1. Introduction
Sentinel lymph node biopsy (SLNB) is considered the gold standard for axillary staging in early-stage breast cancer as it indicates the need for axillary lymph node dissection (ALND) and adjuvant therapy [
1]. While this method provides useful prognostic information, its benefits in early-stage breast cancer, especially for elderly patients, are limited, particularly for those at low risk of axillary lymph node metastasis [
2].
Trials such as SOUND and CALGB 9343 have shown that, in carefully selected older women with hormone receptor–positive tumors, omission of SLNB does not compromise oncologic outcomes while reducing surgical burden [
3,
4]. These data have reinforced a broader tendency toward de-escalating axillary surgery in the elderly. At the same time, several studies suggest that tumor biology and comorbidities may be more relevant than nodal status in determining survival in this population [
5].
This perspective is especially relevant given the potential risks associated with the procedure. Patients undergoing SLNB are exposed to complications such as lymphedema, seroma formation, wound infections, reduced arm mobility, and sensory disturbances. These complications, while often considered manageable, are not negligible, even among older cohorts, and can substantially impair the quality of life of potentially already frail patients [
6,
7].
Considering this evidence, it appears safe to say that there is a need for personalized, tailored surgical approaches and more generally toward individualized therapy in elderly breast cancer patients. The primary goal is to limit unnecessary procedures, reduce hospitalization time, and minimize treatment-related complications without compromising oncological results. However, certain high-risk subtypes, such as HER2+ and TNBC, are associated with a higher risk of recurrence [
8,
9,
10,
11], and patients with these diagnoses often continue to undergo axillary staging.
In an effort to support a more balanced, patient-centered strategy, we retrospectively analysed outcomes in a cohort of very elderly women (≥80 years) with HER2+ or TNBC breast cancer treated surgically without SLNB or ALND. The aim of this exploratory study is not to provide definitive evidence but rather to generate preliminary data and contextualize results within the existing literature. In doing so, we seek to contribute to the ongoing discussion on tailored axillary management strategies for elderly patients with higher-risk subtypes.
2. Materials and Methods
2.1. Study Design and Population
This study is a single-center, retrospective observational analysis conducted at the Breast Surgery Unit of San Martino Hospital in Genoa, Italy. It focused on women aged 80 years and older who were diagnosed with either HER2+ or TNBC and treated surgically between March 2016 and October 2024 (n = 39). The study aimed to evaluate the outcomes of omitting SLNB in this elderly population, a group often underrepresented in clinical trials.
Eligible patients included those with a histologically confirmed diagnosis of HER2+ or TNBC who were clinically node-negative (cN0) based on axillary ultrasound assessment. Preoperative axillary ultrasound was performed in all patients using high-frequency linear transducers (≥10 MHz) in the supine position with the ipsilateral arm abducted, and radiologists assessed nodal morphology (cortical thickness, hilum preservation, shape, size). Any suspicious findings were documented, and if indicated, additional targeted imaging or biopsy was performed. All participating radiologists adhered to the same standardized criteria to minimize interobserver variability.
All patients underwent definitive surgical management, which included either breast-conserving surgery (quadrantectomy) or mastectomy, without any form of axillary surgery. To the purpose of outcome evaluation, only patients with at least one year of follow-up data were included in the survival analyses.
Exclusion criteria were defined to maintain a homogeneous study population and to reduce potential confounding factors. Specifically, we excluded patients younger than 80 years, those with luminal breast cancer subtypes, patients with clinically positive axillary lymph nodes, individuals who underwent axillary surgical procedures, and those with incomplete tumor staging or missing follow-up data. These criteria were applied to focus the study on a well-characterized elderly cohort in which the potential benefits and risks of omitting axillary surgery could be meaningfully assessed.
All eligible and consenting patients were included. Patients who met diagnostic inclusion criteria but elected to undergo SLNB were excluded, as were patients requiring SLNB or ALND based on diagnostic findings or those with a prior history of these procedures.
Patients were enrolled consecutively as part of routine clinical care, without randomization or experimental intervention. Informed consent for the use of medical data for research purposes was obtained during preoperative evaluations. Throughout the study, patient privacy and confidentiality were rigorously maintained, with all data anonymized and managed in compliance with General Data Protection Regulation (GDPR) guidelines.
2.2. Data Collection
Comprehensive data were retrospectively collected from the hospital’s electronic medical records and cross-verified by two independent reviewers to ensure accuracy. Collected variables included patient demographics (age at diagnosis), tumor characteristics such as size, histological grade, receptor status, proliferation index (Ki-67), and overall tumor biology (HER2+ or TNBC). Treatment-related information included details of the surgical procedure (quadrantectomy or mastectomy) and any perioperative or postoperative complications, including wound infections, seroma, hematoma, lymphedema, or functional limitations affecting arm mobility.
Oncological outcomes recorded included locoregional recurrence, distant metastasis, overall survival (OS), and disease-free survival (DFS). Follow-up information was obtained from routine outpatient visits and imaging studies.
2.3. Definitions
Overall survival (OS) was defined as the interval between the date of surgery and the date of death from any cause. Patients still alive at the data cut-off (7 April 2025) were censored at that date.
Disease-free survival (DFS) was defined as the time from surgery to the first documented breast cancer recurrence (local, regional, or distant) or death from any cause. Patients without an event were censored at the end of follow-up (7 April 2025).
