Cardiac Amyloidosis: Tribulations and New Frontiers
Abstract
1. Introduction
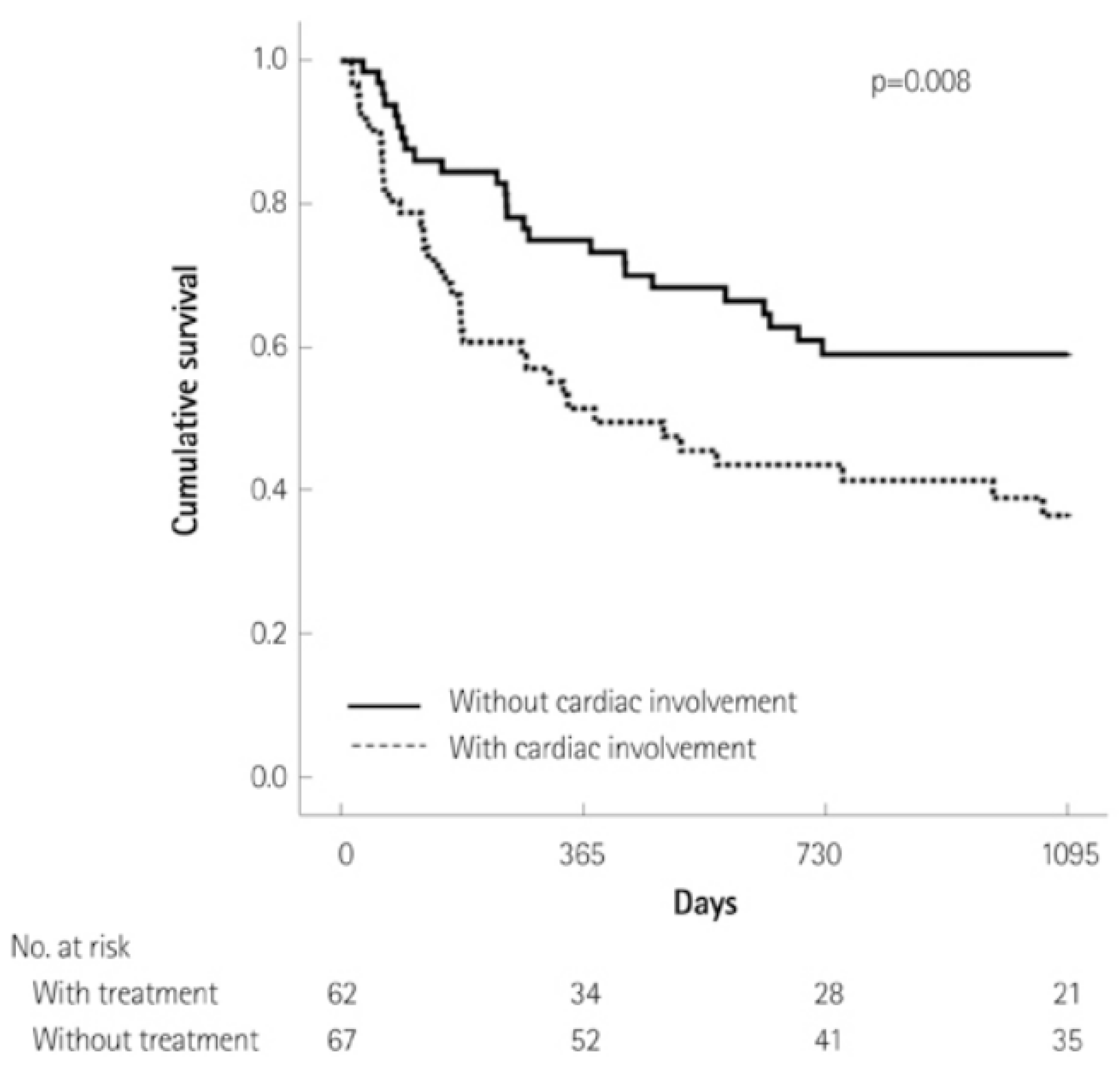
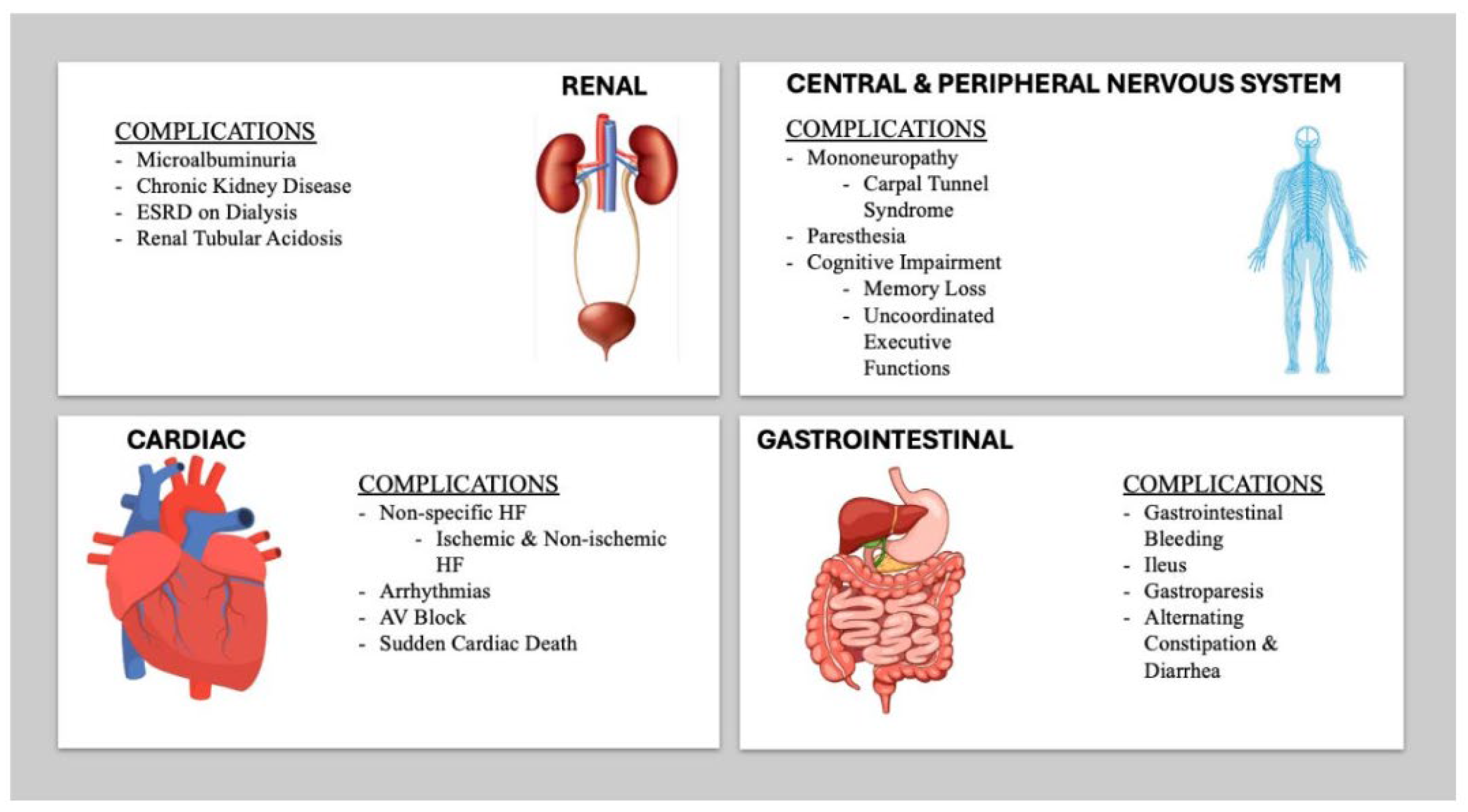
2. Clinical Presentation of Cardiac Amyloidosis
3. Diagnosis of ATTR-CA
3.1. Overview of Diagnostic Modalities
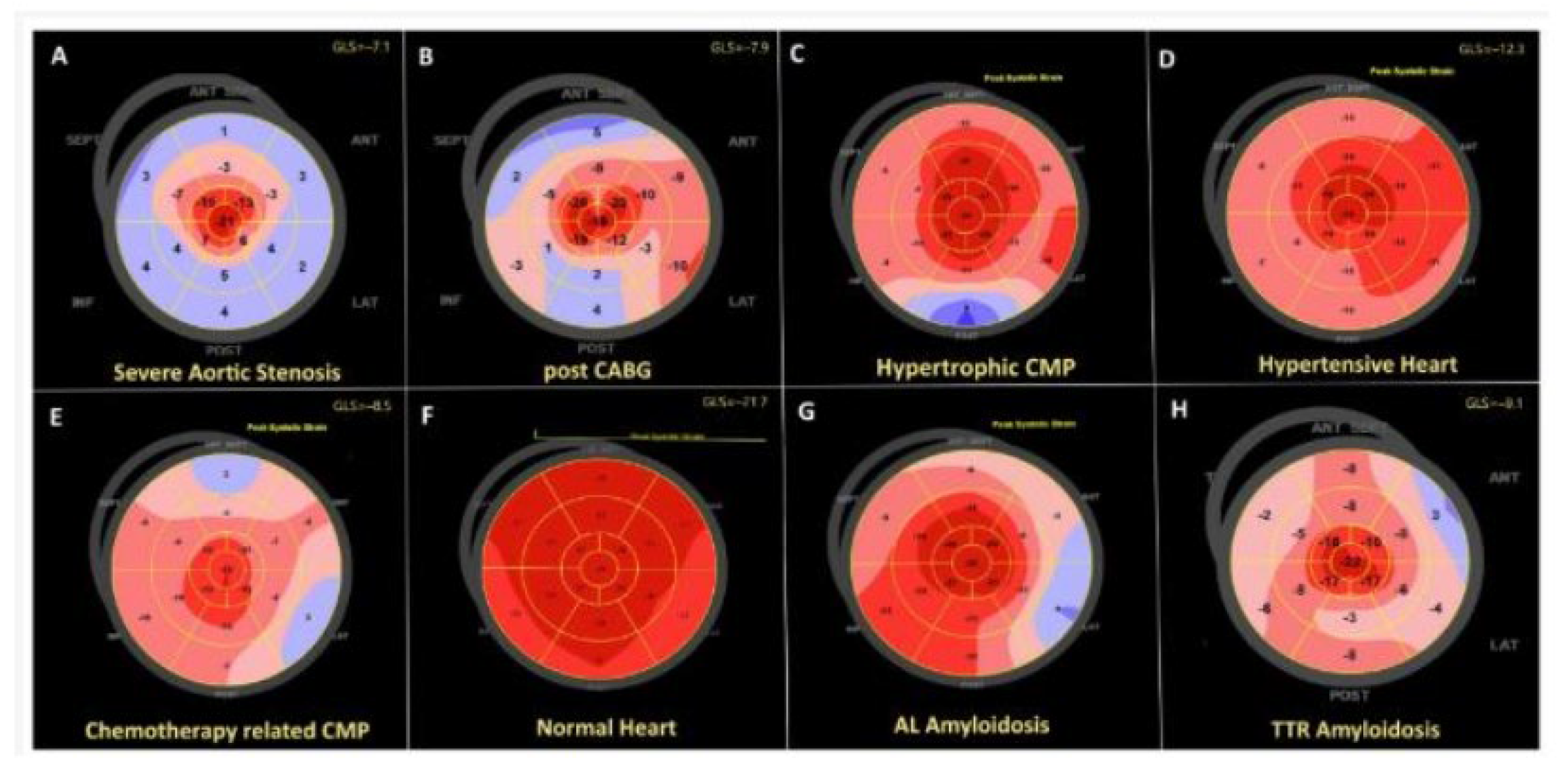
3.2. Cardiac Magnetic Resonance Imaging
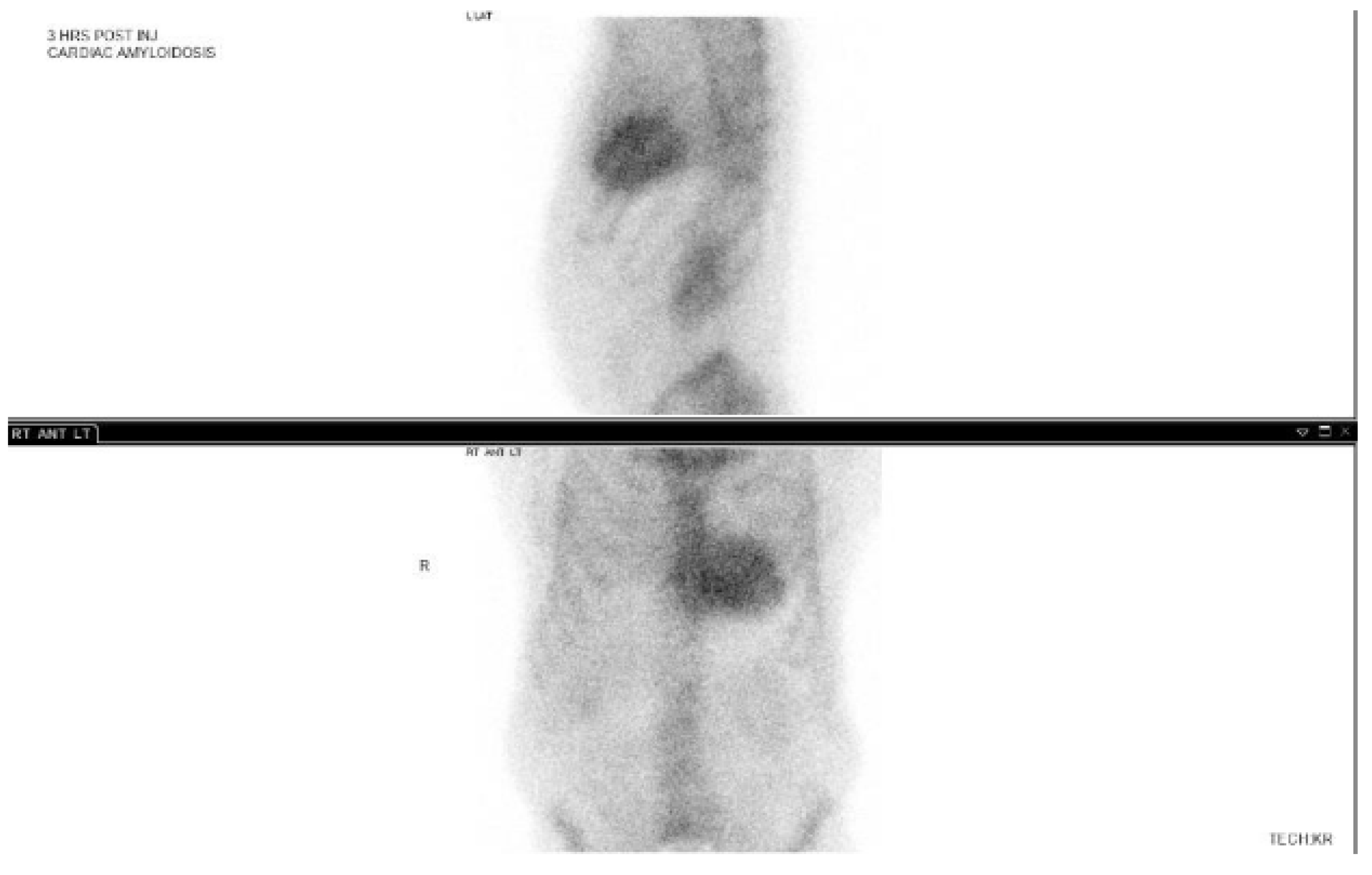
3.3. 99-Technetium Pyrophosphate Imaging
3.4. Diagnosis of AL-Amyloidosis
3.5. Role of Biopsy
3.6. Discussion: A Comparison of Different Diagnostic Techniques
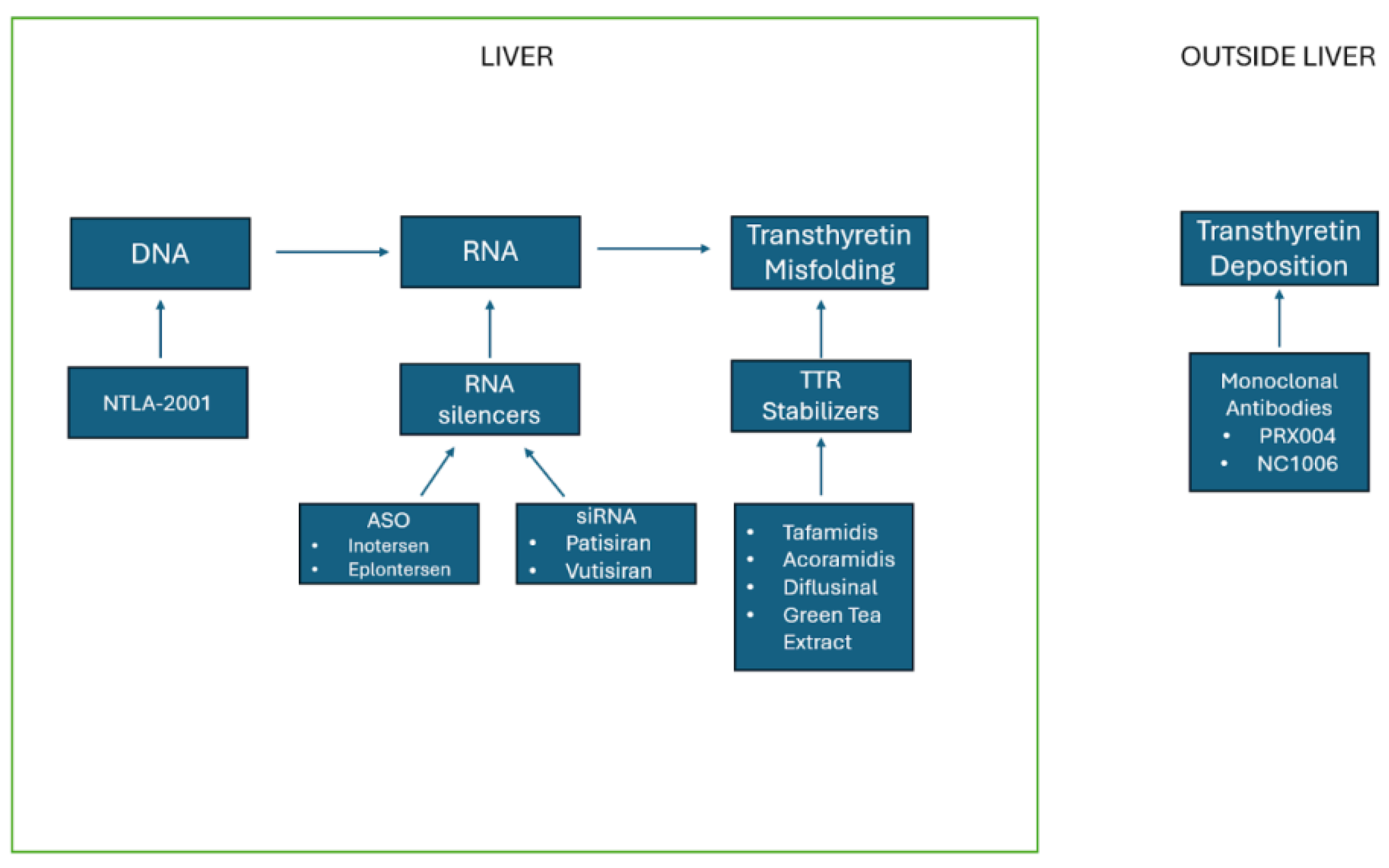
4. Management of Transthyretin Amyloidosis
4.1. TTR Stabilizers
4.2. Tafamidis
4.3. Acoramidis
4.4. Diflunisal
4.5. Green Tea Extract
4.6. TTR Silencers
4.7. Inotersen
4.8. Patisiran
4.9. Vutrisiran
4.10. Eplontersen
4.11. Monoclonal Antibodies
4.12. Gene Editing
5. Medical Management of Heart Failure
6. Device Therapy
7. Management of AL-Amyloidosis: Chemotherapy and Stem Cell Transplantation
8. Heart Transplantation
9. Importance of Multidisciplinary Approach
10. Future Directions
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Devesa, A.; Blasco, A.C.; Lázaro, A.M.P.; Askari, E.; Lapeña, G.; Talavera, S.G.; Urquía, M.T.; Olleros, C.R.; Tuñón, J.; Ibáñez, B.; et al. Prevalence of transthyretin amyloidosis in patients with heart failure and no left ventricular hypertrophy. ESC Heart Fail. 2021, 8, 2856–2865. [Google Scholar] [CrossRef]
- González-López, E.; Gallego-Delgado, M.; Guzzo-Merello, G.; de Haro-Del Moral, F.J.; Cobo-Marcos, M.; Robles, C.; Bornstein, B.; Salas, C.; Lara-Pezzi, E.; Alonso-Pulpon, L.; et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur. Heart J. 2015, 36, 2585–2594. [Google Scholar] [CrossRef]
- Gilstrap, L.G.; Dominici, F.; Wang, Y.; El-Sady, M.S.; Singh, A.; Di Carli, M.F.; Falk, R.H.; Dorbala, S. Epidemiology of Cardiac Amyloidosis–Associated Heart Failure Hospitalizations Among Fee-for-Service Medicare Beneficiaries in the United States. Circ. Heart Fail. 2019, 12, e005407. [Google Scholar] [CrossRef]
- Jacobson, D.R.; Pastore, R.D.; Yaghoubian, R.; Kane, I.; Gallo, G.; Buck, F.S.; Buxbaum, J.N. Variant-Sequence Transthyretin (Isoleucine 122) in Late-Onset Cardiac Amyloidosis in Black Americans. N. Engl. J. Med. 1997, 336, 466–473. [Google Scholar] [CrossRef]
- Jacobson, D.; Alexander, A.; Tagoe, C.; Buxbaum, J. Prevalence of the amyloidogenic transthyretin (TTR) V122I allele in 14 333 African–Americans. Amyloid 2015, 3, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.S.; Hanna, M.; Grogan, M.; Dispenzieri, A.; Witteles, R.; Drachman, B.; Judge, D.P.; Lenihan, D.J.; Gottlieb, S.S.; Shah, S.J.; et al. Genotype and phenotype of transthyretin cardiac amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey). J. Am. Coll. Cardiol. 2016, 68, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Damy, T.; Kristen, A.V.; Suhr, O.B.; Maurer, M.S.; Planté-Bordeneuve, V.; Yu, C.-R.; Ong, M.-L.; Coelho, T.; Rapezzi, C.; THAOS Investigators. Transthyretin cardiac amyloidosis in continental Western Europe: An insight through the Transthyretin Amyloidosis Outcomes Survey (THAOS). Eur. Heart J. 2022, 43, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Reilly, M.; Staunton, H.; Harding, A. Familial amyloid polyneuropathy (TTR ala 60) in north west Ireland: A clinical, genetic, and epidemiological study. J. Neurol. Neurosurg. Psychiatry 1995, 59, 45–49. [Google Scholar] [CrossRef]
- Sattianayagam, P.T.; Hahn, A.F.; Whelan, C.J.; Gibbs, S.D.; Pinney, J.H.; Stangou, A.J.; Rowczenio, D.; Pflugfelder, P.W.; Fox, Z.; Lachmann, H.J.; et al. Cardiac phenotype and clinical outcome of familial amyloid polyneuropathy associated with transthyretin alanine 60 variant. Eur. Heart J. 2012, 33, 1120–1127. [Google Scholar] [CrossRef]
- Chen, Z.; Koh, J.S.; Saini, M.; Tay, K.S.S.; Jayne Tan, Y.; Chai, J.Y.H.; Fam, S.R.; Juraidah, A.; Lim, P.K.; Ng, A.S.L.; et al. Hereditary transthyretin amyloidosis- clinical and genetic characteristics of a multiracial South-East Asian cohort in Singapore. J. Neuromuscul. Disord. 2021, 8, 723–733. [Google Scholar] [CrossRef]
