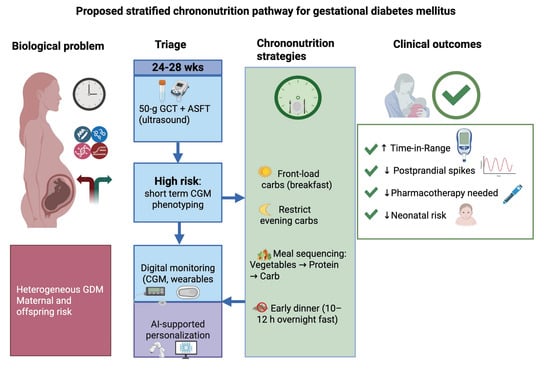
Chrononutrition in Gestational Diabetes: Toward Precision Timing in Maternal Care
Abstract
1. Introduction
2. Methods
3. Clinical Nutrition in GDM: What We Know and Do No Not Know
- Patient A is a South Asian woman with early morning hyperglycemia despite a low-carbohydrate evening meal; further exploration reveals late-night snacking and a strong evening chronotype.
- Patient B, who is obese, presents with minimal postprandial glucose excursions but persistently elevated fasting glucose levels—suggesting a need for targeted nocturnal nutritional adjustments.
- Patient C, a Latina woman, follows culturally normative patterns of late dinners and small breakfasts. Her glycemic control improves only after redistributing caloric intake earlier in the day.
4. Chrononutrition in Pregnancy and GDM
4.1. Circadian Physiology in Pregnancy
- Chronotype: An individual’s characteristic timing of sleep and activity (often “morning” vs. “evening” types). It reflects personal circadian phase: “early” chronotypes wake/sleep earlier (shorter intrinsic cycle) and “late” chronotypes prefer later hours [15].
- Chrononutrition: The study of how meal timing and dietary composition interact with the circadian system. This includes timing of food intake and specific nutrients that can synchronize or disrupt molecular clocks [15].
- Time restricted eating (TRE): A dietary approach in which all daily caloric intake is restricted to a consistent window of 4 to 12 hours each day, without specific guidelines on calorie or nutrient restriction during that period. The remaining hours are spent fasting. TRE aims to align eating patterns with circadian rhythms and typically does not require changes to the amount or quality of food consumed, only the timing of intake [58,59].
4.2. Human Studies on Meal Timing, Chronotype, and GDM
4.3. Translational Implications and Interventions
| Study (Design and Population) | Chrononutrition Exposure/Intervention | Main Findings |
|---|---|---|
| Chandler-Laney et al. (2016)—Observational (n = 40, stratified by BMI) [72]. | Late-night carbohydrate intake (3rd-trimester food diaries). | In women with obesity: higher nighttime carbohydrate intake → higher 2 h OGTT glucose and lower insulin secretion. |
| Loy et al. (2017)—Cross-sectional (n = 1237–1061 completed) [70]. | Meal frequency and overnight fasting duration. | Shorter overnight fasting and more frequent eating → higher maternal glucose concentrations |
| Deniz et al. (2019)—Cross-sectional (n = 148) [73] | Night eating syndrome (NES) vs. no NES. | NES → higher fasting insulin, HOMA-IR, and HbA1c. |
| Dong et al. (2020)—Prospective cohort (n = 84,669 pregnancies—1935 cases of GDM) [69]. | Breakfast frequency (skipping vs. eating) | Skipping breakfast before or during early pregnancy → higher GDM risk (OR ≈ 1.21). |
| Rasmussen et al. (2020)—Randomized crossover (n = 12) [74]. | High vs. low morning carbohydrate intake. | Higher morning carbohydrate intake → lower average glucose, despite modest rise in variability |
| Morris et al. (2019)—Prospective observational pilot (n = 200; 101 completed) [75]. | Meal and snack frequency/distribution. | Three meals + three snacks/day → better glycemic control, especially fasting glucose, vs. lower-frequency eating. |
| AlMogbel et al. (2022)—Retrospective cohort (n = 345) [76]. | Ramadan fasting duration and timing. | Increased neonatal hyperbilirubinemia, decreased neonatal hypoglycemia, birth weight unaffected |
| Facanha et al. (2022)—Cross-sectional (n = 305) [17]. | Chronotype classification. | Evening chronotype → higher risk of preeclampsia and NICU admission |
| Yong et al. (2022)—Intervention trial (n = 12—but 10 completed the study) [77]. | Meal sequencing and meal frequency (five patterns tested: carbs first, protein/veg first, soup first, 3 meals vs. 6 meals) | Protein/vegetables first or soup first → lower mean and peak glucose vs. carbs first; Carb-first meals → larger excursions; increasing meal frequency (6 vs. 3 meals/day) → reduced peaks and excursions at equal calories. |
| Murugesan et al. (2025)—Interventional pilot (n = 27) [40]. | Meal sequencing (vegetables/protein first, carbohydrates last) with short-term CGM feedback to individualize advice. | Reduced postprandial excursions, ↑ CGM time-in-range, ↓ glycemic variability vs. standard dietetic advice |
| Nakano et al. (2025)—Cross-sectional (n = 144) [78]. | Overnight fasting duration and meal frequency. | Longer overnight fasting → lower glycated albumin |
| Messika et al. (2024)—Prospective cohort (n = 208, GDM) [79]. | Breakfast timing, evening carbohydrate intake, sleep quality. | Late breakfast + high evening carbohydrate intake → poor glycemic control, ↑ LGA risk |
5. Translating Chrononutrition into Practice
5.1. Front-Loading Carbohydrates
5.2. Consistent Overnight Fasting
5.3. Meal Sequencing
- Patient A (28 y, 26 wk gestation)
- Patient B (32 y, 24 wk gestation)
- Patient C (35 y, 28 wk gestation)
6. Integrating Molecular and Digital Tools for Precision Maternal Care
6.1. Molecular Stratification and Triage
6.2. Wearables and Real-Time Monitoring
6.3. AI-Driven Decision Support
6.4. Translational Implications: From Bench to Bedside
6.4.1. Clinical Implementation Roadmap
6.4.2. Guidelines and Regulatory Considerations
6.4.3. Patient Engagement and Education
7. Challenges, Gaps, and Future Directions
- How can chrononutrition interventions be adapted for women with varying work schedules, cultural diets and socioeconomic constraints?
