Functional Impairment in Behavioral Variant Frontotemporal Dementia: Cognitive, Behavioral, Personality, and Brain Perfusion Contributions
Abstract
1. Introduction
1.1. Background
1.2. Goals and Hypotheses of the Study
2. Materials and Methods
2.1. Participants
2.2. Procedures
Protocol Approvals and Patient Consents
2.3. Neuropsychological Assessment
2.4. Neuroimaging: Brain Perfusion SPECT Scans
2.5. Statistical Analyses
3. Results
3.1. Demographic and Clinical Characteristics
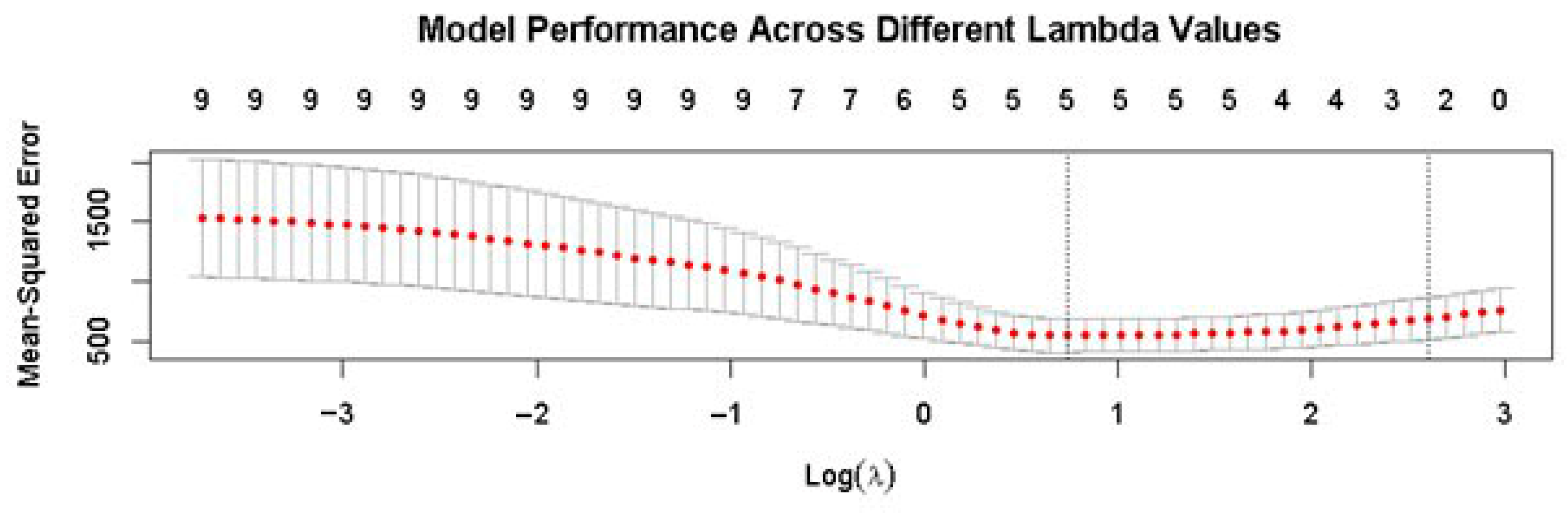
3.2. Selection of Variables for Inclusion in the Penalized LASSO Regression Analysis
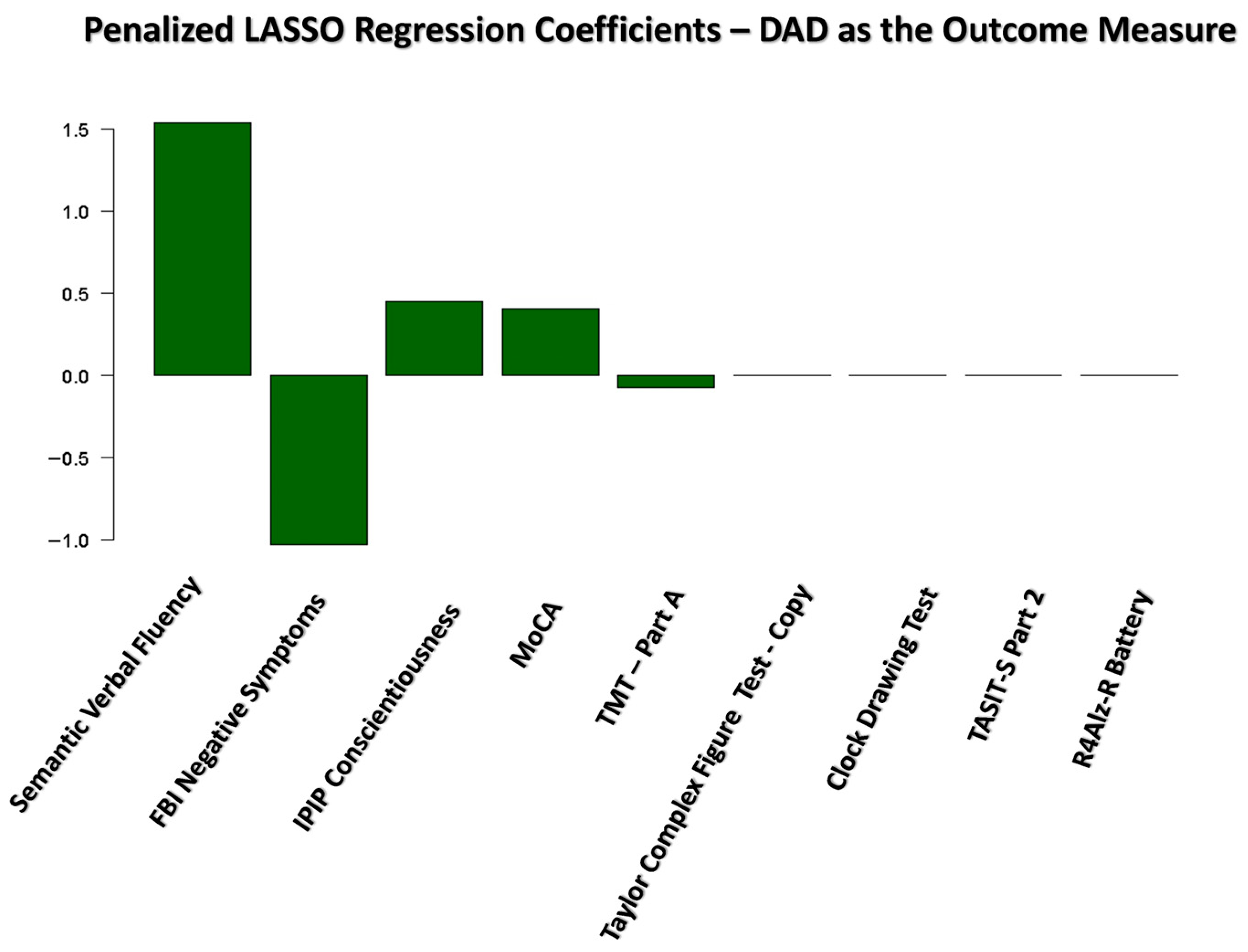
3.3. Results of the Penalized LASSO Regression Analysis
3.4. Brain Perfusion Contributions to Functional Status in bvFTD
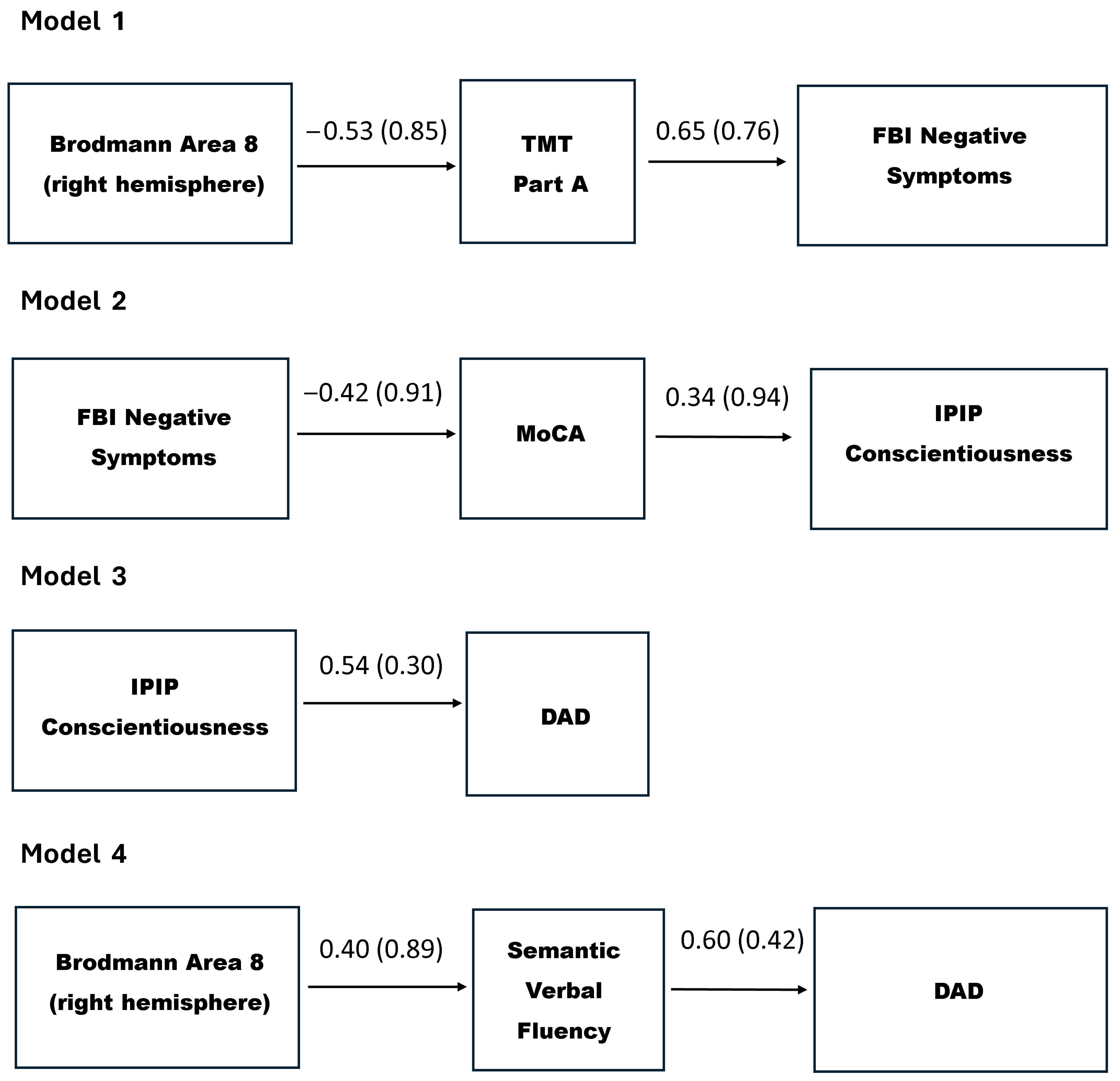
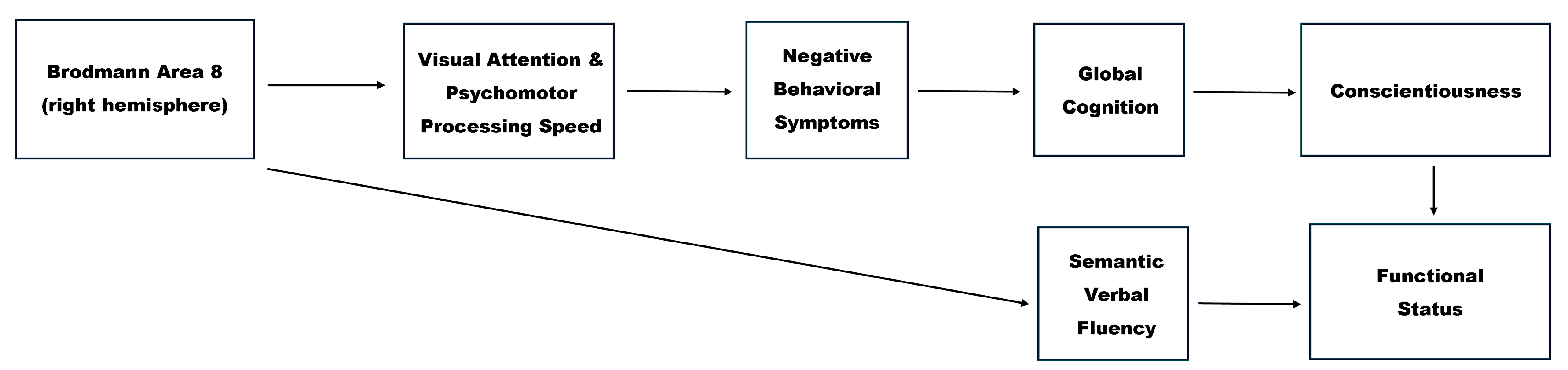
3.5. Path Analysis Results
4. Discussion
4.1. Clinical Implications
4.2. Limitations
4.3. Strengths and Unique Contribution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| ADLs | Activities of daily living |
| AUTh | Aristotle University of Thessaloniki |
| BA | Brodmann area |
| BADLs | Basic activities of daily living |
| bvFTD | Behavioral variant frontotemporal dementia |
| CDR | Clinical Dementia Rating Scale |
| CDT | Clock Drawing Test |
| CFI | Comparative Fit Index |
| CSC | Controlled Semantic Cognition model |
| CT | Computed tomography |
| DAD | Disability Assessment for Dementia |
| EANM | European Association of Nuclear Medicine |
| EET | Emotion Evaluation Test |
| EU | European Union |
| FAQ | Functional Activities Questionnaire |
| FBI | Frontal Behavioral Inventory |
| FES | FRONTIER Executive Screen battery |
| FRS | Frontotemporal Dementia Rating Scale |
| FTDC | Frontotemporal Dementia Criteria Consortium |
| FTLD | Frontotemporal Lobar Degeneration |
| FTLD-CDR | Frontotemporal Lobar Degeneration-Modified Clinical Dementia Rating Scale |
| FWER | Family-wise error rate |
| GDPR | General Data Protection Regulation |
| IADLs | Instrumental activities of daily living |
| IPIP | Goldberg’s International Personality Item Pool Big-Five Questionnaire |
| LASSO | Penalized Least Absolute Shrinkage and Selection Operator regression analysis |
| MoCA | Montreal Cognitive Assessment |
| MRI | Magnetic Resonance Imaging |
| MSE | Mean Squared Error |
| rCBF | Regional cerebral blood flow |
| RMSEA | Root Mean Square Error of Approximation |
| R4Alz-R | REMEDES for Alzheimer-Revised battery |
| SD | Standard deviation |
| SEM | Structural equation modeling |
| SI-M | Social Inference-Minimal Subtest |
| SPECT | Single Photon Emission Computed Tomography |
| SRMR | Standardized Root Mean Square Residual |
| TASIT-S | The Awareness of Social Inference Test—Short Form |
| TMT | Trail Making Test |
| χ2 | Chi-square test |
| 99mTc-HMPAO | Hexamethyl Propylene Amine Oxime labeled with Technique-99 m |
References
- Logroscino, G.; Piccininni, M.; Graff, C.; Hardiman, O.; Ludolph, A.C.; Moreno, F.; Otto, M.; Remes, A.M.; Rowe, J.B.; Seelaar, H.; et al. Incidence of syndromes associated with frontotemporal lobar degeneration in 9 European countries. JAMA Neurol. 2023, 80, 279. [Google Scholar] [CrossRef]
- Antonioni, A.; Raho, E.M.; Lopriore, P.; Pace, A.P.; Latino, R.R.; Assogna, M.; Mancuso, M.; Gragnaniello, D.; Granieri, E.; Pugliatti, M.; et al. Frontotemporal dementia, where do we stand? A narrative review. Int. J. Mol. Sci. 2023, 24, 11732. [Google Scholar] [CrossRef] [PubMed]
- Rascovsky, K.; Hodges, J.R.; Knopman, D.; Mendez, M.F.; Kramer, J.H.; Neuhaus, J.; Van Swieten, J.C.; Seelaar, H.; Dopper, E.G.P.; Onyike, C.U.; et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011, 134, 2456–2477. [Google Scholar] [CrossRef]
- Piguet, O.; Clarke, A.J.; Foxe, D.; Tse, N.Y.; Teng, H.; Burrell, J.R.; Cheung, S.C.; Carrick, J.; Devenney, E.; Halliday, G.M.; et al. Fourteen years later: Reviewing the diagnostic criteria for behavioral-variant frontotemporal dementia. Alzheimer’s Dement. 2025, 21, e70604. [Google Scholar] [CrossRef]
- Chatzidimitriou, E.; Ioannidis, P.; Aretouli, E.; Papaliagkas, V.; Moraitou, D. Correlates of Functional Impairment in Patients with the Behavioral Variant of Frontotemporal Dementia: A PRISMA-Compliant Systematic Review. Int. J. Mol. Sci. 2023, 24, 13810. [Google Scholar] [CrossRef]