- Du, K.; Li, F.; Wang, H.; Miao, Y.; Lv, H.; Zhang, W.; Wang, Z.; Yuan, Y.; Meng, L. Hereditary transthyretin amyloidosis in mainland China: A unicentric retrospective study. Ann. Transl. Neurol. 2021, 8, 831–841. [Google Scholar] [CrossRef]
- Ikura, H.; Endo, J.; Kitakata, H.; Moriyama, H.; Sano, M.; Fukuda, K. Molecular mechanism of pathogenesis and treatment strategies for al amyloidosis. Int. J. Mol. Sci. 2022, 23, 6336. [Google Scholar] [CrossRef] [PubMed]
- Laires, P.A.; Evans, J.; Thompson, J.; Manwani, R.; Mudumby, P.; Field, M.; Fang, S. Prevalence, Incidence, and Characterization of light chain amyloidosis in the USA: A real-world analysis utilizing electronic health records (EHR). Blood 2023, 142, 6767. [Google Scholar] [CrossRef]
- Staron, A.; Conners, L.H.; Zheng, L.; Doros, G.; Sanchorawala, V. Race/ethnicity in systemic AL amyloidosis: Perspectives on disease and outcome disparities. Blood Cancer J. 2020, 10, 118. [Google Scholar] [CrossRef]
- Kumar, N.; Zhang, N.J.; Cherepanov, D.; Romanus, D.; Hughes, M.; Faller, D.V. Global epidemiology of amyloid light-chain amyloidosis. Orphanet J. Rare Dis. 2022, 17, 278. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lee, S.; Kim, Y.; Sohn, D. Incidence, Diagnosis and Prognosis of Cardiac Amyloidosis. Korean Circ. J. 2013, 43, 752–760. [Google Scholar] [CrossRef]
- Escher, F.; Senoner, M.; Doerler, J.; Zaruba, M.M.; Messner, M.; Mussner-Seeber, C.; Ebert, M.; Ensinger, C.; Mair, A.; Kroiss, A.; et al. When and how do patients with cardiac amyloidosis die? Clin. Res. Cardiol. 2020, 109, 78–88. [Google Scholar] [CrossRef]
- Yamada, T.; Takashio, S.; Arima, Y.; Nishi, M.; Morioka, M.; Hirakawa, K.; Hanatani, S.; Fujisue, K.; Yamanaga, K.; Kanazawa, H.; et al. Clinical characteristics and natural history of wild-type transthyretin amyloid cardiomyopathy in Japan. ESC Heart Fail. 2020, 5, 2829–2837. [Google Scholar] [CrossRef]
- Pinney, J.; Whelan, C. Senile Systemic Amyloidosis: Clinical Features at Presentation and Outcome. J. Am. Heart Assoc. 2013, 2, e000098. [Google Scholar] [CrossRef]
- Russell, A.; Hahn, C.; Chhibber, S.; Korngut, L.; Fine, N.M. Utility of Neuropathy Screening for Wild-Type Transthyretin Amyloidosis Patients. Can. J. Neurol. Sci./J. Can. Des. Sci. Neurol. 2021, 5, 607–615. [Google Scholar] [CrossRef]
- Dahiya, D.; Kichloo, A.; Singh, J.; Albosta, M.; Wani, F. Gastrointestinal amyloidosis: A focused review. World J. Gastrointest. Endosc. 2021, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yen, T.; Chen, F.W.; Witteles, R.M.; Liedtke, M.; Nguyen, L.A. Clinical implications of gastrointestinal symptoms in systemic amyloidosis. Neurogastroenterol. Motil. 2018, 30, e13229. [Google Scholar] [CrossRef] [PubMed]
- Guirl, M.J.; Högenauer, C.; Ana, C.S.A.; Porter, J.L.; Little, K.H.; Stone, M.J.; Fordtran, J.S. Rapid intestinal transit as a primary cause of severe chronic diarrhea in patients with amyloidosis. Am. J. Gastroenterol. 2003, 98, 2219–2225. [Google Scholar] [CrossRef] [PubMed]
- Lobato, L.; Beirão, I.; Silva, M.; Bravo, F.; Silvestre, F.; Guimarães, S.; Sousa, A.; Noël, L.; Sequeiros, J. Familial ATTR amyloidosis: Microalbuminuria as a predictor of symptomatic disease and clinical nephropathy. Nephrol. Dial. Transplant. 2003, 18, 532–538. [Google Scholar] [CrossRef]
- Lobato, L.; Beirão, I.; Silva, M.; Fonseca, I.; Queirós, J.; Rocha, G.; Sarmento, A.M.; Sousa, A.; Sequeiros, J. End-stage renal disease and dialysis in hereditary amyloidosis TTR V30M: Presentation, survival and prognostic factors. Amyloid 2004, 11, 27–37. [Google Scholar] [CrossRef]
- Giancaterino, S.; Urey, M.A.; Darden, D.; Hsu, J.C. Management of Arrhythmias in Cardiac Amyloidosis. J. Am. Coll. Cardiol. EP 2020, 4, 351–361. [Google Scholar] [CrossRef]
- Longhi, S.; Quarta, C.C. Atrial fibrillation in amyloidotic cardiomyopathy: Prevalence, incidence, risk factors and prognostic role. Amyloid 2014, 3, 147–155. [Google Scholar] [CrossRef]
- Briasoulis, A.; Kourek, C.; Papamichail, A.; Loritis, K.; Bampatsias, D.; Repasos, E.; Xanthopoulos, A.; Tsougos, E.; Paraskevaidis, I. Arrhythmias in patients with cardiac amyloidosis: A comprehensive review on clinical management and devices. J. Cardiovasc. Dev. Dis. 2023, 10, 337. [Google Scholar] [CrossRef]
