, 12, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Massart, J.; Sjögren, R.J.O.; Lundell, L.S.; Mudry, J.M.; Franck, N.; O’Gorman, D.J.; Egan, B.; Zierath, J.R.; Krook, A. Altered miR-29 Expression in Type 2 Diabetes Influences Glucose and Lipid Metabolism in Skeletal Muscle. Diabetes 2017, 66, 1807–1818. [Google Scholar] [CrossRef]
- García-Jacobo, R.E.; Uresti-Rivera, E.E.; Portales-Pérez, D.P.; González-Amaro, R.; Lara-Ramírez, E.E.; Enciso-Moreno, J.A.; García-Hernández, M.H. Circulating miR-146a, miR-34a and miR-375 in Type 2 Diabetes Patients, Pre-Diabetic and Normal-Glycaemic Individuals in Relation to β-Cell Function, Insulin Resistance and Metabolic Parameters. Clin. Exp. Pharmacol. Physiol. 2019, 46, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, M.; Sadeghi, F.; Mirzapour, A.; Khafri, S.; Korani, B.; Al-E-Ahmad, A.; Parsian, H. Association of MicroRNA-103 Expression with Type 2 Diabetes Mellitus. Casp. J. Intern. Med. 2025, 16, 106–113. [Google Scholar]
- Latreille, M.; Herrmanns, K.; Renwick, N.; Tuschl, T.; Malecki, M.T.; McCarthy, M.I.; Owen, K.R.; Rülicke, T.; Stoffel, M. miR-375 Gene Dosage in Pancreatic β-Cells: Implications for Regulation of β-Cell Mass and Biomarker Development. J. Mol. Med. 2015, 93, 1159–1169. [Google Scholar] [CrossRef]
- He, X.; Kuang, G.; Wu, Y.; Ou, C. Emerging Roles of Exosomal miRNAs in Diabetes Mellitus. Clin. Transl. Med. 2021, 11, e468. [Google Scholar] [CrossRef]
- Miao, X.; Davoudi, M.; Alitotonchi, Z.; Ahmadi, E.S.; Amraee, F.; Alemi, A.; Afrisham, R. Managing Cardiovascular Events, Hyperglycemia, and Obesity in Type 2 Diabetes through microRNA Regulation Linked to Glucagon-like Peptide-1 Receptor Agonists. Diabetol. Metab. Syndr. 2025, 17, 13. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Bustin, S.A.; Ruijter, J.M.; van den Hoff, M.J.; Kubista, M.; Pfaffl, M.W.; Shipley, G.L.; Tran, N.; Rödiger, S.; Untergasser, A.; Mueller, R.; et al. MIQE 2.0: Revision of the Minimum Information for Publication of Quantitative Real-Time PCR Experiments Guidelines. Clin. Chem. 2025, 71, 634–651. [Google Scholar] [CrossRef]
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the Grade Approach (Updated October 2013); The Grade Working Group: Freiburg, Germany, 2013. [Google Scholar]
- Hong, S.J.; Choi, S.C.; Cho, J.Y.; Joo, H.J.; Park, J.H.; Yu, C.W.; Lim, D.-S. Pioglitazone Increases Circulating MicroRNA-24 with Decrease in Coronary Neointimal Hyperplasia in Type 2 Diabetic Patients–Optical Coherence Tomography Analysis–. Circ. J. 2015, 79, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Demirsoy, İ.H.; Ertural, D.Y.; Balci, Ş.; Çınkır, Ü.; Sezer, K.; Tamer, L.; Aras, N. Profiles of Circulating MiRNAs Following Metformin Treatment in Patients with Type 2 Diabetes. J. Med. Biochem. 2018, 37, 499. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, G.; Besharat, Z.M.; Chiacchiarini, M.; Abballe, L.; Sabato, C.; Vacca, A.; Borgiani, P.; Dotta, F.; Tesauro, M.; Po, A.; et al. Circulating microRNAs in Elderly Type 2 Diabetic Patients. Int. J. Endocrinol. 2018, 2018, 6872635. [Google Scholar] [CrossRef]
