Leveraging Synergy: A Review of the Therapeutic Potential of SN-38 and Immune Checkpoint Blockade in Breast and Prostate Cancer Treatment
Abstract
1. Introduction
2. SN-38: Mechanism of Action and Current Applications
Applications of SN-38 in Oncology
3. Immunotherapy in Breast and Prostate Cancer
3.1. Advances in Immunotherapy
3.2. Challenges in Hormone-Driven Cancers
3.3. Opportunities for Combination Therapies
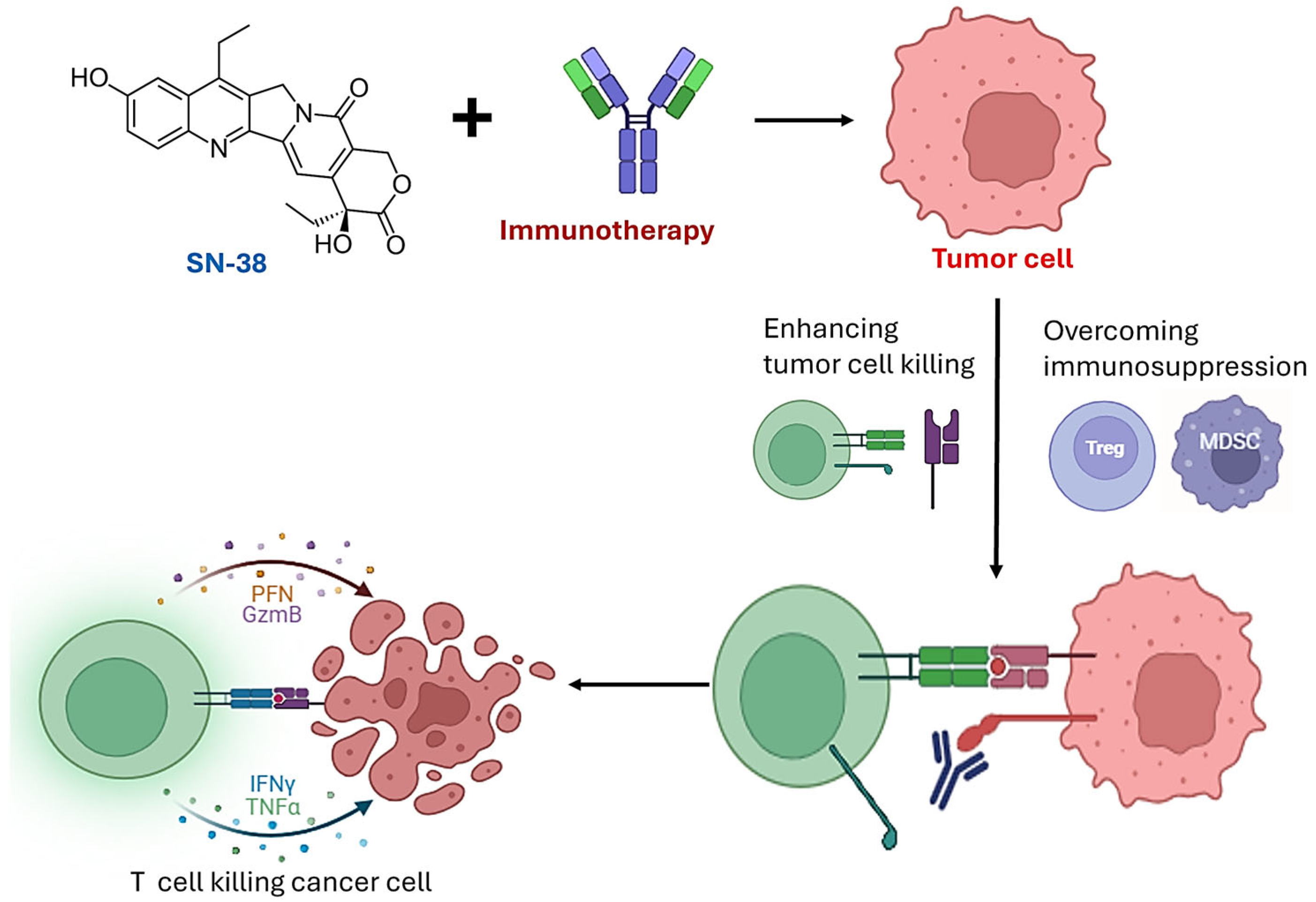
4. Synergy Between SN-38 and Immunotherapy
4.1. Immunogenic Effects of SN-38
4.2. Modulation of the Tumor Microenvironment
4.3. Overcoming Resistance Mechanisms
5. Therapeutic Potential, Obstacles, and Future Perspectives
5.1. Emerging Preclinical Evidence of SN-38 Synergy with Immunotherapy
5.2. Clinical and Formulation Limitations of SN-38
5.3. Biomarker-Driven Strategies for Optimizing SN-38 and Immunotherapy Combinations
5.4. Toxicity, Side Effects and Resistance to Combination Therapy
5.5. Expanding the Applications of SN-38 and Immunotherapy Combinations
6. Future Perspective and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCa | prostate cancer |
| ER | estrogen receptor |
| AR | androgen receptor |
| TME | tumor microenvironment |
| UGT1A1 | UDP-glucuronosyltransferase 1A1 |
| Nal-IRI | Nanoliposomal irinotecan |
| ADCs | antibody-drug conjugates |
| TNBC | triple-negative breast cancer |
| TILs | tumor-infiltrating lymphocytes |
| HR+ | hormone receptor-positive |
| CRPC | castration-resistant prostate cancer |
| mTNBC | metastatic triple-negative breast cancer |
| ICI | immune checkpoint inhibitor |
| BiTEs | bispecific T-cell engagers |
| DAMPs | damage-associated molecular patterns |
| ICD | immunogenic cell death |
| HMGB1 | high mobility group box 1 |
| CTL | cytotoxic T lymphocyte |
| CRT | Calreticulin |
| APCs | antigen-presenting cells |
| NK | Natural killer |
| HNSCC | head and neck squamous cell carcinoma |
| Tregs | regulatory T cells |
| LPS | Lipopolysaccharide |
| APM | antigen-processing machinery |
| ICIs | immune checkpoint inhibitors |
| TROP-2 | trophoblast cell surface antigen 2 |
| ROS | reactive oxygen species |
| aPDL1 | anti-PD-L1 |
| CED | convection-enhanced delivery |
| PDX | patient-derived xenograft |
| HAS | human serum albumin |
| HA | hyaluronic acid |
| PSA | Prostate-specific antigen |
| PR | progesterone receptor |
| IrAEs | immune-related adverse events |
References
- Benitez Fuentes, J.D.; Morgan, E.; De Luna Aguilar, A.; Mafra, A.; Shah, R.; Giusti, F.; Vignat, J.; Znaor, A.; Musetti, C.; Yip, C.-H.; et al. Global Stage Distribution of Breast Cancer at Diagnosis: A Systematic Review and Meta-Analysis. JAMA Oncol. 2024, 10, 71. [Google Scholar] [CrossRef]
- Emons, G. Hormone-Dependent Cancers: Molecular Mechanisms and Therapeutical Implications. Cells 2022, 12, 110. [Google Scholar] [CrossRef]
- Wang, L.; Lu, B.; He, M.; Wang, Y.; Wang, Z.; Du, L. Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries From 2000 to 2019. Front. Public Health 2022, 10, 811044. [Google Scholar] [CrossRef]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.; McManus, J.M.; Sharifi, N. Hormonal Therapy for Prostate Cancer. Endocr. Rev. 2021, 42, 354–373. [Google Scholar] [CrossRef]
- Masoud, V.; Pagès, G. Targeted Therapies in Breast Cancer: New Challenges to Fight against Resistance. World J. Clin. Oncol. 2017, 8, 120. [Google Scholar] [CrossRef]
- Adekiya, T.A.; Moore, M.; Thomas, M.; Lake, G.; Hudson, T.; Adesina, S.K. Preparation, Optimization, and In-Vitro Evaluation of Brusatol- and Docetaxel-Loaded Nanoparticles for the Treatment of Prostate Cancer. Pharmaceutics 2024, 16, 114. [Google Scholar] [CrossRef]