Tumor subgroups were defined according to standard pathological and biomarker criteria. Tumor size categories were defined as ≤pT1c versus ≥pT2, and proliferation index was categorized as low (Ki-67 ≤ 25%) versus high (Ki-67 > 25%) for stratified analyses.
2.4. Statistical Analysis
Survival analyses were conducted using Kaplan–Meier methods to estimate OS and DFS, with survival curves compared using the log-rank test. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using Cox proportional hazards regression models to evaluate the association of clinical and pathological factors with survival outcomes.
Subgroup analyses were performed to investigate potential differences based on tumor biomarker subtype (HER2+ vs. TNBC), tumor size (≤pT1c vs. ≥pT2), and Ki-67 proliferation index (≤25% vs. >25%). All statistical tests were two-sided, and a
p-value < 0.05 was considered statistically significant. Statistical analyses were conducted using R software (version 4.2.2; R Foundation for Statistical Computing, Vienna, Austria). Survival analyses, including Kaplan–Meier estimates and Cox proportional hazards models, were performed using the survival package (version 3.8-3) [
12]. Survival curves were visualized using the survminer package (version 0.5.1) [
13] and ggplot2 (version 4.0.0) [
14]. All analyses were performed according to standard statistical practices and guidelines.
3. Results
3.1. Patient Characteristics
A total of 39 women aged ≥80 years (mean age at surgery: 85.8 ± 4.0 years; range: 80.3–97.4) were included. Among them, 28 (71.8%) had HER2+ tumors and 11 (28.2%) TNBC. All were clinically node-negative and underwent surgery without SLNB or ALND. About 47.1% had a prior history of breast cancer (ipsilateral or contralateral).
The median tumor size was 17 mm (IQR: 12–24; mean: 20.7 mm, SD: 18.4). Most were high grade (59% G3), and the majority were classified as pT1c (48.7%) or pT2 (28.2%). The median Ki-67 index was 27.0% (IQR: 21.9–58.1), with 47.2% ≤25%.
The surgical approach was mostly conservative, with quadrantectomy performed in 27 cases (69.2%).
During follow-up, five recurrences occurred (12.8%): three local (managed with mastectomy) and two axillary (managed with ALND). The median follow-up was 36.5 months (IQR: 21–49), based on all 39 patients.
Only two complications arose (5.1%): a wound dehiscence (which required another surgery) and a wound infection (which was successfully managed with antibiotics and outpatient care).
The characteristics of the cohort are summarized in
Table 1.
3.2. Survival Outcomes
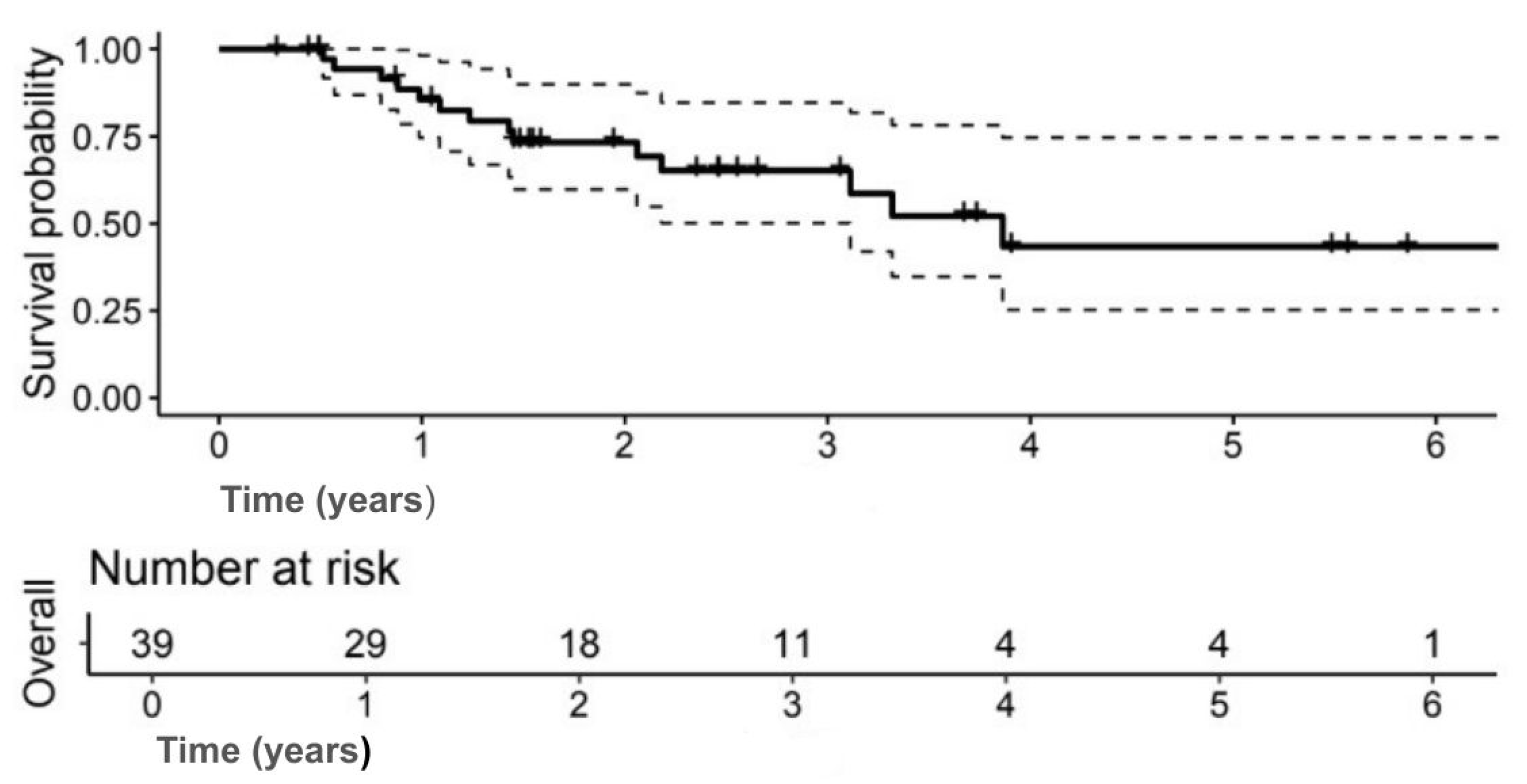
The median OS was 3.9 years (95% CI: 3.1–NA); 5-year OS: 43.4% (95% CI: 25.3–74.6) (
Figure 1). The 5-year DFS was 37.7% (95% CI: 21.5–66.2) (
Figure 2).
