ICU Readmission and In-Hospital Mortality Rates for Patients Discharged from the ICU—Risk Factors and Validation of a New Predictive Model: The Worse Outcome Score (WOScore)
Abstract
1. Background
2. Methods
2.1. Study Design
2.1.1. Outcome Definitions
2.1.2. Ethical Considerations
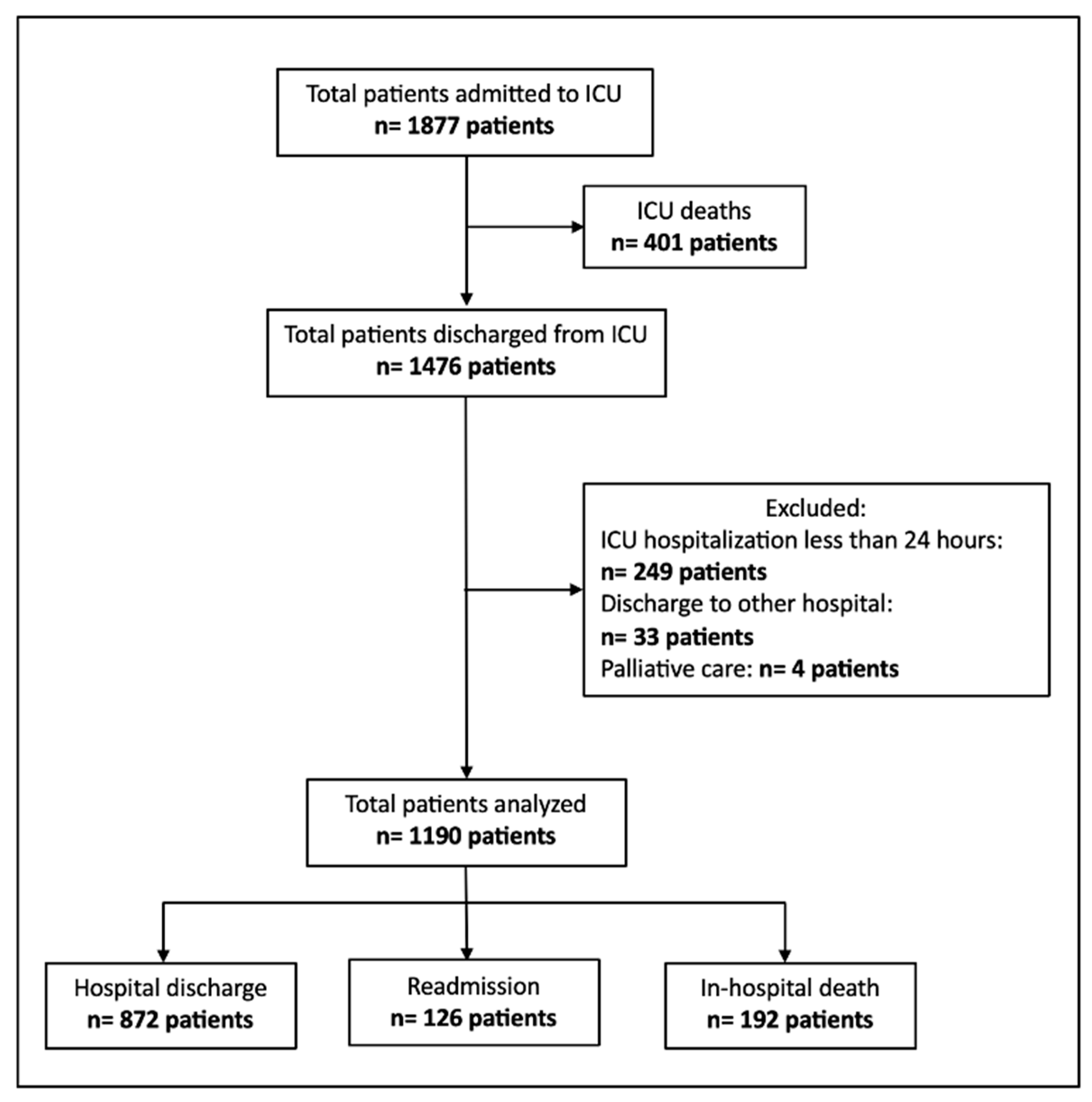
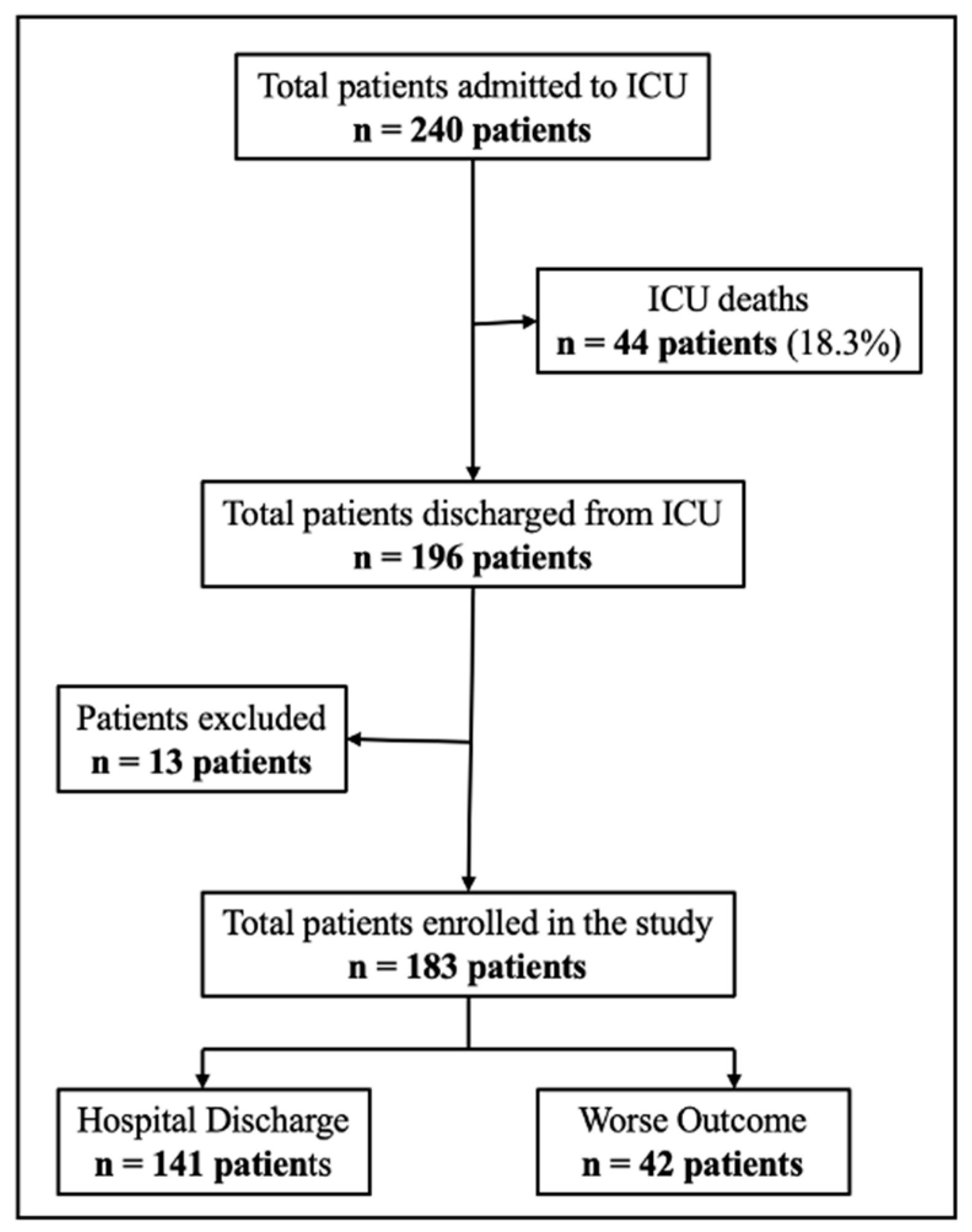
2.2. Population and Data Collection
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Variable Selection
2.4. Statistical Modeling
2.5. Score Construction and Validation
3. Results
3.1. Risk Factors for ICU Readmission
3.2. Risk Factors for In-Hospital Mortality
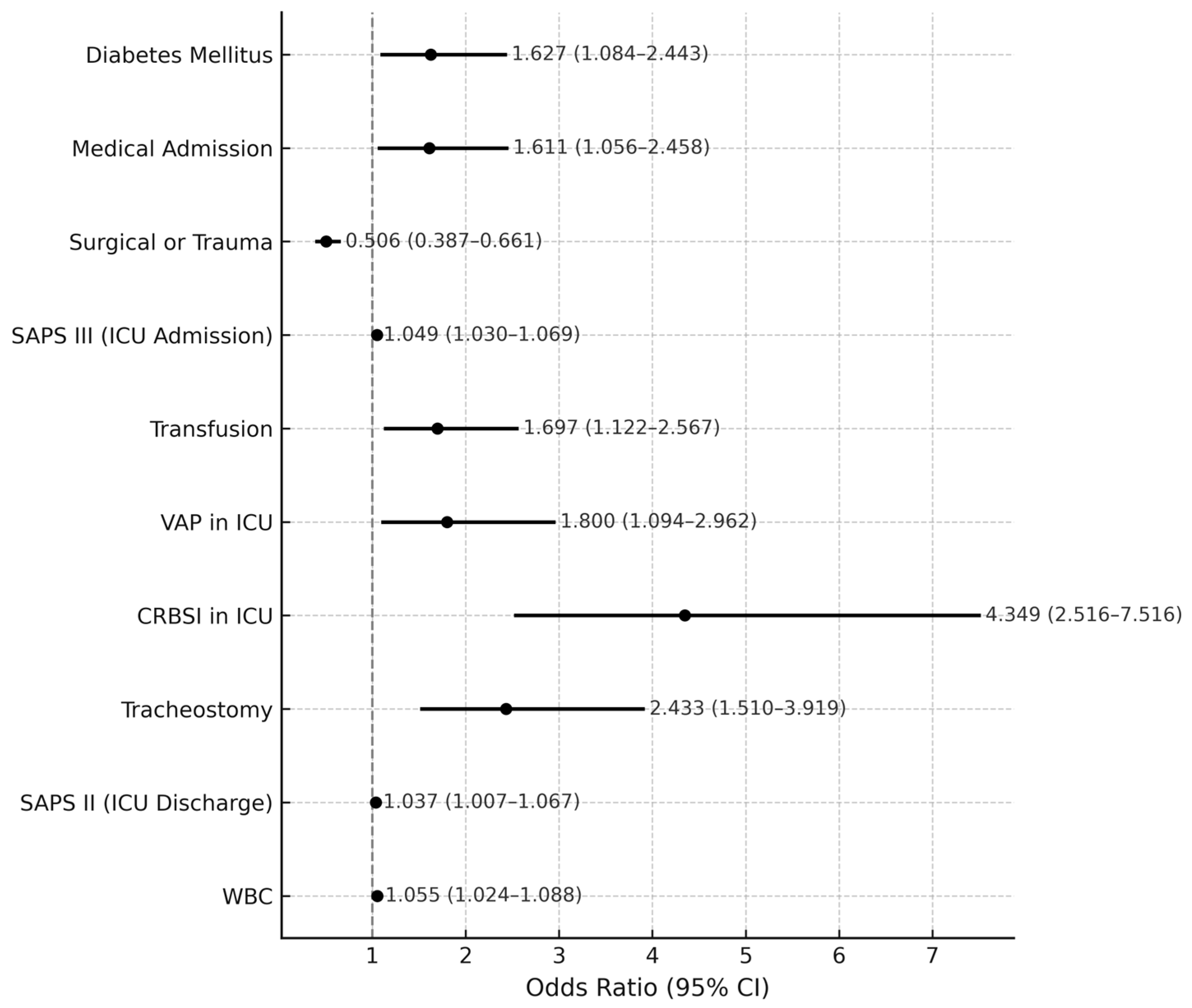
3.3. Risk Factors for Worse Outcome (ICU Readmission or In-Hospital Mortality)
3.4. Model Construction (Derivation Cohort)
3.4.1. Comparison of WOS by Outcome Groups in the Derivation Cohort
3.4.2. Cut-Off Validation and Classification Performance
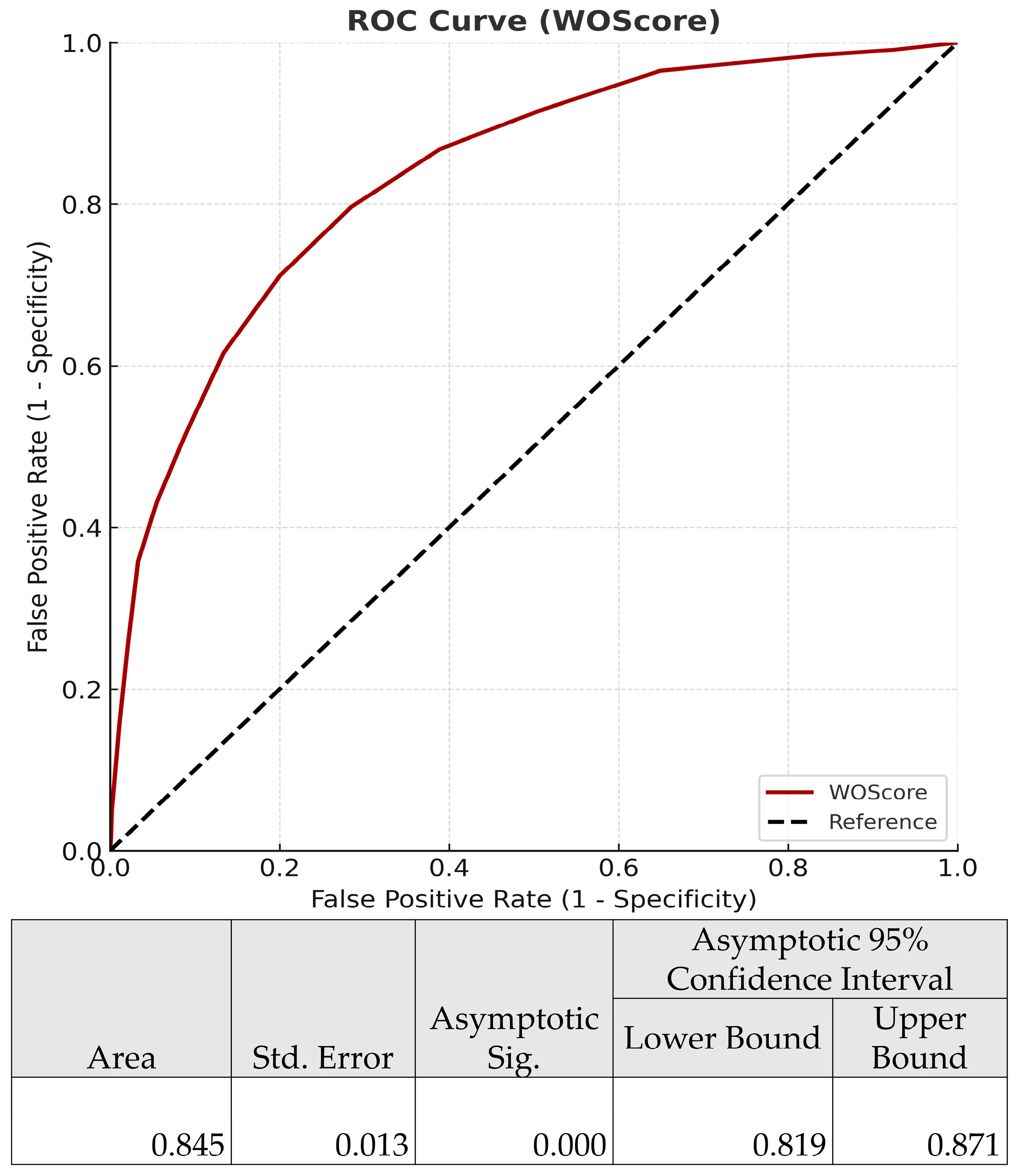
3.4.3. Predictive Performance of the WOS
3.5. Model Validation
3.5.1. Prospective Validation of the Worse Outcome Score (WOS)
3.5.2. Comparison of WOS by Outcome Groups in the Validation Cohort
3.5.3. Discriminative Ability, Calibration and Cut-Off Analysis of WOS
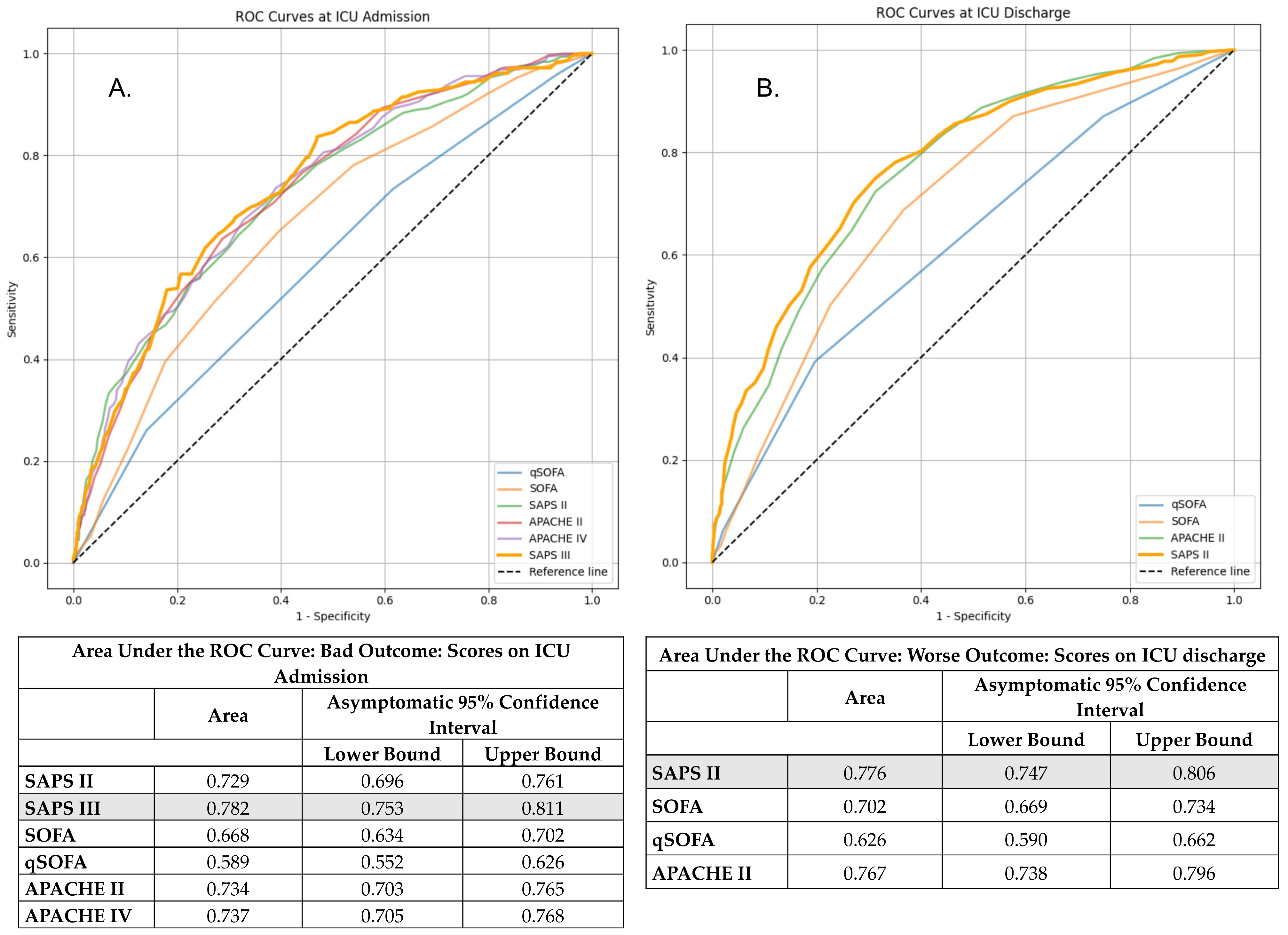
3.6. Comparative Performance of Risk Scores
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Sluisveld, N.; Bakhshi-Raiez, F.; de Keizer, N.; Holman, R.; Wester, G.; Wollersheim, H.; van der Hoeven, J.G.; Zegers, M. Variation in rates of ICU readmissions and post-ICU in-hospital mortality and their association with ICU discharge practices. BMC Health Serv. Res. 2017, 17, 281. [Google Scholar] [CrossRef]
- Madotto, F.; McNicholas, B.; Rezoagli, E.; Pham, T.; Laffey, J.G.; Bellani, G. Death in hospital following ICU discharge: Insights from the LUNG SAFE study. Crit. Care 2021, 25, 144. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, B.P.; Arshad, Z.; Srivastava, V.K.; Prakash, R.; Singh, M.K. Determinants of Readmission in the Intensive Care Unit: A Prospective Observational Study. Cureus 2024, 16, e62840. [Google Scholar] [CrossRef]
- Ponzoni, C.R.; Corrêa, T.D.; Filho, R.R.; Neto, A.S.; Assunção, M.S.C.; Pardini, A.; Schettino, G.P.P. Readmission to the Intensive Care Unit: Incidence, Risk Factors, Resource Use, and Outcomes. A Retrospective Cohort Study. Ann. Am. Thorac. Soc. 2017, 14, 1312–1319. [Google Scholar] [CrossRef]