- Nunez Lopez, Y.O.; Retnakaran, R.; Zinman, B.; Pratley, R.E.; Seyhan, A.A. Predicting and Understanding the Response to Short-Term Intensive Insulin Therapy in People with Early Type 2 Diabetes. Mol. Metab. 2019, 20, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Solini, A.; Seghieri, M.; Giannini, L.; Biancalana, E.; Parolini, F.; Rossi, C.; Dardano, A.; Taddei, S.; Ghiadoni, L.; Bruno, R.M. The Effects of Dapagliflozin on Systemic and Renal Vascular Function Display an Epigenetic Signature. J. Clin. Endocrinol. Metab. 2019, 104, 4253–4263. [Google Scholar] [CrossRef]
- Giglio, R.V.; Nikolic, D.; Volti, G.L.; Stoian, A.P.; Banerjee, Y.; Magan-Fernandez, A.; Castellino, G.; Patti, A.M.; Chianetta, R.; Castracani, C.C.; et al. Liraglutide Increases Serum Levels of microRNA-27b,-130a and-210 in Patients with Type 2 Diabetes Mellitus: A Novel Epigenetic Effect. Metabolites 2020, 10, 391. [Google Scholar] [CrossRef]
- Gaborit, B.; Julla, J.-B.; Besbes, S.; Proust, M.; Vincentelli, C.; Alos, B.; Ancel, P.; Alzaid, F.; Garcia, R.; Mailly, P.; et al. Glucagon-like Peptide 1 Receptor Agonists, Diabetic Retinopathy and Angiogenesis: The AngioSafe Type 2 Diabetes Study. J. Clin. Endocrinol. Metab. 2020, 105, e1549–e1560. [Google Scholar] [CrossRef]
- Cho, N.-J.; Kim, D.-Y.; Kwon, S.H.; Ha, T.W.; Kim, H.K.; Lee, M.R.; Chun, S.W.; Park, S.; Lee, E.; Gil, H.-W. Urinary Exosomal microRNA Profiling in Type 2 Diabetes Patients Taking Dipeptidyl Peptidase-4 Inhibitor Compared with Sulfonylurea. Kidney Res. Clin. Pract. 2021, 40, 383. [Google Scholar] [CrossRef] [PubMed]
- Formichi, C.; Fignani, D.; Nigi, L.; Grieco, G.E.; Brusco, N.; Licata, G.; Sabato, C.; Ferretti, E.; Sebastiani, G.; Dotta, F. Circulating microRNAs Signature for Predicting Response to GLP1-RA Therapy in Type 2 Diabetic Patients: A Pilot Study. Int. J. Mol. Sci. 2021, 22, 9454. [Google Scholar] [CrossRef] [PubMed]
- Nunez Lopez, Y.O.; Casu, A.; Kovacova, Z.; Petrilli, A.M.; Sideleva, O.; Tharp, W.G.; Pratley, R.E. Coordinated Regulation of Gene Expression and microRNA Changes in Adipose Tissue and Circulating Extracellular Vesicles in Response to Pioglitazone Treatment in Humans with Type 2 Diabetes. Front. Endocrinol. 2022, 13, 955593. [Google Scholar] [CrossRef]
- Mone, P.; Lombardi, A.; Kansakar, U.; Varzideh, F.; Jankauskas, S.S.; Pansini, A.; Marzocco, S.; De Gennaro, S.; Famiglietti, M.; Macina, G.; et al. Empagliflozin Improves the MicroRNA Signature of Endothelial Dysfunction in Patients with Heart Failure with Preserved Ejection Fraction and Diabetes. J. Pharmacol. Exp. Ther. 2023, 384, 116–122. [Google Scholar] [CrossRef]
- Tian, F.; Guo, Y.; Zhou, L.; Yao, Q.; Liang, X.; Lu, J.; He, A.; Shen, J. Comparison of Glimepiride and Linagliptin in the Treatment of Non-Alcoholic Hepatic Disease with Type 2 Diabetes Mellitus. Arch. Med. Sci. AMS 2023, 20, 1407. [Google Scholar] [CrossRef]