3.3. Subgroup Analyses
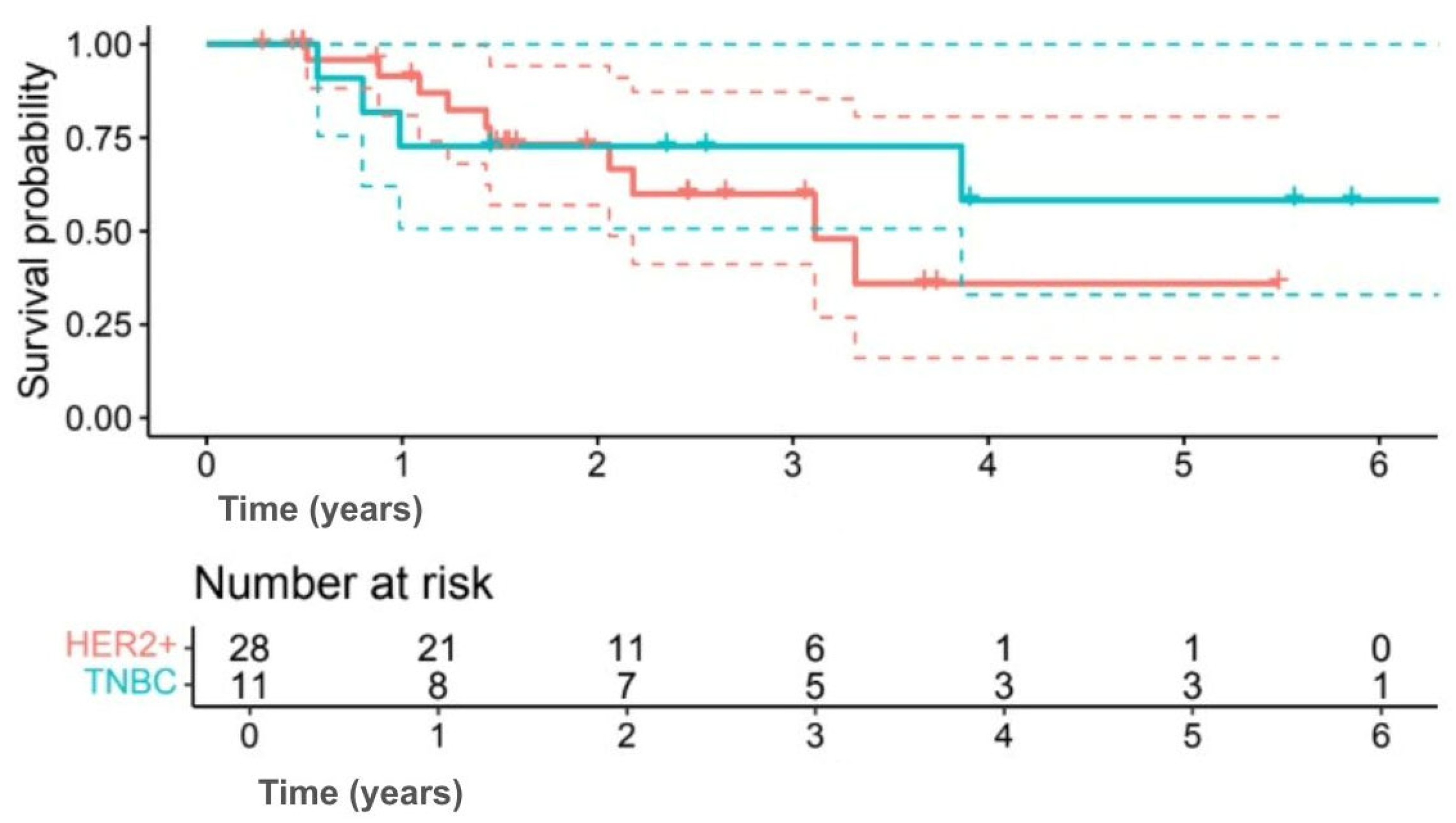
Exploratory subgroup analyses suggested differences in survival outcomes, although none were statistically significant. Among subtypes, 5-year OS was 58.2% for TNBC compared with 35.9% for HER2+ (HR 0.60, 95% CI 0.17–2.09;
