Setting Up Our Lab-in-a-Box: Paving the Road Towards Remote Data Collection for Scalable Personalized Biometrics
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Materials and Equipment
2.3. Procedures
2.3.1. Study 1—Facial Universal Micro Expressions (FUME) Assessment
Task to Characterize Facial Micro-Motions Underlying Instructed Emotions
2.3.2. Study 2—PD Evaluation
2.3.3. Study 3—Dysregulation Screening
The Resting Task
The Pointing Task
The Walking Task
Daily vs. Sleep Task
Lab-in-a-Box Procedure
2.3.4. Data Analysis
Motor Activity (Wearable Sensors Accelerometer Data)
Cardiac Activity (Wearable ECG Data)
ECG—Time Domain Analysis
ECG—Frequency Domain Analysis
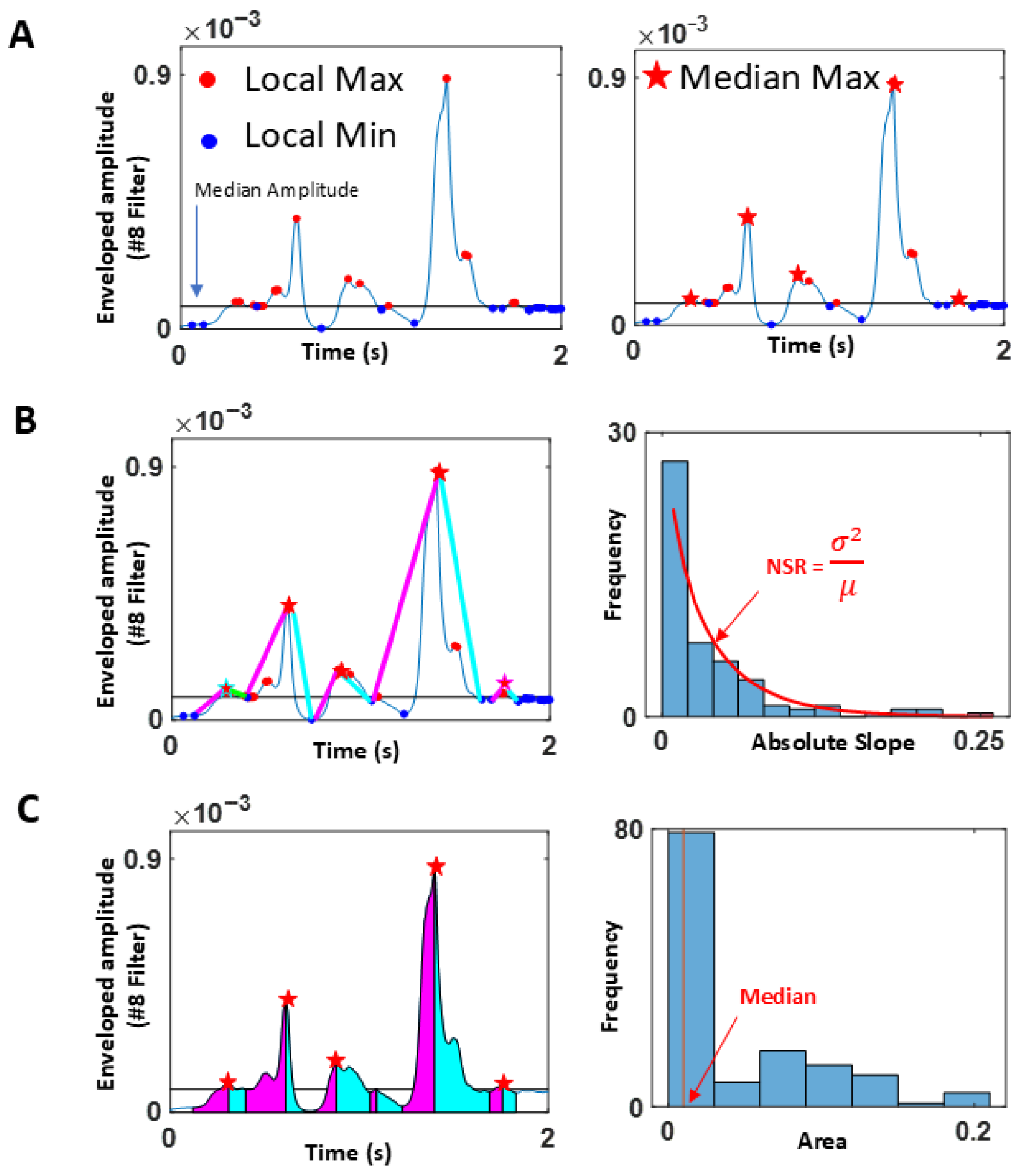
Facial Activity (Proxy Video Data)
Voice Activity (Proxy Audio Data)
Stochastic Characterization of Upwards (Attack) and Downwards (Decay) Phases of Speech
2.3.5. Statistical Tests
2.3.6. Measuring Distance in Probability Space
3. Results
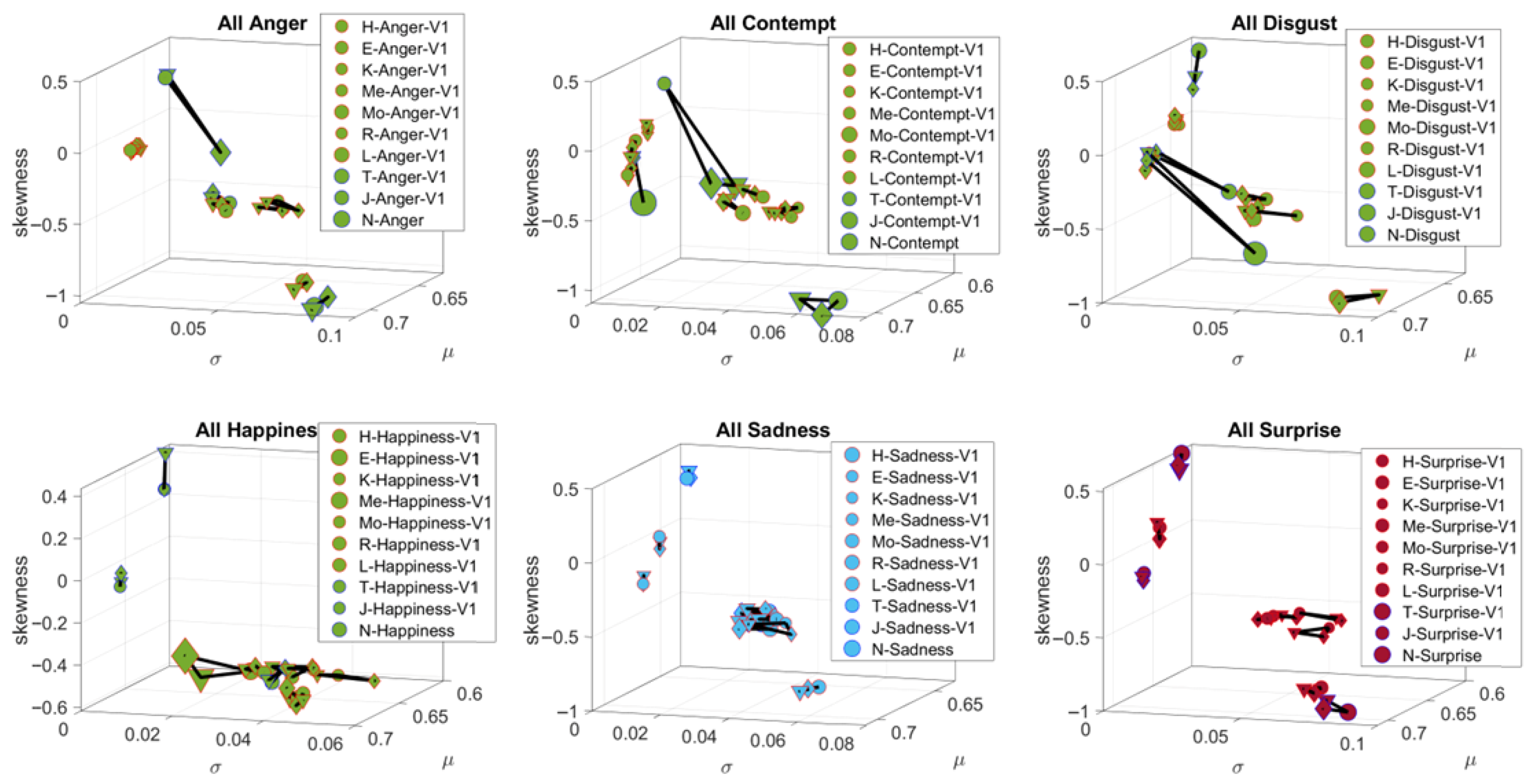
3.1. Study 1—FUME Assessment (Facial Activity)
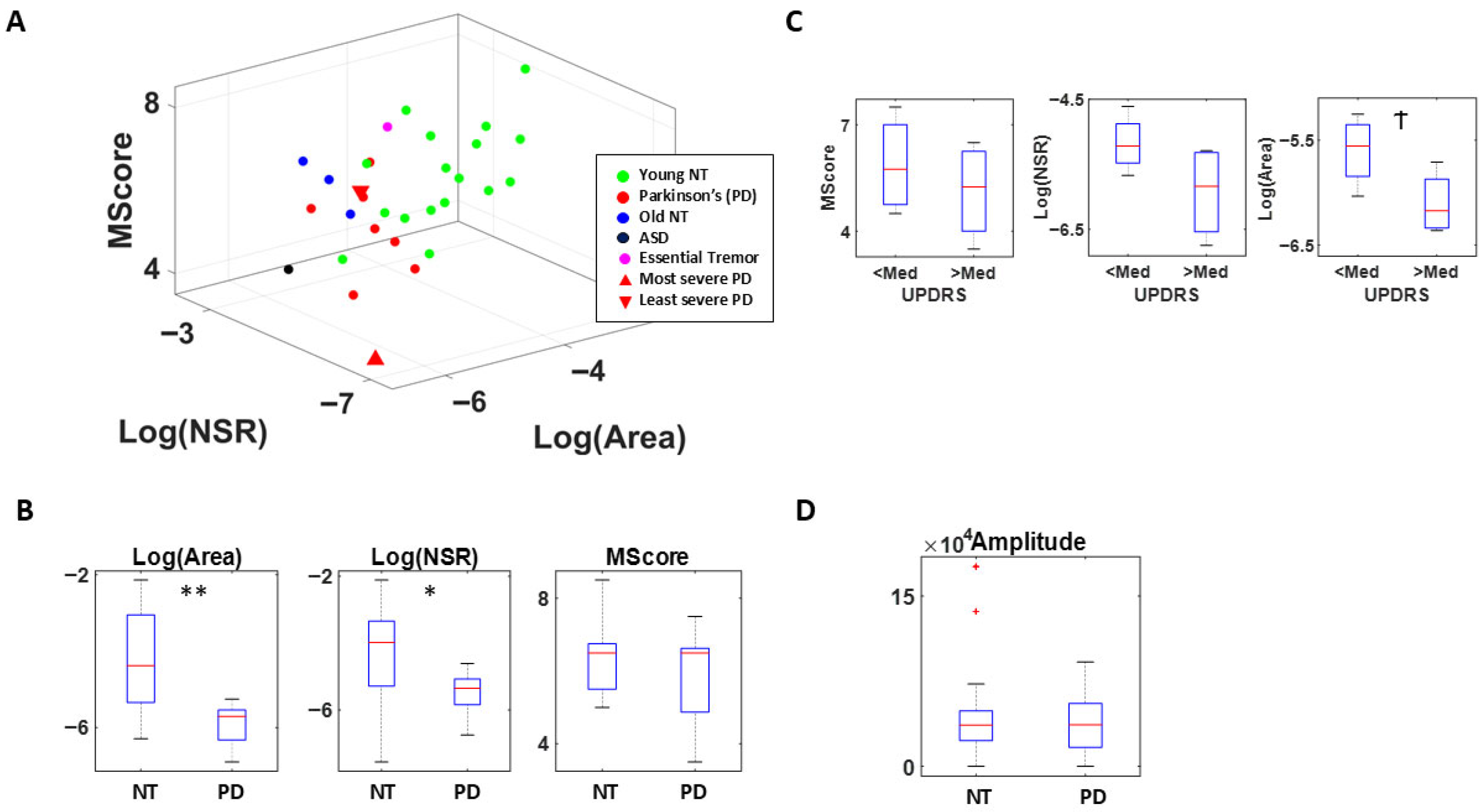
3.2. Study 2—PD Evaluation (Voice Activity)
3.3. Study 3—Dysregulation Screening (Cardiac, Motor, and Facial Activity)
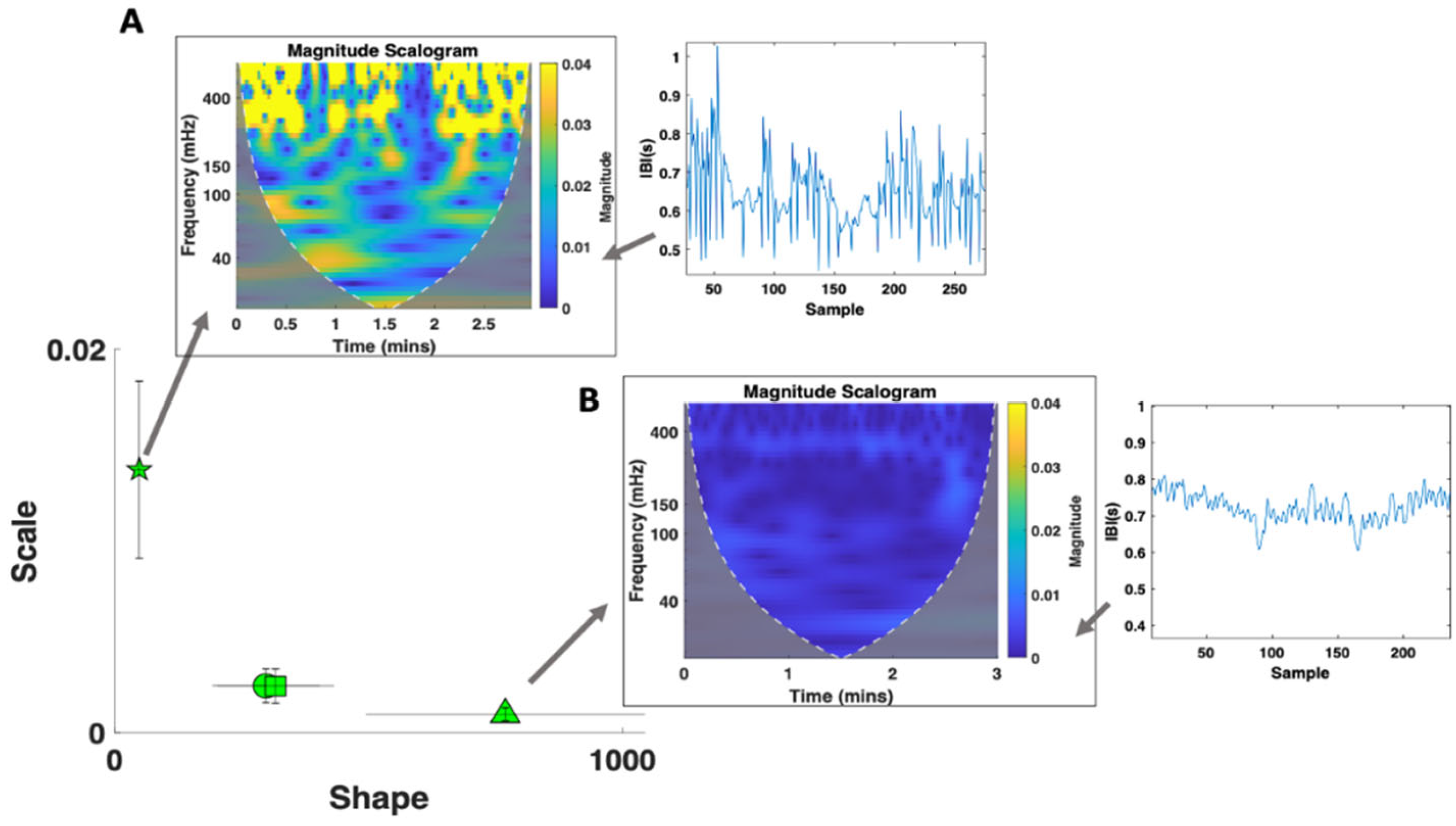
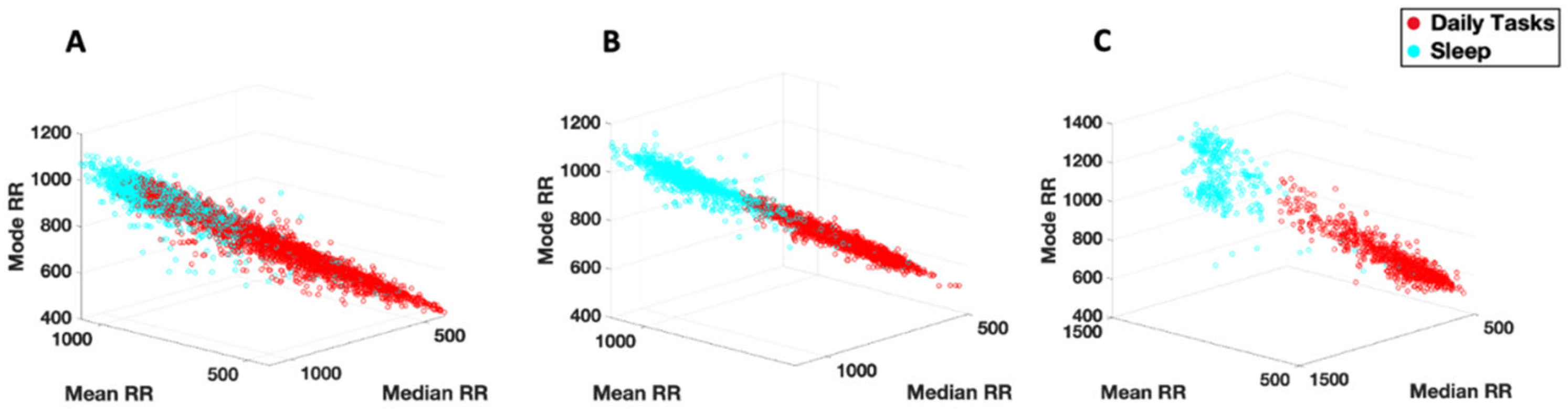
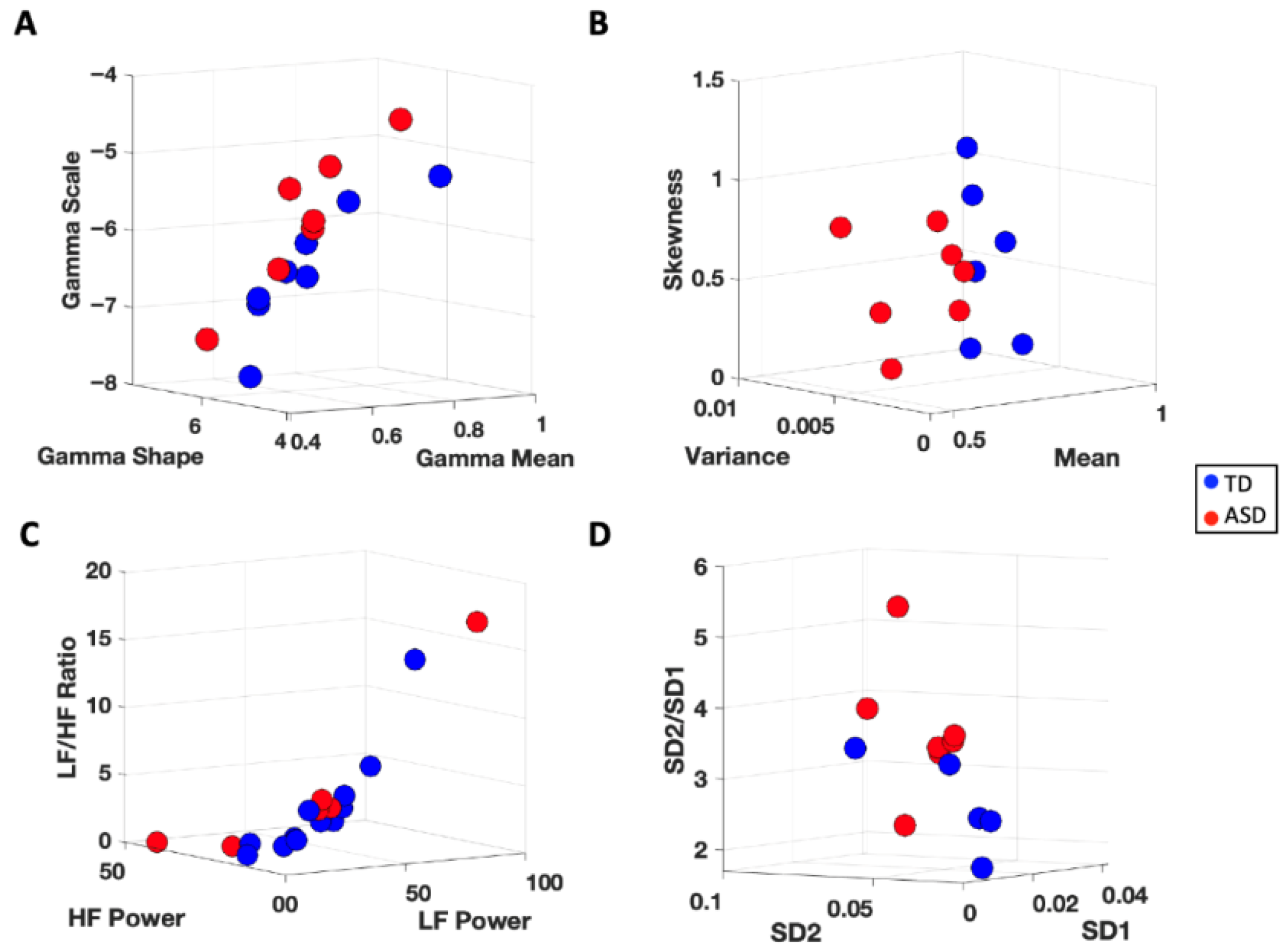
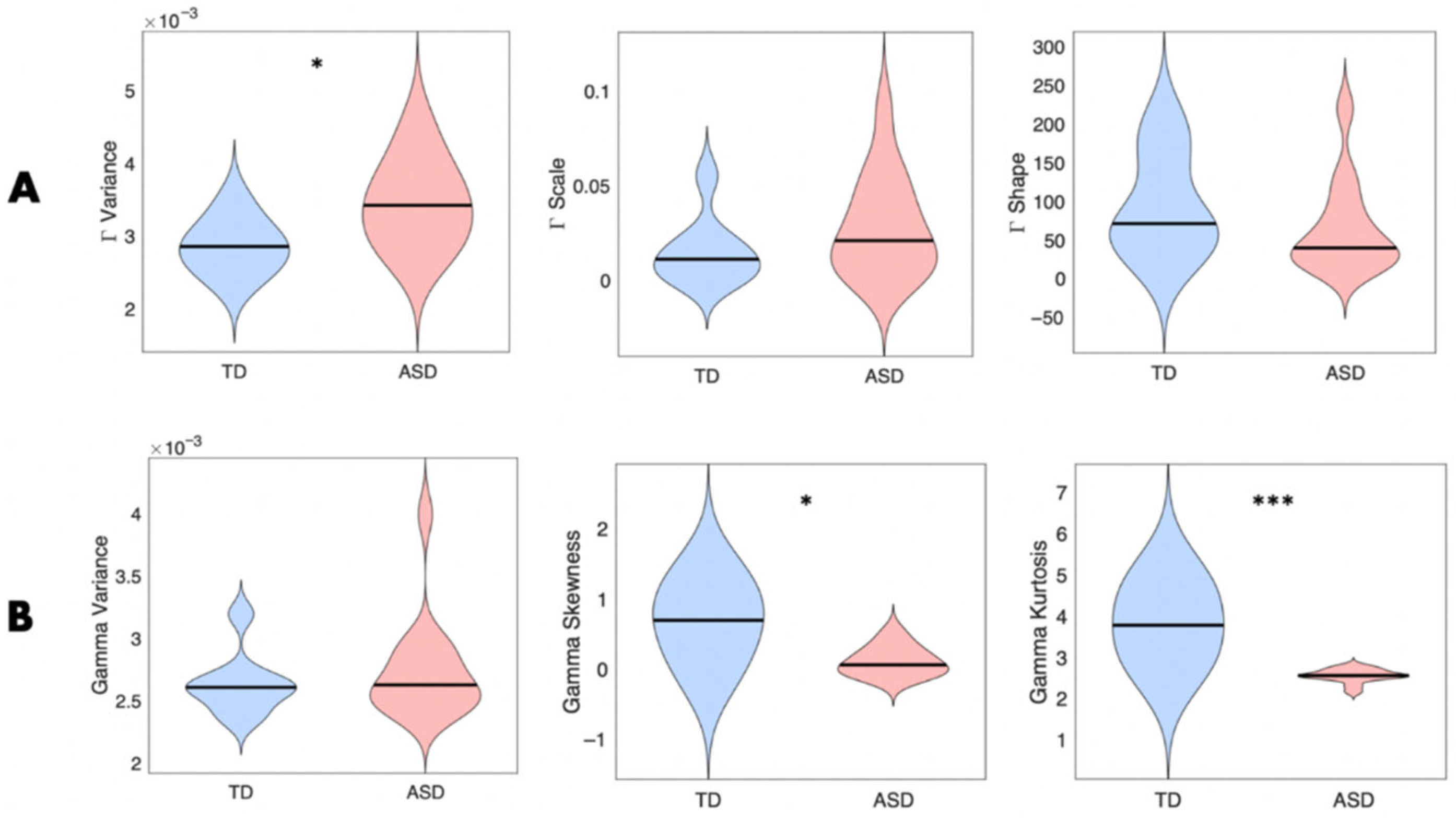
3.3.1. Cardiac Activity—Comparison with Atypical Populations
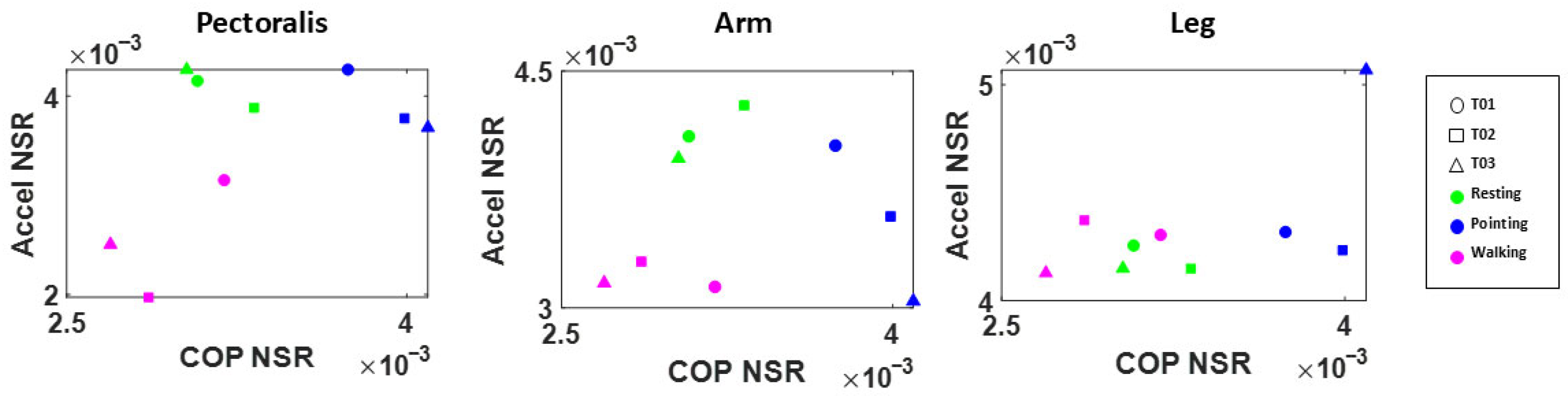
3.3.2. Motor Activity—Arm Movements During a Simple Pointing Task
3.3.3. Facial Activity—Resting State Facial Affect and Muscle Movement in ASD vs. TD Participants
4. Discussion
4.1. Cardiac Activity
4.2. Facial Activity
4.3. Voice Activity
4.4. Motor Activities
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1
- Vanessa Van Edwards videos to obtain reference emotions.https://www.youtube.com/watch?v=dX7zIpHaAXE (accessed on 20 August 2025)https://www.youtube.com/watch?v=B0ouAnmsO1Y (accessed on 20 August 2025)
