Navigating the Decision to Discontinue Intravitreal Injection Therapy in End-Stage Neovascular Age-Related Macular Degeneration
Abstract
1. Introduction
2. Materials and Methods
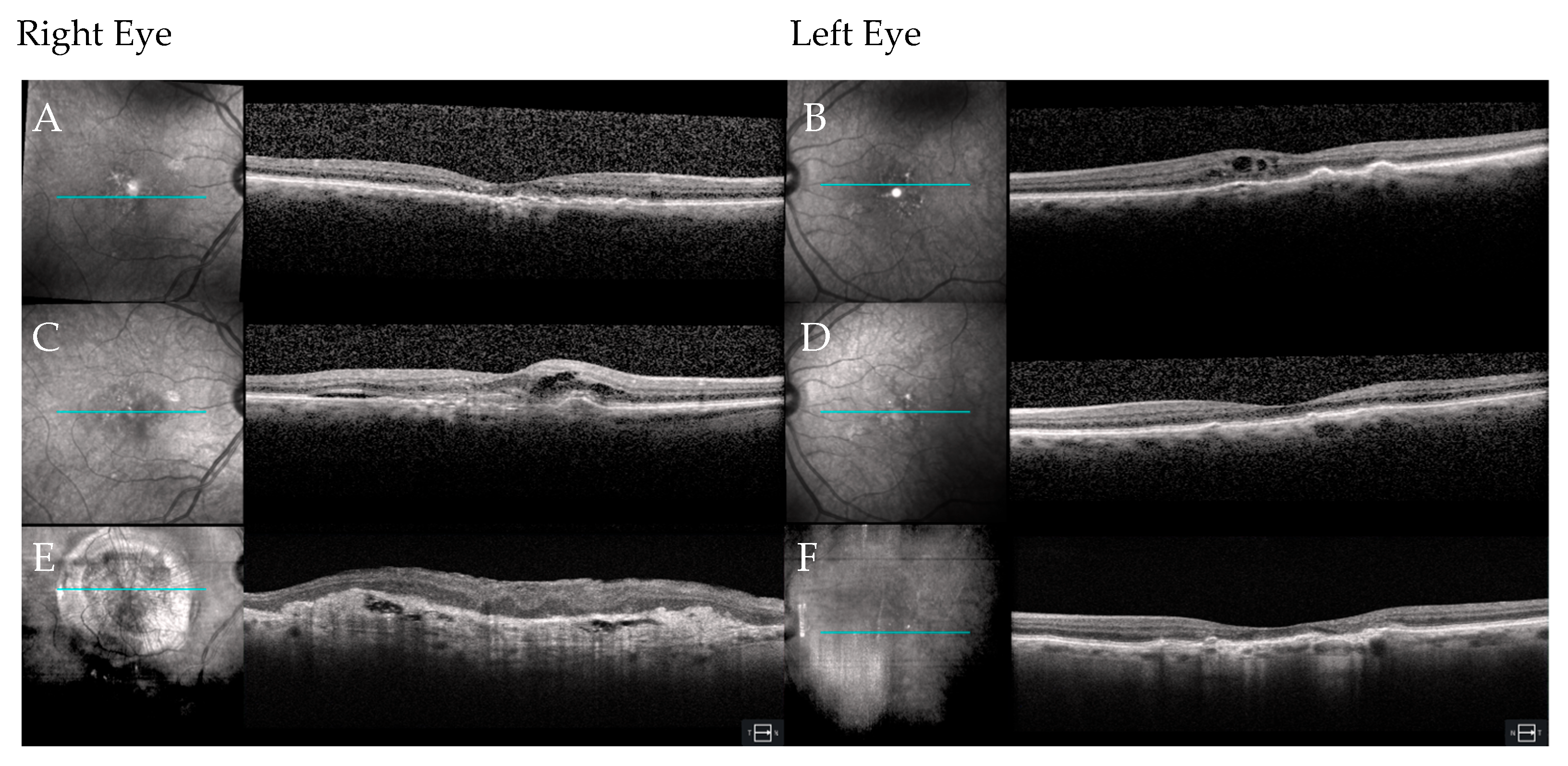
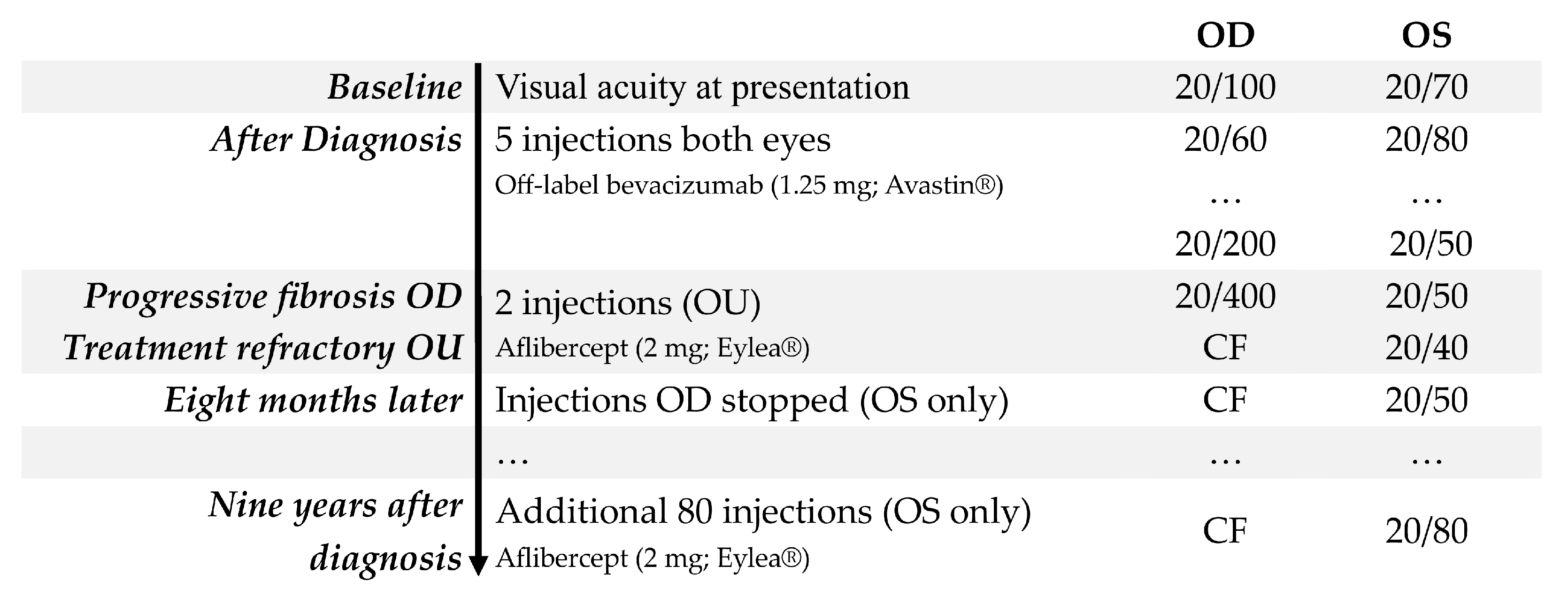
3. Results
3.1. Case 1
3.2. Case 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMD | Age-related macular degeneration |
| nAMD | Neovascular age-related macular degeneration |
| VEGF | Vascular endothelial growth factor |
| CARE | CAse REport |
| CF | Counting fingers |
| RPE | Retinal pigment epithelium |
| BCVA | Best-corrected visual acuity |
| OCT | Optical coherence tomography |
| MARINA | Minimally classic/Occult trial of the Anti-VEGF antibody Ranibizumab in the treatment of neovascular age-related macular degeneration. |
| VIEW | Vascular Endothelial Growth Factor Trap-Eye: Investigation of Efficacy and Safety in Wet Age-Related Macular Degeneration |
References
- Wong, T.Y.; Ferreira, A.; Hughes, R.; Carter, G.; Mitchell, P. Epidemiology and disease burden of pathologic myopia and myopic choroidal neovascularization: An evidence-based systematic review. Am. J. Ophthalmol. 2014, 157, 9.e12–25.e12. [Google Scholar] [CrossRef]
- Maguire, M.G. Comparing treatments for age-related macular degeneration: Safety, effectiveness and cost. LDI Issue Brief 2012, 17, 1–4. [Google Scholar] [PubMed]
- Gilligan, A.K.; Ramsey, D.J. Beyond longer intervals: Advocating for regular treatment of neovascular AMD. J. Clin. Med. 2024, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y.; MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Daniel, E.; Toth, C.A.; Grunwald, J.E.; Jaffe, G.J.; Martin, D.F.; Fine, S.L.; Huang, J.; Ying, G.S.; Hagstrom, S.A.; Winter, K.; et al. Comparison of age-related macular degeneration treatments trials research group. Risk of scar in the comparison of age-related macular degeneration treatments trials. Ophthalmology 2014, 121, 656–666. [Google Scholar] [CrossRef]