p = 0.414) (
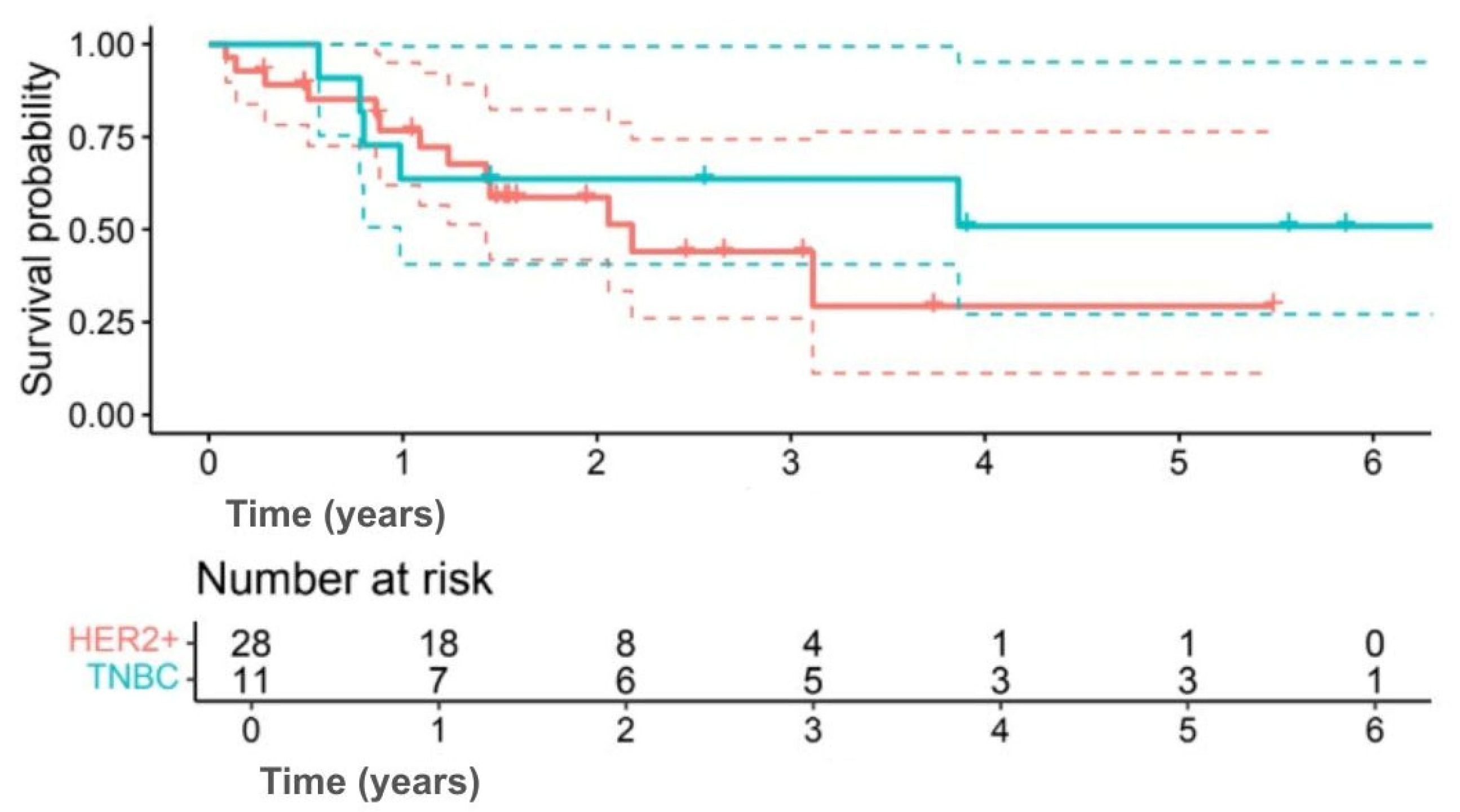
Figure 3), and 5-year DFS was 50.9% versus 29.4%, respectively (HR 0.63, 95% CI 0.19–2.12;
p = 0.402) (
Figure 4). Interpretation was limited by small sample numbers.