- Assays to measure motor function in the Lab Environment:
- Resting and then Pointing with non-dominant handhttps://youtu.be/yrXLUFaD02Y (accessed on 20 August 2025)
- Pointing with dominant hand (lab setting)https://www.youtube.com/watch?v=0zQ2DBNA6Eo (accessed on 20 August 2025)
- Walking (lab setting)https://www.youtube.com/watch?v=rC6vmTxHr5g (accessed on 20 August 2025)
- Assays to measure motor function in the Home Environment:
- Pointing with dominant hand (at home)https://www.youtube.com/watch?v=z6af8tnQCBg (accessed on 20 August 2025)
- Walking (at home)https://www.youtube.com/watch?v=uYCMVHWmKME (accessed on 20 August 2025)
- Resting (at home)https://www.youtube.com/watch?v=hZrH5phNxx0&list=PLTvO_IuPVliEkaITZuHLRuhWh3kx75Wzn (accessed on 20 August 2025)
Appendix A.2


References
- Hawgood, S.; Hook-Barnard, I.G.; O’Brien, T.C.; Yamamoto, K.R. Precision medicine: Beyond the inflection point. Sci. Transl. Med. 2015, 7, 300ps317. [Google Scholar] [CrossRef]
- Atkeson, C.G.; Hollerbach, J.M. Kinematic features of unrestrained vertical arm movements. J. Neurosci. 1985, 5, 2318–2330. [Google Scholar] [CrossRef] [PubMed]
- Viviani, P.; Flash, T. Minimum-jerk, two-thirds power law, and isochrony: Converging approaches to movement planning. J. Exp. Psychol. Hum. Percept. Perform 1995, 21, 32–53. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, K.; Murray, S.; Flanders, M. Do arm postures vary with the speed of reaching? J. Neurophysiol. 1999, 81, 2582–2586. [Google Scholar] [CrossRef]
- Tanaka, H.; Sejnowski, T.J. Motor adaptation and generalization of reaching movements using motor primitives based on spatial coordinates. J. Neurophysiol. 2015, 113, 1217–1233. [Google Scholar] [CrossRef]
- Torres, E.B. Two classes of movements in motor control. Exp. Brain Res. 2011, 215, 269–283. [Google Scholar] [CrossRef]