- Gemenetzi, M.; Lotery, A.J.; Patel, P.J. Risk of geographic atrophy in age-related macular degeneration patients treated with intravitreal anti-VEGF agents. Eye 2017, 31, 1–9. [Google Scholar] [CrossRef]
- Keenan, T.D.; Agrón, E.; Domalpally, A.; Clemons, T.E.; van Asten, F.; Wong, W.T.; Danis, R.G.; Sadda, S.; Rosenfeld, P.J.; Klein, M.L.; et al. Progression of geographic atrophy in age-related macular degeneration: AREDS2 Report Number 16. Ophthalmology 2018, 125, 1913–1928. [Google Scholar] [CrossRef]
- Armendariz, B.G.; Chakravarthy, U. Fibrosis in age-related neovascular macular degeneration in the anti-VEGF era. Eye 2024, 38, 3243–3251. [Google Scholar] [CrossRef]
- Comparison of Age-Related Macular Degeneration Treatments Trials (CATT) Research Group; Writing Committee; Martin, D.F.; Maguire, M.G.; Fine, S.L.; Ying, G.S.; Jaffe, G.J.; Grunwald, J.E.; Toth, C.; Redford, M.; et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: Two-year results. Ophthalmology 2020, 127, S135–S145. [Google Scholar] [CrossRef]
- Aslanis, S.; Amrén, U.; Lindberg, C.; Epstein, D. Recurrent neovascular age-related macular degeneration after discontinuation of vascular endothelial growth factor inhibitors managed in a treat-and-extend regimen. Ophthalmol. Retina 2022, 6, 15–20. [Google Scholar] [CrossRef]
- Willoughby, A.S.; Ying, G.S.; Toth, C.A.; Maguire, M.G.; Burns, R.E.; Grunwald, J.E.; Daniel, E.; Jaffe, G.J. Comparison of age-related macular degeneration treatments trials research group. Subretinal hyperreflective material in the comparison of age-related macular degeneration treatments trials. Ophthalmology 2015, 122, 1846–1853.e5. [Google Scholar] [CrossRef]
- Li, E.; Donati, S.; Lindsley, K.B.; Krzystolik, M.G.; Virgili, G. Treatment regimens for administration of anti-vascular endothelial growth factor agents for neovascular age-related macular degeneration. Cochrane Database Syst. Rev. 2020, 5, CD012208. [Google Scholar] [CrossRef]