- Redling, D.; Bialak, S.; El Ghormli, L.; Chernausek, S.D.; Jones, K.; Tryggestad, J.B. Circulating MicroRNAs as Predictors of Beta Cell Function in Youth-Onset Type 2 Diabetes: The TODAY Study. J. Clin. Endocrinol. Metab. 2024, 109, 3027–3035. [Google Scholar] [CrossRef]
- Liu, Y.; Nie, D.; Lou, X. The Cardiovascular Benefits of Glucagon-like Peptide-1 Receptor Agonists as Novel Diabetes Drugs Are Mediated via the Suppression of miR-203a-3p and miR-429 Expression. DNA Cell Biol. 2024, 43, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Al Zamily, A.M. Effect of GLP-1 Receptor Agonists (Liraglutide) on Glycemic Parameters and Circulating miRNA Expression in Type 2 Diabetes Mellitus. Med. Mod. Mod. Med. 2025, 32, 143–150. [Google Scholar] [CrossRef]
- Pehlivan, D.Y.; Yildiz, H.; Kaya, G. Dapagliflozin Modulates MicroRNA Expression in Type 2 Diabetes Patients with Diabetic Nephropathy: A Retrospective Study. Eur. J. Ther. 2025, 31, 1–10. [Google Scholar] [CrossRef]
- Iacobellis, G.; Goldberger, J.J.; Lamelas, J.; Martinez, C.A.; Sterling, C.M.; Bodenstab, M.; Frasca, D. Liraglutide Effects on Epicardial Adipose Tissue Micro-RNAs and Intra-Operative Glucose Control. Nutr. Metab. Cardiovasc. Dis. 2025, 35, 103726. [Google Scholar] [CrossRef] [PubMed]
- Top, W.M.; Kooy, A.; Stehouwer, C.D. Metformin: A Narrative Review of Its Potential Benefits for Cardiovascular Disease, Cancer and Dementia. Pharmaceuticals 2022, 15, 312. [Google Scholar] [CrossRef]
- Pillai, A.A.; Melo, L.; Frishman, W.H.; Aronow, W.S. The Effects of Metformin on Weight Loss, Cardiovascular Health, and Longevity. Cardiol. Rev. 2024; online ahead of print. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48, S181–S206. [Google Scholar] [CrossRef]
- Handgraaf, S.; Dusaulcy, R.; Visentin, F.; Philippe, J.; Gosmain, Y. Let-7e-5p Regulates GLP-1 Content and Basal Release from Enteroendocrine L Cells from DIO Male Mice. Endocrinology 2020, 161, bqz037. [Google Scholar] [CrossRef]
- Shi, R.; Chen, L.; Wang, W.; Deng, Y.; Liu, Y.; Zhou, H.; Lin, R. Plasma miR-26a-5p Is a Biomarker for Retinal Neurodegeneration of Early Diabetic Retinopathy. Eye 2021, 35, 1587–1599. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, X.; Jiang, L.; Mo, C.; Luo, M.; Hu, K. circFTO Upregulates Transforming Growth Factor-Alpha through Sponging miR-148a-3p to Regulate High Glucose-Induced ARPE-19 Cells Injury. Bioengineered 2022, 13, 11489–11502. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.-Y.; Peng, C.-T.; Wang, H.-J. MicroRNA-146a-5p Mediates High Glucose-Induced Endothelial Inflammation via Targeting Interleukin-1 Receptor-Associated Kinase 1 Expression. Front. Physiol. 2017, 8, 551. [Google Scholar] [CrossRef]
- Fu, D.; Zhao, H.; Huang, Y.; Li, J.; Feng, H.; Li, A.; Liu, Y.; He, L. Metformin Regulates the Effects of IR and IGF-1R Methylation on Mast Cell Activation and Airway Reactivity in Diabetic Rats with Asthma through miR-152-3p/DNMT1 Axis. Cell Biol. Toxicol. 2023, 39, 1851–1872. [Google Scholar] [CrossRef]