- Torres, E.B.; Yanovich, P.; Metaxas, D.N. Give spontaneity and self-discovery a chance in ASD: Spontaneous peripheral limb variability as a proxy to evoke centrally driven intentional acts. Front. Integr. Neurosci. 2013, 7, 46. [Google Scholar] [CrossRef]
- Ryu, J.; Vero, J.; Dobkin, R.D.; Torres, E.B. Dynamic Digital Biomarkers of Motor and Cognitive Function in Parkinson’s Disease. J. Vis. Exp. 2019, 149, e59827. [Google Scholar] [CrossRef]
- Torres, E.B.; Brincker, M.; Isenhower, R.W.; Yanovich, P.; Stigler, K.A.; Nurnberger, J.I.; Metaxas, D.N.; José, J.V. Autism: The micro-movement perspective. Front. Integr. Neurosci. 2013, 7, 32. [Google Scholar] [CrossRef]
- Cao, Z.; Hidalgo, G.; Simon, T.; Wei, S.E.; Sheikh, Y.A. OpenPose: Realtime Multi-Person 2D Pose Estimation Using Part Affinity Fields. arXiv 2019, arXiv:1812.08008. [Google Scholar] [CrossRef]
- Torres, E.B.; Vero, J.; Rai, R. Statistical Platform for Individualized Behavioral Analyses Using Biophysical Micro-Movement Spikes. Sensors 2018, 18, 1025. [Google Scholar] [CrossRef]
- Casco-Rodriguez, J.; Alemohammad, S.; Luzi, L.; Imtiaz, A.; Babaei, H.; LeJeune, D.; Siahkoohi, A.; Baraniuk, R. Self-Consuming Generative Models Go MAD. arXiv 2023, arXiv:2307.01850v1. [Google Scholar] [CrossRef]
- De Los Reyes, A.; Aldao, A. Introduction to the special issue: Toward implementing physiological measures in clinical child and adolescent assessments. J. Clin. Child Adolesc. Psychol. 2015, 44, 221–237. [Google Scholar] [CrossRef]
- Sun, Y.M.; Wang, Z.Y.; Liang, Y.Y.; Hao, C.W.; Shi, C.H. Digital biomarkers for precision diagnosis and monitoring in Parkinson’s disease. NPJ Digit. Med. 2024, 7, 218. [Google Scholar] [CrossRef]
- Youngstrom, E.A.; De Los Reyes, A. Commentary: Moving toward cost-effectiveness in using psychophysiological measures in clinical assessment: Validity, decision making, and adding value. J. Clin. Child Adolesc. Psychol. 