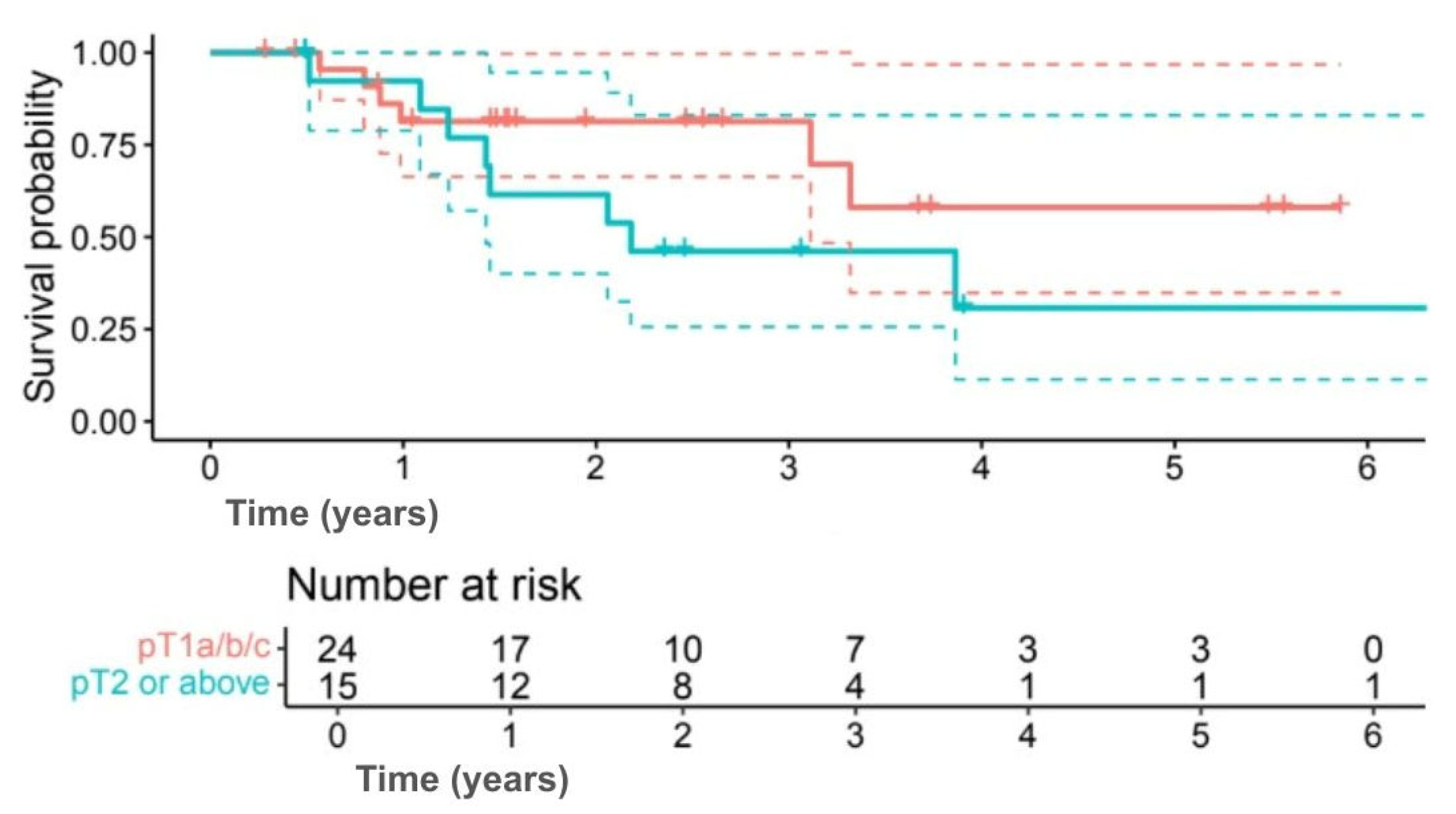
Tumor size showed similar results, with 5-year OS of 58.1% for tumors ≤ pT1c compared with 30.8% for tumors ≥ pT2 (
p = 0.118) (
Figure 5), and DFS of 51.9% versus 25.6% (
p = 0.133) (
Figure 6).
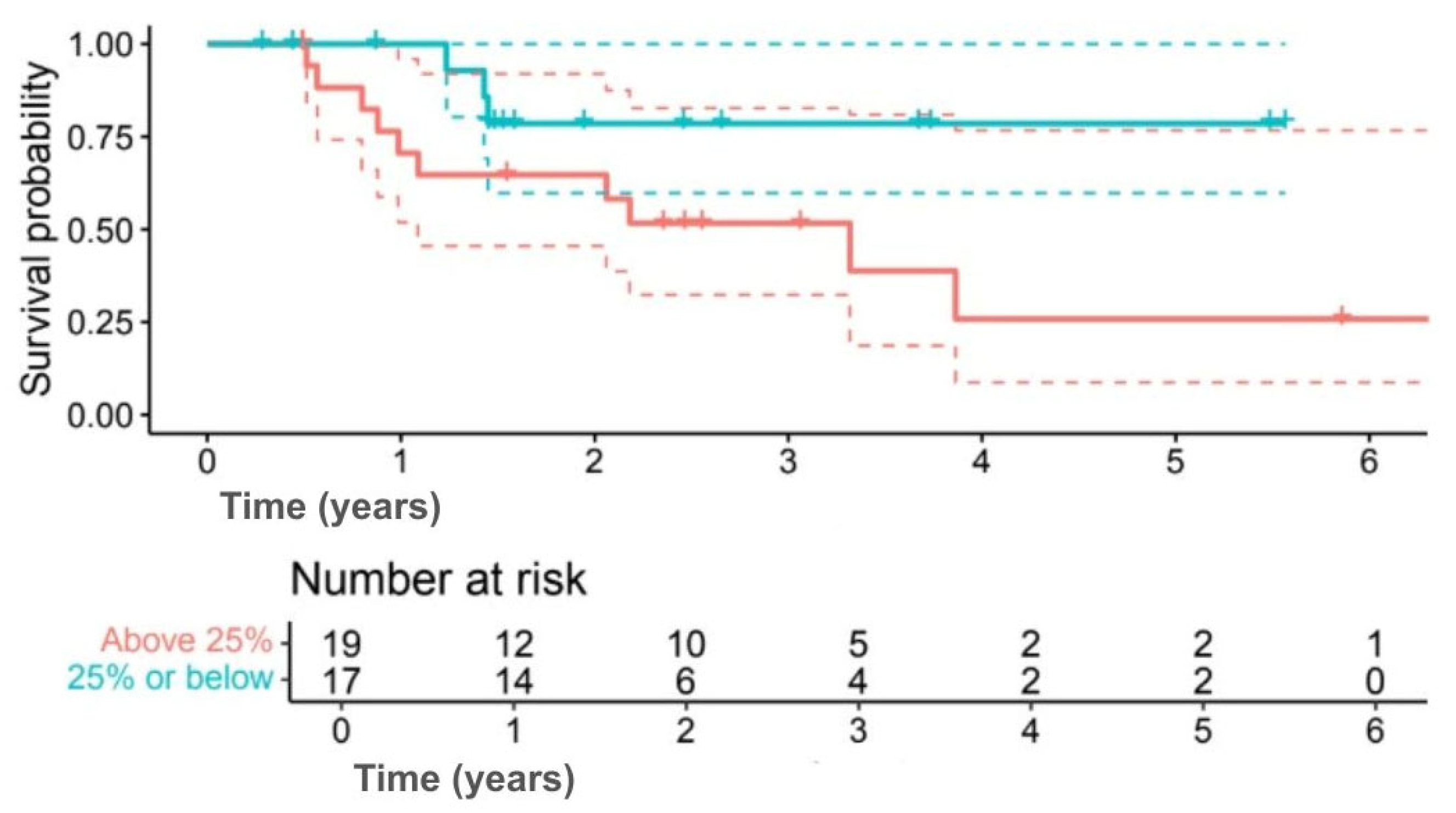
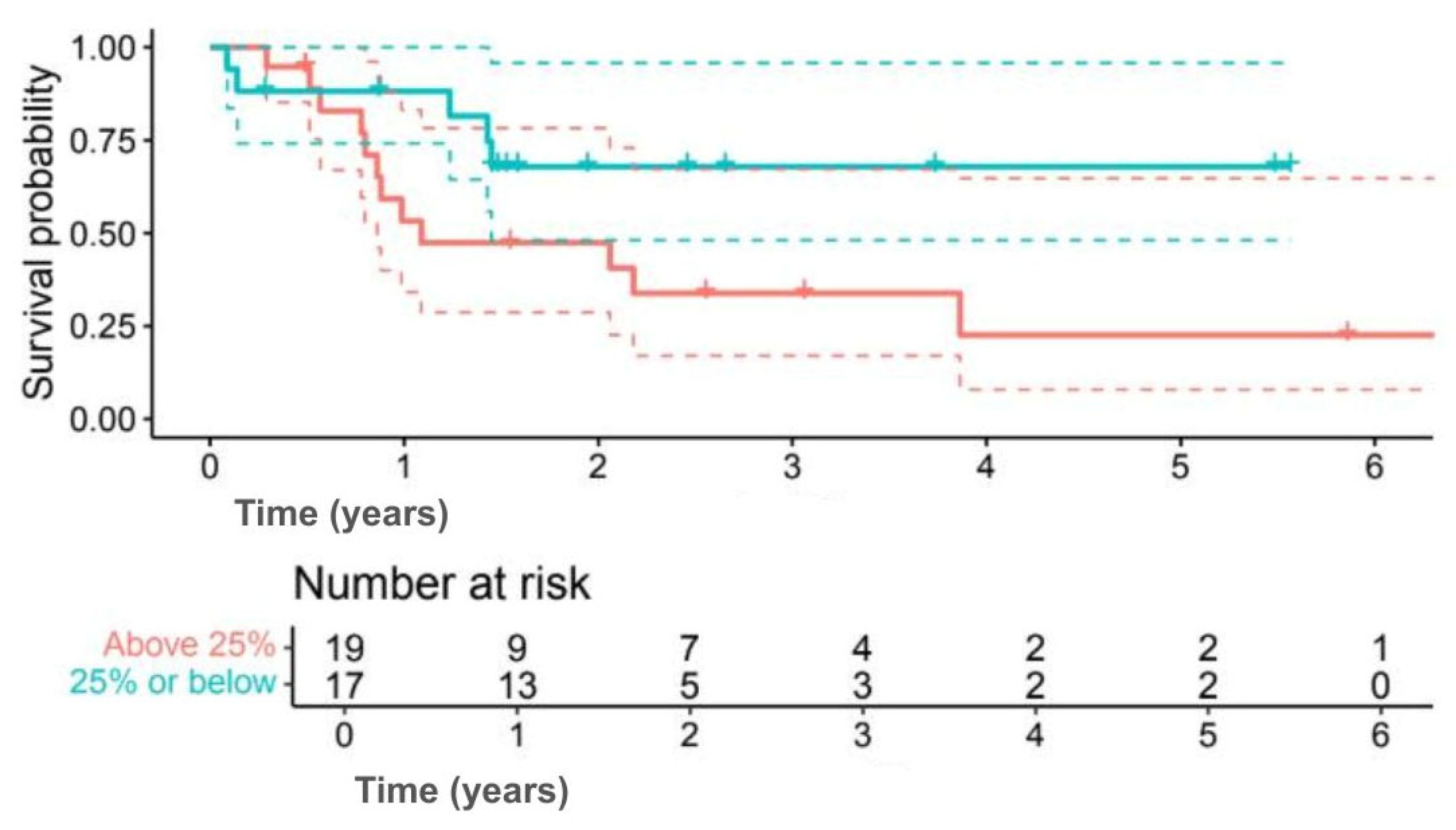
Analysis by Ki-67 index indicated numerically better outcomes for lower proliferation. Five-year OS was 78.6% for Ki-67 ≤ 25% compared with 25.9% for Ki-67 > 25% (
p = 0.080) (
Figure 7), while 5-year DFS was 67.9% versus 22.6% (
p = 0.092) (
Figure 8). Although outcomes consistently favoured the low Ki-67 group, differences were not statistically significant.