- Adekiya, T.A.; Owoseni, O. Emerging Frontiers in Nanomedicine Targeted Therapy for Prostate Cancer. Cancer Treat. Res. Commun. 2023, 37, 100778. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Kim, S.-B. HER2-Low Breast Cancer: Now and in the Future. Cancer Res. Treat. 2024, 56, 700–720. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, M.; Ahlawat, P.; Srinivas, N.R. Irinotecan and Its Active Metabolite, SN-38: Review of Bioanalytical Methods and Recent Update from Clinical Pharmacology Perspectives. Biomed. Chromatogr. 2010, 24, 104–123. [Google Scholar] [CrossRef]
- Cao, Y.; Jin, Z.-X.; Tong, X.-P.; Yue, S.; Sakai, T.; Kawanami, T.; Sawaki, T.; Miki, M.; Iwao, H.; Nakajima, A.; et al. Synergistic Effects of Topoisomerase I Inhibitor, SN38, on Fas-Mediated Apoptosis. Anticancer Res. 2010, 30, 3911–3917. [Google Scholar] [PubMed]
- Shin, W.S.; Han, J.; Kumar, R.; Lee, G.G.; Sessler, J.L.; Kim, J.-H.; Kim, J.S. Programmed Activation of Cancer Cell Apoptosis: A Tumor-Targeted Phototherapeutic Topoisomerase I Inhibitor. Sci. Rep. 2016, 6, 29018. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Tian, H.; Yue, B.; Zhai, B.; Zhao, F. Research Progress of SN38 Drug Delivery System in Cancer Treatment. Int. J. Nanomed. 2024, 19, 945–964. [Google Scholar] [CrossRef]
- Yu, C.; Huang, F.; Wang, K.; Liu, M.; Chow, W.A.; Ling, X.; Li, F.; Causey, J.L.; Huang, X.; Cook-Wiens, G.; et al. Single Protein Encapsulated SN38 for Tumor-Targeting Treatment. J. Transl. Med. 2023, 21, 897. [Google Scholar] [CrossRef]
- Mosallaei, N.; Mahmoudi, A.; Ghandehari, H.; Yellepeddi, V.K.; Jaafari, M.R.; Malaekeh-Nikouei, B. Solid Lipid Nanoparticles Containing 7-Ethyl-10-Hydroxycamptothecin SN38: Preparation, Characterization, in Vitro, and in Vivo Evaluations. Eur. J. Pharm. Biopharm. 2016, 104, 42–50. [Google Scholar] [CrossRef]
- Bataille Backer, P.; Adekiya, T.A.; Kim, Y.; Reid, T.-E.R.; Thomas, M.; Adesina, S.K. Development of a Targeted SN-38-Conjugate for the Treatment of Glioblastoma. ACS Omega 2024, 9, 2615–2628. [Google Scholar] [CrossRef]
- Mukherjee, A.G.; Wanjari, U.R.; Namachivayam, A.; Murali, R.; Prabakaran, D.S.; Ganesan, R.; Renu, K.; Dey, A.; Vellingiri, B.; Ramanathan, G.; et al. Role of Immune Cells and Receptors in Cancer Treatment: An Immunotherapeutic Approach. Vaccines 2022, 10, 1493. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, J.; Chen, S.; Wang, X.; Xi, Q.; Shen, H.; Zhang, R. Immune Checkpoint Inhibitors: Breakthroughs in Cancer Treatment. Cancer Biol. Med. 2024, 21, 451–472. [Google Scholar] [CrossRef]
- Javed, S.A.; Najmi, A.; Ahsan, W.; Zoghebi, K. Targeting PD-1/PD-L-1 Immune Checkpoint Inhibition for Cancer Immunotherapy: Success and Challenges. Front. Immunol. 2024, 15, 1383456. [Google Scholar] [CrossRef]
- Zagami, P.; Cortés, J.; Carey, L.A.; Curigliano, G. Immunotherapy in the Treatment Landscape of Hormone Receptor-Positive HR+ Early Breast Cancer: Is New Data Clinical Practice Changing? ESMO Open 2024, 9, 103695. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Stover, D.G.; Guerriero, J.L.; Dillon, D.; Barry, W.T.; Gjini, E.; Hartl, C.; Lo, W.; Savoie, J.; Brock, J.; et al. The Immune Microenvironment in Hormone Receptor–Positive Breast Cancer Before and After Preoperative Chemotherapy. Clin. Cancer Res. 2019, 25, 4644–4655. [Google Scholar] [CrossRef]
- Ma, M.; McLeod, H. Lessons Learned from the Irinotecan Metabolic Pathway. Curr. Med. Chem. 2003, 10, 41–49. [Google Scholar] [CrossRef]
- Fujita, K.; Sparreboom, A. Pharmacogenetics of Irinotecan Disposition and Toxicity: A Review. Curr. Clin. Pharmacol. 2010, 5, 209–217. [Google Scholar] [CrossRef]