2015, 44, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Halter, M.J.; Rolin-Kenny, D.; Dzurec, L.C. An overview of the DSM-5: Changes, controversy, and implications for psychiatric nursing. J. Psychosoc. Nurs. Ment. Health Serv. 2013, 51, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Volkmar, F.R.; McPartland, J.C. From Kanner to DSM-5: Autism as an evolving diagnostic concept. Annu. Rev. Clin. Psychol. 2014, 10, 193–212. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Risi, S.; Lambrecht, L.; Cook, E.H.; Leventhal, B.L.; DiLavore, P.C.; Pickles, A.; Rutter, M. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000, 30, 205–223. [Google Scholar] [CrossRef]
- Baltrušaitis, T.; Robinson, P.; Morency, L.P. OpenFace: An open source facial behavior analysis toolkit. In Proceedings of the IEEE Winter Conference on Applications of Computer Vision (WACV), Lake Placid, NY, USA, 7–10 March 2016; pp. 1–10. [Google Scholar]
- Ekman, P. Emotion in the Human Face, 2nd ed.; Editions de la Maison des Sciences de l’Homme; Cambridge University Press: Cambridge, UK, 1982. [Google Scholar]
- Jeyabalan, N.; Clement, J.P. SYNGAP1: Mind the Gap. Front. Cell. Neurosci. 2016, 10, 32. [Google Scholar] [CrossRef]
- Torres, E.B.; Denisova, K. Motor noise is rich signal in autism research and pharmacological treatments. Sci. Rep. 2016, 6, 37422. [Google Scholar] [CrossRef]
- Bermperidis, T.; Rai, R.; Ryu, J.; Zanotto, D.; Agrawal, S.K.; Lalwani, A.K.; Torres, E.B. Optimal time lags from causal prediction model help stratify and forecast nervous system pathology. Sci. Rep. 2021, 11, 20904. [Google Scholar] [CrossRef] [PubMed]
- Buchegger, J.; Meier-Koll, A. Motor learning and ultradian sleep cycle: An electroencephalographic study of trampoliners. Percept. Mot. Ski. 1988, 67, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Ziv, I.; Avni, I.; Dinstein, I.; Meiri, G.; Bonneh, Y.S. Oculomotor randomness is higher in autistic children and increases with the severity of symptoms. Autism Res. 2024, 17, 249–265. [Google Scholar] [CrossRef]