- Okamura, T.; Okada, H.; Hashimoto, Y.; Majima, S.; Senmaru, T.; Nakanishi, N.; Asano, M.; Yamazaki, M.; Hamaguchi, M.; Fukui, M. Let-7e-5p Regulates IGF2BP2, and Induces Muscle Atrophy. Front. Endocrinol. 2021, 12, 791363. [Google Scholar] [CrossRef]
- Chen, W.; Lin, G.; Yao, Y.; Chen, J.; Shui, H.; Yang, Q.; Wang, X.; Weng, X.; Sun, L.; Chen, F.; et al. MicroRNA Hsa-Let-7e-5p as a Potential Prognosis Marker for Rectal Carcinoma with Liver Metastases. Oncol. Lett. 2018, 15, 6913–6924. [Google Scholar] [CrossRef]
- Tie, Y.; Chen, C.; Yang, Y.; Qian, Z.; Yuan, H.; Wang, H.; Tang, H.; Peng, Y.; Du, X.; Liu, B. Upregulation of Let-7f-5p Promotes Chemotherapeutic Resistance in Colorectal Cancer by Directly Repressing Several pro-Apoptotic Proteins. Oncol. Lett. 2018, 15, 8695–8702. [Google Scholar] [CrossRef]
- Kerimis, D.; Kontos, C.K.; Christodoulou, S.; Papadopoulos, I.N.; Scorilas, A. Elevated Expression of miR-24-3p Is a Potentially Adverse Prognostic Factor in Colorectal Adenocarcinoma. Clin. Biochem. 2017, 50, 285–292. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, J.; Zhang, L.; Jiang, G.; Wang, B.; Jiang, L. Extracellular Vesicle-Derived miR-26b-5p Is up-Regulated in the Serum of Patients with Diabetic Retinopathy. Comb. Chem. High Throughput Screen. 2022, 25, 877–882. [Google Scholar]
- Jung, S.E.; Kim, S.W.; Jeong, S.; Moon, H.; Choi, W.S.; Lim, S.; Lee, S.; Hwang, K.-C.; Choi, J.-W. MicroRNA-26a/b-5p Promotes Myocardial Infarction-Induced Cell Death by Downregulating Cytochrome c Oxidase 5a. Exp. Mol. Med. 2021, 53, 1332–1343. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Zou, Y.; Zhang, H. MicroRNA-126-5p Facilitates Hypoxia-Induced Vascular Endothelial Cell Injury via HIPK2. Ann. Clin. Lab. Sci. 2022, 52, 918–926. [Google Scholar]
- Wang, Y.; Feng, Y.; Zhang, H.; Niu, Q.; Liang, K.; Bian, C.; Li, H. Clinical value and role of miR-129-5p in non-alcoholic fatty liver disease. Horm. Metab. Res. 2021, 53, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Yang, M.; Li, H.; Xu, R.; Liu, J. miR-130b Regulates PTEN to Activate the PI3K/Akt Signaling Pathway and Attenuate Oxidative Stress-Induced Injury in Diabetic Encephalopathy. Int. J. Mol. Med. 2021, 48, 141. [Google Scholar] [CrossRef] [PubMed]
- Jensterle, M.; Rizzo, M.; Haluzík, M.; Janež, A. Efficacy of GLP-1 RA Approved for Weight Management in Patients with or without Diabetes: A Narrative Review. Adv. Ther. 2022, 39, 2452–2467. [Google Scholar] [CrossRef]
- Abhilasha, A.; Mitra, P.; Suri, S.; Saxena, I.; Shukla, R.K.; Shukla, K.K.; Sharma, P. Downregulation of miR-24-3p and miR-198 in Newly Diagnosed Type 2 Diabetes Mellitus. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Sánchez-Ceinos, J.; Rangel-Zuñiga, O.A.; Clemente-Postigo, M.; Podadera-Herreros, A.; Camargo, A.; Alcalá-Diaz, J.F.; Guzmán-Ruiz, R.; López-Miranda, J.; Malagón, M.M. miR-223-3p as a Potential Biomarker and Player for Adipose Tissue Dysfunction Preceding Type 2 Diabetes Onset. Mol. Ther. Nucleic Acids 2021, 23, 1035–1052. [Google Scholar] [CrossRef] [PubMed]