- What are the key circadian and multi-omic biomarkers of GDM risk and progression, and how can they be validated in large, diverse cohorts?
- Which digital health architectures can integrate EHRs, wearable data and laboratory omics in a privacy-preserving, interoperable way for maternal care?
- What methods (e.g., explainable AI, participatory design) will build trust and ensure bias mitigation in GDM prediction and feedback tools?
- What is the cost-effectiveness of implementing chrononutrition-based strategies in prenatal care programs across different healthcare systems?
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Trimester | Applicability to GDM | Key Physiologic Point | Food Group and Timing Recommendation | Evidence Notes |
|---|---|---|---|---|
| First trimester (0–13 wk) | Not applicable (GDM rarely diagnosed at this stage) | Insulin resistance low Nausea, variable appetite | Focus on nutrient sufficiency: vegetables (non-starchy), lean protein (lean meat, eggs, tofu, legumes, dairy), carbohydrates (whole grains, low-GI fruits), nuts, seeds, healthy fats. Avoid prolonged fasting. If nausea, small frequent meals. Do not start the day with a carbohydrate-only meal; prefer vegetables/protein first. | No evidence; GDM not identified this early |
| Second trimester (14–27 wk) | Primary window for actionable timing strategies | Rising insulin resistance from placental hormones; appetite improves | Front-load carbohydrates earlier in the day but do not start the day with carbs alone. Practical rule: breakfast = protein + vegetables (then carbs if needed). Emphasize whole grains, legumes, non-starchy vegetables, lean protein, unsweetened dairy. Nuts, seeds, and healthy fats useful as carbs “buffers” in meals. Avoid SSBs and large late-night carb loads. | No trimester-specific studies. Evidence from RCTs, pilot trials, and cohorts and systematic review in women with GDM [40,52,70,74,77,80,82,83,84] |
| Third trimester (28 wk–delivery) | Ongoing GDM management | Peak insulin resistance Gastric emptying slows Higher risk of postprandial hyperglycemia Fetal growth acceleration | Continue 2nd-trimester strategies: Front-load carbs earlier, vegetable → protein → carb sequencing, limit late-evening carbs. Avoid late-night snacking unless medically required (e.g., insulin). Continue nuts, seeds, and healthy fats as meal addition—they can buffer carb absorption. Individualize overnight fasting length and monitor with CGM when changing patterns. Avoid prolonged caloric restriction. | No trimester-specific studies. Evidence from RCTs, pilot trials, cohorts and systematic review in women with GDM [40,52,70,74,77,80,82,83,84] |
References
- Buckley, B.S.; Harreiter, J.; Damm, P.; Corcoy, R.; Chico, A.; Simmons, D.; Vellinga, A.; Dunne, F.; on behalf of the DALI Core Investigator Group. Gestational diabetes mellitus in Europe: Prevalence, current screening practice and barriers to screening. A review. Diabet Med. 2012, 29, 844–854. [Google Scholar] [CrossRef]
- Langer, O. Management of gestational diabetes: Pharmacologic treatment options and glycemic control. Endocrinol. Metab. Clin. 2006, 35, 53–78. [Google Scholar] [CrossRef] [PubMed]
- Sweeting, A.; Hannah, W.; Backman, H.; Catalano, P.; Feghali, M.; Herman, W.H.; Hivert, M.-F.; Immanuel, J.; Meek, C.; Oppermann, M.L. Epidemiology and management of gestational diabetes. Lancet 2024, 404, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Farahvar, S.; Walfisch, A.; Sheiner, E. Gestational diabetes risk factors and long-term consequences for both mother and offspring: A literature review. Expert Rev. Endocrinol. Metab. 2019, 14, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Kaaja, R.; Rönnemaa, T. Gestational diabetes: Pathogenesis and consequences to mother and offspring. Rev. Diabet. Stud. RDS 2009, 5, 194. [Google Scholar] [CrossRef]
- Lee, J.; Lee, N.K.; Moon, J.H. Gestational Diabetes Mellitus: Mechanisms Underlying Maternal and Fetal Complications. Endocrinol. Metab. 2025, 40, 10–25. [Google Scholar] [CrossRef]
- Ren, J.; Xiang, A.H.; Trigo, E.; Takayanagi, M.; Beale, E.; Lawrence, J.M.; Hartiala, J.; Richey, J.M.; Allayee, H.; Buchanan, T.A. Genetic variation in MTNR1B is associated with gestational diabetes mellitus and contributes only to the absolute level of beta cell compensation in Mexican Americans. Diabetologia 2014, 57, 1391–1399. [Google Scholar] [CrossRef]