- Lleonart, J.; Salat, J.; Torres, G.J. Removing allometric effects of body size in morphological analysis. J. Theor. Biol. 2000, 205, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Kameenoff, J. Signal processing techniques for removing noise from ecg signals. Biomed. Eng. Res. 2017, 1, 1. [Google Scholar]
- Das, M.; Suszko, A.M.; Nayyar, S.; Viswanathan, K.; Spears, D.A.; Tomlinson, G.; Pinter, A.; Crystal, E.; Dalvi, R.; Krishnan, S.; et al. Automated Quantification of Low-Amplitude Abnormal QRS Peaks From High-Resolution ECG Recordings Predicts Arrhythmic Events in Patients With Cardiomyopathy. Circ. Arrhythm. Electrophysiol. 2017, 10, 1–10. [Google Scholar] [CrossRef]
- Fedotov, A.A. Selection of Parameters of Bandpass Filtering of the ECG Signal for Heart Rhythm Monitoring Systems. Biomed. Eng. 2016, 50, 114–118. [Google Scholar] [CrossRef]
- Teich, M.C.; Lowen, S.B.; Jost, B.M.; Vibe-Rheymer, K.; Heneghan, C. Heart rate variability: Measures and models. Nonlinear Biomed. Signal Process. Dyn. Anal. Model. 2000, 2, 159–213. [Google Scholar]
- Hsu, C.-H.; Tsai, M.-Y.; Huang, G.-S.; Lin, T.-C.; Chen, K.-P.; Ho, S.-T.; Shyu, L.-Y.; Li, C.-Y. Poincaré plot indexes of heart rate variability detect dynamic autonomic modulation during general anesthesia induction. Acta Anaesthesiol. Taiwanica 2012, 50, 12–18. [Google Scholar] [CrossRef]
- Brennan, M.; Palaniswami, M.; Kamen, P. Do existing measures of Poincare plot geometry reflect nonlinear features of heart rate variability? IEEE Trans. Biomed. Eng. 2002, 48, 1342–1347. [Google Scholar] [CrossRef]
- Vest, A.N.; Da Poian, G.; Li, Q.; Liu, C.; Nemati, S.; Shah, A.J.; Clifford, G.D. An open source benchmarked toolbox for cardiovascular waveform and interval analysis. Physiol. Meas. 2018, 39, 105004. [Google Scholar] [CrossRef]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research—Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Front. Psychol. 2017, 8, 213. [Google Scholar] [CrossRef]