- Sedgeman, L.; Beysen, C.; Ramirez Solano, M.; Michell, D.; Sheng, Q.; Zhao, S.; Turner, S.; Linton, M.F.; Vickers, K.C. Beta Cell Secretion of miR-375 to HDL Is Inversely Associated with Insulin Secretion. Sci. Rep. 2019, 9, 3803. [Google Scholar] [CrossRef]
- Olivieri, F.; Spazzafumo, L.; Bonafè, M.; Recchioni, R.; Prattichizzo, F.; Marcheselli, F.; Micolucci, L.; Mensà, E.; Giuliani, A.; Santini, G.; et al. MiR-21-5p and miR-126a-3p Levels in Plasma and Circulating Angiogenic Cells: Relationship with Type 2 Diabetes Complications. Oncotarget 2015, 6, 35372. [Google Scholar] [CrossRef]
- Shi, C.; Huang, F.; Gu, X.; Zhang, M.; Wen, J.; Wang, X.; You, L.; Cui, X.; Ji, C.; Guo, X. Adipogenic miRNA and Meta-Signature miRNAs Involved in Human Adipocyte Differentiation and Obesity. Oncotarget 2016, 7, 40830. [Google Scholar] [CrossRef]
- Ono, K.; Igata, M.; Kondo, T.; Kitano, S.; Takaki, Y.; Hanatani, S.; Sakaguchi, M.; Goto, R.; Senokuchi, T.; Kawashima, J.; et al. Identification of microRNA That Represses IRS-1 Expression in Liver. PLoS ONE 2018, 13, e0191553. [Google Scholar] [CrossRef]
- Rong, Y.; Bao, W.; Shan, Z.; Liu, J.; Yu, X.; Xia, S.; Gao, H.; Wang, X.; Yao, P.; Hu, F.B.; et al. Increased microRNA-146a Levels in Plasma of Patients with Newly Diagnosed Type 2 Diabetes Mellitus. PLoS ONE 2013, 8, e73272. [Google Scholar] [CrossRef]
- Liu, R.; Liu, C.; He, X.; Sun, P.; Zhang, B.; Yang, H.; Shi, W.; Ruan, Q. MicroRNA-21 Promotes Pancreatic β Cell Function through Modulating Glucose Uptake. Nat. Commun. 2022, 13, 3545. [Google Scholar] [CrossRef]
- Ghanem, Y.M.; Moursy, E.Y.; Elsayed, E.T.; El Sayed, N.Y.; Mahmoud, R.F. Expression of microRNA-27b in Type 2 Diabetic Patients and Relation to Microvascular Complications. Alex. J. Med. 2024, 60, 336–341. [Google Scholar] [CrossRef]
- Ye, M.; Li, D.; Yang, J.; Xie, J.; Yu, F.; Ma, Y.; Zhu, X.; Zhao, J.; Lv, Z. MicroRNA-130a Targets MAP3K12 to Modulate Diabetic Endothelial Progenitor Cell Function. Cell. Physiol. Biochem. 2015, 36, 712–726. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.-Y.; Cu, W.; Sun, Y.-H.; Chen, X. MiRNA-203a-3p Inhibits Inflammatory Response in Preeclampsia through Regulating IL24. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5223–5230. [Google Scholar]
- Ke, Z.; Chen, Y.; Lin, X.; Jiang, J. MicroRNA-429 Inhibits Microglial Inflammation by Targeting IKKβ Through the NF-κB Pathway. Mediators Inflamm. 2025, 2025, 7165782. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.; Yan, H.; Liang, J.; Du, C.; Zhao, X.; Sun, L.; Chen, Y. MicroRNA-21 Regulates Hepatic Glucose Metabolism by Targeting FOXO1. Gene 2017, 627, 194–201. [Google Scholar] [CrossRef]
- Faheem, A.; Rehman, K.; Jabeen, K.; Akash, M.S.H. Nicotine-Mediated Upregulation of microRNA-141 Expression Determines Adipokine-Intervened Insulin Resistance. Environ. Toxicol. Pharmacol. 2020, 80, 103506. [Google Scholar] [CrossRef]
