A First Diastolic Function Evaluation in the Personalized Exercise Prescription Program for Solid Organs Transplanted Subjects: Is Atrial Strain Useful?
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol Study
2.2. Inclusion/Exclusion Criteria
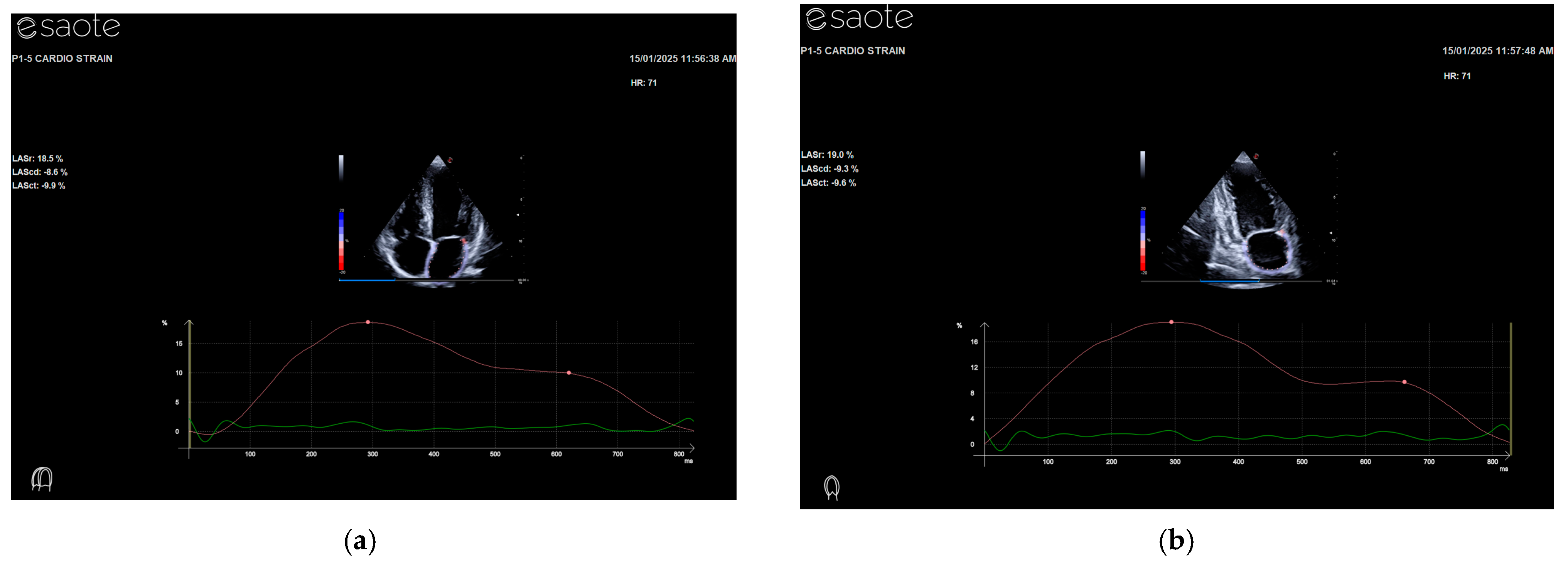
2.3. Echocardiographic Exam
2.4. Cardiopulmonary Test
2.5. Statistical Analysis
3. Results
4. Discussion
Limitation of the Study
5. Conclusions
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hansen, D.; Niebauer, J.; Cornelissen, V.; Barna, O.; Neunhäuserer, D.; Stettler, C.; Tonoli, C.; Greco, E.; Fagard, R.; Coninx, K.; et al. Exercise Prescription in Patients with Different Combinations of Cardiovascular Disease Risk Factors: A Consensus Statement from the EXPERT Working Group. Sports Med. 2018, 48, 1781–1797. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.; Abreu, A.; Ambrosetti, M.; Cornelissen, V.; Gevaert, A.; Kemps, H.; Laukkanen, J.A.; Pedretti, R.; Simonenko, M.; Wilhelm, M.; et al. Exercise intensity assessment and prescription in cardiovascular rehabilitation and beyond: Why and how: A position statement from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur. J. Prev. Cardiol. 2022, 29, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Tian, X.; Zhang, H.; Huang, R.; Li, N.; Chen, P.; Wang, R. Exercise as a prescription for patients with various diseases. J. Sport Health Sci. 2019, 8, 422–441. [Google Scholar] [CrossRef] [PubMed Central]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25, 1–72. [Google Scholar] [CrossRef] [PubMed]
- Gitto, S.; Golfieri, L.; Gabrielli, F.; Falcini, M.; Sofi, F.; Tamè, M.R.; De Maria, N.; Marzi, L.; Mega, A.; Valente, G.; et al. MEDITRA Research Group. Physical activity in liver transplant recipients: A large multicenter study. Intern. Emerg. Med. 2024, 19, 343–352. [Google Scholar] [CrossRef] [PubMed Central]
- Polara, G.; Montagnoli, A.; Palazzo, R.; Orlandi, M.; Mascherini, G.; Corsi, M.; Falconi, E.; Stefani, L. Cardiorespiratory Performance in Kidney and Liver Transplant Recipients: The Dilemma to Combine Lifestyle and Fitness. J. Funct. Morphol. Kinesiol. 2024, 9, 44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Orlandi, M.; Bini, V.; Leone, B.; Zappelli, E.; Pedrizzetti, G.; Stefani, L. Home-based exercise program improves normal right ventricle function in renal transplant recipients. J. Sports Med. Phys. Fit. 2022, 62, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.V.H.; Kroll, P.C.; Kroll, R.T.M.; Carvalho, V.N. Cirrhotic cardiomyopathy: The liver affects the heart. Braz. J. Med. Biol. Res. 2019, 52, e7809. [Google Scholar] [CrossRef]
- Obokata, M.; Reddy, Y.N.V.; Borlaug, B.A. Diastolic dysfunction and heart failure with preserved ejection fraction: Understanding mechanisms by using noninvasive methods. JACC Cardiovasc. Imaging 2020, 13, 245–257. [Google Scholar] [CrossRef]