- Kim, T.W.; Innocenti, F. Insights, Challenges, and Future Directions in Irinogenetics. Ther. Drug Monit. 2007, 29, 265–270. [Google Scholar] [CrossRef]
- Marangon, E.; Posocco, B.; Mazzega, E.; Toffoli, G. Development and Validation of a High-Performance Liquid Chromatography–Tandem Mass Spectrometry Method for the Simultaneous Determination of Irinotecan and Its Main Metabolites in Human Plasma and Its Application in a Clinical Pharmacokinetic Study. PLoS ONE 2015, 10, e0118194. [Google Scholar] [CrossRef]
- Adam, C.; Pérez-López, A.M.; Hamilton, L.; Rubio-Ruiz, B.; Bray, T.L.; Sieger, D.; Brennan, P.M.; Unciti-Broceta, A. Bioorthogonal Uncaging of the Active Metabolite of Irinotecan by Palladium-Functionalized Microdevices. Chem. A Eur. J. 2018, 24, 16783–16790. [Google Scholar] [CrossRef]
- Goldenberg, D.M.; Sharkey, R.M. Antibody-Drug Conjugates Targeting TROP-2 and Incorporating SN-38: A Case Study of Anti-TROP-2 Sacituzumab Govitecan. mAbs 2019, 11, 987–995. [Google Scholar] [CrossRef]
- Nakatsu, S.; Kondo, S.; Kondo, Y.; Yin, D.; Peterson, J.W.; Kaakaji, R.; Morimura, T.; Kikuchi, H.; Takeuchi, J.; Barnett, G.H. Induction of Apoptosis in Multi-Drug Resistant MDR Human Glioblastoma Cells by SN-38, a Metabolite of the Camptothecin Derivative CPT-11. Cancer Chemother. Pharmacol. 1997, 39, 417–423. [Google Scholar] [CrossRef]
- Tahara, M.; Inoue, T.; Sato, F.; Miyakura, Y.; Horie, H.; Yasuda, Y.; Fujii, H.; Kotake, K.; Sugano, K. The Use of Olaparib AZD2281 Potentiates SN-38 Cytotoxicity in Colon Cancer Cells by Indirect Inhibition of Rad51-Mediated Repair of DNA Double-Strand Breaks. Mol. Cancer Ther. 2014, 13, 1170–1180. [Google Scholar] [CrossRef]
- Hahn, R.Z.; Antunes, M.V.; Verza, S.G.; Perassolo, M.S.; Suyenaga, E.S.; Schwartsmann, G.; Linden, R. Pharmacokinetic and Pharmacogenetic Markers of Irinotecan Toxicity. Curr. Med. Chem. 2019, 26, 2085–2107. [Google Scholar] [CrossRef]
- Abdou, Y.; Goudarzi, A.; Yu, J.X.; Upadhaya, S.; Vincent, B.; Carey, L.A. Immunotherapy in Triple Negative Breast Cancer: Beyond Checkpoint Inhibitors. NPJ Breast Cancer 2022, 8, 121. [Google Scholar] [CrossRef]
- Heater, N.K.; Warrior, S.; Lu, J. Current and Future Immunotherapy for Breast Cancer. J. Hematol. Oncol. 2024, 17, 131. [Google Scholar] [CrossRef]
- Runcie, K.D.; Dallos, M.C. Prostate Cancer Immunotherapy—Finally in From the Cold? Curr. Oncol. Rep. 2021, 23, 88. [Google Scholar] [CrossRef]
- Sooi, K.; Walsh, R.; Kumarakulasinghe, N.; Wong, A.; Ngoi, N. A Review of Strategies to Overcome Immune Resistance in the Treatment of Advanced Prostate Cancer. Cancer Drug Resist. 2023, 6, 656–673. [Google Scholar] [CrossRef]
- Venkatachalam, S.; McFarland, T.R.; Agarwal, N.; Swami, U. Immune Checkpoint Inhibitors in Prostate Cancer. Cancers 2021, 13, 2187. [Google Scholar] [CrossRef]
- Dvir, K.; Giordano, S.; Leone, J.P. Immunotherapy in Breast Cancer. Int. J. Mol. Sci. 2024, 25, 7517. [Google Scholar] [CrossRef]
- Fay, E.K.; Graff, J.N. Immunotherapy in Prostate Cancer. Cancers 2020, 12, 1752. [Google Scholar] [CrossRef]
- Torres, E.T.R.; Emens, L.A. Emerging Combination Immunotherapy Strategies for Breast Cancer: Dual Immune Checkpoint Modulation, Antibody–Drug Conjugates and Bispecific Antibodies. Breast Cancer Res. Treat. 2022, 191, 291–302. [Google Scholar] [CrossRef]
- Sun, B.L. Immunotherapy in Treatment of Metastatic Prostate Cancer: An Approach to Circumvent Immunosuppressive Tumor Microenvironment. Prostate 2021, 81, 1125–1134. [Google Scholar] [CrossRef]
- Thomas, C.J.; Delgado, K.; Sawant, K.; Roy, J.; Gupta, U.; Song, C.S.; Poojary, R.; De Figueiredo, P.; Song, J. Harnessing Bacterial Agents to Modulate the Tumor Microenvironment and Enhance Cancer Immunotherapy. Cancers 2024, 16, 3810. [Google Scholar] [CrossRef]