- Chatzidimitriou, E.; Ioannidis, P.; Moraitou, D.; Konstantinopoulou, E.; Aretouli, E. The cognitive and behavioral correlates of functional status in patients with frontotemporal dementia: A pilot study. Front. Hum. Neurosci. 2023, 17, 1087765. [Google Scholar] [CrossRef]
- Besser, L.M.; Galvin, J.E. Perceived burden among caregivers of patients with frontotemporal degeneration in the United States. Int. Psychogeriatr. 2018, 31, 1191–1201. [Google Scholar] [CrossRef]
- Mioshi, E.; Kipps, C.M.; Dawson, K.; Mitchell, J.; Graham, A.; Hodges, J.R. Activities of daily living in frontotemporal dementia and Alzheimer disease. Neurology 2007, 68, 2077–2084. [Google Scholar] [CrossRef]
- Lima-Silva, T.B.; Bahia, V.S.; Carvalho, V.A.; Guimarães, H.C.; Caramelli, P.; Balthazar, M.; Damasceno, B.; De Campos Bottino, C.M.; Brucki, S.M.D.; Nitrini, R.; et al. Functional profile of patients with behavioral variant frontotemporal dementia (bvFTD) compared to patients with Alzheimer’s disease and normal controls. Dement. Neuropsychol. 2013, 7, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Mioshi, E.; Hodges, J.R. Rate of change of functional abilities in frontotemporal dementia. Dement. Geriatr. Cogn. Disord. 2009, 28, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Devenney, E.; Bartley, L.; Hoon, C.; O’Callaghan, C.; Kumfor, F.; Hornberger, M.; Kwok, J.B.; Halliday, G.M.; Kiernan, M.C.; Piguet, O.; et al. Progression in Behavioral Variant Frontotemporal Dementia. JAMA Neurol. 2015, 72, 1501. [Google Scholar] [CrossRef]
- Josephs, K.A.; Whitwell, J.L.; Weigand, S.D.; Senjem, M.L.; Boeve, B.F.; Knopman, D.S.; Smith, G.E.; Ivnik, R.J.; Jack, C.R.; Petersen, R.C. Predicting functional decline in behavioural variant frontotemporal dementia. Brain 2011, 134, 432–448. [Google Scholar] [CrossRef]
- Premi, E.; Gualeni, V.; Costa, P.; Cosseddu, M.; Gasparotti, R.; Padovani, A.; Borroni, B. Looking for measures of disease severity in the frontotemporal dementia continuum. J. Alzheimer’s Dis. 2016, 52, 1227–1235. [Google Scholar] [CrossRef]
- Amanzio, M.; D’Agata, F.; Palermo, S.; Rubino, E.; Zucca, M.; Galati, A.; Pinessi, L.; Castellano, G.; Rainero, I. Neural correlates of reduced awareness in instrumental activities of daily living in frontotemporal dementia. Exp. Gerontol. 2016, 83, 158–164. [Google Scholar] [CrossRef]
- Steinacker, P.; Anderl-Straub, S.; Diehl-Schmid, J.; Semler, E.; Uttner, I.; Von Arnim, C.A.F.; Barthel, H.; Danek, A.; Fassbender, K.; Fliessbach, K.; et al. Serum neurofilament light chain in behavioral variant frontotemporal dementia. Neurology 2018, 91, e1390–e1401. [Google Scholar] [CrossRef]
- Benussi, A.; Dell’Era, V.; Cantoni, V.; Cotelli, M.S.; Cosseddu, M.; Spallazzi, M.; Micheli, A.; Turrone, R.; Alberici, A.; Borroni, B. TMS for staging and predicting functional decline in frontotemporal dementia. Brain Stimul. 2019, 13, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Yassuda, M.S.; Da Silva, T.B.L.; O’Connor, C.M.; Mekala, S.; Alladi, S.; Bahia, V.S.; Almaral-Carvalho, V.; Guimaraes, H.C.; Caramelli, P.; Balthazar, M.L.F.; et al. Apathy and functional disability in behavioral variant frontotemporal dementia. Neurol. Clin. Pract. 2018, 8, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Lima-Silva, T.B.; Bahia, V.S.; Carvalho, V.A.; Guimarães, H.C.; Caramelli, P.; Balthazar, M.L.F.; Damasceno, B.; De Campos Bottino, C.M.; Brucki, S.M.D.; Nitrini, R.; et al. Direct and indirect assessments of activities of daily living in behavioral variant frontotemporal dementia and Alzheimer disease. J. Geriatr. Psychiatry Neurol. 2014, 28, 19–26. [Google Scholar] [CrossRef]
- Moheb, N.; Mendez, M.F.; Kremen, S.A.; Teng, E. Executive Dysfunction and Behavioral Symptoms Are Associated with Deficits in Instrumental Activities of Daily Living in Frontotemporal Dementia. Dement. Geriatr. Cogn. Disord. 2017, 43, 89–99. [Google Scholar] [CrossRef]
- Salech, G.M.; Lillo, P.; Van Der Hiele, K.; Méndez-Orellana, C.; Ibáñez, A.; Slachevsky, A. Apathy, executive function, and emotion recognition are the main drivers of functional impairment in behavioral variant of frontotemporal dementia. Front. Neurol. 2022, 12, 734251. [Google Scholar] [CrossRef] [PubMed]