- Malobert, A.; Ciampi, C.; Politi, F.; Fabbri, S.; Musca, F.; Giannattasio, C. Cardiac amyloidosis red flags: What all the cardiologist have to know. Int. J. Cardiol. Cardiovasc. Risk Prev. 2024, 21, 200271. [Google Scholar] [CrossRef]
- Rozenbaum, M.H.; Large, S.; Bhambri, R.; Stewart, M.; Whelan, J.; van Doornewaard, A.; Dasgupta, N.; Masri, A.; Nativi-Nicolau, J. Impact of Delayed Diagnosis and Misdiagnosis for Patients with Transthyretin Amyloid Cardiomyopathy (ATTR-CM): A Targeted Literature Review. Cardiol. Ther. 2021, 10, 141–159. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhu, K.; Tian, Z.; Zhao, D.; Cui, Q.; Fang, Q. The findings of electrocardiography in patients with cardiac amyloidosis. Ann. Noninvasive Electrocardiol. 2013, 18, 157–162. [Google Scholar] [CrossRef]
- Cyrille, N.B.; Goldsmith, J.; Alvarex, J.; Maurer, M.S. Prevalence and Prognostic Significance of Low QRS Voltage Among the Three Main Types of Cardiac Amyloidosis. Am. J. Cardiol. 2014, 114, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Polo, J.M.; Barrenechea, A.R.U.; Martí, P.R.; Pérez-Palacios, R.; Gutiérrez, A.G.; Juana, E.B.; Gracia, A.A.; Ayala, S.A.; Arregui, M.Á.A. Echocardiographic markers of cardiac amyloidosis in patients with heart failure and left ventricular hypertrophy. Cardiol. J. 2023, 30, 266–275. [Google Scholar] [CrossRef]
- Luis, S.A.; Pellikka, P.A. Is speckle tracking imaging ready for prime time in current echo clinical practice? Prog. Cardiovasc. Dis. 2018, 61, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Phelon, D.; Collier, P.; Thavendiranathan, P.; Popović, Z.B.; Hanna, M.; Plana, J.C.; Marwick, T.H.; Thomas, J.D. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart 2012, 19, 1442–1448. [Google Scholar] [CrossRef]
- Leitman, M.; Tyomkin, V. Apical Sparing in Routine Echocardiography: Occurrence and Clinical Significance. J. Cardiovasc. Dev. Dis. 2024, 11, 262. [Google Scholar] [CrossRef]
- Yang, H.; Wright, L.; Negishi, T.; Negishi, K.; Liu, J.; Marwick, T.H. Research to Practice: Assessment of Left Ventricular Global Longitudinal Strain for Surveillance of Cancer Chemotherapeutic-Related Cardiac Dysfunction. JACC Cardiovasc. Imaging 2018, 8, 1196–1201. [Google Scholar] [CrossRef]
- Pagourelias, E.D.; Mirea, O.; Duchenne, J.; Van Cleemput, J.; Delforge, M.; Bogaert, J.; Kuznetsova, T.; Voigt, J.-U. Echo Parameters for Differential Diagnosis in Cardiac Amyloidosis: A Head-to-Head Comparison of Deformation and Nondeformation Parameters. Circ. Cardiovasc. Imaging 2017, 10, e005588. [Google Scholar] [CrossRef]
- Maciera, A.M.; Joshi, J.; Prasad, S.K.; Moon, J.C.; Perugini, E.; Harding, I.; Sheppard, M.N.; Poole-Wilson, P.A.; Hawkins, P.N.; Pennell, D.J. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation 2005, 111, 186–193. [Google Scholar] [CrossRef]
- Kim, R.J.; Shah, D.J.; Judd, R.M. How we perform delayed enhancement imaging. J. Cardiovasc. Magn. Reson. 2003, 5, 613–615. [Google Scholar] [CrossRef]
- Franco, A.; Javidi, S.; Ruehm, S.G. Delayed Myocardial Enhancement in Cardiac Magnetic Resonance Imaging. J. Radiol. Case Rep. 2015, 9, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Piechnik, S.K.; Ferreira, V.M.; Dall’Armellina, E.; Cochlin, L.E.; Greiser, A.; Neubauer, S.; Robson, M.D. Shortened Modified Look-Locker Inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J. Cardiovasc. Magn. Reson. 2010, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- Karamitsos, T.D.; Piechnik, S.K.; Banypersad, S.M.; Fontana, M.; Ntusi, N.B.; Ferreira, V.M.; Whelan, C.J.; Myerson, S.G.; Robson, M.D.; Hawkins, P.N.; et al. Noncontrast T1 Mapping for the Diagnosis of Cardiac Amyloidosis. J. Am. Coll. Cardiol. Cardiovasc. Imaging 2013, 6, 488–497. [Google Scholar] [CrossRef]
- Haaf, P.; Garg, P.; Messroghli, D.R.; Broadbent, D.A.; Greenwood, J.P.; Plein, S. Cardiac T1 Mapping and Extracellular Volume (ECV) in clinical practice: A comprehensive review. J. Cardiovasc. Magn. Reson. 2017, 18, 89. [Google Scholar] [CrossRef]