- Aryal, D.; Paneru, H.R.; Koirala, S.; Khanal, S.; Acharya, S.P.; Karki, A.; Dona, D.G.; Haniffa, R.; Beane, A.; Salluh, J.I.F. Incidence, risk and impact of ICU readmission on patient outcomes and resource utilisation in tertiary level ICUs in Nepal: A cohort study. Wellcome Open Res. 2025, 7, 272. [Google Scholar] [CrossRef]
- Bekele, T.G.; Melaku, B.; Demisse, L.B.; Abza, L.F.; Assen, A.S. Outcomes and factors associated with prolonged stays among patients admitted to adult intensive care unit in a resource-limited setting: A multicenter chart review. Sci. Rep. 2024, 14, 13960. [Google Scholar] [CrossRef]
- Kramer, A.A.; Dasta, J.F.; Kane-Gill, S.L. The Impact of Mortality on Total Costs Within the ICU. Crit. Care Med. 2017, 45, 1457–1463. [Google Scholar] [CrossRef]
- Lee, S.I.; Koh, Y.; Huh, J.W.; Hong, S.B.; Lim, C.M. Factors and Outcomes of Intensive Care Unit Readmission in Elderly Patients. Gerontology 2022, 68, 280–288. [Google Scholar] [CrossRef]
- Lee, H.; Lim, C.W.; Hong, H.P.; Ju, J.W.; Jeon, Y.T.; Hwang, J.W.; Park, H.P. Efficacy of the APACHE II Score at ICU Discharge in Predicting Post-ICU Mortality and ICU Readmission in Critically Ill Surgical Patients. Anaesth. Intensive Care. 2015, 43, 175–186. [Google Scholar] [CrossRef]
- Yin, Y.-L.; Sun, M.-R.; Zhang, K.; Chen, Y.-H.; Zhang, J.; Zhang, S.-K.; Zhou, L.-L.; Wu, Y.-S.; Gao, P.; Shen, K.-K.; et al. Status and Risk Factors in Patients Requiring Unplanned Intensive Care Unit Readmission Within 48 Hours: A Retrospective Propensity-Matched Study in China. Risk Manag. Healthc. Policy 2023, 16, 383–391. [Google Scholar] [CrossRef]
- Kareliusson, F.; De Geer, L.; Tibblin, A.O. Risk prediction of ICU readmission in a mixed surgical and medical population. J. Intensive Care 2015, 3, 30. [Google Scholar] [CrossRef]
- Kim, H.B.; Na, S.; Paik, H.C.; Joo, H.; Kim, J. Risk factors for intensive care unit readmission after lung transplantation: A retrospective cohort study. Acute Crit. Care 2021, 36, 99–108. [Google Scholar] [CrossRef]
- Maharaj, R.; Terblanche, M.; Vlachos, S. The Utility of ICU Readmission as a Quality Indicator and the Effect of Selection. Crit. Care Med. 2018, 46, 749–756. [Google Scholar] [CrossRef]