- Melo, C.M.; Vidotto, T.; Chaves, L.P.; Lautert-Dutra, W.; Reis, R.B.D.; Squire, J.A. The Role of Somatic Mutations on the Immune Response of the Tumor Microenvironment in Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 9550. [Google Scholar] [CrossRef]
- Movassaghi, M.; Chung, R.; Anderson, C.B.; Stein, M.; Saenger, Y.; Faiena, I. Overcoming Immune Resistance in Prostate Cancer: Challenges and Advances. Cancers 2021, 13, 4757. [Google Scholar] [CrossRef]
- Ayoub, N.M.; Al-Shami, K.M.; Yaghan, R.J. Immunotherapy for HER2-Positive Breast Cancer: Recent Advances and Combination Therapeutic Approaches. Breast Cancer Targets Ther. 2019, 11, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Bou-Dargham, M.J.; Draughon, S.; Cantrell, V.; Khamis, Z.I.; Sang, Q.-X.A. Advancements in Human Breast Cancer Targeted Therapy and Immunotherapy. J. Cancer 2021, 12, 6949–6963. [Google Scholar] [CrossRef]
- Adams, S.; Gatti-Mays, M.E.; Kalinsky, K.; Korde, L.A.; Sharon, E.; Amiri-Kordestani, L.; Bear, H.; McArthur, H.L.; Frank, E.; Perlmutter, J.; et al. Current Landscape of Immunotherapy in Breast Cancer: A Review. JAMA Oncol. 2019, 5, 1205. [Google Scholar] [CrossRef]
- Naik, A.; Monjazeb, A.M.; Decock, J. The Obesity Paradox in Cancer, Tumor Immunology, and Immunotherapy: Potential Therapeutic Implications in Triple Negative Breast Cancer. Front. Immunol. 2019, 10, 1940. [Google Scholar] [CrossRef]
- Xu, P.; Wasielewski, L.J.; Yang, J.C.; Cai, D.; Evans, C.P.; Murphy, W.J.; Liu, C. The Immunotherapy and Immunosuppressive Signaling in Therapy-Resistant Prostate Cancer. Biomedicines 2022, 10, 1778. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, C.; Zu, C.; Rong, X.; Yu, Q.; Jiang, J. Synergistic Potential of Nanomedicine in Prostate Cancer Immunotherapy: Breakthroughs and Prospects. Int. J. Nanomed. 2024, 19, 9459–9486. [Google Scholar] [CrossRef]
- Rizzo, A.; Ricci, A.D.; Lanotte, L.; Lombardi, L.; Di Federico, A.; Brandi, G.; Gadaleta-Caldarola, G. Immune-Based Combinations for Metastatic Triple Negative Breast Cancer in Clinical Trials: Current Knowledge and Therapeutic Prospects. Expert Opin. Investig. Drugs 2022, 31, 557–565. [Google Scholar] [CrossRef]
- Bahreyni, A.; Mohamud, Y.; Luo, H. Emerging Nanomedicines for Effective Breast Cancer Immunotherapy. J. Nanobiotechnol. 2020, 18, 180. [Google Scholar] [CrossRef]
- Evans, S.T.; Jani, Y.; Jansen, C.S.; Yildirim, A.; Kalemoglu, E.; Bilen, M.A. Understanding and Overcoming Resistance to Immunotherapy in Genitourinary Cancers. Cancer Biol. Ther. 2024, 25, 2342599. [Google Scholar] [CrossRef]
- Coletti, R.; Leonardelli, L.; Parolo, S.; Marchetti, L. A QSP Model of Prostate Cancer Immunotherapy to Identify Effective Combination Therapies. Sci. Rep. 2020, 10, 9063. [Google Scholar] [CrossRef]
- Sonnenburg, D.W.; Morgans, A.K. Emerging Therapies in Metastatic Prostate Cancer. Curr. Oncol. Rep. 2018, 20, 46. [Google Scholar] [CrossRef]
- Chao, P.-H.; Chan, V.; Li, S.-D. Nanomedicines Modulate the Tumor Immune Microenvironment for Cancer Therapy. Expert Opin. Drug Deliv. 2024, 21, 1719–1733. [Google Scholar] [CrossRef]
- Papalexis, P.; Georgakopoulou, V.; Drossos, P.; Thymara, E.; Nonni, A.; Lazaris, A.; Zografos, G.; Spandidos, D.; Kavantzas, N.; Thomopoulou, G.E. Precision Medicine in Breast Cancer Review. Mol. Clin. Oncol. 2024, 21, 78. [Google Scholar] [CrossRef]
- Simão, D.C.; Zarrabi, K.K.; Mendes, J.L.; Luz, R.; Garcia, J.A.; Kelly, W.K.; Barata, P.C. Bispecific T-Cell Engagers Therapies in Solid Tumors: Focusing on Prostate Cancer. Cancers 2023, 15, 1412. [Google Scholar] [CrossRef]