- Chatzidimitriou, E.; Chen, Y.; Moraitou, D.; Ioannidis, P.; Aretouli, E.; Kramer, J.H.; Miller, B.L.; Gorno-Tempini, M.; Seeley, W.W.; Rosen, H.J.; et al. The predictive value of social cognition assessment for 1-year follow-up functional outcomes in behavioral variant frontotemporal dementia. J. Neurol. 2025, 272, 526. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Landin-Romero, R.; Clemson, L.; Kaizik, C.; Daveson, N.; Hodges, J.R.; Hsieh, S.; Piguet, O.; Mioshi, E. Behavioral-variant frontotemporal dementia. Neurology 2017, 89, 570–577. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Clemson, L.; Hornberger, M.; Leyton, C.E.; Hodges, J.R.; Piguet, O.; Mioshi, E. Longitudinal change in everyday function and behavioral symptoms in frontotemporal dementia. Neurol. Clin. Pract. 2016, 6, 419–428. [Google Scholar] [CrossRef]
- Kipps, C.M.; Mioshi, E.; Hodges, J.R. Emotion, social functioning and activities of daily living in frontotemporal dementia. In Emotions in Neurological Disease; Psychology Press eBooks: East Sussex, UK, 2020; pp. 182–189. [Google Scholar] [CrossRef]
- De Medeiros Correia Marin, S.; Mansur, L.L.; De Oliveira, F.F.; Marin, L.F.; Wajman, J.R.; Bahia, V.S.; Bertolucci, P.H.F. Swallowing in behavioral variant frontotemporal dementia. Arq. Neuro-Psiquiatr. 2021, 79, 8–14. [Google Scholar] [CrossRef]
- Reverberi, C.; Cherubini, P.; Baldinelli, S.; Luzzi, S. Semantic fluency: Cognitive basis and diagnostic performance in focal dementias and Alzheimer’s disease. Cortex 2014, 54, 150–164. [Google Scholar] [CrossRef] [PubMed]
- Laisney, M.; Matuszewski, V.; Mézenge, F.; Belliard, S.; Sayette, V.; Eustache, F.; Desgranges, B. The underlying mechanisms of verbal fluency deficit in frontotemporal dementia and semantic dementia. J. Neurol. 2009, 256, 1083–1094. [Google Scholar] [CrossRef]
- Wilson, R.S.; Boyle, P.A.; Yu, L.; Segawa, E.; Sytsma, J.; Bennett, D.A. Conscientiousness, dementia related pathology, and trajectories of cognitive aging. Psychol. Aging 2015, 30, 74–82. [Google Scholar] [CrossRef]
- Gao, Y.; Amin, N.; Van Duijn, C.; Littlejohns, T.J. Association of neuroticism with incident dementia, neuroimaging outcomes, and cognitive function. Alzheimer’s Dement. 2024, 20, 5578–5589. [Google Scholar] [CrossRef] [PubMed]
- Low, L.-F.; Harrison, F.; Lackersteen, S.M. Does Personality affect Risk for dementia? A Systematic Review and Meta-Analysis. Am. J. Geriatr. Psychiatry 2013, 21, 713–728. [Google Scholar] [CrossRef]
- Kaup, A.R.; Harmell, A.L.; Yaffe, K. Conscientiousness Is Associated with Lower Risk of Dementia among Black and White Older Adults. Neuroepidemiology 2019, 52, 86–92. [Google Scholar] [CrossRef]
- Sutin, A.R.; Stephan, Y.; Terracciano, A. Facets of Conscientiousness and risk of dementia. Psychol. Med. 2017, 48, 974–982. [Google Scholar] [CrossRef]
- Valotassiou, V.; Papatriantafyllou, J.; Sifakis, N.; Tzavara, C.; Tsougos, I.; Psimadas, D.; Kapsalaki, E.; Fezoulidis, I.; Hadjigeorgiou, G.; Georgoulias, P. Brain Perfusion SPECT with Brodmann Areas Analysis in Differentiating Frontotemporal Dementia Subtypes. Curr. Alzheimer Res. 2014, 11, 941–954. [Google Scholar] [CrossRef]
- Valotassiou, V.; Sifakis, N.; Tzavara, C.; Lykou, E.; Tsinia, N.; Kamtsadeli, V.; Sali, D.; Angelidis, G.; Psimadas, D.; Tsougos, I.; et al. Eating Disorders in Frontotemporal Dementia and Alzheimer’s Disease: Evaluation of Brain Perfusion Correlates Using 99mTc-HMPAO SPECT with Brodmann Areas Analysis. J. Alzheimer’s Dis. 2021, 80, 1657–1667. [Google Scholar] [CrossRef]
- Valotassiou, V.; Sifakis, N.; Tzavara, C.; Lykou, E.; Tsinia, N.; Kamtsadeli, V.; Sali, D.; Angelidis, G.; Psimadas, D.; Theodorou, E.; et al. Anosognosia in dementia: Evaluation of perfusion correlates using 99MTC-HMPAO SPECT and Automated Brodmann areas analysis. Diagnostics 2022, 12, 1136. [Google Scholar] [CrossRef]
- Valotassiou, V.; Sifakis, N.; Tzavara, C.; Lykou, E.; Tsinia, N.; Kamtsadeli, V.; Sali, D.; Angelidis, G.; Psimadas, D.; Tsougos, I.; et al. Differences of apathy perfusion correlates between Alzheimer’s disease and frontotemporal dementia. A 99mTc-HMPAO SPECT study with automated Brodmann areas analysis. Int. J. Psychiatry Clin. Pract. 2020, 26, 14–22. [Google Scholar] [CrossRef]
- Bang, J.; Spina, S.; Miller, B.L. Frontotemporal dementia. Lancet 2015, 386, 1672–1682. [Google Scholar] [CrossRef]
- Ekiz, E.; Videler, A.C.; Van Alphen, S.P.J. Feasibility of the Cognitive Model for Behavioral Interventions in Older Adults with Behavioral and Psychological Symptoms of Dementia. Clin. Gerontol. 2020, 45, 903–914. [Google Scholar] [CrossRef] [PubMed]
- European Union. Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation). Off. J. Eur. Union 2016, 119, 10–13. Available online: http://data.europa.eu/eli/reg/2016/679/2016-05-04 (accessed on 2 October 2022).