- Czajka, S.; Ziębińska, K.; Marczenko, K.; Posmyk, B.; Szczepańska, A.J.; Krzych, Ł.J. Validation of APACHE II, APACHE III and SAPS II scores in in-hospital and one year mortality prediction in a mixed intensive care unit in Poland: A cohort study. BMC Anesthesiol. 2020, 20, 296. [Google Scholar] [CrossRef] [PubMed]
- Stoian, M.; Andone, A.; Bândilă, S.R.; Onișor, D.; Laszlo, S.Ș.; Lupu, G.; Danielescu, A.; Baba, D.-F.; Văsieșiu, A.M.; Manea, A.; et al. Mechanical Ventilator-Associated Pneumonia in the COVID-19 Pandemic Era: A Critical Challenge in the Intensive Care Units. Antibiotics 2025, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Stoian, M.; Azamfirei, L.; Andone, A.; Văsieșiu, A.-M.; Stîngaciu, A.; Huțanu, A.; Bândilă, S.R.; Dobru, D.; Manea, A.; Stoian, A. Incidence and Risk Factors of Secondary Infections in Critically III SARS-CoV-2 Patients: A Retrospective Study in an Intensive Care Unit. Biomedicines 2025, 13, 1333. [Google Scholar] [CrossRef]
- Nates, J.L.; Nunnally, M.; Kleinpell, R.; Blosser, S.; Goldner, J.; Birriel, B.; Fowler, C.S.; Byrum, D.; Miles, W.S.; Bailey, H.; et al. ICU Admission, Discharge, and Triage Guidelines: A Framework to Enhance Clinical Operations, Development of Institutional Policies, and Further Research. Crit. Care Med. 2016, 44, 1553–1602. [Google Scholar] [CrossRef]
- Hiller, M.; Burisch, C.; Wittmann, M.; Bracht, H.; Kaltwasser, A.; Bakker, J. The current state of intensive care unit discharge practices-Results of an international survey study. Front. Med. 2024, 11, 1377902. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanzadeh, K.; Ebadi, A.; Hosseini, M.; Madah, S.S.B.; Khankeh, H. Challenges of the patient transition process from the intensive care unit: A qualitative study. Acute Crit. Care 2021, 36, 133–142. [Google Scholar] [CrossRef]
- Niven, D.J.; Bastos, J.F.; Stelfox, H.T. Critical Care Transition Programs and the Risk of Readmission or Death After Discharge From an ICU: A Systematic Review and Meta-Analysis. Crit. Care Med. 2014, 42, 179–187. [Google Scholar] [CrossRef]
- Österlind, J.; Gerhardsson, J.; Myrberg, T. Critical care transition programs on readmission or death: A systematic review and meta-analysis. Acta Anaesthesiol. Scand. 2020, 64, 870–883. [Google Scholar] [CrossRef] [PubMed]
- Stelfox, H.T.; Bastos, J.; Niven, D.J.; Bagshaw, S.M.; Turin, T.C.; Gao, S. Critical care transition programs and the risk of readmission or death after discharge from ICU. Intensive Care Med. 2016, 42, 401–410. [Google Scholar] [CrossRef]