4. Discussion
Our results align with those of previous studies and give no indication that SLNB omission negatively affects OS or DFS. To contextualize these findings, we reviewed relevant literature examining older breast cancer patients (≥70–75 years) who underwent SLNB. Gu et al. (2022) showed no significant DFS difference in patients >70 years based on SLNB status [
2]. Chung et al. demonstrated that SLNB in women ≥65 years often escalates treatment without survival benefit [
15]. Reimer et al., analyzing over 5000 women in the INSEMA trial confirmed no OS disadvantage when SLNB was omitted in low-risk older patients [
16]. Collectively, these data emphasize that tumor biology and comorbidities may outweigh nodal status in determining prognosis [
17]. In this context, our results suggest that omission of SLNB may be safe even in higher-risk subtypes.
Notably, TNBC patients in our series did not show worse outcomes than HER2+ cases, though this must be interpreted with caution given the very small numbers and curve crossing in survival analyses.
Emerging literature also indicates that systemic therapy plays a dominant role in outcomes for these subtypes [
18]. For HER2+ disease, large databases show nodal positivity is exceedingly rare among those achieving pathological complete response after neoadjuvant HER2-targeted therapy, limiting the incremental value of SLNB [
19]. For TNBC, studies in older patients highlight that undertreatment (especially chemotherapy omission) is associated with worse outcomes, while aggressive local therapy confers limited benefit in very elderly patients [
11,
20]. Together, this supports the notion that when systemic therapy is already indicated, the additional prognostic contribution of SLNB may be limited in this age group.
It is also important to consider the natural life expectancy of women in this age group. For example, in many high-income countries, women aged 85–90 years have a median life expectancy of approximately 5–7 years [
21]. This reality underscores the necessity of balancing the potential benefits of invasive staging procedures against the risks of complications, recovery time, and impact on overall well-being, particularly when the likelihood of breast cancer–related mortality may be lower than competing risks.
We acknowledge several limitations in our study, including its retrospective design, single-center nature, and relatively small sample size (reflecting strict age-based eligibility). Importantly, the absence of an internal control group of elderly patients who underwent SLNB precludes causal inference; comparisons were drawn from existing literature. Furthermore, cause-of-death data were unavailable, preventing analysis of cancer-specific survival. As for the results, we recognize that subgroup analyses were underpowered; they are presented as exploratory data. Hazard ratios are reported descriptively but should not be overinterpreted.
Nevertheless, we believe that these findings provide support for personalized, biomarker-driven surgical strategies in elderly breast cancer patients, highlighting the potential to tailor axillary management to individual risk profiles and optimize quality of life without compromising oncological outcomes.
5. Conclusions
Omitting SLNB in very elderly patients (≥80 years) with HER2+ or TNBC and no clinical nodal involvement may represent a reasonable option in carefully selected cases. This strategy may be particularly appropriate for patients with smaller tumors and low Ki-67 proliferation indices, where the risk of axillary metastasis is relatively low. By reducing the extent of surgical intervention, clinicians can ethically minimize the risk of procedure-related complications, including lymphedema, wound infections, and impaired arm function, while still maintaining effective oncological outcomes for this vulnerable patient population.
Importantly, the decision to omit SLNB should be made following a comprehensive assessment by a multidisciplinary team, accounting for tumor biology, patient comorbidities, and overall functional status. Such individualized planning ensures that the benefits of de-escalated surgery are balanced against potential oncological risks.
Our results should be interpreted as preliminary rather than definitive. Nonetheless, they highlight the potential role of individualized surgical de-escalation strategies in elderly patients.
To further validate these observations and support evidence-based clinical practice, larger multicenter studies are needed. These studies should aim to confirm the safety and long-term outcomes of omitting SLNB in elderly patients with high-risk breast cancer subtypes, ultimately contributing to the development of more personalized and patient-centered approaches to breast cancer management in older populations.
Author Contributions
Conceptualization, A.C.d. and P.F.; methodology, A.C.d., P.F., R.D. and R.A.; data curation, A.C.d., R.A. and R.D.; writing—original draft preparation, A.C.d., R.A. and P.F.; writing—review and editing, A.C.d., R.D. and P.F.; supervision, P.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the analysis of retrospective data. All data were anonymized and managed in accordance with the guidelines of the General Data Protection Regulation (GDPR).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data supporting reported results are available in institutional database.