- Hunt, K.J.; Fankhauser, S.E. Heart rate control during treadmill exercise using input-sensitivity shaping for disturb-ance rejection of very-low-frequency heart rate variability. Biomed. Signal Process. Control 2016, 30, 31–42. [Google Scholar] [CrossRef]
- Rani, P.; Sims, J.; Brackin, R.; Sarkar, N. Online stress detection using psychophysiological signals for implicit human-robot cooperation. Robotica 2002, 20, 673–685. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Laguna, P.; Moody, G.B.; Mark, R.G.; Laguna, P.; Moody, G.; Mark, R. Power spectral density of unevenly sampled data by least-square analysis: Performance and application to heart rate signals. IEEE Trans. Biomed. Eng. 1998, 45, 698–715. [Google Scholar] [CrossRef] [PubMed]
- Wachowiak, M.P.; Wachowiak-Smolíková, R.; Johnson, M.J.; Hay, D.C.; Power, K.E.; Williams-Bell, F.M. Quantitative feature analysis of continuous analytic wavelet transforms of electrocardiography and electromyography. Philos. Trans. A Math. Phys. Eng. Sci. 2018, 376, 20170250. [Google Scholar] [CrossRef]
- Torres, E.B.; Vero, J.; Drain, N.; Rai, R.; Bermperidis, T. Hidden social and emotional competencies in autism spectrum disorders captured through the digital lens. Front. Psychiatry 2025, 16, 1559202. [Google Scholar] [CrossRef]
- Patterson, R.D.; Unoki, M.; Irino, T. Extending the domain of center frequencies for the compressive gammachirp auditory filter. J. Acoust. Soc. Am. 2003, 114, 1529–1542. [Google Scholar] [CrossRef]
- Elsayed, M. Framework of Interpretable Biometrics to Assess Internal Psychophysiological States of Distress in Autism and the General Population. Ph.D. Thesis, Rutgers the State University of New Jersey, New Brunswick, NJ, USA, 2024. [Google Scholar]
- Kim, H.G.; Cheon, E.J.; Bai, D.S.; Lee, Y.H.; Koo, B.H. Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef]
- Joosten, A.V.; Bundy, A.C. Sensory processing and stereotypical and repetitive behaviour in children with autism and intellectual disability. Aust. Occup. Ther. J. 2010, 57, 366–372. [Google Scholar] [CrossRef]