- Ruppert, M.M.; Loftus, T.J.; Small, C.B.; Li, H.B.; Ozrazgat-Baslanti, T.; Balch, J.; Holmes, R.B.; Tighe, P.J.; Upchurch, G.R.J.M.; Efron, P.A.M.; et al. Predictive Modeling for Readmission to Intensive Care: A Systematic Review. Crit. Care Explor. 2023, 5, e0848. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Wang, M.; Li, W.; Cheng, J.; Yuan, M.; Zhong, M.; Zhang, Z.; Zhang, C. The risk assessment tool for intensive care unit readmission: A systematic review and meta-analysis. Intensive Crit. Care Nurs. 2023, 76, 103378. [Google Scholar] [CrossRef]
- Hammer, M.; Grabitz, S.D.; Teja, B.; Wongtangman, K.; Serrano, M.; Neves, S.; Siddiqui, S.; Xu, X.; Eikermann, M. A Tool to Predict Readmission to the Intensive Care Unit in Surgical Critical Care Patients—The RISC Score. J. Intensive Care Med. 2021, 36, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Mcneill, H.; Khairat, S. Impact of Intensive Care Unit Readmissions on Patient Outcomes and the Evaluation of the National Early Warning Score to Prevent Readmissions: Literature Review. JMIR Perioper. Med. 2020, 3, e13782. [Google Scholar] [CrossRef]
- Amagai, S.; Chaudhari, V.; Chhikara, K.; Ingraham, N.E.; Hochberg, C.H.; Barker, A.K.; Mao, C.; Ortiz, A.C.; Weissman, G.E.; Schmid, B.E.; et al. The Epidemiology of Intensive Care Unit Readmissions Across Ten Health Systems. medRxiv 2025. medRxiv: 2025.03.10.25323672. [Google Scholar] [CrossRef]
- Cumberworth, J.; Chequers, M.; Bremner, S.; Boyd, O.; Philips, B. Mortality and readmission rates of patients discharged in-hours and out-of-hours from a British ICU over a 3-year period. Sci. Rep. 2022, 12, 6659. [Google Scholar] [CrossRef]
- Tejerina Álvarez, E.E.; Gómez Mediavilla, K.A.; Rodríguez Solís, C.; Valero González, N.; Lorente Balanza, J.Á. Risk factors for readmission to ICU and analysis of intra-hospital mortality. Med. Clínica (Engl. Ed.) 2022, 158, 58–64. [Google Scholar] [CrossRef]
- Hasswa, M.K.; Elshazly, M.; Osman, M.N.; Tantawy, A.A. Risk factors and associated outcomes of respiratory ICU readmission. Egypt. J. Bronchol. 2025, 19, 45. [Google Scholar] [CrossRef]