Acknowledgments
The authors would like to thank Letizia Cuniolo, Federica Murelli, Chiara Cornacchia, Francesca Depaoli, Marco Gipponi, Cecilia Margarino, Chiara Boccardo, Simonetta Franchelli, Marianna Pesce, Martina Cossu and Saad Abdallah for their dedicated support and valuable contribution in data collection and curation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Manca, G.; Tardelli, E.; Rubello, D.; Gennaro, M.; Marzola, M.C.; Cook, G.J.; Volterrani, D. Sentinel lymph node biopsy in breast cancer: A technical and clinical appraisal. Nucl. Med. Commun. 2016, 37, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Chen, X.; Wang, L.; He, Y.; Ouyang, T.; Li, J.; Wang, T.; Fan, T.; Fan, Z. Impact of sentinel lymph node biopsy on treatment decision and survival in patients aged ≥70 years with breast cancer: A retrospective study. Technol. Cancer Res. Treat. 2022, 21, 15330338221137216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gentilini, O.D.; Botteri, E.; Sangalli, C.; Galimberti, V.; Porpiglia, M.; Agresti, R.; Luini, A.; Viale, G.; Cassano, E.; Peradze, N.; et al. Sentinel lymph node biopsy vs. no axillary surgery in patients with small breast cancer and negative results on ultrasonography of axillary lymph nodes: The SOUND randomized clinical trial. JAMA Oncol. 2023, 9, 1557–1564. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hughes, K.S.; Schnaper, L.A.; Bellon, J.R.; Cirrincione, C.T.; Berry, D.A.; McCormick, B.; Muss, H.B.; Smith, B.L.; Hudis, C.A.; Winer, E.P.; et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: Long-term follow-up of CALGB 9343. J. Clin. Oncol. 2013, 31, 2382–2387. [Google Scholar] [CrossRef] [PubMed]
- van la Parra, R.F.; Peer, P.G.; Ernst, M.F.; Bosscha, K. Meta-analysis of predictive factors for non-sentinel lymph node metastases in breast cancer patients with a positive SLN. Eur. J. Surg. Oncol. 2011, 37, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Fregatti, P.; Gipponi, M.; Giacchino, M.; Sparavigna, M.; Murelli, F.; Toni, M.L.; Calabrò, M.T.; Orsino, L.; Friedman, D. Breast cancer surgery during the COVID-19 pandemic: An observational clinical study of the breast surgery clinic at Ospedale Policlinico San Martino—Genoa, Italy. In Vivo 2020, 34 (Suppl. 3), 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Killelea, B.K.; Long, J.B.; Dang, W.; Mougalian, S.S.; Evans, S.B.; Gross, C.P.; Wang, S.Y. Associations between sentinel lymph node biopsy and complications for patients with ductal carcinoma in situ. Ann. Surg. Oncol. 2018, 25, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.J.; Kim, S.H.; Kang, B.J.; Gil Choi, B.; Kim, H.S.; Cha, E.S.; Song, B.J. Breast cancer recurrence according to molecular subtype. Asian Pac. J. Cancer Prev. 2014, 15, 5539–5544. [Google Scholar] [CrossRef] [PubMed]
- Cirmena, G.; Dameri, M.; Ravera, F.; Fregatti, P.; Ballestrero, A.; Zoppoli, G. Assessment of circulating nucleic acids in cancer: From current status to future perspectives and potential clinical applications. Cancers 2021, 13, 3460. [Google Scholar] [CrossRef] [PubMed]
- Zoppoli, G.; Garuti, A.; Cirmena, G.; di Cantogno, L.V.; Botta, C.; Gallo, M.; Ferraioli, D.; Carminati, E.; Baccini, P.; Curto, M.; et al. HER2 assessment using quantitative reverse transcriptase polymerase chain reaction reliably identifies HER2 overexpression without amplification in breast cancer cases. J. Transl. Med. 2017, 15, 91. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Knapp, G.; Quan, M.L.; Bouchard-Fortier, A. Cancer-specific outcomes in the elderly with triple-negative breast cancer: A systematic review. Curr. Oncol. 2021, 28, 2337–2345. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Therneau, T.M. A Package for Survival Analysis in R. R Package Survival. CRAN. 2020. Available online: https://CRAN.R-project.org/package=survival (accessed on 28 July 2025).
- Kassambara, A.; Kosinski, M.; Biecek, P. Survminer: Drawing Survival Curves Using ‘ggplot2’. R Package Survminer. CRAN’. 2019. Available online: https://CRAN.R-project.org/package=survminer (accessed on 26 May 2021).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 28 July 2025).
- Chung, A.P.; Dang, C.M.; Karlan, S.R.; Amersi, F.F.; Phillips, E.M.; Boyle, M.K.; Cui, Y.; Giuliano, A.E. A Prospective Study of Sentinel Node Biopsy Omission in Women Age ≥65 Years with ER+ Breast Cancer. Ann. Surg. Oncol. 2024, 31, 3160–3167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reimer, T.; Stachs, A.; Veselinovic, K.; Kühn, T.; Heil, J.; Polata, S.; Marmé, F.; Müller, T.; Hildebrandt, G.; Krug, D.; et al. Axillary Surgery in Breast Cancer—Primary Results of the INSEMA Trial. N. Engl. J. Med. 2024, 391, 1011–1022. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Finkelman, B.S.; Zhang, H.; Hicks, D.G.; Turner, B.M. The evolution of Ki-67 and breast carcinoma: Past observations, present directions, and future considerations. Cancers 2023, 15, 808. [Google Scholar] [CrossRef] [PubMed]
- Baranova, A.; Krasnoselskyi, M.; Starikov, V.; Kartashov, S.; Zhulkevych, I.; Vlasenko, V.; Oleshko, K.; Bilodid, O.; Sadchikova, M.; Vinnyk, Y. Triple-negative breast cancer: Current treatment strategies and factors of negative prognosis. J. Med. Life 2022, 15, 153–161. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dalton, J.C.; Crowell, K.-A.; Ntowe, K.W.; van den Bruele, A.B.; DiNome, M.L.; Rosenberger, L.H.; Thomas, S.M.; Wang, T.; Hwang, E.S.; Plichta, J.K. Utility of axillary staging in older patients with HER2-positive breast cancer. Ann. Surg. Oncol. 2024, 31, 7621–7633. [Google Scholar] [CrossRef] [PubMed]
- Xiu, M.; Zhang, P. Treatment and prognosis analysis of elderly patients with nonmetastatic triple-negative breast cancer. J. Clin. Oncol. 2019, 37 (Suppl. 15), e12038. [Google Scholar] [CrossRef]
- OECD. Society at a Glance 2024: OECD Social Indicators; OECD Publishing: Paris, France, 2024. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).