- Qin, Q.; Cui, L.; Zhou, Z.; Zhang, Z.; Wang, Y.; Zhou, C. Inhibition of microRNA-141-3p Reduces Hypoxia-Induced Apoptosis in H9c2 Rat Cardiomyocytes by Activating the RP105-Dependent PI3K/AKT Signaling Pathway. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 7016. [Google Scholar] [CrossRef]
- Zhu, X.-D.; Chi, J.-Y.; Liang, H.-H.; Huangfu, L.-T.; Guo, Z.-D.; Zou, H.; Yin, X.-H. MicroRNA-377 Mediates Cardiomyocyte Apoptosis Induced by Cyclosporin A. Can. J. Cardiol. 2016, 32, 1249–1259. [Google Scholar] [CrossRef]
- Yang, G.; Li, X.; Wu, Y.; Zhong, S. MicroRNA-377 Knockdown Suppresses High Glucose-Induced Proliferation and Inflammation in Human Mesangial Cells. Chin. J. Nephrol. 2018, 34, 446–452. [Google Scholar]
- Chen, L.-Y.; Xia, X.-D.; Zhao, Z.-W.; Gong, D.; Ma, X.-F.; Yu, X.-H.; Zhang, Q.; Wang, S.-Q.; Dai, X.-Y.; Zheng, X.-L.; et al. MicroRNA-377 Inhibits Atherosclerosis by Regulating Triglyceride Metabolism through the DNA Methyltransferase 1 in Apolipoprotein E-Knockout Mice. Circ. J. 2018, 82, 2861–2871. [Google Scholar] [CrossRef]
- Qian, H.; Zhu, Y.-F.; Shen, N.-N. The Expression Profiles and Roles of microRNAs in Cardiac Glucose Metabolism. Front. Endocrinol. 2025, 16, 1565385. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-B.; Wu, Q.; Liu, J.; Fan, Y.-Z.; Yu, K.-F.; Cai, Y. miR-199a-3p Is Involved in the Pathogenesis and Progression of Diabetic Neuropathy through Downregulation of SerpinE2. Mol. Med. Rep. 2017, 16, 2417–2424. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Modi, A.; Khokhar, M.; Sankanagoudar, S.; Yadav, D.; Sharma, S.; Purohit, P.; Sharma, P. MicroRNA 21 Emerging Role in Diabetic Complications: A Critical Update. Curr. Diabetes Rev. 2021, 17, 122–135. [Google Scholar]
- Wang, W.-Y.; Zheng, Y.-S.; Li, Z.-G.; Cui, Y.-M.; Jiang, J.-C. MiR-92a Contributes to the Cardiovascular Disease Development in Diabetes Mellitus through NF-κB and Downstream Inflammatory Pathways. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3070–3079. [Google Scholar]
- Giglio, R.V.; Papanas, N.; Rizvi, A.A.; Ciaccio, M.; Patti, A.M.; Ilias, I.; Pantea Stoian, A.; Sahebkar, A.; Janez, A.; Rizzo, M. An Update on the Current and Emerging Use of Thiazolidinediones for Type 2 Diabetes. Medicina 2022, 58, 1475. [Google Scholar] [CrossRef]
- Ndakotsu, A.; Vivekanandan, G. The Role of Thiazolidinediones in the Amelioration of Nonalcoholic Fatty Liver Disease: A Systematic Review. Cureus 2022, 14, e25380. [Google Scholar] [CrossRef]
- Saeidi, L.; Shahrokhi, S.Z.; Sadatamini, M.; Jafarzadeh, M.; Kazerouni, F. Can Circulating miR-7-1-5p, and miR-33a-5p Be Used as Markers of T2D Patients? Arch. Physiol. Biochem. 2023, 129, 771–777. [Google Scholar] [CrossRef]
- Katayama, M.; Wiklander, O.P.; Fritz, T.; Caidahl, K.; El-Andaloussi, S.; Zierath, J.R.; Krook, A. Circulating Exosomal miR-20b-5p Is Elevated in Type 2 Diabetes and Could Impair Insulin Action in Human Skeletal Muscle. Diabetes 2019, 68, 515–526. [Google Scholar] [CrossRef]
- Wong, L.L.; Saw, E.L.; Lilyanna, S.; Martinez, E.C.; Lam, C.S.; Richards, A.M. Circulating miR-503 and miR-374b-5p Are Associated with Heart Failure. Circulation 2015, 132, A15090. [Google Scholar] [CrossRef]