- Martinez-Naharro, A.; Kotecha, T.; Norrington, K.; Boldrini, M.; Rezk, T.; Quarta, C.; Treibel, T.A.; Whelan, C.J.; Knight, D.S.; Kellman, P.; et al. Native T1 and Extracellular Volume in Transthyretin Amyloidosis. JACC Cardiovasc. Imaging 2019, 12, 810–819. [Google Scholar] [CrossRef]
- Korthals, D.; Chatzantonis, G.; Bietenbeck, M.; Meier, C.; Stalling, P.; Yilmaz, A. CMR-based T1-mapping offers superior diagnostic value compared to longitudinal strain-based assessment of relative apical sparing in cardiac amyloidosis. Sci. Rep. 2021, 11, 15521. [Google Scholar] [CrossRef]
- Kidoh, M.; Oda, S.; Takashio, S.; Morioka, M.; Kuyama, N.; Oguni, T.; Nakaura, T.; Nagayama, Y.; Izumiya, Y.; Tsujita, K.; et al. MRI-Extracellular Volume Fraction Versus Histological Amyloid Load in Cardiac Amyloidosis: The Importance of T2 Mapping. Circ. Cardiovasc. Imaging 2025, 18, e017427. [Google Scholar] [CrossRef]
- Grazzini, G.; Pradella, S.; Bani, R.; Fornaciari, C.; Cappelli, F.; Perfetto, F.; Cozzi, D.; Giovannelli, S.; Sica, G.; Miele, V.; et al. The Role of T2 Mapping in Cardiac Amyloidosis. Diagnostics 2024, 14, 1048. [Google Scholar] [CrossRef]
- Ridouani, F.; Damy, T.; Tacher, V.; Derbel, H.; Legou, F.; Sifaoui, I.; Audureau, E.; Bodez, D.; Rahmouni, A.; Deux, J.-F. Myocardial native T2 measurement to differentiate light-chain and transthyretin cardiac amyloidosis and assess prognosis. J. Cardiovasc. Magn. Reson. 2018, 20, 58. [Google Scholar] [CrossRef]
- Mori, A.; Saito, Y.; Nakamura, K.; Iida, T.; Akagi, S.; Yoshida, M.; Taniyama, M.; Miyoshi, T.; Ito, H. Microcalcification and 99mTc-Pyrophosphate Uptake without Increased Bone Metabolism in Cardiac Tissue from Patients with Transthyretin Cardiac Amyloidosis. Int. J. Mol. Sci. 2023, 24, 1921. [Google Scholar] [CrossRef]
- Masri, A.; Bukhari, S. Efficient 1-Hour Technetium-99 m Pyrophosphate Imaging Protocol for the Diagnosis of Transthyretin Cardiac Amyloidosis. Circ. Cardiovasc. Imaging 2020, 13, e010249. [Google Scholar] [CrossRef]
- Asif, T.; Gomez, J.; Singh, V.; Doukky, R.; Nedeltcheva, A.; Malhotra, S. Comparison of planar with tomographic pyrophosphate scintigraphy for transthyretin cardiac amyloidosis: Perils and pitfalls. J. Nucl. Cardiol. 2021, 28, 104–111. [Google Scholar] [CrossRef]
- Dorbala, S.; Ando, Y.; Bokhari, S.; Dispenzieri, A.; Falk, R.H.; Ferrari, V.A.; Fontana, M.; Gheysens, O.; Gillmore, J.D.; Glaudemans, A.W.; et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI Expert Consensus Recommendations for Multimodality Imaging in Cardiac Amyloidosis: Part 1 of 2—Evidence Base and Standardized Methods of Imaging. J. Card. Fail. 2019, 25, e1–e39. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, S.; Castano, A. 99mTc-Pyrophosphate Scintigraphy for Differentiating Light-Chain Cardiac Amyloidosis From the Transthyretin-Related Familial and Senile Cardiac Amyloidoses. Circ. Cardiovasc. Imaging. 2013, 6, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Long, T.E.; Indridason, O.S.; Palsson, R.; Rognvaldsson, S.; Love, T.J.; Thorsteinsdottir, S.; Sverrisdottir, I.S.; Vidarsson, B.; Onundarson, P.T.; Agnarsson, B.A.; et al. Defining new reference intervals for serum free light chains in individuals with chronic kidney disease: Results of the iStopMM study. Blood Cancer J. 2022, 12, 133. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.; Maurer, M. Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation 2016, 133, 2404–2412. [Google Scholar] [CrossRef]
- Witteles, R.M.; Liedtke, M.L. AL Amyloidosis for the Cardiologist and Oncologist: Epidemiology, Diagnosis, and Management. J. Am. Coll. Cardiol. CardioOncology 2019, 1, 117–130. [Google Scholar]
- Sipe, J.D.; Benson, M.D.; Buxbaum, J.N.; Ikeda, S.-I.; Merlini, G.; Saraiva, M.J.M.; Westermark, P. Amyloid fibril proteins and amyloidosis: Chemical identification and clinical classification International Society of Amyloidosis 2016 Nomenclature Guidelines. Amyloid 2016, 23, 203–213. [Google Scholar] [CrossRef]