- Di Salvo, G.; Caso, P.; Lo Piccolo, R.; Fusco, A.; Martiniello, A.R.; Russo, M.G.; D’Onofrio, A.; Severino, S.; Calabró, P.; Pacileo, G.; et al. Atrial myocardial deformation properties predict maintenance of sinus rhythm after external cardioversion of recent-onset lone atrial fibrillation: A color Doppler myocardial imaging and transthoracic and transesophageal echocardiographic study. Circulation 2005, 112, 387–395. [Google Scholar] [CrossRef]
- Silva, M.R.; Sampaio, F.; Braga, J.; Ribeiro, J.; Fontes-Carvalho, R. Left atrial strain evaluation to assess left ventricle diastolic dysfunction and heart failure with preserved ejection fraction: A guide to clinical practice: Left atrial strain and diastolic function. Int. J. Cardiovasc. Imaging 2023, 39, 1083–1096. [Google Scholar] [CrossRef] [PubMed]
- Mandoli, G.E.; Sisti, N.; Mondillo, S.; Cameli, M. Left atrial strain in left ventricular diastolic dysfunction: Have we finally found the missing piece of the puzzle? Heart Fail. Rev. 2020, 25, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, G.; Sofi, F.; Moscarelli, L.; Cirami, L.; Mancini, S.; Stefani, L. Exercise Prescription in Renal Transplant Recipients: From Sports Medicine Toward Multidisciplinary Aspects: A Pilot Study. J. Funct. Morphol. Kinesiol. 2020, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Gómez, A.M.A.; Sierra, L.T.; Mora, N.M.; Toledo, E.; Alonso, A.; Uriarte, M.G.; Sanchez, C.S.; Portillo, M.P.; Rodriguez, L.L.; Arellano, E.E.; et al. Left atrial strain improves echocardiographic classification of diastolic function in patients with metabolic syndrome and overweight-obesity. Int. J. Cardiol. 2022, 348, 169–174. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed]
- Jae, K.O.; William, R.M.; Jared, G.B.; Garvan, C.K.; Sherif, F.N. The 2016 Diastolic Function Guideline: Is it Already Time to Revisit or Revise Them? JACC Cardiovasc. Imaging 2016, 13, 327–335. [Google Scholar]
- Bouthoorn, S.; Valstar, G.B.; Gohar, A.; den Ruijter, H.M.; Reitsma, H.B.; Hoes, A.W.; Rutten, F.H. The prevalence of left ventricular diastolic dysfunction and heart failure with preserved ejection fraction in men and women with type 2 diabetes: A systematic review and meta-analysis. Diabetes Vasc. Dis. Res. 2018, 15, 477–493. [Google Scholar] [CrossRef] [PubMed Central]
- Zhang, L.; Liebelt, J.J.; Madan, N.; Shan, J.; Taub, C.C. Comparison of Predictors of Heart Failure With Preserved Versus Reduced Ejection Fraction in a Multiracial Cohort of Preclinical Left Ventricular Diastolic Dysfunction. Am. J. Cardiol. 2017, 119, 1815–1820. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.H.; Vogel, M.W.; Chen, H.H. Pre-clinical diastolic dysfunction. J. Am. Coll. Cardiol. 2014, 63, 407–416. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gary, L. ACSM’s Guidelines for Exercise Testing and Prescription; American College of Sports Medicine (ACSM): Indianapolis, IN, USA, 2021; ISBN 9781975150181. [Google Scholar]