- Ciulla, T.A.; Huang, F.; Westby, K.; Williams, D.F.; Zaveri, S.; Patel, S.C. Real-world outcomes of anti-vascular endothelial growth factor therapy in neovascular age-related macular degeneration in the United States. Ophthalmol. Retina 2018, 2, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.S.; Brown, D.M.; Chong, V.; Korobelnik, J.F.; Kaiser, P.K.; Nguyen, Q.D.; Kirchhof, B.; Ho, A.; Ogura, Y.; Yancopoulos, G.D.; et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012, 119, 2537–2548. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.S.; Khanani, A.M.; Quezada Ruiz, C.; Basu, K.; Ferrone, P.J.; Brittain, C.; Figueroa, M.S.; Lin, H.; Holz, F.G.; Patel, V.; et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): Two randomised, double-masked, phase 3, non-inferiority trials. Lancet 2022, 399, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Ziemssen, F.; Feltgen, N.; Holz, F.G.; Guthoff, R.; Ringwald, A.; Bertelmann, T.; Wiedon, A.; Korb, C.; OCEAN Study Group. Demographics of patients receiving intravitreal anti-VEGF treatment in real-world practice: Healthcare research data versus randomized controlled trials. BMC Ophthalmol. 2017, 17, 7. [Google Scholar] [CrossRef]
- Cao, X.; Sanchez, J.C.; Patel, T.P.; Yang, Z.; Guo, C.; Malik, D.; Sopeyin, A.; Montaner, S.; Sodhi, A. Aflibercept more effectively weans patients with neovascular age-related macular degeneration off therapy compared with bevacizumab. J. Clin. Investig. 2023, 133, e159125. [Google Scholar] [CrossRef]
- Channa, R.; Sophie, R.; Bagheri, S.; Shah, S.M.; Wang, J.; Adeyemo, O.; Sodhi, A.; Wenick, A.; Ying, H.S.; Campochiaro, P.A. Regression of choroidal neovascularization results in macular atrophy in anti-vascular endothelial growth factor-treated eyes. Am. J. Ophthalmol. 2015, 159, 9–19.e2. [Google Scholar] [CrossRef]