- Kittleson, M.; Ruberg, F.L.; Ambardekar, A.V.; Brannagan, T.H.; Cheng, R.K.; Clarke, J.O.; Dember, L.M.; Frantz, J.G.; Hershberger, R.E.; Maurer, M.S.; et al. 2023 ACC expert consensus decision pathway on comprehensive multidisciplinary care for the patient with cardiac amyloidosis: A report of the american college of cardiology solution set oversight committee. J. Am. Coll. Cardiol. 2023, 81, 1076–1126. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Gabrovsek, A.; Sul, L.; Cotta, C.; Rodriguez, E.R.; Tan, C.D.; Hanna, M. Evidence of Concurrent Light Chain and Transthyretin Cardiac Amyloidosis in 2 Patients. J. Am. Coll. Cardiol. CardioOncology 2020, 2, 127–130. [Google Scholar] [CrossRef]
- Kanelidis, A.J.; Miller, P.; Prabhu, N.; Cruz, M.J.D.; Alenghat, F.J.; McMullen, P.; Sarswat, N.; Derman, B.A.; Polonsky, T.S.; DeCara, J.M. ATTR Cardiomyopathy Meets Multiple Myeloma. J. Am. Coll. Cardiol. CardioOncology 2021, 3, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Sidiqi, M.H.; McPhail, E.D.; Theis, J.D.; Dasari, S.; Vrana, J.A.; Drosou, M.E.; Leung, N.; Hayman, S.; Rajkumar, S.V.; Warsame, R.; et al. Two types of amyloidosis presenting in a single patient: A case series. Nat. Blood Cancer J. 2019, 9, 30. [Google Scholar] [CrossRef]
- Dorbala, S.; Cuddy, S.; Falk, R.H. How to Image Cardiac Amyloidosis: A Practical Approach. J. Am. Coll. Cardiol. Cardiovasc. Imaging 2020, 13, 1368–1383. [Google Scholar]
- Madias, J.E. Low QRS voltage and its causes. J. Electrocardiol. 2008, 41, 498–500. [Google Scholar] [CrossRef] [PubMed]
- Buss, S.; Emami, M.; Mereles, D.; Korosoglou, G.; Kristen, A.V.; Voss, A.; Schellberg, D.; Zugck, C.; Galuschky, C.; Giannitsis, E.; et al. Longitudinal Left Ventricular Function for Prediction of Survival in Systemic Light-Chain Amyloidosis: Incremental Value Compared with Clinical and Biochemical Markers. J. Am. Coll. Cardiol. 2012, 12, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Barros-Gomes, S.; Williams, B.; Nhola, L.; Grogan, M.; Maalouf, J.F.; Dispenzieri, A.; Pellikka, P.A.; Villarraga, H.R. Prognosis of Light Chain Amyloidosis With Preserved LVEF: Added Value of 2D Speckle-Tracking Echocardiography to the Current Prognostic Staging System. J. Am. Coll. Cardiol. Imaging 2017, 4, 398–407. [Google Scholar] [CrossRef]
- Ghadimi, M.; Sapra, A. Magnetic Resonance Imaging Contraindications; StatPearls: Treasure Island, FL, USA, 2019. [Google Scholar]
- Quarta, C.C.; Gonzalez-Lopez, E.; Gilbertson, J.A.; Botcher, N.; Rowczenio, D.; Petrie, A.; Rezk, T.; Youngstein, T.; Mahmood, S.; Sachchithanantham, S.; et al. Diagnostic sensitivity of abdominal fat aspiration in cardiac amyloidosis. Eur. Heart J. 2017, 24, 1905–1908. [Google Scholar] [CrossRef]
- Muchtar, E.; Dispenzieri, A.; Lacy, M.Q.; Buadi, F.K.; Kapoor, P.; Hayman, S.R.; Gonsalves, W.; Warsame, R.; Kourelis, T.V.; Chakraborty, R.; et al. Overuse of organ biopsies in immunoglobulin light chain amyloidosis (AL): The consequence of failure of early recognition. Ann. Med. 2017, 49, 545–551. [Google Scholar] [CrossRef]
- Palladini, G.; Milani, P.; Merlini, G. Management of AL amyloidosis in 2020. Blood 2020, 136, 2620–2627. [Google Scholar] [CrossRef]
- Maurer, M.S.; Schwartz, J.H.; Gundapaneni, B.; Elliott, P.M.; Merlini, G.; Waddington-Cruz, M.; Kristen, A.V.; Grogan, M.; Witteles, R.; Damy, T.; et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N. Engl. J. Med. 2018, 379, 1007–1016. [Google Scholar] [CrossRef]
- Shah, S.J.; Fine, N.; Garcia-Pavia, P.; Klein, A.L.; Fernandes, F.; Weissman, N.J.; Maurer, M.S.; Boman, K.; Gundapaneni, B.; Sultan, M.B.; et al. Effect of tafamidis on cardiac function in patients with transthyretin amyloid cardiomyopathy: A post hoc analysis of the ATTR-ACT randomized clinical trial. JAMA Cardiol. 2024, 9, 25–34. [Google Scholar] [CrossRef]
- Elliott, P.; Drachman, B.M.; Gottlieb, S.S.; Hoffman, J.E.; Hummel, S.L.; Lenihan, D.J.; Ebede, B.; Gundapaneni, B.; Li, B.; Sultan, M.B.; et al. Long-Term Survival With Tafamidis in Patients With Transthyretin Amyloid Cardiomyopathy. Circ. Heart Fail. 2021, 15, e008193. [Google Scholar] [CrossRef] [PubMed]
- Canadian Agency for Drugs and Technologies in Health. Pharmacoeconomic Review Report: Tafamidis (Vyndaqel). Available online: https://www.ncbi.nlm.nih.gov/books/NBK563701/ (accessed on 27 June 2025).