- Hallal, P.C.; Victora, C.G. Reliability and validity of the international physical activity questionnaire (IPAQ). Med. Sci. Sports Exerc. 2004, 36, 556. [Google Scholar] [CrossRef]
- Yabe, H.; Kono, K.; Onoyama, A.; Kiyota, A.; Moriyama, Y.; Okada, K.; Kasuga, H. Predicting a target exercise heart rate that reflects the anaerobic threshold in non beta-blocked hemodialysis patients: The Karvonen and heart rate reserve formulas. Ther. Apher. Dial. 2021, 25, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Goodyear, M.D.E.; Krleza-Jeric, K.; Lemmens, T. The declaration of Helsinki. BMJ 2007, 335, 624–625. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.E14. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.; et al. Recommendations for chamber quantification. Eur. J. Echocardiogr. 2006, 7, 79–108. [Google Scholar] [CrossRef] [PubMed]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Reviewers: This document was reviewed by members of the 2016–2018 EACVI Scientific Documents Committee. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzi, F.; Cameli, M.; Padeletti, M.; Lisi, M.; Zacà, V.; Natali, B.; Malandrino, A.; Alvino, F.; Morelli, M.; Vassallo, G.M.; et al. Characterization of right atrial function and dimension in top-level athletes: A speckle tracking study. Int. J. Cardiovasc. Imaging 2013, 29, 87–94. [Google Scholar] [CrossRef]
- Kurt, M.; Wang, J.; Torre-Amione, G.; Nagueh, S.F. Left atrial function in diastolic heart failure. Circ. Cardiovasc. Imaging 2009, 2, 10–15. [Google Scholar] [CrossRef]
- Guazzi, M.; Arena, R.; Halle, M.; Piepoli, M.F.; Myers, J.; Lavie, C.J. Focused update: Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation 2012, 126, 2261–2274. [Google Scholar] [CrossRef]
- Ney, M.; Haykowsky, M.J.; Vandermeer, B.; Shah, A.; Ow, M.; Tandon, P. Systematic review: Pre- and post-operative prognostic value of cardiopulmonary exercise testing in liver transplant candidates. Aliment. Pharmacol. Ther. 2016, 44, 796–806. [Google Scholar] [CrossRef]
- Guazzi, M.; Bandera, F.; Ozemek, C.; Systrom, D.; Arena, R. Cardiopulmonary exercise testing: What is its value? J. Am. Coll. Cardiol. 2017, 70, 1618–1636. [Google Scholar] [CrossRef]