- Lewis, K.A.; Stroebel, B.M.; Zhang, L.; Aouizerat, B.; Mattis, A.N.; Flowers, E. MicroRNAs Associated with Metformin Treatment in the Diabetes Prevention Program. Int. J. Mol. Sci. 2024, 25, 5684. [Google Scholar] [CrossRef]
- Zheng, H.; Yu, Z.; Wang, H.; Liu, H.; Chen, X. MicroRNA-195-5p Facilitates Endothelial Dysfunction by Inhibiting Vascular Endothelial Growth Factor A in Gestational Diabetes Mellitus. Reprod. Biol. 2022, 22, 100605. [Google Scholar] [CrossRef] [PubMed]
- Rastegar-Moghaddam, S.H.; Bigham, M.; Lombardi, G.; Mohammadipour, A.; Malvandi, A.M. MicroRNA-24 Therapeutic Potentials in Infarction, Stroke, and Diabetic Complications. Mol. Biol. Rep. 2024, 51, 1137. [Google Scholar] [CrossRef] [PubMed]
- Saini, K.; Sharma, S.; Khan, Y. DPP-4 Inhibitors for Treating T2DM-Hype or Hope? An Analysis Based on the Current Literature. Front. Mol. Biosci. 2023, 10, 1130625. [Google Scholar] [CrossRef]
- Martinez-Arroyo, O.; Ortega, A.; Flores-Chova, A.; Sanchez-Garcia, B.; Garcia-Garcia, A.B.; Chaves, F.J.; Martin-Escudero, J.C.; Forner, M.J.; Redon, J.; Cortes, R. High miR-126-3p Levels Associated with Cardiovascular Events in a General Population. Eur. J. Intern. Med. 2023, 113, 49–56. [Google Scholar] [CrossRef]
- Guo, X.; Huang, M.; Yang, D.; Luo, Z. Expression and Clinical Significance of Plasma miR-223 in Patients with Diabetic Nephropathy. Int. J. Endocrinol. 2023, 2023, 9663320. [Google Scholar] [CrossRef]
- Tang, P.; Xu, Y.; Zhang, J.; Nan, J.; Zhong, R.; Luo, J.; Xu, D.; Shi, S.; Zhang, L. miR-223-3p Mediates the Diabetic Kidney Disease Progression by Targeting IL6ST/STAT3 Pathway. Biochem. Biophys. Res. Commun. 2023, 648, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Ishida, M.; Shimabukuro, M.; Yagi, S.; Nishimoto, S.; Kozuka, C.; Fukuda, D.; Soeki, T.; Masuzaki, H.; Tsutsui, M.; Sata, M. MicroRNA-378 Regulates Adiponectin Expression in Adipose Tissue: A New Plausible Mechanism. PLoS ONE 2014, 9, e111537. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jia, Z.; Zhao, X.; Xu, M.; Chen, M. Expression of miR-210 in the Peripheral Blood of Patients with Newly Diagnosed Type 2 Diabetes Mellitus and Its Effect on the Number and Function of Endothelial Progenitor Cells. Microvasc. Res. 2020, 131, 104032. [Google Scholar] [CrossRef]
- Altabas, V.; Altabas, K.; Berković-Cigrovski, M.; Maloševac, S.; Vrkljan, M.; Nikolić Heitzler, V. Glucose Metabolism Disorders in Patients with Acute Coronary Syndromes. Acta Clin. Croat. 2012, 51, 71–77. [Google Scholar] [PubMed]
- Collado, A.; Jiao, T.; Kontidou, E.; Carvalho, L.R.R.A.; Chernogubova, E.; Yang, J.; Zaccagnini, G.; Zhao, A.; Tengbom, J.; Zheng, X.; et al. miR-210 as a Therapeutic Target in Diabetes-Associated Endothelial Dysfunction. Br. J. Pharmacol. 2025, 182, 417–431. [Google Scholar] [CrossRef]