- Landa, G.; Su, E.; Garcia, P.M.; Seiple, W.H.; Rosen, R.B. Inner segment-outer segment junctional layer integrity and corresponding retinal sensitivity in dry and wet forms of age-related macular degeneration. Retina 2011, 31, 364–370. [Google Scholar] [CrossRef]
- Archambault, S.D.; Sweeny, C.; Bhardwaj, M.; Ramsey, D.J. Low vision rehabilitation referral characteristics for patients with neovascular age-related macular degeneration. Healthcare 2025, 13, 64. [Google Scholar] [CrossRef]
- Awh, K.C.; Mahmoudzadeh, R.; Salabati, M.; Mansour, H.A.; Bechay, J.; Magagna, J.; Regillo, C.D.; Ho, A.C.; Garg, S.J.; Hsu, J. Outcomes of intentionally suspending treatment in eyes with advanced neovascular age-related macular degeneration. Am. J. Ophthalmol. 2023, 256, 20–26. [Google Scholar] [CrossRef]
- Chujo, S.; Matsubara, H.; Mase, Y.; Kato, K.; Kondo, M. Recurrence rate during 5-year period after suspension of anti-vascular endothelial growth factor treatment for neovascular age-related macular degeneration. J. Clin. Med. 2024, 13, 4317. [Google Scholar] [CrossRef]
- Borrelli, E.; Foti, C.; Ulla, L.; Porreca, A.; Introini, U.; Grassi, M.O.; Viggiano, P.; Peronetti, M.; Toscani, R.; Boscia, G.; et al. Incidence and reasons for discontinuation of anti-VEGF treatment in neovascular age-related macular degeneration. Br. J. Ophthalmol. 2025, 109, 875–881. [Google Scholar] [CrossRef]
- Canadian Agency for Drugs and Technologies in Health. Aflibercept (Eylea): Treatment of Neovascular (Wet) Age-Related Macular Degeneration (wAMD). Available online: https://www.ncbi.nlm.nih.gov/books/NBK349613/ (accessed on 4 August 2025).
- U.S. Centers for Disease Control and Prevention. Vision and Eye Health Surveillance System (VEHSS). VEHSS Modeled Estimates: Age-Related Macular Degeneration (AMD). Available online: https://www.cdc.gov/vision-health-data/prevalence-estimates/amd-prevalence.html (accessed on 4 August 2025).