- Rosa, R.G.; Falavigna, M.; Robinson, C.C.; Sanchez, E.C.; Kochhann, R.; Schneider, D.; Sganzerla, D.; Dietrich, C.; Barbosa, M.G.; de Souza, D.; et al. Early and Late Mortality Following Discharge From the ICU: A Multicenter Prospective Cohort Study. Crit. Care Med. 2020, 48, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, P.A.; Czech, I.J.; Krzych, Ł.J. The Pros and Cons of the Prediction Game: The Never-ending Debate of Mortality in the Intensive Care Unit. Int. J. Environ. Res. Public Health 2019, 16, 3394. [Google Scholar] [CrossRef] [PubMed]



| Variable | Univariate OR (95% CI) | p-Value | Multivariate OR (95% CI) | p-Value |
|---|---|---|---|---|
| Diabetes Mellitus | 1.742 (1.182–2.57) | 0.005 | 1.569 (1.182–2.507) | 0.047 |
| SAPS III on ICU admission | 1.035 (1.021–1.049) | <0.001 | 1.022 (1.002–1.042) | 0.029 |
| VAP during ICU stay | 2.702 (1.75–4.173) | <0.001 | 1.749 (1.040–2.943) | 0.035 |
| CRBSI during ICU stay | 3.684 (2.360–5.751) | <0.001 | 2.520 (1.494–4.251) | <0.001 |
| WBC count at ICU discharge | 1.046 (1.022–1.093) | 0.003 | 1.050 (1.017–1.085) | 0.003 |
| Variable | Univariate OR (95% CI) | p-Value | Multivariate OR (95% CI) | p-Value |
|---|---|---|---|---|
| Medical admission | 2.224 (1.594–3.104) | <0.001 | 2.247 (1.294–3.904) | 0.004 |
| Lactate at ICU admission | 1.009 (1.003–1.015) | 0.002 | 1.014 (1.005–1.023) | 0.003 |
| Lactate clearance at 48 h | 1.514 (1.132–2.026) | 0.005 | 1.750 (1.070–2.864) | 0.026 |
| AKI during ICU stay | 3.529 (2.568–4.848) | <0.001 | 1.671 (1.005–2.777) | 0.048 |
| Transfusion | 3.685 (2.663–5.100) | <0.001 | 2.240 (1.360–3.690) | 0.002 |
| CRBSI during ICU stay | 4.460 (3.015–6.598) | <0.001 | 1.947 (1.103–3.437) | 0.021 |
| Tracheostomy at discharge | 8.412 (5.986–11.821) | <0.001 | 3.956 (2.275–6.879) | <0.001 |
| GCS at discharge | 0.764 (0.730–0.799) | 0.001 | 0.882 (0.817–0.951) | 0.001 |
| SAPS III at admission | 1.098 (1.081–1.115) | <0.001 | 1.046 (1.024–1.069) | <0.001 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Patients’ Characteristics | ||||||
| Age | 1.027 | 1.019–1.036 | <0.001 | 0.993 | 0.976–1.011 | 0.453 |
| Heart Failure | 1.451 | 1.008–2.088 | 0.045 | 0.883 | 0.527–1.480 | 0.638 |
| CAD | 1.424 | 1.029–1.972 | 0.033 | 0.809 | 0.504–1.297 | 0.378 |
| Metastatic Cancer | 2.697 | 1.585–4.592 | <0.001 | 1.284 | 0.534–3.089 | 0.577 |
| Diabetes Mellites | 2.161 | 1.637–2.853 | <0.001 | 1.627 | 1.084–2.443 | 0.019 |