- McGill, J.B.; Hirsch, I.B.; Parkin, C.G.; Aleppo, G.; Levy, C.J.; Gavin, J.R. 3rd. The Current and Future Role of Insulin Therapy in the Management of Type 2 Diabetes: A Narrative Review. Diabetes Ther. Res. Treat. Educ. Diabetes Relat. Disord. 2024, 15, 1085–1098. [Google Scholar] [CrossRef]
- Wang, S.; Yi, P.; Wang, N.; Song, M.; Li, W.; Zheng, Y. LncRNA TUG1/miR-29c-3p/SIRT1 Axis Regulates Endoplasmic Reticulum Stress-Mediated Renal Epithelial Cells Injury in Diabetic Nephropathy Model In Vitro. PLoS ONE 2021, 16, e0252761. [Google Scholar] [CrossRef]
- Zahari Sham, S.Y.; Ng, C.T.; Azwar, S.; Yip, W.K.; Abdullah, M.; Thevandran, K.; Osman, M.; Seow, H.F. Circulating miRNAs in Type 2 Diabetic Patients with and without Albuminuria in Malaysia. Kidney Blood Press. Res. 2022, 47, 81–93. [Google Scholar] [CrossRef]
- Fan, S.; Li, N. Obesity-Induced Adipocytes Promote Diabetes Mellitus by Regulating β Islet Cell Function through Exosome miR-138-5p. Sci. Rep. 2025, 15, 17275. [Google Scholar] [CrossRef]
- Fu, X.-L.; He, F.-T.; Li, M.-H.; Fu, C.-Y.; Chen, J.-Z. Up-Regulation of miR-192-5p Inhibits the ELAVL1/PI3Kδ Axis and Attenuates Microvascular Endothelial Cell Proliferation, Migration and Angiogenesis in Diabetic Retinopathy. Diabet. Med. 2023, 40, e15077. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Yao, J.; Xie, H.; Wang, C.; Chen, J.; Wei, K.; Ji, Y.; Liu, L. MicroRNA-195-5p Downregulation Inhibits Endothelial Mesenchymal Transition and Myocardial Fibrosis in Diabetic Cardiomyopathy by Targeting Smad7 and Inhibiting Transforming Growth Factor Beta 1-Smads-Snail Pathway. Front. Physiol. 2021, 12, 709123. [Google Scholar] [CrossRef]
- Zhang, R.; Qin, Y.; Zhu, G.; Li, Y.; Xue, J. Low Serum miR-320b Expression as a Novel Indicator of Carotid Atherosclerosis. J. Clin. Neurosci. 2016, 33, 252–258. [Google Scholar] [CrossRef]
- Xu, W.; Liang, Y.; Zhuang, Y.; Yuan, Z. Identification of miRNA-mRNA Regulatory Networks Associated with Diabetic Retinopathy Using Bioinformatics Analysis. Endocr. Metab. Immune Disord. Drug Targets 2023, 23, 1628–1636. [Google Scholar] [CrossRef]
- Pushkaryov, V.; Sokolova, L.; Kovzun, O.; Belchina, Y.; Vatseba, T.; Pushkaryov, V.; Tronko, M. The miRNA-126 content in the blood serum of patients with type 2 diabetes after treatment with certain hypoglycemic drugs. Probl. Endocr. Pathol. 2020, 73, 81–88. [Google Scholar] [CrossRef]
- Xing, Q.; Feng, B.; Yong, Z.; Fengqin, X.; Min, C.; Wen, H. Effect of Dapagliflozin on Urinary Exosomal microRNA in Early-Stage Type 2 Diabetes Mellitus Diabetic Kidney Disease. Chin. J. Diabetes Mellit. 2021, 13, 73. [Google Scholar]
- Shi, Y.; Zhu, Y.; Xie, A.; Xiang, W.; Huang, X.; Li, N.; Wu, S. Effect of Metformin on miRNA Expression in Type 2 Diabetic Patients and Potential Targets. Chin. J. Endocrinol. Metab. 2021, 782–788. [Google Scholar] [CrossRef]
- Chen, R.; Liu, F.; Tan, H.; Han, S.; Chen, Y.; Su, C. Effects of Dapagliflozin on the Expression of MicroRNA-423-5p and Cardiac Function in Patients with Type 2 Diabetes Mellitus and Chronic Heart Failure. Chin. Gen. Pract. 2023, 26, 1733. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).