- Debien, V.; De Caluwé, A.; Wang, X.; Piccart-Gebhart, M.; Tuohy, V.K.; Romano, E.; Buisseret, L. Immunotherapy in Breast Cancer: An Overview of Current Strategies and Perspectives. NPJ Breast Cancer 2023, 9, 7. [Google Scholar] [CrossRef]
- Cha, H.-R.; Lee, J.H.; Ponnazhagan, S. Revisiting Immunotherapy: A Focus on Prostate Cancer. Cancer Res. 2020, 80, 1615–1623. [Google Scholar] [CrossRef]
- Meng, L.; Yang, Y.; Mortazavi, A.; Zhang, J. Emerging Immunotherapy Approaches for Treating Prostate Cancer. Int. J. Mol. Sci. 2023, 24, 14347. [Google Scholar] [CrossRef]
- Chen, M.; Hu, S.; Li, Y.; Jiang, T.T.; Jin, H.; Feng, L. Targeting Nuclear Acid-Mediated Immunity in Cancer Immune Checkpoint Inhibitor Therapies. Sig. Transduct. Target. Ther. 2020, 5, 270. [Google Scholar] [CrossRef]
- Li, A.; Fang, J. Anti-angiogenic Therapy Enhances Cancer Immunotherapy: Mechanism and Clinical Application. Interdiscip. Med. 2024, 2, e20230025. [Google Scholar] [CrossRef]
- Hu, Z.I.; McArthur, H.L. Immunotherapy in Breast Cancer: The New Frontier. Curr. Breast Cancer Rep. 2018, 10, 35–40. [Google Scholar] [CrossRef]
- Tallarida, R.J. Quantitative Methods for Assessing Drug Synergism. Genes Cancer 2011, 2, 1003–1008. [Google Scholar] [CrossRef]
- Plana, D.; Palmer, A.C.; Sorger, P.K. Independent Drug Action in Combination Therapy: Implications for Precision Oncology. Cancer Discov. 2022, 12, 606–624. [Google Scholar] [CrossRef]
- Gong, Y.; Chen, M.; Tan, Y.; Shen, J.; Jin, Q.; Deng, W.; Sun, J.; Wang, C.; Liu, Z.; Chen, Q. Injectable Reactive Oxygen Species-Responsive SN38 Prodrug Scaffold with Checkpoint Inhibitors for Combined Chemoimmunotherapy. ACS Appl. Mater. Interfaces 2020, 12, 50248–50259. [Google Scholar] [CrossRef]
- Jiang, X.; Lee, M.; Xia, J.; Luo, T.; Liu, J.; Rodriguez, M.; Lin, W. Two-Stage SN38 Release from a Core–Shell Nanoparticle Enhances Tumor Deposition and Antitumor Efficacy for Synergistic Combination with Immune Checkpoint Blockade. ACS Nano 2022, 16, 21417–21430. [Google Scholar] [CrossRef]
- Chung, Y.M.; Khan, P.P.; Wang, H.; Tsai, W.-B.; Qiao, Y.; Yu, B.; Larrick, J.W.; Hu, M.C.-T. Sensitizing Tumors to Anti-PD-1 Therapy by Promoting NK and CD8+ T Cells via Pharmacological Activation of FOXO3. J. Immunother. Cancer 2021, 9, e002772. [Google Scholar] [CrossRef]
- Lee, Y.; Chen, Y.; Ou, D.; Hsu, C.; Liu, J.; Ko, J.; Hu, M.C.; Tan, C. SN-38, an Active Metabolite of Irinotecan, Enhances anti-PD11 Treatment Efficacy in Head and Neck Squamous Cell Carcinoma. J. Pathol. 2023, 259, 428–440. [Google Scholar] [CrossRef]
- Iwai, T.; Sugimoto, M.; Wakita, D.; Yorozu, K.; Kurasawa, M.; Yamamoto, K. Topoisomerase I Inhibitor, Irinotecan, Depletes Regulatory T Cells and up-Regulates MHC Class I and PD-L1 Expression, Resulting in a Supra-Additive Antitumor Effect When Combined with Anti-PD-L1 Antibodies. Oncotarget 2018, 9, 31411–31421. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, Z.; Dang, Q.; Xu, H.; Lv, J.; Li, H.; Han, X. Immunosuppression in Tumor Immune Microenvironment and Its Optimization from CAR-T Cell Therapy. Theranostics 2022, 12, 6273–6290. [Google Scholar] [CrossRef]
- Wong, D.V.T.; Ribeiro-Filho, H.V.; Wanderley, C.W.S.; Leite, C.A.V.G.; Lima, J.B.; Assef, A.N.B.; Cajado, A.G.; Batista, G.L.P.; González, R.H.; Silva, K.O.; et al. SN-38, the Active Metabolite of Irinotecan, Inhibits the Acute Inflammatory Response by Targeting Toll-like Receptor 4. Cancer Chemother. Pharmacol. 2019, 84, 287–298. [Google Scholar] [CrossRef]
- Kuzmich, N.; Sivak, K.; Chubarev, V.; Porozov, Y.; Savateeva-Lyubimova, T.; Peri, F. TLR4 Signaling Pathway Modulators as Potential Therapeutics in Inflammation and Sepsis. Vaccines 2017, 5, 34. [Google Scholar] [CrossRef]