- Ahmed, A.; Ali, M.; Dun, C.; Cai, C.X.; Makary, M.A.; Woreta, F.A. Geographic distribution of US ophthalmic surgical subspecialists. JAMA Ophthalmol. 2025, 143, 117–124. [Google Scholar] [CrossRef]
- Sivaprasad, S.; Bailey, C.; Downey, L.; Gilbert, R.; Gale, R.; Kotagiri, A.; Mahmood, S.; Morgan-Warren, P.; Napier, J.; Narendran, N.; et al. Real-world service costs for neovascular-AMD clinics in the United Kingdom: Structured literature review and scenario analysis. Curr. Med. Res. Opin. 2024, 40, 1221–1233. [Google Scholar] [CrossRef]
- Brown, G.C.; Brown, M.M.; Stein, J.D.; Smiddy, W.E.; Ophthalmic Utility Research Study Group. Vision-related quality of life associated with unilateral and bilateral ocular conditions. Ophthalmology 2018, 125, 965–971. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, G.; Fenu, E.; Bennett, N.; Almond, C. Intravitreal ranibizumab for the treatment of visual impairment due to choroidal neovascularization associated with rare diseases: Cost-effectiveness in the UK. Adv. Ther. 2019, 36, 632–644. [Google Scholar] [CrossRef]
- Calvo, P.; Abadia, B.; Ferreras, A.; Ruiz-Moreno, O.; Leciñena, J.; Torrón, C. Long-term visual outcome in wet age-related macular degeneration patients depending on the number of ranibizumab injections. J. Ophthalmol. 2015, 2015, 820605. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.C.; Kleinman, D.M.; Lum, F.C.; Heier, J.S.; Lindstrom, R.L.; Orr, S.C.; Chang, G.C.; Smith, E.L.; Pollack, J.S. Baseline visual acuity at wet AMD diagnosis predicts long-term vision outcomes: An analysis of the IRIS Registry. Ophthalmic Surg. Lasers Imaging Retina 2020, 51, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, N.; Upasani, D.; Ghanchi, F.D. Patterns of treatment discontinuation in patients receiving anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Indian J. Ophthalmol. 2022, 70, 2065–2070. [Google Scholar] [CrossRef]
- Kusenda, P.; Caprnda, M.; Gabrielova, Z.; Kukova, N.; Pavlovic, S.; Stefanickova, J. Understanding loss to follow-up in AMD patients receiving VEGF inhibitor therapy: Associated factors and underlying reasons. Diagnostics 2024, 14, 400. [Google Scholar] [CrossRef]
- Munzar, R.; Roh, S.; Ramsey, D.J. Factors associated with loss to follow-up in patients with advanced age-related macular degeneration: A telehealth recall initiative. Ophthalmic Physiol. Opt. 2024, 44, 626–633. [Google Scholar] [CrossRef]
- Garweg, J.G.; Traine, P.G.; Garweg, R.A.; Wons, J.; Gerhardt, C.; Pfister, I.B. Continued anti-VEGF treatment does not prevent recurrences in eyes with stable neovascular age-related macular degeneration using a treat-and-extend regimen: A retrospective case series. Eye 2022, 36, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Archambault, S.D.; Nichols, M.M.; McCullum, J.C.; Zhang, Y.; Steinberger, E.E.; Ramsey, D.J. Patient adherence to therapy after switch to aflibercept from bevacizumab or ranibizumab for treatment-refractory neovascular age-related macular degeneration. Indian J. Ophthalmol. 2024, 72, S101–S105. [Google Scholar] [CrossRef]
- Savant, S.V.; Kwan, J.T.; Barouch, F.; Chang, J.; Ramsey, D.J.; Marx, J.; Blaha, G.; Klein-Mascia, K. Durability and efficacy of faricimab in treatment-resistant retinal edema utilizing "real-world" dosing regimens. J. Ophthalmol. 2024, 2024, 8583348. [Google Scholar] [CrossRef]