- Gillmore, J.D.; Judge, D.P.; Cappelli, F.; Fontana, M.; Garcia-Pavia, P.; Gibbs, S.; Grogan, M.; Hanna, M.; Hoffman, J.; Masri, A.; et al. Efficacy and Safety of Acoramidis in Transthyretin Amyloid Cardiomyopathy. N. Engl. J. Med. 2024, 390, 132–142. [Google Scholar] [CrossRef]
- Castaño, A.; Helmke, S.; Alvarez, J.; Delisle, S.; Maurer, M.S. Diflunisal for ATTR Cardiac Amyloidosis. Congest. Heart Fail. 2012, 18, 315–319. [Google Scholar] [CrossRef]
- Ferreira, N.; Cardoso, I.; Domingues, M.R.; Vitorino, R.; Bastos, M.; Bai, G.; Saraiva, M.J.; Almeida, M.R. Binding of epigallocatechin-3-gallate to transthyretin modulates its amyloidogenicity. FEBS Lett. 2009, 583, 3569–3576. [Google Scholar] [CrossRef]
- Kristen, A.V.; Lehrke, S.; Buss, S.; Mereles, D.; Steen, H.; Ehlermann, P.; Hardt, S.; Giannitsis, E.; Schreiner, R.; Haberkorn, U.; et al. Green tea halts progression of cardiac transthyretin amyloidosis: An observational report. Clin. Res. Cardiol. 2012, 101, 805–813. [Google Scholar] [CrossRef]
- Canadian Agency for Drugs and Technologies in Health. Pharmacoeconomic Review Report: Patisiran (Onpattro). Available online: https://www.ncbi.nlm.nih.gov/books/NBK549697/ (accessed on 27 June 2025).
- Canadian Agency for Drugs and Technologies in Health. Pharmacoeconomic Review Report: Inotersen (Tegsedi). Available online: https://www.ncbi.nlm.nih.gov/books/NBK558353/ (accessed on 27 June 2025).
- Prata, A.A.; Katsuyama, E.S.; Scardini, P.G.; Covre, A.C.; Neto, W.F.; Fernandes, J.M.; Barbosa, G.S.; Fukunaga, C.; Pinheiro, R.P.; Antunes, V.L.J.; et al. The efficacy and safety of specific therapies for cardiac Transthyretin-mediated amyloidosis: A systematic review and meta-analysis of randomized trials. BMC Cardiovasc. Discord. 2025, 25, 296. [Google Scholar] [CrossRef]
- Benson, M.D.; Waddington-Cruz, M.; Berk, J.L.; Polydefkis, M.; Dyck, P.J.; Wang, A.K.; Planté-Bordeneuve, V.; Barroso, F.A.; Merlini, G.; Obici, L.; et al. Inotersen Treatment for Patients with Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 22–31. [Google Scholar] [CrossRef]
- Karam, C.; Brown, D.; Yang, M.; Done, N.; Zhu, J.J.; Greatsinger, A.; Bozas, A.; Vera-Llonch, M.; Signorovitch, J. Long-term treatment effects of inotersen on health-related quality of life in patients with hATTR amyloidosis with polyneuropathy: Analysis of the open-label extension of the NEURO-TTR trial. Muscle Nerve 2022, 66, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.S.; Kale, P.; Fontana, M.; Berk, J.L.; Grogan, M.; Gustafsson, F.; Hung, R.R.; Gottlieb, R.L.; Damy, T.; González-Duarte, A.; et al. Patisiran Treatment in Patients with Transthyretin Cardiac Amyloidosis. N. Engl. J. Med. 2023, 387, 1553–1567. [Google Scholar] [CrossRef] [PubMed]
- Alnylam. Alnylam Announces Receipt of Complete Response Letter from, U.S. FDA for Supplemental New Drug Application for Patisiran for the Treatment of the Cardiomyopathy of ATTR Amyloidosis. Available online: https://investors.alnylam.com/press-release?id=27741 (accessed on 27 June 2025).
- Cllinicaltrails.gov. HELIOS-A: A Study of Vutrisiran (ALN-TTRSC02) in Patients with Hereditary Transthyretin Amyloidosis (ATTRv Amyloidosis). Available online: https://clinicaltrials.gov/study/NCT03759379?term=helios&cond=cardiac%20amyloidosis&rank=1 (accessed on 27 June 2025).
- Clinicaltrials.gov. HELIOS-B: A Study to Evaluate Vutrisiran in Patients with Transthyretin Amyloidosis with Cardiomyopathy. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04153149 (accessed on 27 June 2025).
- Food Drug Administration. Developing Products for Rare Diseases and Conditions. Available online: https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=827521 (accessed on 27 June 2025).
- Coelho, T.; Marques, W.; Dasgupta, N.R.; Chao, C.-C.; Parman, Y.; França, M.C.; Guo, Y.-C.; Wixner, J.; Ro, L.-S.; Calandra, C.R.; et al. Eplontersen for Hereditary Transthyretin Amyloidosis With Polyneuropathy. J. Am. Med. Assoc. 2023, 330, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.B.; Li, Y.; Geng, J.M.; Zhou, H.M.; Liu, L.; Peng, X. Recent Progress in the Development and Clinical Application of New Drugs for Transthyretin Cardiac Amyloidosis. J. Cardiovasc. Pharmacol. 2023, 82, 427–437. [Google Scholar] [CrossRef]
- Rare Disease Advisor N1006 Has Acceptable Safety Profile in Patients with, A.T.T.R.-C.M. Available online: https://www.rarediseaseadvisor.com/news/n1006-safety-profile-transthyretin-amyloid-cardiomyopathy/ (accessed on 27 June 2025).