- Minetti, E.; Klika, R.; Ingletto, C.; Mascherini, G.; Pedrizzetti, G.; Stefani, L. Changes in global longitudinal strain in renal transplant recipients following 12 months of exercise. Intern. Emerg. Med. 2018, 13, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Mascherini, G.; Corsi, M.; Falconi, E.; Cebrián-Ponce, Á.; Checcucci, P.; Pinazzi, A.; Russo, D.; Gitto, S.; Sofi, F.; Stefani, L. Unsupervised Exercise Intervention vs. Adherence to a Mediterranean Diet Alone: The Role of Bioelectrical Impedance Vector Analysis and Cardiovascular Performance in Liver-Transplanted Recipients. Nutrients 2024, 16, 190. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.T.; Guo, Y.K.; Liu, X.; Ren, Y.; Jiang, L.; Xie, L.J.; Gao, Y.; Zhang, Y.; Deng, M.Y.; Li, Y.; et al. Impact of BMI on Left Atrial Strain and Abnormal Atrioventricular Interaction in Patients With Type 2 Diabetes Mellitus: A Cardiac Magnetic Resonance Feature Tracking Study. J. Magn. Reson. Imaging 2022, 55, 1461–1475. [Google Scholar] [CrossRef] [PubMed]
- Chin, J.F.; Aga, Y.S.; Abou Kamar, S.; Snelder, S.M.; Kardys, I.; de Boer, R.A.; Brugts, J.J.; van Dalen, B.M. Exploring risk Indicators of atrial fibrillation in severe Obesity: Left atrial cardiomyopathy and premature atrial contractions. Int. J. Cardiol. Heart Vasc. 2024, 55, 101555. [Google Scholar] [CrossRef]
- Pucci, M.; Gammaldi, V.; Capece, L.M.; Paoletta, D.; Iervolino, A.; Pontoriero, M.; Iacono, M.; Megaro, P.; Esposito, R. Association between Obesity and Atrial Function in Patients with Non-Valvular Atrial Fibrillation: An Echocardiographic Study. J. Clin. Med. 2024, 13, 2895. [Google Scholar] [CrossRef]
- Cameli, M.; Sparla, S.; Losito, M.; Righini, F.M.; Menci, D.; Lisi, M.; D’Ascenzi, F.; Focardi, M.; Favilli, R.; Pierli, C.; et al. Correlation of Left Atrial Strain and Doppler Measurements with Invasive Measurement of Left Ventricular End-Diastolic Pressure in Patients Stratified for Different Values of Ejection Fraction. Echocardiography 2016, 33, 398–405. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzi, F.; Caselli, S.; Solari, M.; Pellicciao, A.; Cameli, M.; Focardi, M.; Padeletti, M.; Corrado, D.; Bonifazi, M.; Mondillo, S. Novel echocardiographic techniques for the evaluation of athletes’ heart: A focus on speckle-tracking echocardiography. Eur. J. Prev. Cardiol. 2016, 23, 437–446. [Google Scholar] [CrossRef]
- Lakatos, B.K.; Molnár, A.Á.; Kiss, O.; Sydó, N.; Tokodi, M.; Solymossi, B.; Fábián, A.; Dohy, Z.; Vágó, H.; Babity, M.; et al. Relationship between Cardiac Remodeling and Exercise Capacity in Elite Athletes: Incremental Value of Left Atrial Morphology and Function Assessed by Three-Dimensional Echocardiography. J. Am. Soc. Echocardiogr. 2020, 33, 101–109.e1. [Google Scholar] [CrossRef] [PubMed]
| OTR (n = 54) | HS (n = 44) | p | |
|---|---|---|---|
| Age (y) | 59.43 ± 8.75 | 36.52 ± 12.24 | <0.001 |
| BMI (kg/m2) | 25.79 ± 2.92 | 22.25 ± 2.45 | <0.001 |
| IPAQ (METs/week) | 1053.87 ± 1024.30 | 1974.45 ± 1438.87 | 0.02 |
| VO2 max (mL/kg/min) | 22.90 ± 7.30 | 38.87 ± 28.65 | 0.01 |
| VO2 max (%) | 85.3 ± 23.62 | 91.74 ± 12.43 | 0.02 |