- Obeid, A.; Gao, X.; Ali, F.S.; Aderman, C.M.; Shahlaee, A.; Adam, M.K.; Kasi, S.K.; Hyman, L.; Ho, A.C.; Hsu, J. Loss to follow-up among patients with neovascular age-related macular degeneration who received intravitreal anti-vascular endothelial growth factor injections. JAMA Ophthalmol. 2018, 136, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Sarver, J.; Baker, D.W. Effect of language barriers on follow-up appointments after an emergency department visit. J. Gen. Intern. Med. 2000, 15, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Khanani, A.M.; Kotecha, A.; Chang, A.; Chen, S.J.; Chen, Y.; Guymer, R.; Heier, J.S.; Holz, F.G.; Iida, T.; Ives, J.A.; et al. TENAYA and LUCERNE: Two-year results from the phase 3 neovascular age-related macular degeneration trials of faricimab with treat-and-extend dosing in year 2. Ophthalmology 2024, 131, 914–926. [Google Scholar] [CrossRef]
- Yuzawa, M.; Fujita, K.; Wittrup-Jensen, K.U.; Norenberg, C.; Zeitz, O.; Adachi, K.; Wang, E.C.; Heier, J.; Kaiser, P.; Chong, V.; et al. Improvement in vision-related function with intravitreal aflibercept: Data from phase 3 studies in wet age-related macular degeneration. Ophthalmology 2015, 122, 571–578. [Google Scholar] [CrossRef]
- Jackson, M.L.; Virgili, G.; Shepherd, J.D.; Di Nome, M.A.; Fletcher, D.C.; Kaleem, M.A.; Lam, L.A.; Lawrence, L.M.; Sunness, J.S.; Riddering, A.T. Vision Rehabilitation Preferred Practice Pattern®. Ophthalmology 2023, 130, P271–P335. [Google Scholar] [CrossRef]
- World Health Organization. World Report on Vision. Available online: https://www.who.int/publications/i/item/world-report-on-vision (accessed on 31 July 2025).
- Brown, M.M.; Brown, G.C.; Sharma, S.; Landy, J. Health care economic analyses and value-based medicine. Surv. Ophthalmol. 2003, 48, 204–223. [Google Scholar] [CrossRef] [PubMed]
- Moshtaghion, S.M.M.; Locri, F.; Reyes, A.P.; Plastino, F.; Kvanta, A.; Morillo-Sanchez, M.J.; Rodríguez-de-la-Rúa, E.; Gutierrez-Sanchez, E.; Montero-Sánchez, A.; Lucena-Padros, H.; et al. VEGF in tears as a biomarker for exudative age-related macular degeneration: Molecular dynamics in a mouse model and human samples. Int. J. Mol. Sci. 2025, 26, 3855. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bennie, J.; Ramsey, D.J. Navigating the Decision to Discontinue Intravitreal Injection Therapy in End-Stage Neovascular Age-Related Macular Degeneration. J. Pers. Med. 2025, 15, 487. https://doi.org/10.3390/jpm15100487
Bennie J, Ramsey DJ. Navigating the Decision to Discontinue Intravitreal Injection Therapy in End-Stage Neovascular Age-Related Macular Degeneration. Journal of Personalized Medicine. 2025; 15(10):487. https://doi.org/10.3390/jpm15100487
Chicago/Turabian StyleBennie, Justin, and David J. Ramsey. 2025. "Navigating the Decision to Discontinue Intravitreal Injection Therapy in End-Stage Neovascular Age-Related Macular Degeneration" Journal of Personalized Medicine 15, no. 10: 487. https://doi.org/10.3390/jpm15100487
APA StyleBennie, J., & Ramsey, D. J. (2025). Navigating the Decision to Discontinue Intravitreal Injection Therapy in End-Stage Neovascular Age-Related Macular Degeneration. Journal of Personalized Medicine, 15(10), 487. https://doi.org/10.3390/jpm15100487