- Jacobsen, T.; Hernandez, P.; Chahine, N. Inhibition of Toll-like Receptor 4 Protects against Inflammation-Induced Mechanobiological Alterations to Intervertebral Disc Cells. Eur. Cells Mater. 2021, 41, 576–591. [Google Scholar] [CrossRef]
- Rialdi, A.; Campisi, L.; Zhao, N.; Lagda, A.C.; Pietzsch, C.; Ho, J.S.Y.; Martinez-Gil, L.; Fenouil, R.; Chen, X.; Edwards, M.; et al. Topoisomerase 1 Inhibition Suppresses Inflammatory Genes and Protects from Death by Inflammation. Science 2016, 352, aad7993. [Google Scholar] [CrossRef]
- Böttcher, J.P.; Bonavita, E.; Chakravarty, P.; Blees, H.; Cabeza-Cabrerizo, M.; Sammicheli, S.; Rogers, N.C.; Sahai, E.; Zelenay, S.; Reis E Sousa, C. NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell 2018, 172, 1022–1037.e14. [Google Scholar] [CrossRef]
- Li, J.; Dong, T.; Wu, Z.; Zhu, D.; Gu, H. The Effects of MYC on Tumor Immunity and Immunotherapy. Cell Death Discov. 2023, 9, 103. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Y.; Wang, S.; Shen, Q.; Zhou, X. The Role of STAT3 in Leading the Crosstalk between Human Cancers and the Immune System. Cancer Lett. 2018, 415, 117–128. [Google Scholar] [CrossRef]
- Zou, S.; Tong, Q.; Liu, B.; Huang, W.; Tian, Y.; Fu, X. Targeting STAT3 in Cancer Immunotherapy. Mol. Cancer 2020, 19, 145. [Google Scholar] [CrossRef]
- Dhatchinamoorthy, K.; Colbert, J.D.; Rock, K.L. Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Front. Immunol. 2021, 12, 636568. [Google Scholar] [CrossRef]
- Wu, X.; Li, T.; Jiang, R.; Yang, X.; Guo, H.; Yang, R. Targeting MHC-I Molecules for Cancer: Function, Mechanism, and Therapeutic Prospects. Mol. Cancer 2023, 22, 194. [Google Scholar] [CrossRef]
- Liang, Y.-H.; Tsai, J.-H.; Cheng, Y.-M.; Chan, K.-Y.; Hsu, W.-L.; Lee, C.-C.; Chen, K.-H.; Yeh, K.-H. Chemotherapy Agents Stimulate Dendritic Cells against Human Colon Cancer Cells through Upregulation of the Transporter Associated with Antigen Processing. Sci. Rep. 2021, 11, 9080. [Google Scholar] [CrossRef]
- Donati, G.; Amati, B. MYC and Therapy Resistance in Cancer: Risks and Opportunities. Mol. Oncol. 2022, 16, 3828–3854. [Google Scholar] [CrossRef]
- Cyberski, T.F.; Singh, A.; Korzinkin, M.; Mishra, V.; Pun, F.; Shen, L.; Wing, C.; Cheng, X.; Baird, B.; Miao, Y.; et al. Acquired Resistance to Immunotherapy and Chemoradiation in MYC Amplified Head and Neck Cancer. NPJ Precis. Oncol. 2024, 8, 114. [Google Scholar] [CrossRef]
- Chen, M.; Wang, T.; Tian, D.; Hai, C.; Qiu, Z. Induction, Growth, Drug Resistance, and Metastasis: A Comprehensive Summary of the Relationship between STAT3 and Gastric Cancer. Heliyon 2024, 10, e37263. [Google Scholar] [CrossRef]
- Fares, C.M.; Van Allen, E.M.; Drake, C.G.; Allison, J.P.; Hu-Lieskovan, S. Mechanisms of Resistance to Immune Checkpoint Blockade: Why Does Checkpoint Inhibitor Immunotherapy Not Work for All Patients? Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 147–164. [Google Scholar] [CrossRef]
- Bai, R.; Chen, N.; Li, L.; Du, N.; Bai, L.; Lv, Z.; Tian, H.; Cui, J. Mechanisms of Cancer Resistance to Immunotherapy. Front. Oncol. 2020, 10, 1290. [Google Scholar] [CrossRef]
- Jiang, M.; Jia, K.; Wang, L.; Li, W.; Chen, B.; Liu, Y.; Wang, H.; Zhao, S.; He, Y.; Zhou, C. Alterations of DNA Damage Repair in Cancer: From Mechanisms to Applications. Ann. Transl. Med. 2020, 8, 1685. [Google Scholar] [CrossRef]
- Jiang, M.; Jia, K.; Wang, L.; Li, W.; Chen, B.; Liu, Y.; Wang, H.; Zhao, S.; He, Y.; Zhou, C. Alterations of DNA Damage Response Pathway: Biomarker and Therapeutic Strategy for Cancer Immunotherapy. Acta Pharm. Sin. B 2021, 11, 2983–2994. [Google Scholar] [CrossRef]