- Zeng, Q.; Liu, X.; Liu, J.; Wu, Y.; Zhang, Y.; He, Z.; Wei, Y.; Zeng, G.; Zou, D.; Guo, R. MTNR1B variants increase gestational diabetes mellitus risk in young Chinese pregnant women. Sci. Rep. 2025, 15, 19643. [Google Scholar] [CrossRef] [PubMed]
- Cruciat, G.; Florian, A.R.; Chaikh-Sulaiman, M.-S.; Staicu, A.; Caracostea, G.V.; Procopciuc, L.M.; Stamatian, F.; Muresan, D. TCF7L2 Polymorphism rs7903146 (C/T) and Gestational Diabetes Influence on Obstetric Outcome: A Romanian Case–Control Study. Int. J. Mol. Sci. 2024, 25, 4039. [Google Scholar] [CrossRef]
- Chang, S.; Wang, Z.; Wu, L.; Lu, X.; Shangguan, S.; Xin, Y.; Li, L.; Wang, L. Association between TCF 7L2 polymorphisms and gestational diabetes mellitus: A meta-analysis. J. Diabetes Investig. 2017, 8, 560–570. [Google Scholar] [CrossRef]
- Powe, C.E.; Hivert, M.-F.; Udler, M.S. Defining heterogeneity among women with gestational diabetes mellitus. Diabetes 2020, 69, 2064–2074. [Google Scholar] [CrossRef]
- Huvinen, E.; Eriksson, J.G.; Stach-Lempinen, B.; Tiitinen, A.; Koivusalo, S.B. Heterogeneity of gestational diabetes (GDM) and challenges in developing a GDM risk score. Acta Diabetol. 2018, 55, 1251–1259. [Google Scholar] [CrossRef]
- Ray, G.W.; Zeng, Q.; Kusi, P.; Zhang, H.; Shao, T.; Yang, T.; Wei, Y.; Li, M.; Che, X.; Guo, R. Genetic and inflammatory factors underlying gestational diabetes mellitus: A review. Front. Endocrinol. 2024, 15, 1399694. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 15. Management of Diabetes in Pregnancy: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48 (Suppl. S1), S306–S320. [Google Scholar] [CrossRef]
- Ahluwalia, M.K. Chrononutrition—When we eat is of the essence in tackling obesity. Nutrients 2022, 14, 5080. [Google Scholar] [CrossRef]
- Facanha, C.F.S.; De Bruin, V.S.; Alencar, V.S.; Machado, P.S.; Rocha, T.M.; Lopes, F.H.A.; Macedo, R.B.; Viana, A.B.; De Bruin, P.F. Maternal chronotype and pregnancy outcomes in gestational diabetes. J. Endocr. Soc. 2021, 5 (Suppl. S1), A434. [Google Scholar] [CrossRef]
- Facanha, C.F.S.; Alencar, V.S.; Machado, P.S.; Macêdo, R.B.L.; Bruin, P.F.C.d.; Forti, A.C.E.; Rocha, T.M.; Bruin, V.M.S.d. Morningness/eveningness in gestational diabetes mellitus: Clinical characteristics and maternal-neonatal outcomes. Arch. Endocrinol. Metab. 2022, 67, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Lesniara-Stachon, A.; Treviño Montemayor, M.; Collet, T.-H.; Andrey, M.; Quansah, D.Y.; Puder, J.J. Eating Patterns, Chronotypes, and Their Relationship with Metabolic Health in the Early Postpartum Period in Women after Gestational Diabetes Mellitus. Nutrients 2024, 16, 1588. [Google Scholar] [CrossRef]
- Messika, A.; Toledano, Y.; Hadar, E.; Shmuel, E.; Tauman, R.; Shamir, R.; Froy, O. Relationship among chrononutrition, sleep, and glycemic control in women with gestational diabetes mellitus: A randomized controlled trial. Am. J. Obstet. Gynecol. MFM 2022, 4, 100660. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Contreras, B.; Armella, A.; Appel, J.; Mennickent, D.; Araya, J.; González, M.; Castro, E.; Obregón, A.; Lamperti, L.; Gutiérrez, J. Pathophysiological role of genetic factors associated with gestational diabetes mellitus. Front. Physiol. 2022, 13, 769924. [Google Scholar] [CrossRef] [PubMed]
- Abu Samra, N.; Jelinek, H.F.; Alsafar, H.; Asghar, F.; Seoud, M.; Hussein, S.M.; Mubarak, H.M.; Anwar, S.; Memon, M.; Afify, N. Genomics and epigenomics of gestational diabetes mellitus: Understanding the molecular pathways of the disease pathogenesis. Int. J. Mol. Sci. 2022, 23, 3514. [Google Scholar] [CrossRef]
- Lizárraga, D.; Gómez-Gil, B.; García-Gasca, T.; Ávalos-Soriano, A.; Casarini, L.; Salazar-Oroz, A.; García-Gasca, A. Gestational diabetes mellitus: Genetic factors, epigenetic alterations, and microbial composition. Acta Diabetol. 2024, 61, 1–17. [Google Scholar] [CrossRef]
- Veeraswamy, S.; Vijayam, B.; Gupta, V.K.; Kapur, A. Gestational diabetes: The public health relevance and approach. Diabetes Res. Clin. Pract. 2012, 97, 350–358. [Google Scholar] [CrossRef]
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat. Rev. Dis. Primers 2019, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.C.H.; Sugiyama, T.; Ma, R.C.W. Recent updates and future perspectives on gestational diabetes mellitus: An important public health challenge. J. Diabetes Investig. 2021, 12, 1944. [Google Scholar] [CrossRef] [PubMed]