- Jones, W.; Klaiman, C.; Richardson, S.; Aoki, C.; Smith, C.; Minjarez, M.; Bernier, R.; Pedapati, E.; Bishop, S.; Ence, W.; et al. Eye-Tracking-Based Measurement of Social Visual Engagement Compared With Expert Clinical Diagnosis of Autism. JAMA 2023, 330, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, K.L.H.; Hahemi, J.; Campbell, K.; Lippmann, S.J.; Baker, J.P.; Egger, H.L.; Espinosa, S.; Vermeer, S.; Sapiro, G.; Dawson, G. Digital Behavioral Phenotyping Detects Atypical Pattern of Facial Expression in Toddlers with Autism. Autism Res. 2021, 14, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Sucksmith, E.; Allison, C.; Baron-Cohen, S.; Chakrabarti, B.; Hoekstra, R.A. Empathy and emotion recognition in people with autism, first-degree relatives, and controls. Neuropsychologia 2013, 51, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Grove, R.; Baillie, A.; Allison, C.; Baron-Cohen, S.; Hoekstra, R.A. Exploring the quantitative nature of empathy, systemising and autistic traits using factor mixture modelling. Br. J. Psychiatry 2015, 207, 400–406. [Google Scholar] [CrossRef]
- Gamboa, J.; Jiménez-Jiménez, F.J.; Nieto, A.; Montojo, J.; Ortí-Pareja, M.; Molina, J.A.; García-Albea, E.; Cobeta, I. Acoustic voice analysis in patients with Parkinson’s disease treated with dopaminergic drugs. J. Voice 1997, 11, 314–320. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nishio, M.; Niimi, S. Vocal acoustic characteristics of patients with Parkinson’s disease. Folia Phoniatr. Logop. 2011, 63, 223–230. [Google Scholar] [CrossRef]
- Yu, Q.; Zou, X.; Quan, F.; Dong, Z.; Yin, H.; Liu, J.; Zuo, H.; Xu, J.; Han, Y.; Zou, D.; et al. Parkinson’s disease patients with freezing of gait have more severe voice impairment than non-freezers during “ON state”. J. Neural Transm. 2022, 129, 277–286. [Google Scholar] [CrossRef]