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Participants. JAMA 2024, 333, 71–74. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MOCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Poptsi, E.; Moraitou, D.; Eleftheriou, M.; Kounti-Zafeiropoulou, F.; Papasozomenou, C.; Agogiatou, C.; Bakoglidou, E.; Batsila, G.; Liapi, D.; Markou, N.; et al. Normative data for the Montreal Cognitive Assessment in Greek older adults with subjective cognitive decline, mild cognitive impairment and dementia. J. Geriatr. Psychiatry Neurol. 2019, 32, 265–274. [Google Scholar] [CrossRef]
- Freedman, M. Clock Drawing: A Neuropsychological Analysis; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Bozikas, V.P.; Giazkoulidou, A.; Hatzigeorgiadou, M.; Karavatos, A.; Kosmidis, M.H. Do age and education contribute to performance on the clock drawing test? Normative data for the Greek population. J. Clin. Exp. Neuropsychol. 2008, 30, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Kosmidis, M.H.; Bozikas, V.; Vlahou, C.H.; Giaglis, G. Neuropsychological Battery. In Cognitive Neuroscience Laboratory; Aristotle University of Thessaloniki (AUTh): Thessaloniki, Greece, 2011; Unpublished work. [Google Scholar]
- Taylor, E.M. Psychological Appraisal of Children with Cerebral Deficits; Harvard University Press: Cambridge, MA, USA, 1959. [Google Scholar]
- Borod, J.C.; Goodglass, H.; Kaplan, E. Normative data on the boston diagnostic aphasia examination, parietal lobe battery, and the boston naming Test. J. Clin. Neuropsychol. 1980, 2, 209–215. [Google Scholar] [CrossRef]
- Reitan, R.M. Validity of the trail making test as an indicator of organic brain damage. Percept. Mot. Ski. 1958, 8, 271–276. [Google Scholar] [CrossRef]
- Zalonis, I.; Kararizou, E.; Triantafyllou, N.I.; Kapaki, E.; Papageorgiou, S.; Sgouropoulos, P.; Vassilopoulos, D. A normative study of the trail making test A and B in Greek adults. Clin. Neuropsychol. 2008, 22, 842–850. [Google Scholar] [CrossRef]
- Tsatali, M.; Surdu, F.; Konstantinou, A.; Moraitou, D. Normative data for the D-KEFS Color-Word interference and trail making tests adapted in Greek adult population 20–49 years old. NeuroSci 2024, 5, 378–395. [Google Scholar] [CrossRef]
- Lezak, M.; Howieson, M.; Loring, D. Neuropsychological Assessment, 4th ed.; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The unity and diversity of executive functions and their contributions to complex “Frontal lobe” tasks: A latent variable analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef] [PubMed]
- Stroop, J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935, 18, 643–662. [Google Scholar] [CrossRef]
- Zalonis, I.; Christidi, F.; Bonakis, A.; Kararizou, E.; Triantafyllou, N.I.; Paraskevas, G.; Kapaki, E.; Vasilopoulos, D. The Stroop effect in Greek healthy population: Normative data for the Stroop Neuropsychological Screening Test. Arch. Clin. Neuropsychol. 2009, 24, 81–88. [Google Scholar] [CrossRef]
- Kosmidis, M.H.; Vlahou, C.H.; Panagiotaki, P.; Kiosseoglou, G. The verbal fluency task in the Greek population: Normative data, and clustering and switching strategies. J. Int. Neuropsychol. Soc. 2004, 10, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Leslie, F.V.C.; Foxe, D.; Daveson, N.; Flannagan, E.; Hodges, J.R.; Piguet, O. FRONTIER Executive Screen: A brief executive battery to differentiate frontotemporal dementia and Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2015, 87, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Konstantinopoulou, E.; Vilou, I.; Falega, I.; Papadopoulou, V.; Chatzidimitriou, E.; Grigoriadis, N.; Aretouli, E.; Panagiotis, I. Screening for Executive Impairment in Patients with Frontotemporal Dementia: Evidence from the Greek Version of the Frontier Executive Screen. Arch. Clin. Neuropsychol. 2024, 40, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Poptsi, E.; Tsardoulias, E.; Moraitou, D.; Symeonidis, A.L.; Tsolaki, M. REMEDES for Alzheimer-R4ALZ Battery: Design and development of a new tool of Cognitive Control assessment for the diagnosis of minor and major neurocognitive disorders. J. Alzheimer’s Dis. 2019, 72, 783–801. [Google Scholar] [CrossRef]
- Poptsi, E.; Moraitou, D.; Tsardoulias, E.; Symeonidis, A.L.; Tsolaki, M. Subjective Cognitive Impairment Can Be Detected from the Decline of Complex Cognition: Findings from the Examination of Remedes 4 Alzheimer’s (R4Alz) Structural Validity. Brain Sci. 2024, 14, 548. [Google Scholar] [CrossRef]
- Poptsi, E.; Moraitou, D.; Tsardoulias, E.; Symeonidis, A.L.; Tsolaki, M. R4Alz-R: A cutting-edge tool for spotting the very first and subtle signs of aging-related cognitive impairment with high accuracy. GeroScience 2024, 47, 5317–5335. [Google Scholar] [CrossRef] [PubMed]
- Moraitou, D.; Papantoniou, G.; Gkinopoulos, T.; Nigritinou, M. Older adults’ decoding of emotions: Age-related differences in interpreting dynamic emotional displays and the well-preserved ability to recognize happiness. Psychogeriatrics 2013, 13, 139–147. [Google Scholar] [CrossRef]
- McDonald, S.; Flanagan, S.; Honan, C. The Awareness of Social Inference Test–Short (TASIT-S); ASSBI Resources: Sydney, Australia, 2017. [Google Scholar]
- Tsentidou, G.; Moraitou, D.; Tsolaki, M. Similar Theory of Mind Deficits in Community Dwelling Older Adults with Vascular Risk Profile and Patients with Mild Cognitive Impairment: The Case of Paradoxical Sarcasm Comprehension. Brain Sci. 2021, 11, 627. [Google Scholar] [CrossRef]
- Goldberg, L.R.; Johnson, J.A.; Eber, H.W.; Hogan, R.; Ashton, M.C.; Cloninger, C.R.; Gough, H.G. The international personality item pool and the future of public-domain personality measures. J. Res. Personal. 2005, 40, 84–96. [Google Scholar] [CrossRef]
- Costa, P.T.; McCrae, R.R. The Five-Factor model of personality and its relevance to personality disorders. J. Personal. Disord. 1992, 6, 343–359. [Google Scholar] [CrossRef]
- Dutczak, B.; Pachalska, M. Assessment of personality changes in dementia of the Alzheimer’s type and frontotemporal dementia. Ann. Gen. Psychiatry 2008, 7 (Suppl. S1), S297. [Google Scholar] [CrossRef]
- Hunt, A.; Martyr, A.; Gamble, L.D.; Morris, R.G.; Thom, J.M.; Pentecost, C.; Clare, L. The associations between personality traits and quality of life, satisfaction with life, and well-being over time in people with dementia and their caregivers: Findings from the IDEAL programme. BMC Geriatr. 2023, 23, 354. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, M.J.; Paterson, T.S. 89 The Effect of Personality Traits on the Development of Predementia Cognitive States: Results from the Einstein Aging Study. J. Int. Neuropsychol. Soc. 2023, 29 (Suppl. S1), 391–392. [Google Scholar] [CrossRef]