- Alum, E.U.; Ugwu, O.P.; Obeagu, E.I. Beyond pregnancy: Understanding the long-term implications of gestational diabetes mellitus. INOSR Sci. Res. 2024, 11, 63–71. [Google Scholar] [CrossRef]
- Aziz, F.; Khan, M.F.; Moiz, A. Gestational diabetes mellitus, hypertension, and dyslipidemia as the risk factors of preeclampsia. Sci. Rep. 2024, 14, 6182. [Google Scholar] [CrossRef]
- Wei, X.; Zou, H.; Zhang, T.; Huo, Y.; Yang, J.; Wang, Z.; Li, Y.; Zhao, J. Gestational diabetes mellitus: What can medical nutrition therapy do? Nutrients 2024, 16, 1217. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.T.; Hofer, O.J.; Harding, J.E.; Wall, C.R.; Crowther, C.A. Dietary recommendations for women with gestational diabetes mellitus: A systematic review of clinical practice guidelines. Nutr. Rev. 2021, 79, 988–1021. [Google Scholar] [CrossRef]
- Kusinski, L.C.; Jones, D.; Atta, N.; Turner, E.; Smith, S.; Oude Griep, L.M.; Rennie, K.; De Lucia Rolfe, E.; Sharp, S.J.; Farewell, V. Reduced-energy diet in women with gestational diabetes: The dietary intervention in gestational diabetes DiGest randomized clinical trial. Nat. Med. 2025, 31, 514–523. [Google Scholar] [CrossRef]
- Deng, Y.; Yu, J.; Tao, A.; Liu, J.; Wang, Q.; Cao, Y.; Han, S.; Xu, X.; Yan, X.; Fang, X. Effect of low-glycemic index diet advice on pregnant outcomes in women with elevated risk of gestational diabetes mellitus: A meta-analysis of randomized controlled trails. Clin. Nutr. ESPEN 2023, 57, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Izadi, V.; Tehrani, H.; Haghighatdoost, F.; Dehghan, A.; Surkan, P.J.; Azadbakht, L. Adherence to the DASH and Mediterranean diets is associated with decreased risk for gestational diabetes mellitus. Nutrition 2016, 32, 1092–1096. [Google Scholar] [CrossRef]
- Asemi, Z.; Samimi, M.; Tabassi, Z.; Esmaillzadeh, A. The effect of DASH diet on pregnancy outcomes in gestational diabetes: A randomized controlled clinical trial. Eur. J. Clin. Nutr. 2014, 68, 490–495. [Google Scholar] [CrossRef]
- Fan, M.; Chu, Y.; Zheng, Y.; Zhang, Z.; Hou, M. Association of pregnancy diet with metabolic adverse outcomes in pregnant women and their children: A Systematic Review and Meta-Analysis. Ann. Nutr. Metab. 2025, 81, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Phalle, A.; Gokhale, D. Maternal and fetal outcomes in gestational diabetes mellitus: A narrative review of dietary interventions. Front. Glob. Women’s Health 2025, 6, 1510260. [Google Scholar] [CrossRef] [PubMed]
- Arnoriaga-Rodríguez, M.; Serrano, I.; Paz, M.; Barabash, A.; Valerio, J.; Del Valle, L.; O’Connors, R.; Melero, V.; de Miguel, P.; Diaz, Á. A Simplified Screening Model to Predict the Risk of Gestational Diabetes Mellitus in Caucasian and Latin American Pregnant Women. Genes 2024, 15, 482. [Google Scholar] [CrossRef]
- Benny, P.; Ahn, H.J.; Burlingame, J.; Lee, M.-J.; Miller, C.; Chen, J.; Urschitz, J. Genetic risk factors associated with gestational diabetes in a multi-ethnic population. PLoS ONE 2021, 16, e0261137. [Google Scholar] [CrossRef]
- Bennett, G.; Bardon, L.A.; Gibney, E.R. A comparison of dietary patterns and factors influencing food choice among ethnic groups living in one locality: A systematic review. Nutrients 2022, 14, 941. [Google Scholar] [CrossRef]
- Rothman, R.L.; Housam, R.; Weiss, H.; Davis, D.; Gregory, R.; Gebretsadik, T.; Shintani, A.; Elasy, T.A. Patient understanding of food labels: The role of literacy and numeracy. Am. J. Prev. Med. 2006, 31, 391–398. [Google Scholar] [CrossRef]
- Murugesan, R.J.S.K.; Leela, K.; Thiruselvam, S.; Satheesan, A. Improving Glucose Control in Gdm with Food Sequencing: A Cgm-Based Intervention Study; SSRN: New York, NY, USA, 2025. [Google Scholar]
- Patton, A.P.; Hastings, M.H. The suprachiasmatic nucleus. Curr. Biol. 2018, 28, R816–R822. [Google Scholar] [CrossRef]
- Buijs, R.M.; Tinoco, E.C.S.; Alvarado, G.H.; Escobar, C. The circadian system: From clocks to physiology. Handb. Clin. Neurol. 2021, 179, 233–247. [Google Scholar]
- Panda, S. Circadian physiology of metabolism. Science 2016, 354, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Buijs, R.M.; Kalsbeek, A. Hypothalamic integration of central and peripheral clocks. Nat. Rev. Neurosci. 2001, 2, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, U. Timing to perfection: The biology of central and peripheral circadian clocks. Neuron 2012, 74, 246–260. [Google Scholar] [CrossRef]