- Hassan, M.; Trung, V.; Bedi, D.; Shaddox, S.; Gunturu, D.; Yates, C.; Datta, P.; Samuel, T. Interference with Pathways Activated by Topoisomerase Inhibition Alters the Surface Expression of PD-L1 and MHC I in Colon Cancer Cells. Oncol. Lett. 2022, 25, 41. [Google Scholar] [CrossRef]
- Xie, N.; Shen, G.; Gao, W.; Huang, Z.; Huang, C.; Fu, L. Neoantigens: Promising Targets for Cancer Therapy. Signal Transduct. Target. Ther. 2023, 8, 9. [Google Scholar] [CrossRef]
- Chung, Y.M.; Tsai, W.B.; Khan, P.P.; Ma, J.; Berek, J.S.; Larrick, J.W.; Hu, M.C.-T. FOXO3-Dependent Suppression of PD-L1 Promotes Anticancer Immune Responses via Activation of Natural Killer Cells. Am. J. Cancer Res. 2022, 12, 1241–1263. [Google Scholar]
- Koliqi, R.; Dimchevska, S.; Geskovski, N.; Petruševski, G.; Chacorovska, M.; Pejova, B.; Hristov, D.R.; Ugarkovic, S.; Goracinova, K. PEO-PPO-PEO/PolyDL-Lactide-Co-Caprolactone Nanoparticles as Carriers for SN-38: Design, Optimization and Nano-Bio Interface Interactions. Curr. Drug Deliv. 2016, 13, 339–352. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Rouhani, H.; Sepehri, N.; Varshochian, R.; Ghahremani, M.H.; Amini, M.; Gharghabi, M.; Ostad, S.N.; Atyabi, F.; Baharian, A.; et al. Biotin Decorated PLGA Nanoparticles Containing SN-38 Designed for Cancer Therapy. Artif. Cells Nanomed. Biotechnol. 2017, 45, 495–504. [Google Scholar] [CrossRef]
- Monterrubio, C.; Paco, S.; Olaciregui, N.G.; Pascual-Pasto, G.; Vila-Ubach, M.; Cuadrado-Vilanova, M.; Ferrandiz, M.M.; Castillo-Ecija, H.; Glisoni, R.; Kuplennik, N.; et al. Targeted Drug Distribution in Tumor Extracellular Fluid of GD2-Expressing Neuroblastoma Patient-Derived Xenografts Using SN-38-Loaded Nanoparticles Conjugated to the Monoclonal Antibody 3F8. J. Control. Release 2017, 255, 108–119. [Google Scholar] [CrossRef]
- Narsinh, K.H.; Kumar, K.; Bankiewicz, K.; Martin, A.J.; Berger, M.; Clarke, J.; Taylor, J.; Bush, N.A.O.; Molinaro, A.M.; Aghi, M.; et al. A Phase I Study of Convection-Enhanced Delivery CED of Liposomal-Irinotecan Using Real-Time Magnetic Resonance Imaging in Patients with Recurrent High-Grade Glioma. J. Neurooncol. 2025, 172, 219–227. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, X.; Wang, H.; Liu, X.; Zhang, Q.; Li, K.; Chen, Y.; Zhu, Q.; Shen, Y.; Sui, M. A Nanotherapeutic Strategy to Overcome Chemoresistance to Irinotecan/7-Ethyl-10-Hydroxy-Camptothecin in Colorectal Cancer. Acta Biomater. 2022, 137, 262–275. [Google Scholar] [CrossRef]
- Sharkey, R.M.; Govindan, S.V.; Cardillo, T.M.; Goldenberg, D.M. Epratuzumab–SN-38: A New Antibody–Drug Conjugate for the Therapy of Hematologic Malignancies. Mol. Cancer Ther. 2012, 11, 224–234. [Google Scholar] [CrossRef]
- Moon, S.-J.; Govindan, S.V.; Cardillo, T.M.; D’Souza, C.A.; Hansen, H.J.; Goldenberg, D.M. Antibody Conjugates of 7-Ethyl-10-Hydroxycamptothecin SN-38 for Targeted Cancer Chemotherapy. J. Med. Chem. 2008, 51, 6916–6926. [Google Scholar] [CrossRef]
- Yang, S.-J.; Pai, J.-A.; Yao, C.-J.; Huang, C.-H.; Chen, J.L.; Wang, C.-H.; Chen, K.-C.; Shieh, M.-J. SN38-Loaded Nanomedicine Mediates Chemo-Radiotherapy against CD44-Expressing Cancer Growth. Cancer Nanotechnol. 2023, 14, 1. [Google Scholar] [CrossRef]
- Ribeiro, R.; Carvalho, M.J.; Goncalves, J.; Moreira, J.N. Immunotherapy in Triple-Negative Breast Cancer: Insights into Tumor Immune Landscape and Therapeutic Opportunities. Front. Mol. Biosci. 2022, 9, 903065. [Google Scholar] [CrossRef]
- Liu, Y.; Hatano, K.; Nonomura, N. Liquid Biomarkers in Prostate Cancer Diagnosis: Current Status and Emerging Prospects. World J. Mens Health 2025, 43, 8. [Google Scholar] [CrossRef]