| Charlson Comorbidity Index | 1.224 | 1.166–1.285 | <0.001 | 1.107 | 0.977–1.254 | 0.111 |
| Category of Admission | ||||||
| Medical | 1.977 | 1.512–2.586 | <0.001 | 1.611 | 1.056–2.458 | 0.027 |
| Elective Surgery | 0.418 | 0.261–0.670 | <0.001 | |||
| Emergent Surgery | 0.686 | 0.501–0.939 | 0.019 | |||
| Trauma | 0.608 | 0.399–0.926 | 0.020 | |||
| Neurological/Neurosurgical | 1.534 | 1.146–2.055 | 0.004 | 1.187 | 0.715–1.971 | 0.507 |
| Patients’ origin | ||||||
| Ward/Other ICU | 1.785 | 1.376–2.315 | <0.001 | 1.012 | 0.665–1.540 | 0.956 |
| Operating Room | 0.536 | 0.395–0.728 | <0.001 | |||
| Pre-ICU in-hospital days | 1.030 | 1.019–1.041 | <0.001 | 1.012 | 1.000–1.024 | 0.054 |
| Patients’ clinical condition at ICU admission | ||||||
| Respiratory Failure | 1.330 | 1.000–1.768 | 0.050 | 0.959 | 0.594–1.547 | 0.863 |
| Sepsis | 1.755 | 1.249–2.464 | 0.001 | 0.894 | 0.515–1.553 | 0.691 |
| SAPS II | 1.061 | 1.050–1.071 | <0.001 | |||
| SAPS III | 1.092 | 1.078–1.107 | <0.001 | 1.049 | 1.030–1.069 | <0.001 |
| SOFA | 1.221 | 1.165–1.281 | <0.001 | |||
| qSOFA | 1.808 | 1.517–2.156 | <0.001 | |||
| APACHE II | 1.115 | 1.094–1.136 | <0.001 | |||
| APACHE IV | 1.040 | 1.033–1.047 | <0.001 | |||
| GFR | 0.991 | 0.988–0.995 | <0.001 | 1.003 | 0.997–1.009 | 0.285 |
| Data from the Patient’s ICU Stay | ||||||
| ICU LOS | 1.035 | 1.023–1.047 | <0.001 | 0.985 | 0.962–1.008 | 0.207 |
| Duration of MV | 1.002 | 1.002–1.003 | <0.001 | 1.000 | 0.998–1.001 | 0.767 |
| Duration of vasopressors adm. | 1.071 | 1.051–1.090 | <0.001 | 1.001 | 0.986–1.017 | 0.853 |
| Lactate Clearance 48 h | 1.449 | 1.120–1.875 | 0.005 | 1.285 | 0.848–1.949 | 0.237 |
| Transfusion | 2.959 | 2.272–3.854 | <0.001 | 1.697 | 1.122–2.567 | 0.012 |
| Number of Blood products | 1.085 | 1.050–1.122 | <0.001 | 1.010 | 0.964–1.059 | 0.668 |
| Infection in ICU | 2.055 | 1.570–2.689 | <0.001 | 1.249 | 0.790–1.975 | 0.342 |
| VAP in ICU | 4.638 | 3.288–6.542 | <0.001 | 1.800 | 1.094–2.962 | 0.021 |
| CRBSI in ICU | 7.048 | 4.769–10.418 | <0.001 | 4.349 | 2.516–7.516 | <0.001 |
| AKI | 3.044 | 2.332–3.973 | <0.001 | 1.066 | 0.677–1.678 | 0.783 |
| CRRT | 2.872 | 2.114–3.901 | <0.001 | 0.852 | 0.466–1.558 | 0.603 |
| Duration of CRRT | 1.004 | 1.003–1.006 | <0.001 | 1.001 | 0.998–1.003 | 0.580 |
| Total Parenteral Nutrition | 2.530 | 1.847–3.465 | <0.001 | 1.404 | 0.892–2.209 | 0.143 |