- Ypofanti, M.; Zisi, V.; Zourbanos, N.; Mouchtouri, B.; Tzanne, P.; Theodorakis, Y.; Lyrakos, G. Psychometric properties of the International Personality Item Pool Big-Five personality questionnaire for the Greek population. Health Psychol. Res. 2015, 3, 2206. [Google Scholar] [CrossRef]
- Bastin, C.; Giacomelli, F.; Miévis, F.; Lemaire, C.; Guillaume, B.; Salmon, E. Anosognosia in mild cognitive impairment: Lack of awareness of memory difficulties characterizes prodromal Alzheimer’s disease. Front. Psychiatry 2021, 12, 2206. [Google Scholar] [CrossRef]
- Leocadi, M.; Canu, E.; Paldino, A.; Agosta, F.; Filippi, M. Awareness impairment in Alzheimer’s disease and frontotemporal dementia: A systematic MRI review. J. Neurol. 2022, 270, 1880–1907. [Google Scholar] [CrossRef]
- Bracca, V.; Premi, E.; Cotelli, M.S.; Micheli, A.; Altomare, D.; Cantoni, V.; Gasparotti, R.; Borroni, B. Loss of insight in syndromes associated with Frontotemporal Lobar Degeneration: Clinical and imaging features. Am. J. Geriatr. Psychiatry 2024, 33, 450–462. [Google Scholar] [CrossRef]
- Tondelli, M.; Galli, C.; Vinceti, G.; Fiondella, L.; Salemme, S.; Carbone, C.; Molinari, M.A.; Chiari, A.; Zamboni, G. Anosognosia in Early- and Late-Onset dementia and its association with neuropsychiatric symptoms. Front. Psychiatry 2021, 12, 658934. [Google Scholar] [CrossRef] [PubMed]
- Sutin, A.R.; Luchetti, M.; Stephan, Y.; Terracciano, A. Informant-rated change in personality traits, psychological distress, well-being, and social connection with dementia. Arch. Gerontol. Geriatr. 2023, 115, 105218. [Google Scholar] [CrossRef]
- Kertesz, A.; Davidson, W.; Fox, H. Frontal Behavioral Inventory: Diagnostic criteria for frontal lobe dementia. Can. J. Neurol. Sci. 1997, 24, 29–36. [Google Scholar] [CrossRef]
- Neary, D.; Snowden, J.S.; Gustafson, L.; Passant, U.; Stuss, D.; Black, S.; Freedman, M.; Kertesz, A.; Robert, P.H.; Albert, M.; et al. Frontotemporal lobar degeneration. Neurology 1998, 51, 1546–1554. [Google Scholar] [CrossRef]
- Konstantinopoulou, E.; Aretouli, E.; Ioannidis, P.; Karacostas, D.; Kosmidis, M.H. Behavioral disturbances differentiate frontotemporal lobar degeneration subtypes and Alzheimer’s disease: Evidence from the Frontal Behavioral Inventory. Int. J. Geriatr. Psychiatry 2012, 28, 939–946. [Google Scholar] [CrossRef]
- Milan, G.; Lamenza, F.; Iavarone, A.; Galeone, F.; Lorè, E.; De Falco, C.; Sorrentino, P.; Postiglione, A. Frontal Behavioural Inventory in the differential diagnosis of dementia. Acta Neurol. Scand. 2007, 117, 260–265. [Google Scholar] [CrossRef]
- Alberici, A.; Geroldi, C.; Cotelli, M.; Adorni, A.; Calabria, M.; Rossi, G.; Borroni, B.; Padovani, A.; Zanetti, O.; Kertesz, A. The Frontal Behavioural Inventory (Italian version) differentiates frontotemporal lobar degeneration variants from Alzheimer’s disease. Neurol. Sci. 2007, 28, 80–86. [Google Scholar] [CrossRef]
- Kertesz, A.; Nadkarni, N.; Davidson, W.; Thomas, A.W. The Frontal Behavioral Inventory in the differential diagnosis of frontotemporal dementia. J. Int. Neuropsychol. Soc. 2000, 6, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C. The Clinical Dementia Rating (CDR). Neurology 1993, 43, 2412. [Google Scholar] [CrossRef]
- Knopman, D.S.; Kramer, J.H.; Boeve, B.F.; Caselli, R.J.; Graff-Radford, N.R.; Mendez, M.F.; Miller, B.L.; Mercaldo, N. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain 2008, 131, 2957–2968. [Google Scholar] [CrossRef] [PubMed]
- Maiovis, P.; Ioannidis, P.; Gerasimou, G.; Gotzamani-Psarrakou, A.; Karacostas, D. Frontotemporal Lobar Degeneration-Modified Clinical Dementia Rating (FTLD-CDR) scale and Frontotemporal Dementia Rating Scale (FRS) correlation with regional brain perfusion in a series of FTLD patients. J. Neuropsychiatry 2016, 29, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Knopman, D.S.; Weintraub, S.; Pankratz, V.S. Language and behavior domains enhance the value of the clinical dementia rating scale. Alzheimer’s Dement. 2011, 7, 293–299. [Google Scholar] [CrossRef]
- Mioshi, E.; Hsieh, S.; Savage, S.; Hornberger, M.; Hodges, J.R. Clinical staging and disease progression in frontotemporal dementia. Neurology 2010, 74, 1591–1597. [Google Scholar] [CrossRef]
- Pornari, X.; Tsantali, E.; Tsolaki, M.; Kigiaki, E. Do Functional Disability Is a Good Predictor Index for Alzheimer’s Disease? Ann. Gen. Psychiatry 2006, 5 (Suppl. S1), S294. [Google Scholar] [CrossRef]
- Gélinas, I.; Gauthier, L.; McIntyre, M.; Gauthier, S. Development of a functional measure for persons with Alzheimer’s disease: The Disability Assessment for Dementia. Am. J. Occup. Ther. 1999, 53, 471–481. [Google Scholar] [CrossRef]
- Pfeffer, R.I.; Kurosaki, T.T.; Harrah, C.H.; Chance, J.M.; Filos, S. Measurement of functional activities in older adults in the community. J. Gerontol. 1982, 37, 323–329. [Google Scholar] [CrossRef]
- Kapucu, Ö.L.; Nobili, F.; Varrone, A.; Booij, J.; Borght, T.V.; Någren, K.; Darcourt, J.; Tatsch, K.; Van Laere, K.J. EANM procedure guideline for brain perfusion SPECT using 99mTc-labelled radiopharmaceuticals, version 2. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 2093–2102. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025. [Google Scholar]
- Zhou, D.J.; Chahal, R.; Gotlib, I.H.; Liu, S. Comparison of lasso and stepwise regression in psychological data. Methodology 2024, 20, 121–143. [Google Scholar] [CrossRef]
- Kim, J.; An, S.; Hong, S. Exploring the factors that influence academic stress among elementary school students using a LASSO penalty regression model. Asia Pac. Educ. Rev. 2023, 25, 1331–1343. [Google Scholar] [CrossRef]
- Hovens, I.B.; Dalenberg, J.R.; Small, D.M. A Brief Neuropsychological Battery for Measuring Cognitive Functions Associated with Obesity. Obesity 2019, 27, 1988–1996. [Google Scholar] [CrossRef]
- De Silva, D.; Hsieh, S.; Caga, J.; Leslie, F.V.C.; Kiernan, M.C.; Hodges, J.R.; Mioshi, E.; Burrell, J.R. Motor function and behaviour across the ALS-FTD spectrum. Acta Neurol. Scand. 2015, 133, 367–372. [Google Scholar] [CrossRef]
- Bentler, P.M. EQS 6.4 for Windows [Computer Software]; Multivariate Software Inc.: Encino, CA, USA, 2019. [Google Scholar]
- Kline, R.B. Software review: Software programs for structural equation modeling: AMOS, EQS, and LISREL. J. Psychoeduc. Assess. 1998, 16, 343–364. [Google Scholar] [CrossRef]
- O’Bryant, S.E.; Waring, S.C.; Cullum, C.M.; Hall, J.; Lacritz, L.; Massman, P.J.; Lupo, P.J.; Reisch, J.S.; Doody, R. Staging dementia using Clinical dementia rating scale sum of boxes scores. Arch. Neurol. 2008, 65, 1091. [Google Scholar] [CrossRef]
- Ralph, M.A.L.; Jefferies, E.; Patterson, K.; Rogers, T.T. The neural and computational bases of semantic cognition. Nat. Rev. Neurosci. 2016, 18, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Nakhla, M.Z.; Banuelos, D.; Pagán, C.; Olvera, A.G.; Razani, J. Differences between episodic and semantic memory in predicting observation-based activities of daily living in mild cognitive impairment and Alzheimer’s disease. Appl. Neuropsychol. Adult 2021, 29, 1499–1510. [Google Scholar] [CrossRef]