| IVS (mm) | 9.81 ± 1.1 | 9.49 ± 0.94 | Ns |
| PW (mm) | 9.61 ± 1.22 | 9.30 ± 0.86 | Ns |
| LVEDD (mm) | 50.52 ± 4.60 | 49.31 ± 3.61 | Ns |
| EF (%) | 64.39 ± 5.37 | 67.09 ± 6.44 | 0.05 |
| LVMI (gr/m2) | 100 ± 21.4 | 87.62 ± 14 | Ns |
| E/A | 1.01 ± 0.44 | 1.96 ± 0.74 | <0.001 |
| IVRT (ms) | 85.67 ± 20.26 | 71.83 ± 26.60 | 0.01 |
| DTc (ms) | 219.15 ± 79.00 | 195.86 ± 38.47 | Ns |
| E/E’ sep | 9.93 ± 3.06 | 6.91 ± 1.31 | <0.001 |
| E/E’ lat | 7.63 ± 4.66 | 5.11 ± 1.34 | 0.02 |
| E/E’ med | 8.52 ± 3.32 | 5.92 ± 1.5 | 0.03 |
| iLAV (mL) | 24.32 ± 8.65 | 27.22 ± 11.25 | Ns |
| TR vel (m/s) | 2.1 ± 0.4 | 1.9 ± 0.6 | Ns |
| 4C PALS (%) | 33.45 ± 9.57 | 45.36 ± 14,19 | <0.001 |
| 4C PACS (%) | 15.88 ± 6.80 | 11.95 ± 7.48 | 0.003 |
| 2C PALS (%) | 35.45 ± 11.26 | 47.55 ± 14.97 | <0.001 |
| 2C PACS (%) | 17.29 ± 5.09 | 13.16 ± 6.79 | 0.001 |
| 4C PALS | p | 4C PACS | p | 2C PALS | p | 2C PACS | p | |
|---|---|---|---|---|---|---|---|---|
| Age | −0.436 ** | 0.000 | 0.224 * | 0.029 | −0.496 ** | 0.000 | 0.338 ** | 0.003 |
| BMI | −0.406 ** | 0.000 | 0.086 | 0.410 | −0.276 * | 0.013 | 0.188 | 0.101 |
| IVS | −0.009 | 0.933 | 0.035 | 0.737 | 0.000 | 0.999 | 0.132 | 0.254 |
| PW | 0.113 | 0.266 | 0.228 * | 0.026 | 0.097 | 0.394 | 0.187 | 0.103 |
| LVDD | 0.076 | 0.458 | 0.174 | 0.092 | 0.101 | 0.374 | 0.141 | 0.221 |
| EF | 0.184 | 0.069 | 0.025 | 0.808 | 0.080 | 0.478 | 0.118 | 0.305 |
| E | 0.040 | 0.696 | −0.190 | 0.066 | 0.287 ** | 0.010 | −0.198 | 0.085 |
| A | −0.463 ** | 0.000 | 0.286 ** | 0.005 | −0.362 ** | 0.001 | 0.295 ** | 0.009 |
| E/A | 0.378 ** | 0.000 | −0.315 ** | 0.002 | 0.415 ** | 0.000 | −0.346 ** | 0.002 |
| IVRT | −0.291 ** | 0.004 | 0.062 | 0.552 | −0.091 | 0.421 | 0.178 | 0.122 |
| DTc | 0.033 | 0.747 | 0.149 | 0.152 | −0.016 | 0.885 | 0.171 | 0.136 |
| E’ sep | 0.267 ** | 0.008 | −0.245 * | 0.017 | 0.396 ** | 0.000 | −0.376 ** | 0.001 |
| E/E’ sep | −0.355 ** | 0.000 | 0.136 | 0.194 | −0.336 ** | 0.002 | 0.227 * | 0.048 |
| E’ lat | 0.206 | 0.169 | −0.059 | 0.702 | 0.354 * | 0.027 | 0.055 | 0.740 |
| E/E’ lat | −0.574 ** | 0.000 | −0.097 | 0.542 | −0.253 | 0.143 | 0.130 | 0.456 |
| iLAV | −0.028 | 0.786 | −0.076 | 0.466 | 0.155 | 0.172 | 0.057 | 0.622 |
| TR vel | −0.008 | 0.954 | 0.035 | 0.863 | 0.134 | 0.261 | 0.123 | 0.214 |
| 4C PALS | 1.000 | 0.170 | 0.100 | 0.446 ** | 0.000 | −0.094 | 0.416 | |
| 4C PACS | 0.170 | 0.100 | 10.000 | −0.066 | 0.561 | 0.527 ** | 0.000 | |
| 2C PALS | 0.446 ** | 0.000 | −0.066 | 0.561 | 10.000 | 0.183 | 0.111 | |
| 2C PACS | −0.094 | 0.416 | 0.527 ** | 0.000 | 0.183 | 0.111 | 10.000 |
| OTR | HS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 4C PALS | 4C PACS | 2C PALS | 2C PACS | 4C PALS | 4C PACS | 2C PALS | 2C PACS | ||
| Age | R | 0.000 | −0.025 | −0.233 | −0.112 | −0.347 * | 0.108 | −0.213 | 0.308 |