- Lohajová Behulová, R.; Bugalová, A.; Bugala, J.; Struhárňanská, E.; Šafranek, M.; Juráš, I. Circulating Exosomal miRNAs as a Promising Diagnostic Biomarker in Cancer. Physiol. Res. 2023, 72, S193–S207. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Kulkarni, R.P. Circulating Biomarkers Predictive of Tumor Response to Cancer Immunotherapy. Expert Rev. Mol. Diagn. 2019, 19, 895–904. [Google Scholar] [CrossRef]
- Zhou, Y.; Tao, L.; Qiu, J.; Xu, J.; Yang, X.; Zhang, Y.; Tian, X.; Guan, X.; Cen, X.; Zhao, Y. Tumor Biomarkers for Diagnosis, Prognosis and Targeted Therapy. Sig. Transduct. Target. Ther. 2024, 9, 132. [Google Scholar] [CrossRef]
- Lawler, M.; Keeling, P.; Kholmanskikh, O.; Minnaard, W.; Moehlig-Zuttermeister, H.; Normanno, N.; Philip, R.; Popp, C.; Salgado, R.; Santiago-Walker, A.E.; et al. Empowering Effective Biomarker-Driven Precision Oncology: A Call to Action. Eur. J. Cancer 2024, 209, 114225. [Google Scholar] [CrossRef]
- Díaz-Villamarín, X.; Nieto-Sánchez, M.T.; Martínez-Pérez, M.; Novo-González, P.; Fernández-Varón, E.; Torres-García, A.; González Astorga, B.; Blancas, I.; Cabeza-Barrera, J.; Morón, R. Dose-Limiting Toxicities and the Maximum Tolerated Dose of Irinotecan Based on UGT1A1 Genotypes: A Systematic Review. Pharmaceutics 2025, 17, 542. [Google Scholar] [CrossRef]
- Fletcher, K.; Johnson, D.B. Chronic Immune-Related Adverse Events Arising from Immune Checkpoint Inhibitors: An Update. J. Immuno Ther. Cancer 2024, 12, e008591. [Google Scholar] [CrossRef]
- Shalit, A.; Sarantis, P.; Koustas, E.; Trifylli, E.-M.; Matthaios, D.; Karamouzis, M.V. Predictive Biomarkers for Immune-Related Endocrinopathies Following Immune Checkpoint Inhibitors Treatment. Cancers 2023, 15, 375. [Google Scholar] [CrossRef]
- Shalata, W.; Abu-salman, A.; Steckbeck, R.; Mathew Jacob, B.; Massalha, I.; Yakobson, A. Cardiac Toxicity Associated with Immune Checkpoint Inhibitors: A Systematic Review. Cancers 2021, 13, 5218. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, S.; Tang, H.; Meng, X.; Zheng, Q. Molecular Mechanisms of Immunotherapy Resistance in Triple-Negative Breast Cancer. Front. Immunol. 2023, 14, 1153990. [Google Scholar] [CrossRef]
- Ghemrawi, R.; Abuamer, L.; Kremesh, S.; Hussien, G.; Ahmed, R.; Mousa, W.; Khoder, G.; Khair, M. Revolutionizing Cancer Treatment: Recent Advances in Immunotherapy. Biomedicines 2024, 12, 2158. [Google Scholar] [CrossRef] [PubMed]
- Saini, K.S.; Punie, K.; Twelves, C.; Bortini, S.; De Azambuja, E.; Anderson, S.; Criscitiello, C.; Awada, A.; Loi, S. Antibody-Drug Conjugates, Immune-Checkpoint Inhibitors, and Their Combination in Breast Cancer Therapeutics. Expert Opin. Biol. Ther. 2021, 21, 945–962. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adekiya, T.A.; Adesina, S.K. Leveraging Synergy: A Review of the Therapeutic Potential of SN-38 and Immune Checkpoint Blockade in Breast and Prostate Cancer Treatment. J. Pers. Med. 2025, 15, 512. https://doi.org/10.3390/jpm15110512
Adekiya TA, Adesina SK. Leveraging Synergy: A Review of the Therapeutic Potential of SN-38 and Immune Checkpoint Blockade in Breast and Prostate Cancer Treatment. Journal of Personalized Medicine. 2025; 15(11):512. https://doi.org/10.3390/jpm15110512
Chicago/Turabian StyleAdekiya, Tayo A., and Simeon K. Adesina. 2025. "Leveraging Synergy: A Review of the Therapeutic Potential of SN-38 and Immune Checkpoint Blockade in Breast and Prostate Cancer Treatment" Journal of Personalized Medicine 15, no. 11: 512. https://doi.org/10.3390/jpm15110512
APA StyleAdekiya, T. A., & Adesina, S. K. (2025). Leveraging Synergy: A Review of the Therapeutic Potential of SN-38 and Immune Checkpoint Blockade in Breast and Prostate Cancer Treatment. Journal of Personalized Medicine, 15(11), 512. https://doi.org/10.3390/jpm15110512