- Roll, E.E.; Giovannetti, T.; Libon, D.J.; Eppig, J. Everyday task knowledge and everyday function in dementia. J. Neuropsychol. 2017, 13, 96–120. [Google Scholar] [CrossRef]
- Chiou, R.; Humphreys, G.F.; Jung, J.; Ralph, M.A.L. Controlled semantic cognition relies upon dynamic and flexible interactions between the executive ‘semantic control’ and hub-and-spoke ‘semantic representation’ systems. Cortex 2018, 103, 100–116. [Google Scholar] [CrossRef] [PubMed]
- De Boer, L.; Poos, J.M.; Van Den Berg, E.; De Houwer, J.F.H.; Swartenbroekx, T.; Dopper, E.G.P.; Boesjes, P.; Tahboun, N.; Bouzigues, A.; Foster, P.H.; et al. Montreal Cognitive Assessment vs the Mini-Mental State Examination as a Screening Tool for Patients With Genetic Frontotemporal Dementia. Neurology 2025, 104, e213401. [Google Scholar] [CrossRef] [PubMed]
- Costantini, G.; Saraulli, D.; Perugini, M. Uncovering the motivational core of traits: The case of conscientiousness. Eur. J. Personal. 2020, 34, 1073–1094. [Google Scholar] [CrossRef]
- Di Sarno, M.; Costantini, G.; Richetin, J.; Preti, E.; Perugini, M. Why are you (un)conscientious? The dynamic interplay of goals, states, and traits in everyday life. J. Personal. 2022, 91, 977–991. [Google Scholar] [CrossRef]
- Petrides, M. Lateral prefrontal cortex: Architectonic and functional organization. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 781–795. [Google Scholar] [CrossRef]
- Dadario, N.B.; Tanglay, O.; Sughrue, M.E. Deconvoluting human Brodmann area 8 based on its unique structural and functional connectivity. Front. Neuroanat. 2023, 17, 1127143. [Google Scholar] [CrossRef] [PubMed]
- Godefroy, V.; Batrancourt, B.; Charron, S.; Bouzigues, A.; Bendetowicz, D.; Carle, G.; Rametti-Lacroux, A.; Bombois, S.; Cognat, E.; Migliaccio, R.; et al. Functional connectivity correlates of reduced goal-directed behaviors in behavioural variant frontotemporal dementia. Brain Struct. Funct. 2022, 227, 2971–2989. [Google Scholar] [CrossRef]
- Hai, T.; Swansburg, R.; Kahl, C.K.; Frank, H.; Stone, K.; Lemay, J.-F.; MacMaster, F.P. Right superior frontal gyrus cortical thickness in pediatric ADHD. J. Atten. Disord. 2022, 26, 1895–1906. [Google Scholar] [CrossRef]
- Marques, R.C.; Vieira, L.; Marques, D.; Cantilino, A. Transcranial magnetic stimulation of the medial prefrontal cortex for psychiatric disorders: A systematic review. Braz. J. Psychiatry 2019, 41, 447–457. [Google Scholar] [CrossRef]
- Shen, Y.-T.; Yuan, Y.-S.; Wang, M.; Zhi, Y.; Wang, J.-W.; Wang, L.-N.; Ma, K.-W.; Si, Q.-Q.; Zhang, K.-Z. Dysfunction in superior frontal gyrus associated with diphasic dyskinesia in Parkinson’s disease. Npj Park. Dis. 2020, 6, 30. [Google Scholar] [CrossRef]
- Sakurai, Y. Brodmann Areas 39 and 40: Human parietal association area and higher cortical function. PubMed 2017, 69, 461–469. [Google Scholar] [CrossRef]
- Silani, G.; Lamm, C.; Ruff, C.C.; Singer, T. Right supramarginal gyrus is crucial to overcome emotional egocentricity bias in social judgments. J. Neurosci. 2013, 33, 15466–15476. [Google Scholar] [CrossRef] [PubMed]
- Damiani, D.; Nascimento, A.M.; Pereira, L.K. Cortical Brain Functions—The Brodmann legacy in the 21st century. Arq. Bras. Neurocir. Braz. Neurosurg. 2017, 39, 261–270. [Google Scholar] [CrossRef]
- Grossman, M.; Seeley, W.W.; Boxer, A.L.; Hillis, A.E.; Knopman, D.S.; Ljubenov, P.A.; Miller, B.; Piguet, O.; Rademakers, R.; Whitwell, J.L.; et al. Frontotemporal lobar degeneration. Nat. Rev. Dis. Primers 2023, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, F.; Tondo, G.; Corrado, L.; Menegon, F.; Aprile, D.; Anselmi, M.; D’Alfonso, S.; Comi, C.; Mazzini, L. Neuroinflammatory pathways in the ALS-FTD continuum: A focus on genetic variants. Genes 2023, 14, 1658. [Google Scholar] [CrossRef]
- Dev, S.I.; Dickerson, B.C.; Touroutoglou, A. Neuroimaging in Frontotemporal Lobar Degeneration: Research and Clinical utility. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2021; pp. 93–112. [Google Scholar] [CrossRef]
- Duignan, J.A.; Haughey, A.; Kinsella, J.A.; Killeen, R.P. Molecular and anatomical imaging of dementia with lewy bodies and frontotemporal lobar degeneration. Semin. Nucl. Med. 2021, 51, 264–274. [Google Scholar] [CrossRef]
- Ratti, E.; Domoto-Reilly, K.; Caso, C.; Murphy, A.; Brickhouse, M.; Hochberg, D.; Makris, N.; Cudkowicz, M.E.; Dickerson, B.C. Regional prefrontal cortical atrophy predicts specific cognitive-behavioral symptoms in ALS-FTD. Brain Imaging Behav. 2021, 15, 2540–2551. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Trujillo, J. Visual attention in the prefrontal cortex. Annu. Rev. Vis. Sci. 2022, 8, 407–425. [Google Scholar] [CrossRef]
- Jiskoot, L.C.; Panman, J.L.; Van Asseldonk, L.; Franzen, S.; Meeter, L.H.H.; Kaat, L.D.; Van Der Ende, E.L.; Dopper, E.G.P.; Timman, R.; Van Minkelen, R.; et al. Longitudinal cognitive biomarkers predicting symptom onset in presymptomatic frontotemporal dementia. J. Neurol. 2018, 265, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Shuai, D.; Bu, X.; Hu, X.; Tang, S.; Zhang, L.; Li, H.; Hu, X.; Lu, L.; Gong, Q.; et al. Impairments of large-scale functional networks in attention-deficit/hyperactivity disorder: A meta-analysis of resting-state functional connectivity. Psychol. Med. 2019, 49, 2475–2485. [Google Scholar] [CrossRef]
- Rouse, M.A.; Husain, M.; Garrard, P.; Patterson, K.; Rowe, J.B.; Ralph, M.A.L. Behavioural changes in frontotemporal dementia and their cognitive and neuroanatomical correlates. Brain 2025, 148, 2730–2745. [Google Scholar] [CrossRef]
- Eggins, P.; Wong, S.; Wei, G.; Hodges, J.R.; Husain, M.; Piguet, O.; Irish, M.; Kumfor, F. A shared cognitive and neural basis underpinning cognitive apathy and planning in behavioural-variant frontotemporal dementia and Alzheimer’s disease. Cortex 2022, 154, 241–253. [Google Scholar] [CrossRef]
- Johnson, E.; Kumfor, F. Overcoming apathy in frontotemporal dementia: Challenges and future directions. Curr. Opin. Behav. Sci. 2018, 22, 82–89. [Google Scholar] [CrossRef]
- Suchy, Y.; DesRuisseaux, L.A.; Mora, M.G.; Brothers, S.L.; Niermeyer, M.A. Conceptualization of the term “ecological validity” in neuropsychological research on executive function assessment: A systematic review and call to action. J. Int. Neuropsychol. Soc. 2024, 30, 499–522. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.O.; Dores, A.R.; Peixoto, B.; Barbosa, F. Ecological validity in neurocognitive assessment: Systematized review, content analysis, and proposal of an instrument. Appl. Neuropsychol. Adult 2023, 32, 577–594. [Google Scholar] [CrossRef]