| BMI | R | −0.243 | −0.133 | −0.159 | −0.312 * | −0.202 | 0.092 | −0.130 | 0.315 |
| IVS | R | 0.056 | 0.148 | 0.187 | 0.058 | 0.058 | −0.161 | −0.107 | 0.181 |
| PW | R | 0.048 | 0.227 | 0.236 | 0.143 | 0.311 * | 0.154 | −0.011 | 0.176 |
| LVEDD | R | 0.201 | 0.246 | 0.126 | 0.013 | 0.070 | 0.121 | 0.127 | 0.293 |
| EF | R | 0.221 | −0.024 | −0.122 | 0.148 | −0.009 | 0.220 | 0.127 | 0.257 |
| E/A | R | −0.057 | −0.165 | −0.002 | −0.207 | 0.406 ** | 0.032 | 0.375 * | −0.006 |
| IVRT | R | −0.090 | 0.004 | 0.015 | −0.009 | −0.247 | −0.076 | 0.017 | 0.212 |
| DTc | R | 0.084 | 0.106 | 0.145 | 0.221 | 0.125 | 0.017 | 0.051 | −0.067 |
| E/E’ sep | R | −0.119 | −0.148 | −0.169 | 0.020 | −0.081 | 0.027 | 0.190 | 0.138 |
| E/E’ lat | R | −0.459 | −0.262 | −0.159 | −0.090 | −0.414 * | −0.105 | 0.060 | 0.038 |
| iLAV | R | −0.263 | −0.071 | 0.130 | 0.118 | 0.088 | 0.019 | 0.131 | 0.141 |
| TR vel | R | −0.543 | −0.075 | 0.154 | 0.126 | 0.021 | 0.035 | 0.125 | 0.165 |
| 4C PALS | R | 1.000 | 0.488 ** | 0.215 | 0.376 * | 1.000 | 0.183 | 0.338 * | −0.174 |
| 4C PACS | R | 0.488 ** | 1.000 | 0.128 | 0.398 * | 0.183 | 1.000 | 0.138 | 0.433 ** |
| 2C PALS | R | 0.215 | 0.128 | 1.000 | 0.629 ** | 0.338 * | 0.138 | 1.000 | 0.248 |
| 2C PACS | R | 0.376 * | 0.398 * | 0.629 ** | 1.000 | −0.174 | 0.433 ** | 0.248 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlandi, M.; Corsi, M.; Bini, V.; Palazzo, R.; Gitto, S.; Fiorillo, C.; Becatti, M.; Maglione, M.; Stefani, L. A First Diastolic Function Evaluation in the Personalized Exercise Prescription Program for Solid Organs Transplanted Subjects: Is Atrial Strain Useful? J. Pers. Med. 2025, 15, 32. https://doi.org/10.3390/jpm15010032
Orlandi M, Corsi M, Bini V, Palazzo R, Gitto S, Fiorillo C, Becatti M, Maglione M, Stefani L. A First Diastolic Function Evaluation in the Personalized Exercise Prescription Program for Solid Organs Transplanted Subjects: Is Atrial Strain Useful? Journal of Personalized Medicine. 2025; 15(1):32. https://doi.org/10.3390/jpm15010032
Chicago/Turabian StyleOrlandi, Melissa, Marco Corsi, Vittorio Bini, Roberto Palazzo, Stefano Gitto, Claudia Fiorillo, Matteo Becatti, Marco Maglione, and Laura Stefani. 2025. "A First Diastolic Function Evaluation in the Personalized Exercise Prescription Program for Solid Organs Transplanted Subjects: Is Atrial Strain Useful?" Journal of Personalized Medicine 15, no. 1: 32. https://doi.org/10.3390/jpm15010032
APA StyleOrlandi, M., Corsi, M., Bini, V., Palazzo, R., Gitto, S., Fiorillo, C., Becatti, M., Maglione, M., & Stefani, L. (2025). A First Diastolic Function Evaluation in the Personalized Exercise Prescription Program for Solid Organs Transplanted Subjects: Is Atrial Strain Useful? Journal of Personalized Medicine, 15(1), 32. https://doi.org/10.3390/jpm15010032