| Patients’ Clinical Condition at ICU discharge | ||||||
| Tracheostomy | 5.359 | 4.054–7.085 | <0.001 | 2.433 | 1.510–3.919 | <0.001 |
| GCS | 0.795 | 0.761–0.830 | <0.001 | 0.968 | 0.885–1.059 | 0.473 |
| SAPS II | 1.101 | 1.085–1.117 | <0.001 | 1.037 | 1.007–1.067 | 0.014 |
| APACHE II | 1.187 | 1.156–1.218 | <0.001 | |||
| SOFA | 1.311 | 1.230–1.389 | <0.001 | |||
| GFR | 0.999 | 0.996–1.002 | 0.520 | |||
| WBC | 1.039 | 1.016–1.064 | 0.001 | 1.055 | 1.024–1.088 | <0.001 |
| HgB | 0.722 | 0.657–0.795 | <0.001 | 0.936 | 0.817–1.073 | 0.342 |
| Lactate | 1.094 | 1.066–1.124 | <0.001 | 1.027 | 0.991–1.064 | 0.144 |
| Worse Outcome Score | ||
|---|---|---|
| Medical episode | No = −5 pts | Yes = 5 pts |
| Diabetes Meletus Patient’s comorbidity | No = 0 pts | Yes = 10 pts |
| SAPS III On patient’s ICU admission | ≤70 = 0 pts | >70 = 5 pts |
| Transfusion Even 1 unit blood products during ICU stay | No = 0 pts | Yes = 10 pts |
| VAP OR CRBSI During patient’s ICU stay | No = 0 pts | Yes = 20 pts |
| SAPS II On ICU discharge | ≤26 = 0 pts | >26 = 5 pts |
| Tracheostomy On patient’s ICU discharge | No = 0 pts | Yes = 15 pts |
| WBC On patient’s ICU discharge | ≤13 K/μL = 0 pts | >13 K/μL = 5 pts |
| N | Mean | Std. Deviation | Std. Error | 95% Confidence Interval for Mean | Sign | Odds Ratio | 95% CI for OR | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | Lower | Upper | |||||||
| Hospital Discharge | 872 | 15.67 | 14.652 | 0.0496 | 14.70 | 16.65 | <0.001 | 1.082 | 1.072 | 1.093 |
| Worse Outcome | 318 | 38.55 | 17.919 | 1.005 | 36.58 | 42.20 | ||||
| Total | 1190 | 21.79 | 18.589 | 0.0539 | 20.73 | 22.85 | ||||
| Worse Outcome | |||||
|---|---|---|---|---|---|
| Hospital Discharge | Readmission or In-Hospital Death | Total | |||
| WOScore | <20 | n | 533 | 42 | 575 |
| (%) | 61.1% | 13.2% | 48.32% | ||
| ≥20 | n | 339 | 276 | 615 | |
| (%) | 38.9% | 86.8% | 51.68% | ||
| Total | n | 872 | 318 | 1190 | |
| (%) | 100% | 100% | 100% | ||
| Sensitivity | Specificity | ||||
| 86.8% | 61.1% | ||||
| PPV (Positive Predictive Value) | NPV (Negative Predictive Value) | ||||
| 44.9% | 92.7% | ||||
| Worse Outcome | |||||
|---|---|---|---|---|---|
| Hospital Discharge | Readmission or In-Hospital Death | Total | |||
| WOScore | <20 | n | 103 | 5 | 108 |
| (%) | 73.0% | 11.9% | 59.02% | ||
| ≥20 | n | 38 | 37 | 75 | |
| (%) | 27.0% | 88.1% | 40.98% | ||