- Chaytor, N.S.; Barbosa-Leiker, C.; Germine, L.T.; Fonseca, L.M.; McPherson, S.M.; Tuttle, K.R. Construct validity, ecological validity and acceptance of self-administered online neuropsychological assessment in adults. Clin. Neuropsychol. 2020, 35, 148–164. [Google Scholar] [CrossRef]
- Fieldhouse, J.L.P.; Van Paassen, D.N.; Van Engelen, M.-P.E.; De Boer, S.C.M.; Hartog, W.L.; Braak, S.; Schoonmade, L.J.; Schouws, S.N.T.M.; Krudop, W.A.; Oudega, M.L.; et al. The pursuit for markers of disease progression in behavioral variant frontotemporal dementia: A scoping review to optimize outcome measures for clinical trials. Front. Aging Neurosci. 2024, 16, 1382593. [Google Scholar] [CrossRef]
- Zammitt, D.; Brotherhood, E.V.; Fearn, C.; Greaves, C.; Hayes, O.; Harding, E.; Lykourgos, M.; Rohrer, J.D.; Stott, J. Barriers and facilitators to participation in clinical trials related to familial frontotemporal dementia: A Qualitative study. Mol. Genet. Genom. Med. 2024, 12, e70038. [Google Scholar] [CrossRef] [PubMed]
- Ljubenkov, P.A.; Boxer, A. Clinical Trial Development in Frontotemporal Lobar Degeneration; Cambridge University Press eBooks: Cambridge, UK, 2022; pp. 216–231. [Google Scholar] [CrossRef]
- Lee, S.; Cho, E.-J.; Kwak, H.-B. Personalized healthcare for dementia. Healthcare 2021, 9, 128. [Google Scholar] [CrossRef]
- Lee, K.H.; Lee, J.Y.; Kim, B. Person-Centered Care in Persons living with Dementia: A Systematic review and Meta-analysis. Gerontologist 2020, 62, e253–e264. [Google Scholar] [CrossRef]
- Berkovic, D.; Macrae, A.; Gulline, H.; Horsman, P.; Soh, S.-E.; Skouteris, H.; Ayton, D. The Delivery of Person-Centered Care for People Living with Dementia in Residential Aged Care: A Systematic Review and Meta-Analysis. Gerontologist 2023, 64, gnad052. [Google Scholar] [CrossRef]
- Saragih, I.D.; Suarilah, I.; Saragih, I.S.; Pu, L.; Porta, C.M.; Saragih, H.; Lin, Y.; Lin, C. A meta-analysis of person-centered care interventions for improving health outcomes in persons living with dementia. Worldviews Evid. Based Nurs. 2024, 22, e12746. [Google Scholar] [CrossRef]
- Zidén, L.; Erhag, H.F.; Wijk, H. Person-centered care as a tool to reduce behavioral and psychological symptoms in older adults with dementia living in residential care facilities. Geriatr. Nurs. 2024, 57, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Vuic, B.; Konjevod, M.; Tudor, L.; Milos, T.; Perkovic, M.N.; Erjavec, G.N.; Pivac, N.; Uzun, S.; Mimica, N.; Strac, D.S. Tailoring the therapeutic interventions for behavioral and psychological symptoms of dementia. Expert Rev. Neurother. 2022, 22, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Broce, I.J.; Castruita, P.A.; Yokoyama, J.S. Moving toward Patient-Tailored Treatment in ALS and FTD: The Potential of Genomic Assessment as a tool for biological discovery and trial recruitment. Front. Neurosci. 2021, 15, 639078. [Google Scholar] [CrossRef] [PubMed]
- Vlotinou, P.; Tsiakiri, A.; Detsaridou, G.; Nikova, A.; Tsiptsios, D.; Vadikolias, K.; Aggelousis, N. Occupational Therapy Interventions in Patients with Frontotemporal Dementia: A Systematic Review. Med. Sci. 2023, 11, 71. [Google Scholar] [CrossRef]
- Zhang, C.; An, L.; Wulan, N.; Nguyen, K.; Orban, C.; Chen, P.; Chen, C.; Zhou, J.H.; Liu, K.; Yeo, B.T.T. Cross-Dataset Evaluation of Dementia Longitudinal Progression Prediction models. Hum. Brain Mapp. 2025, 46, e70280. [Google Scholar] [CrossRef]
- Foxe, D.; Irish, M.; Cheung, S.C.; D’Mello, M.; Hwang, Y.T.; Muggleton, J.; Cordato, N.J.; Piguet, O. Longitudinal changes in functional capacity in frontotemporal dementia and Alzheimer’s disease. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2024, 16, e70028. [Google Scholar] [CrossRef]
- Long, Z.; Irish, M.; Hodges, J.R.; Piguet, O.; Burrell, J.R. Distinct disease trajectories in frontotemporal dementia–motor neuron disease and behavioural variant frontotemporal dementia: A longitudinal study. Eur. J. Neurol. 2022, 29, 3158–3169. [Google Scholar] [CrossRef] [PubMed]
- Fieldhouse, J.L.P.; Van Engelen, M.E.; Handgraaf, D.; De Boer, S.C.M.; Van ’t Hooft, J.J.; Schouws, S.N.T.M.; Van Grootheest, D.; Kerssens, C.; Duits, F.H.; Van Harten, A.C.; et al. Trajectories of behavior and social cognition in behavioral variant frontotemporal dementia and primary psychiatric disorders: A call for better operationalization of socioemotional changes. Eur. J. Neurol. 2024, 31, e16426. [Google Scholar] [CrossRef]
- Giebel, C.M.; Knopman, D.; Mioshi, E.; Khondoker, M. Distinguishing frontotemporal dementia from Alzheimer disease through Everyday Function Profiles: Trajectories of Change. J. Geriatr. Psychiatry Neurol. 2020, 34, 66–75. [Google Scholar] [CrossRef]
- Zhang, X.; Irish, M.; Piguet, O.; Ahmed, R.M. Behavioural and cognitive profiles in frontotemporal dementia and Alzheimer’s disease: A longitudinal study. J. Neurol. 2025, 272, 279. [Google Scholar] [CrossRef]
- Van Der Ende, E.L.; Bron, E.E.; Poos, J.M.; Jiskoot, L.C.; Panman, J.L.; Papma, J.M.; Meeter, L.H.; Dopper, E.G.P.; Wilke, C.; Synofzik, M.; et al. A data-driven disease progression model of fluid biomarkers in genetic frontotemporal dementia. Brain 2021, 145, 1805–1817. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Liu, T.-C.; Lu, C.-J. Establishing a machine learning dementia progression prediction model with multiple integrated data. BMC Med. Res. Methodol. 2024, 24, 288. [Google Scholar] [CrossRef]
- Tavazzi, E.; Daberdaku, S.; Zandonà, A.; Vasta, R.; Nefussy, B.; Lunetta, C.; Mora, G.; Mandrioli, J.; Grisan, E.; Tarlarini, C.; et al. Predicting functional impairment trajectories in amyotrophic lateral sclerosis: A probabilistic, multifactorial model of disease progression. J. Neurol. 2022, 269, 3858–3878. [Google Scholar] [CrossRef]
- Murley, A.G.; Rouse, M.A.; Coyle-Gilchrist, I.T.S.; Jones, P.S.; Li, W.; Wiggins, J.; Lansdall, C.; Rodríguez, P.V.; Wilcox, A.; Patterson, K.; et al. Predicting loss of independence and mortality in frontotemporal lobar degeneration syndromes. J. Neurol. Neurosurg. Psychiatry 2021, 92, 737–744. [Google Scholar] [CrossRef]
- Ramsey, S.U.; Arnold, R.M. Prognostication in dementia. Handb. Clin. Neurol. 2022, 190, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Anderl-Straub, S.; Lausser, L.; Lombardi, J.; Uttner, I.; Fassbender, K.; Fliessbach, K.; Huppertz, H.; Jahn, H.; Kornhuber, J.; Obrig, H.; et al. Predicting disease progression in behavioral variant frontotemporal dementia. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2021, 13, e12262. [Google Scholar] [CrossRef] [PubMed]
- Dilcher, R.; Malpas, C.B.; O’Brien, T.J.; Vivash, L. Social cognition in behavioral variant frontotemporal dementia and pathological subtypes: A Narrative review. J. Alzheimer’s Dis. 2023, 94, 19–38. [Google Scholar] [CrossRef]