- Garcia-Pavia, P.; Siepen, F.A.D.; Donal, E.; Lairez, O.; van der Meer, P.; Kristen, A.V.; Mercuri, M.F.; Michalon, A.; Frost, R.J.; Grimm, J.; et al. Phase 1 Trial of Antibody NI006 for Depletion of Cardiac Transthyretin Amyloid. N. Engl. J. Med. 2023, 389, 239–250. [Google Scholar] [CrossRef]
- Finn, J.D.; Smith, A.R.; Patel, M.C.; Shaw, L.; Youniss, M.R.; van Heteren, J.; Dirstine, T.; Ciullo, C.; Lescarbeau, R.; Seitzer, J.; et al. A Single Administration of CRISPR/Cas9 Lipid Nanoparticles Achieves Robust and Persistent In Vivo Genome Editing. Cell Rep. 2018, 9, 2227–2235. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Gane, E.; Taubel, J.; Kao, J.; Fontana, M.; Maitland, M.L.; Seitzer, J.; O’Connell, D.; Walsh, K.R.; Wood, K.; et al. CRISPR-Cas9 In Vivo Gene Editing for Transthyretin Amyloidosis. N. Engl. J. Med. 2021, 6, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Fontana, M.; Solomon, S.D.; Kachadourian, J.; Walsh, L.; Rocha, R.; Lebwohl, D.; Smith, D.; Täubel, J.; Gane, E.J.; Pilebro, B.; et al. CRISPR-Cas9 Gene Editing with Nexiguran Ziclumeran for ATTR Cardiomyopathy. N. Engl. J. Med. 2024, 319, 2231–2241. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Cradick, T.J.; Brown, M.T.; Deshmukh, H.; Ranjan, P.; Sarode, N.; Wile, B.M.; Vertino, P.M.; Stewart, F.J.; Bao, G. CRISPR/Cas9 systems have off-target activity with insertions or deletions between target DNA and guide RNA sequences. Nucleic Acids Res. 2014, 11, 7473–7485. [Google Scholar] [CrossRef]
- Kazemian, P.; Yu, S.-Y.; Thomson, S.B.; Birkenshaw, A.; Leavitt, B.R.; Ross, C.J.D. Lipid-Nanoparticle-Based Delivery of CRISPR/Cas9 Genome-Editing Components. Mol. Pharm. 2022, 6, 1669–1686. [Google Scholar] [CrossRef]
- Akinc, A.; Querbes, W.; De, S.; Qin, J.; Frank-Kamenetsky, M.; Jayaprakash, K.N.; Jayaraman, M.; Rajeev, K.G.; Cantley, W.L.; Dorkin, J.R.; et al. Targeted Delivery of RNAi Therapeutics With Endogenous and Exogenous Ligand-Based Mechanisms. Mol. Ther. 2010, 18, 1357–1364. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. MAGNITUDE: A Phase 3 Study of NTLA-2001 in Participants with Transthyretin Amyloidosis with Cardiomyopathy. Available online: https://clinicaltrials.gov/study/NCT06128629 (accessed on 27 June 2025).
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.; Vaduganathan, M.; Claggett, B.; Jhund, P.S.; Desai, A.S.; Henderson, A.D.; Lam, C.S.; Pitt, B.; Senni, M.; et al. Finerenone in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2024, 391, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Kittleson, M.M.; Maurer, M.S.; Ambardekar, A.V.; Bullock-Palmer, R.P.; Chang, P.P.; Eisen, H.J.; Nair, A.P.; Nativi-Nicolau, J.; Ruberg, F.L.; On behalf of the American Heart Association Heart Failure; et al. Cardiac Amyloidosis: Evolving Diagnosis and Management: A Scientific Statement From the American Heart Association. Circulation 2020, 142, E7–E22. [Google Scholar] [CrossRef]
- Donnellan, E.; Wazni, O.M.; Hanna, M.; Elshazly, M.B.; Puri, R.; Saliba, W.; Kanj, M.; Vakamudi, S.; Patel, D.R.; Baranowski, B.; et al. Atrial Fibrillation in Transthyretin Cardiac Amyloidosis. JACC. Clin. Electrophysiol. 2020, 6, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Touboul, O.; Algalarrondo, V.; Oghina, S.; Elbaz, N.; Rouffiac, S.; Hamon, D.; Extramiana, F.; Gandjbakhch, E.; D'HUmieres, T.; Marijon, E.; et al. Electrical cardioversion of atrial arrhythmias with cardiac amyloidosis in the era of direct oral anticogulants. ESC Heart Fail. 2022, 9, 3556–3564. [Google Scholar] [CrossRef]
- Black-Maier, E.; Rehorn, M.; Loungani, R.; Friedman, D.J.; Alenezi, F.; Geurink, K.; Pokorney, S.D.; Daubert, J.P.; Sun, A.Y.; Atwater, B.D.; et al. Catheter ablation of atrial fibrillation in cardiac amyloidosis. Pacing. Clin. Electrophysiol. 2020, 43, 913–921. [Google Scholar] [CrossRef]
- Mints, Y.Y.; Doros, G.; Berk, J.L.; Connors, L.H.; Ruberg, F.L. Features of atrial fibrillation in wild-type transthyretin cardiac amyloidosis: A systematic review and clinical experience. Eur. Soc. Cardiol. 2018, 5, 772–779. [Google Scholar] [CrossRef]
- Donnellan, E.; Elshazly, M.B.; Vakamudi, S.; Wazni, O.M.; Cohen, J.A.; Kanj, M.; Hanna, M.; Baranowski, B.; Saliba, W.; Jaber, W. No Association Between CHADS-VASc Score and Left Atrial Appendage Thrombus in Patients With Transthyretin Amyloidosis. J. Am. Coll. Cardiol. EP 2019, 5, 1473–1474. [Google Scholar] [CrossRef]
- Kristen, A.V.; Dengler, T.J.; Hegenbart, U.; Schonland, S.O.; Goldschmidt, H.; Sack, F.-U.; Voss, F.; Becker, R.; Katus, H.A.; Bauer, A. Prophylactic implantation of cardioverter-defibrillator in patients with severe cardiac amyloidosis and high risk for sudden cardiac death. Heart Rhythm. 2008, 5, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Varr, B.C.; Zarafshar, S.; Coakley, T.; Liedtke, M.; Lafayette, R.A.; Arai, S.; Schrier, S.L.; Witteles, R.M. Implantable cardioverter-defibrillator placement in patients with cardiac amyloidosis. Heart Rhythm. 2013, 11, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Dispenzieri, A.; Kyle, R.; Grogan, M.; Brady, P.A. Implantable Cardioverter Defibrillators in Patients with Cardiac Amyloidosis. J. Cardiovasc. Electrophysiol. 2013, 24, 793–798. [Google Scholar] [CrossRef]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for Management of Patients with Ventricular Arrhythmias and:: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2018, 13, e272–e391. [Google Scholar] [CrossRef] [PubMed]
- Porcari, A.; Rossi, M.; Cappelli, F.; Canepa, M.; Musumeci, B.; Cipriani, A.; Tini, G.; Barbati, G.; Varrà, G.G.; Morelli, C.; et al. Incidence and risk factors for pacemaker implantation in light-chain and transthyretin cardiac amyloidosis. ESC Heart Fail. 2022, 24, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Saturi, G.; De Frutos, F.; Sguazzotti, M.; Gonzalez-Lopez, E.; Nardi, E.; Domínguez, F.; Ponziani, A.; Cabrera, E.; Caponetti, A.G.; Lozano, S.; et al. Predictors and outcomes of pacemaker implantation in patients with cardiac amyloidosis. Heart 2024, 110, 40–48. [Google Scholar] [CrossRef]
- Bukhari, S.; Kasi, A.; Khan, B. Bradyarrhythmias in Cardiac Amyloidosis and Role of Pacemaker. Curr. Probl. Cardiol. 2023, 48, 101912. [Google Scholar] [CrossRef]
- Donnellan, E.; Wazni, O.M.; Hanna, M.; Kanj, M.; Saliba, W.I.; Jaber, W.A. Cardiac Resynchronization Therapy for Transthyretin Cardiac Amyloidosis. J. Am. Heart Assoc. 2020, 9, e017335. [Google Scholar] [CrossRef]
- Fischer, K.; Lellouche, N.; Damy, T.; Martins, R.; Clementy, N.; Bisson, A.; Lesaffre, F.; Espinosa, M.; Garcia, R.; Degand, B.; et al. Cardiovascular outcomes after cardiac resynchronization therapy in cardiac amyloidosis. ESC Heart Fail. 2021, 9, 740–750. [Google Scholar] [CrossRef]
- Cordes, S.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; Dingli, D.; Kumar, S.K.; Hogan, W.J.; Gertz, M.A. Ten-year survival after autologous stem cell transplantation for immunoglobulin light chain amyloidosis. J. Am. Cancer Soc. 2012, 118, 6105–6109. [Google Scholar] [CrossRef]
- Sidana, S.; Sidiqi, M.H.; Dispenzieri, A.; Buadi, F.K.; Lacy, M.Q.; Muchtar, E.; Dingli, D.; Hayman, S.R.; Gonsalves, W.I.; Kapoor, P.; et al. Fifteen year overall survival rates after autologous stem cell transplantation for AL amyloidosis. Am. J. Hematol. 2019, 94, 1020–1026. [Google Scholar] [CrossRef]