- Olcese, J. Circadian Clocks and Pregnancy; Frontiers Media SA: Lausanne, Switzerland, 2014; Volume 5, p. 123. [Google Scholar]
- Wharfe, M.D.; Wyrwoll, C.S.; Waddell, B.J.; Mark, P.J. Pregnancy-induced changes in the circadian expression of hepatic clock genes: Implications for maternal glucose homeostasis. Am. J. Physiol.-Endocrinol. Metab. 2016, 311, E575–E586. [Google Scholar] [CrossRef]
- Spichiger, C.; Torres-Farfan, C.; Galdames, H.A.; Mendez, N.; Alonso-Vazquez, P.; Richter, H.G. Gestation under chronic constant light leads to extensive gene expression changes in the fetal rat liver. Physiol. Genom. 2015, 47, 621–633. [Google Scholar] [CrossRef]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef]
- Partch, C.L.; Green, C.B.; Takahashi, J.S. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014, 24, 90–99. [Google Scholar] [CrossRef]
- Pappa, K.I.; Gazouli, M.; Anastasiou, E.; Iliodromiti, Z.; Antsaklis, A.; Anagnou, N.P. Circadian clock gene expression is impaired in gestational diabetes mellitus. Gynecol. Endocrinol. 2013, 29, 331–335. [Google Scholar] [CrossRef]
- Loy, S.L.; Loo, R.S.X.; Godfrey, K.M.; Chong, Y.-S.; Shek, L.P.-C.; Tan, K.H.; Chong, M.F.-F.; Chan, J.K.Y.; Yap, F. Chrononutrition during pregnancy: A review on maternal night-time eating. Nutrients 2020, 12, 2783. [Google Scholar] [CrossRef]
- Mason, I.C.; Qian, J.; Adler, G.K.; Scheer, F.A. Impact of circadian disruption on glucose metabolism: Implications for type 2 diabetes. Diabetologia 2020, 63, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Stenvers, D.J.; Scheer, F.A.; Schrauwen, P.; la Fleur, S.E.; Kalsbeek, A. Circadian clocks and insulin resistance. Nat. Rev. Endocrinol. 2019, 15, 75–89. [Google Scholar]
- Ribas-Latre, A.; Eckel-Mahan, K. Interdependence of nutrient metabolism and the circadian clock system: Importance for metabolic health. Mol. Metab. 2016, 5, 133–152. [Google Scholar] [CrossRef]
- Gamble, K.L.; Berry, R.; Frank, S.J.; Young, M.E. Circadian clock control of endocrine factors. Nat. Rev. Endocrinol. 2014, 10, 466–475. [Google Scholar] [CrossRef]
- Reddy, S.R.V.; Sharma, S. Physiology, Circadian Rhythm. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519507/#:~:text=The%20regulation%20of%20sleep%20is,This%20biological%20circadian%20system (accessed on 1 May 2023).
- Regmi, P.; Heilbronn, L.K. Time-restricted eating: Benefits, mechanisms, and challenges in translation. iScience 2020, 23, 101161. [Google Scholar] [CrossRef]
- Liu, D.; Huang, Y.; Huang, C.; Yang, S.; Wei, X.; Zhang, P.; Guo, D.; Lin, J.; Xu, B.; Li, C. Calorie restriction with or without time-restricted eating in weight loss. N. Engl. J. Med. 2022, 386, 1495–1504. [Google Scholar] [CrossRef]
- Wharfe, M.D.; Mark, P.J.; Wyrwoll, C.S.; Smith, J.T.; Yap, C.; Clarke, M.W.; Waddell, B.J. Pregnancy-induced adaptations of the central circadian clock and maternal glucocorticoids. J. Endocrinol. 2016, 228, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Gamble, K.L.; Resuehr, D.; Johnson, C.H. Shift work and circadian dysregulation of reproduction. Front. Endocrinol. 2013, 4, 92. [Google Scholar] [CrossRef] [PubMed]
- Hammer, P.; Flachs, E.; Specht, I.; Pinborg, A.; Petersen, S.; Larsen, A.; Hougaard, K.; Hansen, J.; Hansen, Å.; Kolstad, H. Night work and hypertensive disorders of pregnancy: A national register-based cohort study. Scand. J. Work Environ. Health 2018, 44, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Vandermeer, B.; Khurana, R.; Nerenberg, K.; Featherstone, R.; Sebastianski, M.; Davenport, M.H. The impact of occupational shift work and working hours during pregnancy on health outcomes: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2019, 221, 563–576. [Google Scholar] [CrossRef]
- Flores, R.C.; Yaffe, R.; Nhunzwi, M.M.; Nguyen, H.; Rabinovich-Nikitin, I. Maternal shift work during pregnancy and cardiovascular health impacts on mother and offspring. J. Mol. Cell. Cardiol. 2025, 199, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.A.; Reid, K.; Grobman, W.A.; Facco, F.L.; Silver, R.M.; Pien, G.W.; Louis, J.; Zee, P.C.; Redline, S.; Sofer, T. Associations between evening shift work, irregular sleep timing, and gestational diabetes in the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-be (nuMoM2b). Sleep 2023, 46, zsac297. [Google Scholar] [CrossRef] [PubMed]