- Poos, J.M.; Grandpierre, L.D.M.; Van Der Ende, E.L.; Panman, J.L.; Papma, J.M.; Seelaar, H.; Van Den Berg, E.; Van ’t Klooster, R.; Bron, E.; Steketee, R.; et al. Longitudinal brain atrophy rates in presymptomatic carriers of genetic frontotemporal dementia. Neurology 2022, 99, e2661–e2671. [Google Scholar] [CrossRef]
- Buccellato, F.R.; D’Anca, M.; Tartaglia, G.M.; Del Fabbro, M.; Galimberti, D. Frontotemporal dementia: From genetics to therapeutic approaches. Expert Opin. Investig. Drugs 2024, 33, 561–573. [Google Scholar] [CrossRef]
- Jiménez-García, A.M.; Tortorella, M.E.; Nishimura, A.L.; Arias, N. The Differential Effects of Genetic Mutations in ALS and FTD genes on Behavioural and Cognitive Changes: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2025, 26, 6199. [Google Scholar] [CrossRef]
- Weijs, R.W.J.; Shkredova, D.A.; Brekelmans, A.C.M.; Thijssen, D.H.J.; Claassen, J.A.H.R. Longitudinal changes in cerebral blood flow and their relation with cognitive decline in patients with dementia: Current knowledge and future directions. Alzheimer’s Dement. 2022, 19, 532–548. [Google Scholar] [CrossRef]
- Del Campo, M.; Zetterberg, H.; Gandy, S.; Onyike, C.U.; Oliveira, F.; Udeh-Momoh, C.; Lleó, A.; Teunissen, C.E.; Pijnenburg, Y. New developments of biofluid-based biomarkers for routine diagnosis and disease trajectories in frontotemporal dementia. Alzheimer’s Dement. 2022, 18, 2292–2307. [Google Scholar] [CrossRef] [PubMed]
- Swift, I.J.; Sogorb-Esteve, A.; Heller, C.; Synofzik, M.; Otto, M.; Graff, C.; Galimberti, D.; Todd, E.; Heslegrave, A.J.; Van Der Ende, E.L.; et al. Fluid biomarkers in frontotemporal dementia: Past, present and future. J. Neurol. Neurosurg. Psychiatry 2020, 92, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Liampas, I.; Kyriakoulopoulou, P.; Karakoida, V.; Kavvoura, P.A.; Sgantzos, M.; Bogdanos, D.P.; Stamati, P.; Dardiotis, E.; Siokas, V. Blood-Based biomarkers in frontotemporal dementia: A narrative review. Int. J. Mol. Sci. 2024, 25, 11838. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cheng, Q.; Gao, J.; Kim, W.S. Status of biomarker development for frontotemporal dementia and amyotrophic lateral sclerosis. Neural Regen. Res. 2024, 19, 2117–2118. [Google Scholar] [CrossRef]
- Santos, F.; Cabreira, V.; Rocha, S.; Massano, J. Blood biomarkers for the diagnosis of Neurodegenerative Dementia: A Systematic review. J. Geriatr. Psychiatry Neurol. 2022, 36, 267–281. [Google Scholar] [CrossRef]
- Panman, J.L.; Venkatraghavan, V.; Van Der Ende, E.L.; Steketee, R.M.E.; Jiskoot, L.C.; Poos, J.M.; Dopper, E.G.P.; Meeter, L.H.H.; Kaat, L.D.; Rombouts, S.A.R.B.; et al. Modelling the cascade of biomarker changes in GRN-related frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry 2021, 92, 494–501. [Google Scholar] [CrossRef]
- Vignaroli, F.; Mele, A.; Tondo, G.; De Giorgis, V.; Manfredi, M.; Comi, C.; Mazzini, L.; De Marchi, F. The need for biomarkers in the ALS–FTD spectrum: A Clinical point of view on the role of proteomics. Proteomes 2023, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Ntymenou, S.; Tsantzali, I.; Kalamatianos, T.; Voumvourakis, K.I.; Kapaki, E.; Tsivgoulis, G.; Stranjalis, G.; Paraskevas, G.P. Blood Biomarkers in Frontotemporal Dementia: Review and Meta-Analysis. Brain Sci. 2021, 11, 244. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Landin-Romero, R.; Matis, S.; Dalton, M.A.; Piguet, O. Longitudinal volumetric changes in amygdala subregions in frontotemporal dementia. J. Neurol. 2024, 271, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Davy, V.; Dumurgier, J.; Fayosse, A.; Paquet, C.; Cognat, E. Neurofilaments as Emerging Biomarkers of Neuroaxonal Damage to Differentiate Behavioral Frontotemporal Dementia from Primary Psychiatric Disorders: A Systematic Review. Diagnostics 2021, 11, 754. [Google Scholar] [CrossRef]
- Zhu, N.; Santos-Santos, M.; Illán-Gala, I.; Montal, V.; Estellés, T.; Barroeta, I.; Altuna, M.; Arranz, J.; Muñoz, L.; Belbin, O.; et al. Plasma glial fibrillary acidic protein and neurofilament light chain for the diagnostic and prognostic evaluation of frontotemporal dementia. Transl. Neurodegener. 2021, 10, 50. [Google Scholar] [CrossRef]
- Benussi, A.; Borroni, B. Advances in the treatment and management of frontotemporal dementia. Expert Rev. Neurother. 2023, 23, 621–639. [Google Scholar] [CrossRef]
- Lozupone, M.; Dibello, V.; Sardone, R.; Castellana, F.; Zupo, R.; Lampignano, L.; Bortone, I.; Sborgia, G.; Daniele, A.; Solfrizzi, V.; et al. Novel approaches for the treatment of frontotemporal dementia: Is there hope for the future? Expert Rev. Neurother. 2025, 1–14. [Google Scholar] [CrossRef]
- Ljubenkov, P.A.; Boxer, A.L. FTLD treatment: Current practice and future possibilities. In Advances in Experimental Medicine and Biology; Springer Nature: Berlin, Germany, 2021; pp. 297–310. [Google Scholar] [CrossRef]
- Neylan, K.D.; Miller, B.L. New approaches to the treatment of frontotemporal dementia. Neurotherapeutics 2023, 20, 1055–1065. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzidimitriou, E.; Ntritsos, G.; Lagoudaki, R.; Poptsi, E.; Tsardoulias, E.; Symeonidis, A.L.; Tsolaki, M.; Konstantinopoulou, E.; Papadopoulou, K.; Charalambous, P.; et al. Functional Impairment in Behavioral Variant Frontotemporal Dementia: Cognitive, Behavioral, Personality, and Brain Perfusion Contributions. J. Pers. Med. 2025, 15, 466. https://doi.org/10.3390/jpm15100466
Chatzidimitriou E, Ntritsos G, Lagoudaki R, Poptsi E, Tsardoulias E, Symeonidis AL, Tsolaki M, Konstantinopoulou E, Papadopoulou K, Charalambous P, et al. Functional Impairment in Behavioral Variant Frontotemporal Dementia: Cognitive, Behavioral, Personality, and Brain Perfusion Contributions. Journal of Personalized Medicine. 2025; 15(10):466. https://doi.org/10.3390/jpm15100466
Chicago/Turabian StyleChatzidimitriou, Electra, Georgios Ntritsos, Roza Lagoudaki, Eleni Poptsi, Emmanouil Tsardoulias, Andreas L. Symeonidis, Magda Tsolaki, Eleni Konstantinopoulou, Kyriaki Papadopoulou, Panos Charalambous, and et al. 2025. "Functional Impairment in Behavioral Variant Frontotemporal Dementia: Cognitive, Behavioral, Personality, and Brain Perfusion Contributions" Journal of Personalized Medicine 15, no. 10: 466. https://doi.org/10.3390/jpm15100466
APA StyleChatzidimitriou, E., Ntritsos, G., Lagoudaki, R., Poptsi, E., Tsardoulias, E., Symeonidis, A. L., Tsolaki, M., Konstantinopoulou, E., Papadopoulou, K., Charalambous, P., Rankin, K. P., Aretouli, E., Sioka, C., Iakovou, I., Afrantou, T., Ioannidis, P., & Moraitou, D. (2025). Functional Impairment in Behavioral Variant Frontotemporal Dementia: Cognitive, Behavioral, Personality, and Brain Perfusion Contributions. Journal of Personalized Medicine, 15(10), 466. https://doi.org/10.3390/jpm15100466