- Sanchorawala, V.; Boccadoro, M.; Gertz, M.; Hegenbart, U.; Kastritis, E.; Landau, H.; Mollee, P.; Wechalekar, A.; Palladini, G. Guidelines for high dose chemotherapy and stem cell transplantation for systemic AL amyloidosis: EHA-ISA working group guidelines. Amyloid 2022, 29, 1–7. [Google Scholar] [CrossRef]
- Wechalekar, A.D.; Cibeira, M.T.; Gibbs, S.D.; Jaccard, A.; Kumar, S.; Merlini, G.; Palladini, G.; Sanchorawala, V.; Schönland, S.; Venner, C.; et al. Guidelines for non-transplant chemotherapy for treatment of systemic AL amyloidosis: EHA-ISA working group. Amyloid 2022, 30, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Sanchorawala, V.; Sun, F.; Quillen, K.; Sloan, J.M.; Berk, J.L.; Seldin, D.C. Long-term outcome of patients with AL amyloidosis treated with high-dose melphalan and stem cell transplantation: 20-year experience. Blood 2015, 20, 2345–2347. [Google Scholar] [CrossRef][Green Version]
- Bianchi, G.; Zhang, Y.; Comenzo, R.L. AL amyloidosis: Current chemotherapy and immune therapy treatment strategies: Jacc: CardioOncology state-of-the-art review. JACC CardioOncol. 2021, 3, 467–487. [Google Scholar] [CrossRef]
- Mehra, M.R.; Canter, C.E.; Hannan, M.M.; Semigran, M.J.; Uber, P.A.; Baran, D.A.; Danziger-Isakov, L.; Kirklin, J.K.; Kirk, R.; Kushwaha, S.S.; et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J. Heart Lung Transplant. 2016, 35, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Lund, L.H.; Khush, K.K.; Cherikh, W.S.; Goldfarb, S.; Kucheryavaya, A.Y.; Levvey, B.J.; Meiser, B.; Rossano, J.W.; Chambers, D.C.; Yusen, R.D.; et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Heart Transplantation Report—2017; Focus Theme: Allograft ischemic time. J. Heart Lung Transplant. 2017, 10, 1037–1046. [Google Scholar] [CrossRef]
- Barrett, C.; Alexander, K.; Zhao, H.; Haddad, F.; Cheng, P.; Liao, R.; Wheeler, M.T.; Liedtke, M.; Schrier, S.; Arai, S.; et al. Outcomes in Patients with Cardiac Amyloidosis Undergoing Heart Transplantation. Heart Fail. 2020, 6, 461–468. [Google Scholar] [CrossRef]
- Obici, L.; Kuks, J.B.; Buades, J.; Adams, D.; Suhr, O.B.; Coelho, T.; Kyriakides, T. Recommendations for presymptomatic genetic testing and management of individuals at risk for hereditary transthyretin amyloidosis. Curr. Opin. Neurol. 2016, 29, 27–35. [Google Scholar] [CrossRef]
- Schmidt, H.H.; Barroso, F.; González-Duarte, A.; Conceição, I.; Obici, L.; Keohane, D.; Amass, L. Management of asymptomatic gene carriers of transthyretin familial amyloid polyneuropathy. Muscle Nerve 2016, 54, 353–360. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Koike, H.; Akane, A.; Shibata, Y.; Nishiwaki, K.; Sobue, G. Spinal cord stimulation markedly ameliorated refractory neuropathic pain in transthyretin Val30Met familial amyloid polyneuropathy. Amyloid 2011, 18, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Bollée, G.; Guery, B.; Joly, D.; Snanoudj, R.; Terrier, B.; Allouache, M.; Mercadal, L.; Peraldi, M.-N.D.; Viron, B.A.A.; Fumeron, C.; et al. Presentation and Outcome of Patients with Systemic Amyloidosis Undergoing Dialysis. Clin. J. Am. Soc. Nephrol. 2008, 3, 375–381. [Google Scholar] [CrossRef]
- Havasi, A.; Heybeli, C.; Leung, N.; Angel-Korman, A.; Sanchorawala, V.; Cohen, O.; Wechalekar, A.; Bridoux, F.; Jaffer, I.; Gutgarts, V.; et al. Outcomes of renal transplantation in patients with AL amyloidosis: An international collaboration through The International Kidney and Monoclonal Gammopathy Research Group. Blood Cancer J. 2022, 12, 119. [Google Scholar] [CrossRef]
- Angel-Korman, A.; Stern, L.; Sarosiek, S.; Sloan, J.M.; Doros, G.; Sanchorawala, V.; Havasi, A. Long-term outcome of kidney transplantation in AL amyloidosis. Kidney Int. 2019, 95, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Dispenzieri, A. Artificial Intelligence Enhanced ECG to Detect Cardiac Amyloidosis. Available online: https://clinicaltrials.gov/study/NCT05557162?cond=cardiac%20amyloidosis&page=2&rank=11 (accessed on 27 June 2025).
- Ouyang, D. Artificial Intelligence Guided Echocardiographic Screening of Rare Diseases (EchoNet-Screening). Available online: https://clinicaltrials.gov/study/NCT05139797?cond=cardiac%20amyloidosis&term=artificial%20intelligence&rank=1 (accessed on 27 June 2025).
- Barua, I.; Wieszczy, P.; Kudo, S.-E.; Misawa, M.; Holme, Ø.; Gulati, S.; Williams, S.; Mori, K.; Itoh, H.; Takishima, K.; et al. Real-Time Artificial Intelligence–Based Optical Diagnosis of Neoplastic Polyps during Colonoscopy. N. Engl. J. Med. 2022, 1, EVIDoa2200003. [Google Scholar] [CrossRef] [PubMed]
- Meehan, A.M.; Core, M.A.; Ross, J.M.; Rahman, P.A.; Borah, B.J.; Caraballo, P.J. Clinical Implementation of an AI Early Warning System Algorithm: Lessons Learned. Stud. Health Technol. Inform. 2024, 25, 1376–1377. [Google Scholar]




| Study Title | Intervention | Number of Study Participants | Study Progress |
|---|---|---|---|
| HELIOS-A [79] | Efficacy of vutrisiran for hereditary transthyretin amyloid cardiomyopathy | 164 | Estimated Completion: 10/2026 |
| HELIOS-B [80] | Efficacy of vutrisiran for transthyretin amyloid cardiomyopathy | 655 | Estimated Completion: 12/2026 |
| CARDIO-TTRANSFORM [126] | Efficacy of eplontersen for transthyretin amyloid cardiomyopathy | 1438 | Estimated Completion: 11/2025 |
| MAGNITUDE [92] | NTLA-2001 transthyretin amyloid cardiomyopathy | 765 | Estimated Completion: 4/2028 |
| Early Detection of Neuropathy in ATTRv [127] | Comparing different diagnostic modalities for early diagnosis of peripheral neuropathy in patients with ATTRv | 47 | Estimated Completion: 2/2029 |
| Exploring Biomarkers in Hereditary Amyloidosis [128] | Characterizing different biomarkers to better characterize severity and natural course of amyloid disease | 80 | Estimated Completion: 4/2026 |
| Artificial Intelligence Enhanced ECG to Detect Cardiac Amyloidosis [129] | Utilization of artificial intelligence to improve diagnosis of amyloidosis | 200 | Estimated Completion: 12/2024 |
| Artificial Intelligence Guided Echocardiographic Screening of Rare Diseases (EchoNet-Screening) [130] | Utilization of AI to identify patients with LVH. AI will determine whether these patients need additional screening | 300 | Estimated Completion: 6/2025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, D.M.; Ramazani, N.; Sodhi, G.; Tak, T. Cardiac Amyloidosis: Tribulations and New Frontiers. J. Pers. Med. 2025, 15, 472. https://doi.org/10.3390/jpm15100472
Nguyen DM, Ramazani N, Sodhi G, Tak T. Cardiac Amyloidosis: Tribulations and New Frontiers. Journal of Personalized Medicine. 2025; 15(10):472. https://doi.org/10.3390/jpm15100472
Chicago/Turabian StyleNguyen, Darren M., Noyan Ramazani, Gurpreet Sodhi, and Tahir Tak. 2025. "Cardiac Amyloidosis: Tribulations and New Frontiers" Journal of Personalized Medicine 15, no. 10: 472. https://doi.org/10.3390/jpm15100472
APA StyleNguyen, D. M., Ramazani, N., Sodhi, G., & Tak, T. (2025). Cardiac Amyloidosis: Tribulations and New Frontiers. Journal of Personalized Medicine, 15(10), 472. https://doi.org/10.3390/jpm15100472