- Longo-Silva, G.; Serenini, R.; Pedrosa, A.; Lima, M.; Soares, L.; Melo, J.; Menezes, R. Chrononutrition patterns and their association with body weight: Differences across multiple chronotypes. Endocrinol. Diabetes Y Nutr. (Engl. Ed.) 2025, 72, 4–13. [Google Scholar]
- Almoosawi, S.; Vingeliene, S.; Karagounis, L.G.; Pot, G. Chrono-nutrition: A review of current evidence from observational studies on global trends in time-of-day of energy intake and its association with obesity. Proc. Nutr. Soc. 2016, 75, 487–500. [Google Scholar] [CrossRef]
- Ruiz-Lozano, T.; Vidal, J.; De Hollanda, A.; Canteras, M.; Garaulet, M.; Izquierdo-Pulido, M. Evening chronotype associates with obesity in severely obese subjects: Interaction with CLOCK 3111T/C. Int. J. Obes. 2016, 40, 1550–1557. [Google Scholar] [CrossRef]
- Dong, J.-Y.; Ikehara, S.; Kimura, T.; Cui, M.; Kawanishi, Y.; Kimura, T.; Ueda, K.; Iso, H. Skipping breakfast before and during early pregnancy and incidence of gestational diabetes mellitus: The Japan Environment and Children’s Study. Am. J. Clin. Nutr. 2020, 111, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Loy, S.L.; Chan, J.K.Y.; Wee, P.H.; Colega, M.T.; Cheung, Y.B.; Godfrey, K.M.; Kwek, K.; Saw, S.M.; Chong, Y.-S.; Natarajan, P. Maternal circadian eating time and frequency are associated with blood glucose concentrations during pregnancy. J. Nutr. 2017, 147, 70–77. [Google Scholar] [CrossRef]
- Reutrakul, S.; Martyn-Nemeth, P.; Quinn, L.; Rydzon, B.; Priyadarshini, M.; Danielson, K.K.; Baron, K.G.; Duffecy, J. Effects of Sleep-Extend on glucose metabolism in women with a history of gestational diabetes: A pilot randomized trial. Pilot Feasibility Stud. 2022, 8, 119. [Google Scholar] [CrossRef]
- Chandler-Laney, P.C.; Schneider, C.R.; Gower, B.A.; Granger, W.M.; Mancuso, M.S.; Biggio, J.R. Association of late-night carbohydrate intake with glucose tolerance among pregnant A frican A merican women. Matern. Child Nutr. 2016, 12, 688–698. [Google Scholar] [CrossRef]
- Deniz, Ç.D.; Özler, S.; Sayın, F.K.; Eryılmaz, M.A. Associations between night eating syndrome and metabolic parameters in pregnant women. Turk. J. Obstet. Gynecol. 2019, 16, 107. [Google Scholar] [CrossRef]
- Rasmussen, L.; Christensen, M.L.; Poulsen, C.W.; Rud, C.; Christensen, A.S.; Andersen, J.R.; Kampmann, U.; Ovesen, P.G. Effect of high versus low carbohydrate intake in the morning on glycemic variability and glycemic control measured by continuous blood glucose monitoring in women with gestational diabetes mellitus—A randomized crossover study. Nutrients 2020, 12, 475. [Google Scholar] [CrossRef]
- Morris, M.A.; Hutchinson, J.; Gianfrancesco, C.; Alwan, N.A.; Carter, M.C.; Scott, E.M.; Cade, J.E. Relationship of the frequency, distribution, and content of meals/snacks to glycaemic control in gestational diabetes: The myfood24 GDM pilot study. Nutrients 2019, 12, 3. [Google Scholar] [CrossRef]
- AlMogbel, T.A.; Ross, G.; Wu, T.; Molyneaux, L.; Constantino, M.I.; McGill, M.; Harding, A.J.; Pech, C.; Alrasheed, A.A.; Wong, J. Ramadan and gestational diabetes: Maternal and neonatal outcomes. Acta Diabetol. 2022, 59, 21–30. [Google Scholar] [CrossRef]
- Yong, G.; Jing, Q.; Yao, Q.; Yang, K.; Ye, X. Changing meal sequence affects glucose excursions in gestational diabetes mellitus. J. Diabetes Res. 2022, 2022, 7083106. [Google Scholar] [CrossRef]
- Nakano, K.; Tanaka, M.; Nishihara, N.; Usui, Y.; Yonezawa, K.; Hikita, N.; Tahara-Sasagawa, E.; Sasaki, S.; Nagamatsu, T.; Haruna, M. Association of Overnight Fasting Duration and Meal Frequency with Glucose and Lipid Metabolism During Pregnancy: A Cross-Sectional Study. Nutrients 2025, 17, 807. [Google Scholar] [CrossRef]
- Messika, A.; Toledano, Y.; Hadar, E.; Tauman, R.; Froy, O.; Shamir, R. Chronobiological factors influencing glycemic control and birth outcomes in gestational diabetes mellitus. Nutrients 2024, 17, 157. [Google Scholar] [CrossRef]
- Zhu, S.; Surampudi, P.; Field, N.T.; Chondronikola, M. Meal timing and glycemic control during pregnancy—Is there a link? Nutrients 2021, 13, 3379. [Google Scholar] [CrossRef]
- Smith, R.; Borg, R.; Wong, V.; Russell, H.; Mak, K.H. Associations Between Carbohydrate Intake Behaviours and Glycaemia in Gestational Diabetes: A Prospective Observational Study. Nutrients 2025, 17, 400. [Google Scholar] [CrossRef]
- Moses, R.G.; Barker, M.; Winter, M.; Petocz, P.; Brand-Miller, J.C. Can a low–glycemic index diet reduce the need for insulin in gestational diabetes mellitus? A randomized trial. Diabetes Care 2009, 32, 996–1000. [Google Scholar] [CrossRef]