| Total | n | 141 | 42 | 183 | |
| (%) | 100% | 100% | 100% | ||
| Sensitivity | Specificity | ||||
| 88.1% | 73.0% | ||||
| PPV (Positive Predictive Value) | NPV (Negative Predictive Value) | ||||
| 49.3% | 95.4% | ||||
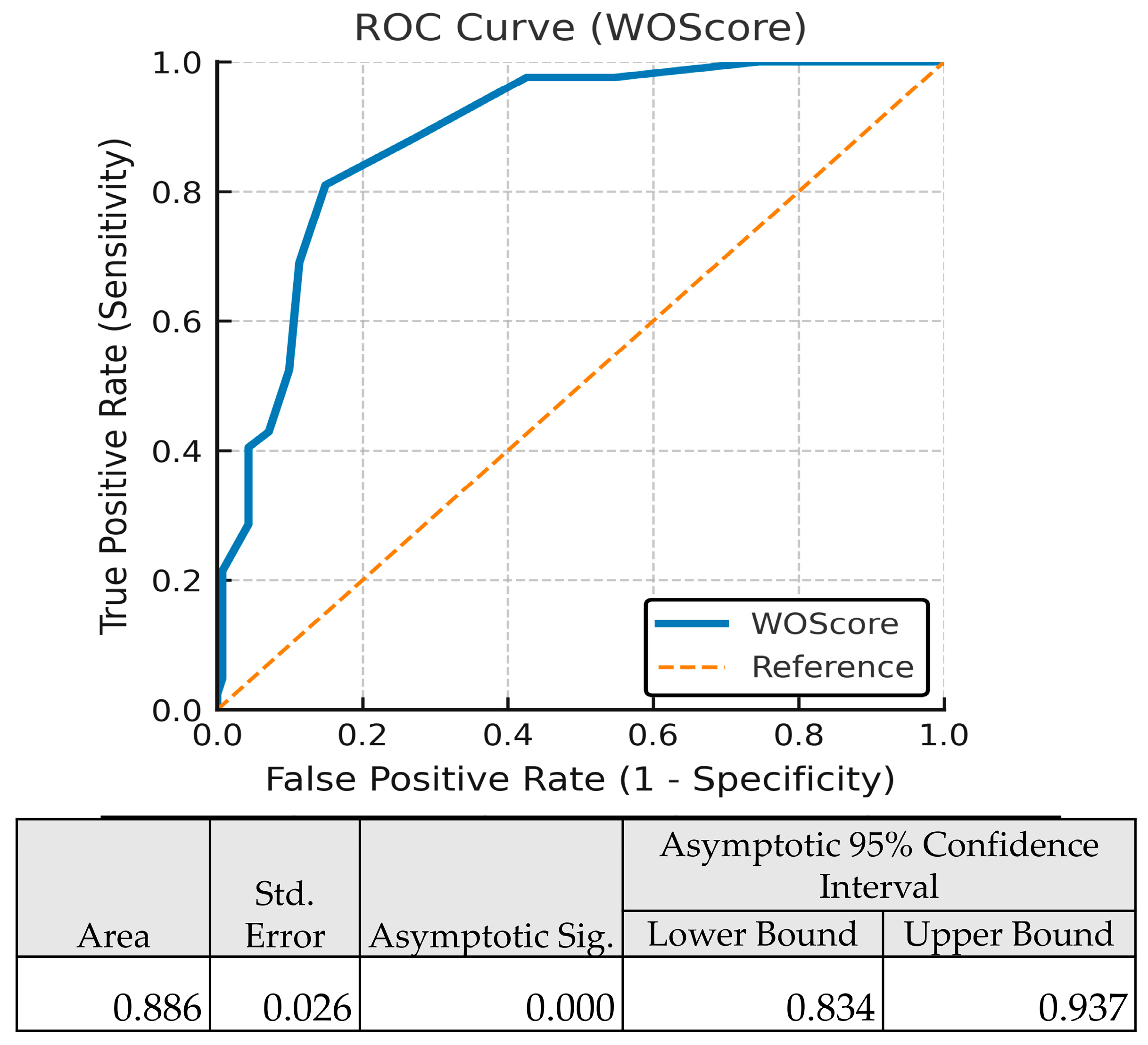
| Score | AUC | Brier Score | Nagelkerke R2 | Calibration Slope | Hosmer–Lemeshow Test p-Value |
|---|---|---|---|---|---|
| WOScore | 0.886 | 0.1042 | 0.521 | 1.0003 | 0.7323 |
| SAPS III (ICU admission) | 0.797 | 0.1064 | 0.45 | 1.0004 | 0.0011 |
| SAPS II (ICU discharge) | 0.762 | 0.1273 | 0.349 | 1.0007 | 0.9781 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadakis, E.; Proklou, A.; Kokkini, S.; Papakitsou, I.; Konstantinou, I.; Konstantinidi, A.; Prinianakis, G.; Intzes, S.; Symeonidou, M.; Kondili, E. ICU Readmission and In-Hospital Mortality Rates for Patients Discharged from the ICU—Risk Factors and Validation of a New Predictive Model: The Worse Outcome Score (WOScore). J. Pers. Med. 2025, 15, 479. https://doi.org/10.3390/jpm15100479
Papadakis E, Proklou A, Kokkini S, Papakitsou I, Konstantinou I, Konstantinidi A, Prinianakis G, Intzes S, Symeonidou M, Kondili E. ICU Readmission and In-Hospital Mortality Rates for Patients Discharged from the ICU—Risk Factors and Validation of a New Predictive Model: The Worse Outcome Score (WOScore). Journal of Personalized Medicine. 2025; 15(10):479. https://doi.org/10.3390/jpm15100479
Chicago/Turabian StylePapadakis, Eleftherios, Athanasia Proklou, Sofia Kokkini, Ioanna Papakitsou, Ioannis Konstantinou, Aggeliki Konstantinidi, Georgios Prinianakis, Stergios Intzes, Marianthi Symeonidou, and Eumorfia Kondili. 2025. "ICU Readmission and In-Hospital Mortality Rates for Patients Discharged from the ICU—Risk Factors and Validation of a New Predictive Model: The Worse Outcome Score (WOScore)" Journal of Personalized Medicine 15, no. 10: 479. https://doi.org/10.3390/jpm15100479
APA StylePapadakis, E., Proklou, A., Kokkini, S., Papakitsou, I., Konstantinou, I., Konstantinidi, A., Prinianakis, G., Intzes, S., Symeonidou, M., & Kondili, E. (2025). ICU Readmission and In-Hospital Mortality Rates for Patients Discharged from the ICU—Risk Factors and Validation of a New Predictive Model: The Worse Outcome Score (WOScore). Journal of Personalized Medicine, 15(10), 479. https://doi.org/10.3390/jpm15100479