- Asghari, S.Z.; Farashi, S.; Bashirian, S.; Jenabi, E. Distinctive prosodic features of people with autism spectrum disorder: A systematic review and meta-analysis study. Sci. Rep. 2021, 11, 23093. [Google Scholar] [CrossRef]
- Talkar, T.; Johnson, K.T.; Narain, J.; Maes, P.; Picard, R.; Quatieri, T.F. Brief Report: Quantifying Speech Production Coordination from Non- and Minimally-Speaking Individuals. J. Autism Dev. Disord. 2024, 1–15. [Google Scholar] [CrossRef]
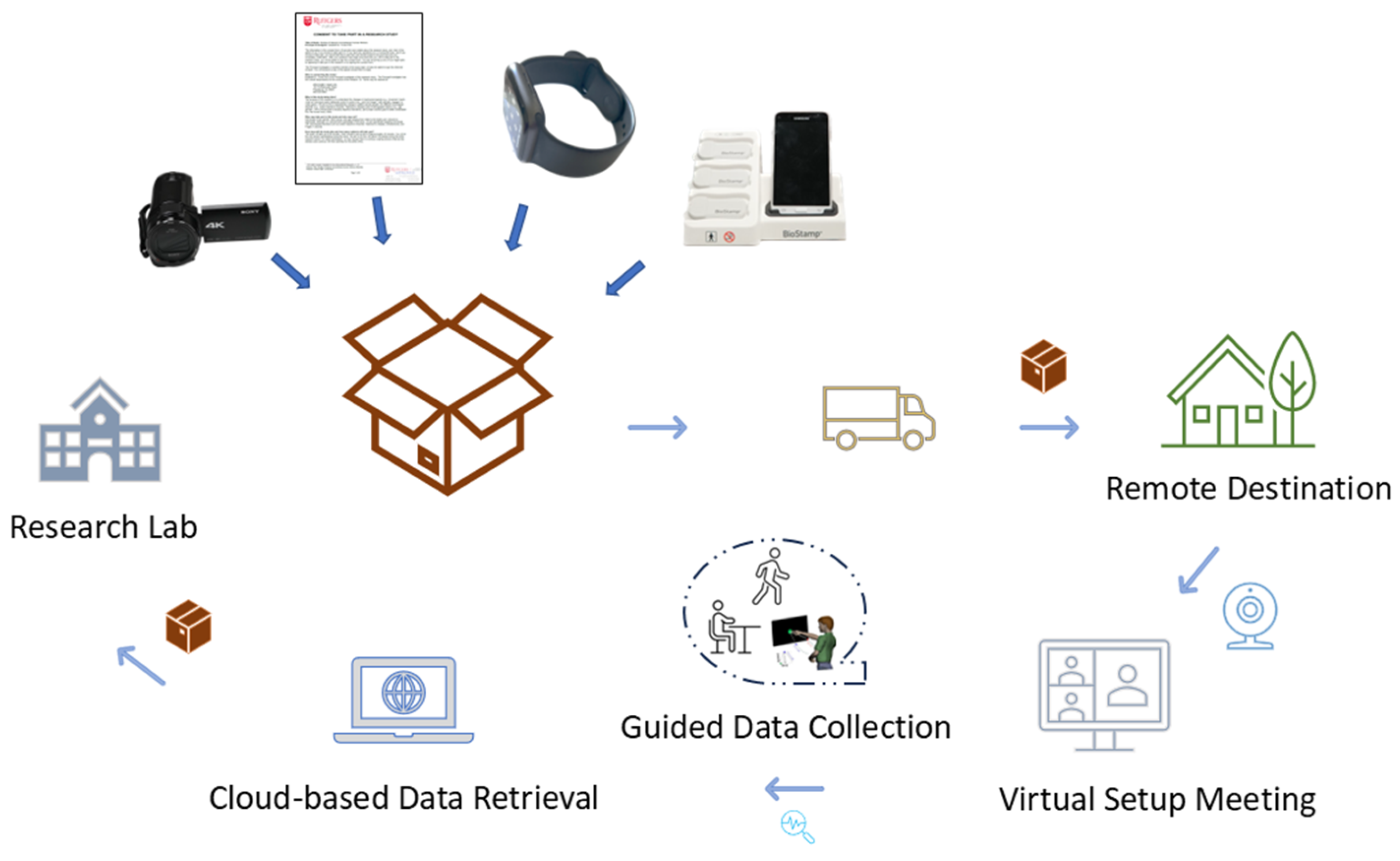
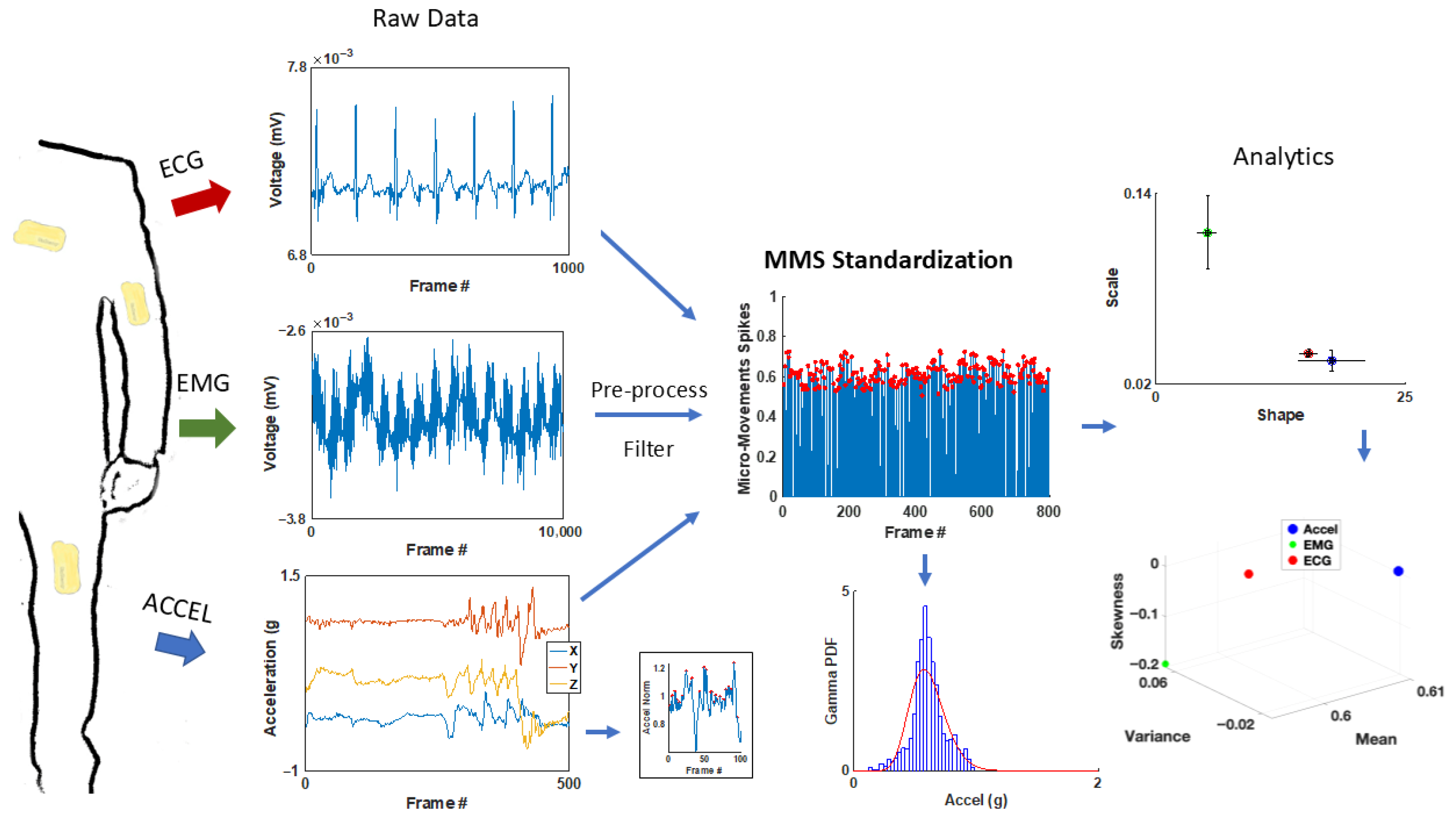


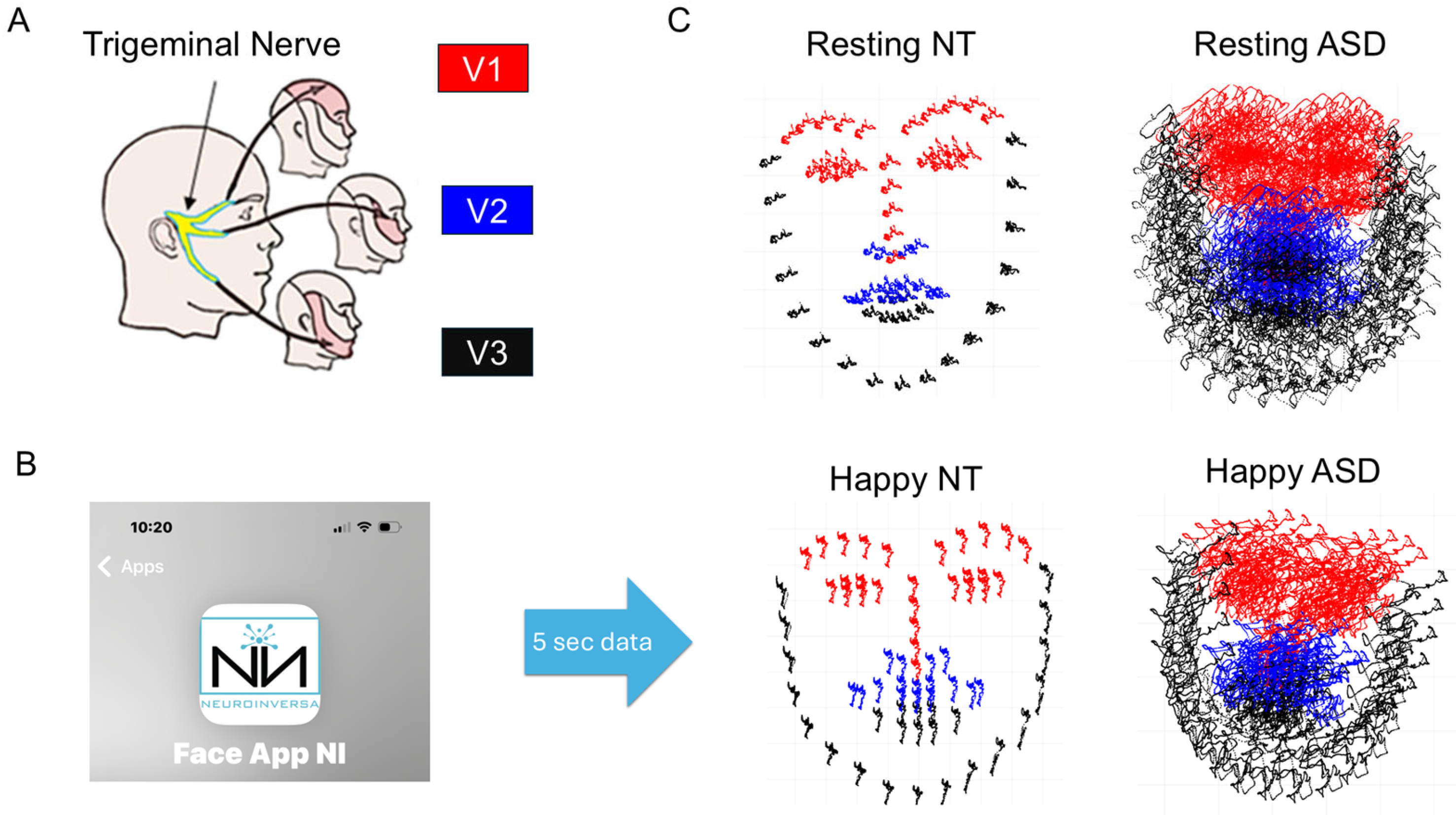
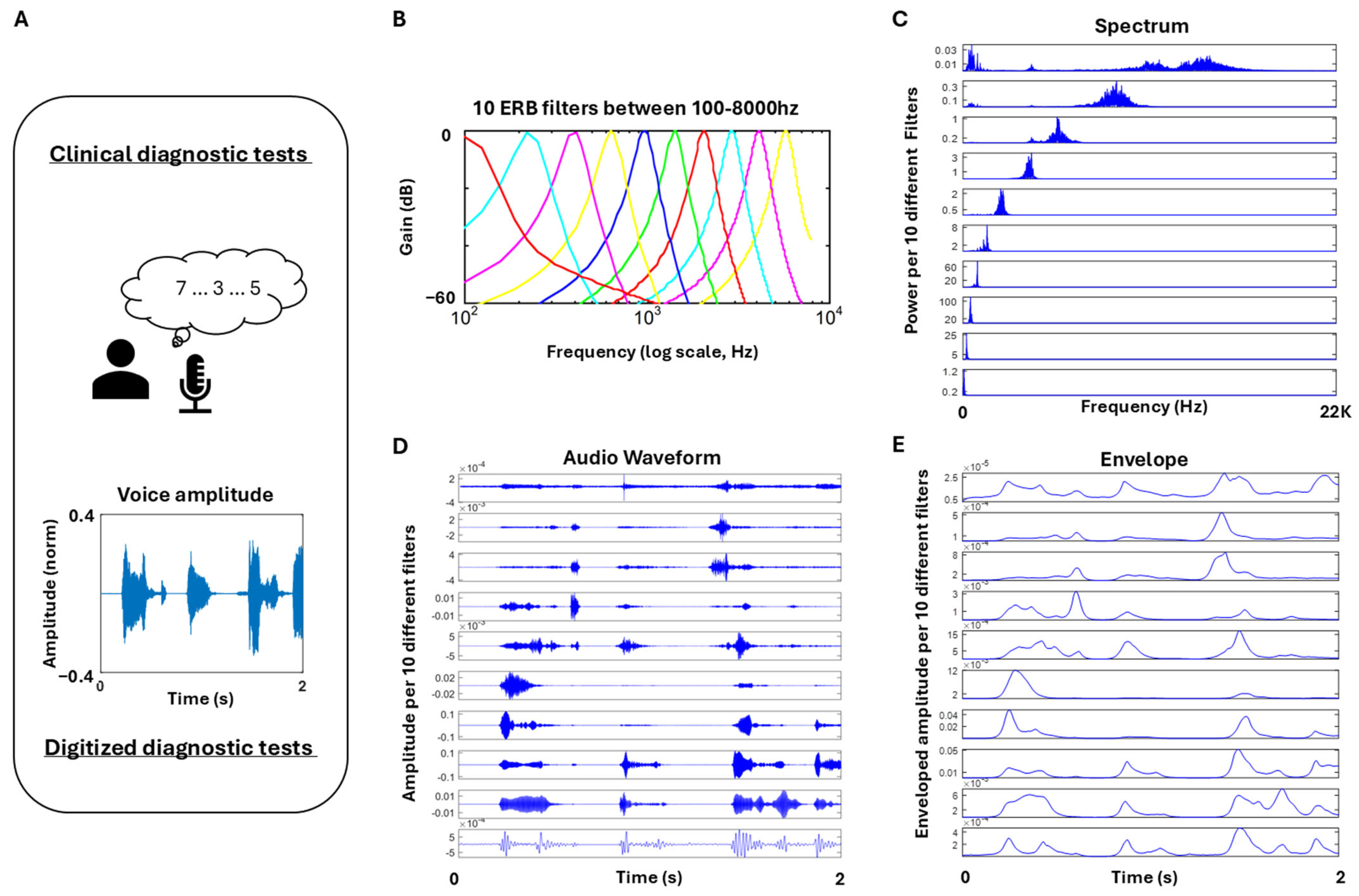




| Study Name | Sample Size | Age|Sex | Diagnoses | Data Type(s) Collected |
|---|---|---|---|---|
| FUME Assessment | 10 | 28 +/− 5 (3 M, 7 F) | 10 TD | Face + |
| PD Evaluation | 30 | 59.8 +/− 10.7 (22 M, 8 F) | 20 TD 8 PD 1 ET, 1ASD | Voice ++ |
| Dysregulation Screening | 18 | 20.2 +/− 12.10 (9 M, 9F) | 10 TD 8 ASD | Face +, Motor +++ Heart ++++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsayed, M.; Ryu, J.; Vero, J.; Torres, E.B. Setting Up Our Lab-in-a-Box: Paving the Road Towards Remote Data Collection for Scalable Personalized Biometrics. J. Pers. Med. 2025, 15, 463. https://doi.org/10.3390/jpm15100463
Elsayed M, Ryu J, Vero J, Torres EB. Setting Up Our Lab-in-a-Box: Paving the Road Towards Remote Data Collection for Scalable Personalized Biometrics. Journal of Personalized Medicine. 2025; 15(10):463. https://doi.org/10.3390/jpm15100463
Chicago/Turabian StyleElsayed, Mona, Jihye Ryu, Joseph Vero, and Elizabeth B. Torres. 2025. "Setting Up Our Lab-in-a-Box: Paving the Road Towards Remote Data Collection for Scalable Personalized Biometrics" Journal of Personalized Medicine 15, no. 10: 463. https://doi.org/10.3390/jpm15100463
APA StyleElsayed, M., Ryu, J., Vero, J., & Torres, E. B. (2025). Setting Up Our Lab-in-a-Box: Paving the Road Towards Remote Data Collection for Scalable Personalized Biometrics. Journal of Personalized Medicine, 15(10), 463. https://doi.org/10.3390/jpm15100463