- Loy, S.L.; Ku, C.W.; Zheng, R.T.; Lim, C.H.F.; Chang, T.Y.; Chen, L.-W.; Cheung, Y.B.; Godfrey, K.M.; Tan, K.H.; Chong, M.F.-F. Associations of predominant night-eating with plasma glycemic status and continuous glucose monitoring measures among pregnant women. Clin. Nutr. 2023, 42, 2320–2327. [Google Scholar] [CrossRef]
- Boege, H.L.; Park, C.; Gagnier, R.; Deierlein, A.L. Timing of Eating and Glycemic Control During Pregnancy: A Systematic Review. Nutr. Metab. Cardiovasc. Dis. 2025, 35, 104094. [Google Scholar] [CrossRef]
- Pervjakova, N.; Moen, G.-H.; Borges, M.-C.; Ferreira, T.; Cook, J.P.; Allard, C.; Beaumont, R.N.; Canouil, M.; Hatem, G.; Heiskala, A. Multi-ancestry genome-wide association study of gestational diabetes mellitus highlights genetic links with type 2 diabetes. Hum. Mol. Genet. 2022, 31, 3377–3391. [Google Scholar] [CrossRef]
- Kwak, S.H.; Kim, S.-H.; Cho, Y.M.; Go, M.J.; Cho, Y.S.; Choi, S.H.; Moon, M.K.; Jung, H.S.; Shin, H.D.; Kang, H.M. A genome-wide association study of gestational diabetes mellitus in Korean women. Diabetes 2012, 61, 531–541. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, L.; Geng, L.; Zhang, X.; Du, M.; Sun, Y.; Zhao, L.; Bai, B.; Li, X. Placental whole transcriptome expression profile in patients with early-onset, late-onset preeclampsia and gestational diabetes mellitus. Sci. Rep. 2025, 15, 19476. [Google Scholar] [CrossRef] [PubMed]
- Radaelli, T.; Varastehpour, A.; Catalano, P.; Hauguel-de Mouzon, S. Gestational diabetes induces placental genes for chronic stress and inflammatory pathways. Diabetes 2003, 52, 2951–2958. [Google Scholar] [CrossRef] [PubMed]
- Alesi, S.; Ghelani, D.; Rassie, K.; Mousa, A. Metabolomic biomarkers in gestational diabetes mellitus: A review of the evidence. Int. J. Mol. Sci. 2021, 22, 5512. [Google Scholar] [CrossRef] [PubMed]
- Popova, P.V.; Isakov, A.O.; Rusanova, A.N.; Sitkin, S.I.; Anopova, A.D.; Vasukova, E.A.; Tkachuk, A.S.; Nemikina, I.S.; Stepanova, E.A.; Eriskovskaya, A.I. Personalized prediction of glycemic responses to food in women with diet-treated gestational diabetes: The role of the gut microbiota. NPJ Biofilms Microbiomes 2025, 11, 25. [Google Scholar] [CrossRef]
- Oğlak, S.C.; Yılmaz, E.Z.; Budak, M.Ş. Abdominal subcutaneous fat thickness combined with a 50-g glucose challenge test at 24-28 weeks of pregnancy in predicting gestational diabetes mellitus. J. Obstet. Gynaecol. 2024, 44, 2329880. [Google Scholar] [CrossRef]
- Durnwald, C.; Beck, R.W.; Li, Z.; Norton, E.; Bergenstal, R.M.; Johnson, M.; Dunnigan, S.; Banfield, M.; Krumwiede, K.; Sibayan, J. Continuous glucose monitoring profiles in pregnancies with and without gestational diabetes mellitus. Diabetes Care 2024, 47, 1333–1341. [Google Scholar] [CrossRef]
- Daley, B.J.; Ni’Man, M.; Neves, M.R.; Bobby Huda, M.S.; Marsh, W.; Fenton, N.E.; Hitman, G.A.; McLachlan, S. mHealth apps for gestational diabetes mellitus that provide clinical decision support or artificial intelligence: A scoping review. Diabet. Med. 2022, 39, e14735. [Google Scholar] [CrossRef]
- Kokori, E.; Olatunji, G.; Aderinto, N.; Muogbo, I.; Ogieuhi, I.J.; Isarinade, D.; Ukoaka, B.; Akinmeji, A.; Ajayi, I.; Chidiogo, E. The role of machine learning algorithms in detection of gestational diabetes; a narrative review of current evidence. Clin. Diabetes Endocrinol. 2024, 10, 18. [Google Scholar] [CrossRef]
- Liu, K.; Clarke, G.S.; Grieger, J.A. The Use of Omics in Untangling the Effect of Lifestyle Factors in Pregnancy and Gestational Diabetes: A Systematic Review. Diabetes/Metab. Res. Rev. 2025, 41, e70026. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Choi, S.H. Advancing Early Prediction of Gestational Diabetes Mellitus with Circular RNA Biomarkers. Diabetes Metab. J. 2025, 49, 403–404. [Google Scholar] [CrossRef]
- Crimmins, S.D.; Ginn-Meadow, A.; Jessel, R.H.; Rosen, J.A. Leveraging technology to improve diabetes care in pregnancy. Clin. Diabetes 2020, 38, 486–494. [Google Scholar] [CrossRef]
- Benham, J.L.; Gingras, V.; McLennan, N.-M.; Most, J.; Yamamoto, J.M.; Aiken, C.E.; Ozanne, S.E.; Reynolds, R.M. Precision gestational diabetes treatment: A systematic review and meta-analyses. Commun. Med. 2023, 3, 135. [Google Scholar] [CrossRef] [PubMed]
- Administration USFDA. Laboratory Developed Tests; U.S. Department of Health and Human Services: Washington, DC, USA, 2024.
- Jurczak, K.M.; van Der Boon, T.A.; Devia-Rodriguez, R.; Schuurmann, R.C.; Sjollema, J.; van Huizen, L.; De Vries, J.P.P.; van Rijn, P. Recent regulatory developments in EU Medical Device Regulation and their impact on biomaterials translation. Bioeng. Transl. Med. 2025, 10, e10721. [Google Scholar] [CrossRef]
- Dunsmuir, D.T.; Payne, B.A.; Cloete, G.; Petersen, C.L.; Görges, M.; Lim, J.; Von Dadelszen, P.; Dumont, G.A.; Ansermino, J.M. Development of mHealth applications for pre-eclampsia triage. IEEE J. Biomed. Health Inform. 2014, 18, 1857–1864. [Google Scholar] [CrossRef]
- Mackillop, L.; Loerup, L.; Bartlett, K.; Farmer, A.; Gibson, O.J.; Hirst, J.E.; Kenworthy, Y.; Kevat, D.A.; Levy, J.C.; Tarassenko, L. Development of a real-time smartphone solution for the management of women with or at high risk of gestational diabetes. J. Diabetes Sci. Technol. 2014, 8, 1105–1114. [Google Scholar] [CrossRef]
- Stroux, L.; Martinez, B.; Coyote Ixen, E.; King, N.; Hall-Clifford, R.; Rohloff, P.; Clifford, G.D. An mHealth monitoring system for traditional birth attendant-led antenatal risk assessment in rural Guatemala. J. Med. Eng. Technol. 2016, 40, 356–371. [Google Scholar] [CrossRef]
- Oxlad, M.; Whitburn, S.; Grieger, J.A. The complexities of managing gestational diabetes in women of culturally and linguistically diverse backgrounds: A qualitative study of Women’s experiences. Nutrients 2023, 15, 1053. [Google Scholar] [CrossRef] [PubMed]
- Ebekozien, O.; Fantasia, K.; Farrokhi, F.; Sabharwal, A.; Kerr, D. Technology and health inequities in diabetes care: How do we widen access to underserved populations and utilize technology to improve outcomes for all? Diabetes Obes. Metab. 2024, 26, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Myneni, S.; Zingg, A.; Singh, T.; Ross, A.; Franklin, A.; Rogith, D.; Refuerzo, J. Digital health technologies for high-risk pregnancy management: Three case studies using Digilego framework. JAMIA Open 2024, 7, ooae022. [Google Scholar] [CrossRef] [PubMed]
- Callahan, A.E. Challenges and Opportunities for Precision and Personalized Nutrition: Proceedings of a Workshop—In Brief; National Academies Press: Washington, DC, USA, 2021. [Google Scholar]
- Pham, Q.; Gamble, A.; Hearn, J.; Cafazzo, J.A. The need for ethnoracial equity in artificial intelligence for diabetes management: Review and recommendations. J. Med. Internet Res. 2021, 23, e22320. [Google Scholar] [CrossRef] [PubMed]
| Behavioral Target | Practical Strategy | Supporting Evidence |
|---|---|---|
| Overnight fasting duration | Aim for an overnight fasting window of ~8–10 h. In individuals with elevated fasting glucose, consider longer fasts (≥12 h) under supervision. Avoid late-night carbohydrate snacks. | Longer overnight fasting (~10–12 h) linked to lower fasting glucose in observational GDM cohorts [80]; however, other studies [81] found extended fasts may worsen glycemic stability, especially when total carbohydrate intake is low or inconsistent. This suggests that overnight fasting duration may need to be tailored to individual glycemic profiles. |
| Front-load carbohydrate intake | Allocate ~50% of daily carbohydrates to breakfast and lunch; reduce carbohydrate intake at dinner. | Morning carb loading improved glycemic control in one RCT supported by observational and pilot studies [74,80,82]. |
| Minimize evening/night eating | Finish the last meal by early evening and avoid high-carb snacks late at night to extend the overnight fast. | Concentrating calorie intake at night worsens glycemic control. Eating >50% of calories after 7pm → higher fasting and mean glucose [83]; systematic review: later meals and shorter overnight fasting → poorer glycemic outcomes in pregnancy [84]. |
| Choose low-GI, balanced meals | Select low-glycemic-index carbs (whole grains, legumes, etc.) and pair them with protein and healthy fats to slow glucose absorption. | High-glycemic-index meals at lunch/dinner → elevated postprandial glucose [81]; low-GI diet in GDM halved insulin requirement [82]. |
| Sequence food intake | Eat high-fiber vegetables first, then protein, and carbohydrates last. | Sequencing meals this way delays carbohydrate absorption, moderates glucose spikes, and improves time-in-range [40,77]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xega, V.; Liu, J.-L. Chrononutrition in Gestational Diabetes: Toward Precision Timing in Maternal Care. J. Pers. Med. 2025, 15, 534. https://doi.org/10.3390/jpm15110534
Xega V, Liu J-L. Chrononutrition in Gestational Diabetes: Toward Precision Timing in Maternal Care. Journal of Personalized Medicine. 2025; 15(11):534. https://doi.org/10.3390/jpm15110534
Chicago/Turabian StyleXega, Viktoria, and Jun-Li Liu. 2025. "Chrononutrition in Gestational Diabetes: Toward Precision Timing in Maternal Care" Journal of Personalized Medicine 15, no. 11: 534. https://doi.org/10.3390/jpm15110534
APA StyleXega, V., & Liu, J.-L. (2025). Chrononutrition in Gestational Diabetes: Toward Precision Timing in Maternal Care. Journal of Personalized Medicine, 15(11), 534. https://doi.org/10.3390/jpm15110534