Intravascular Laser Blood Irradiation (ILIB) Enhances Antioxidant Activity and Energy Metabolism in Aging Ovaries
Abstract
1. Introduction
2. Materials and Methods
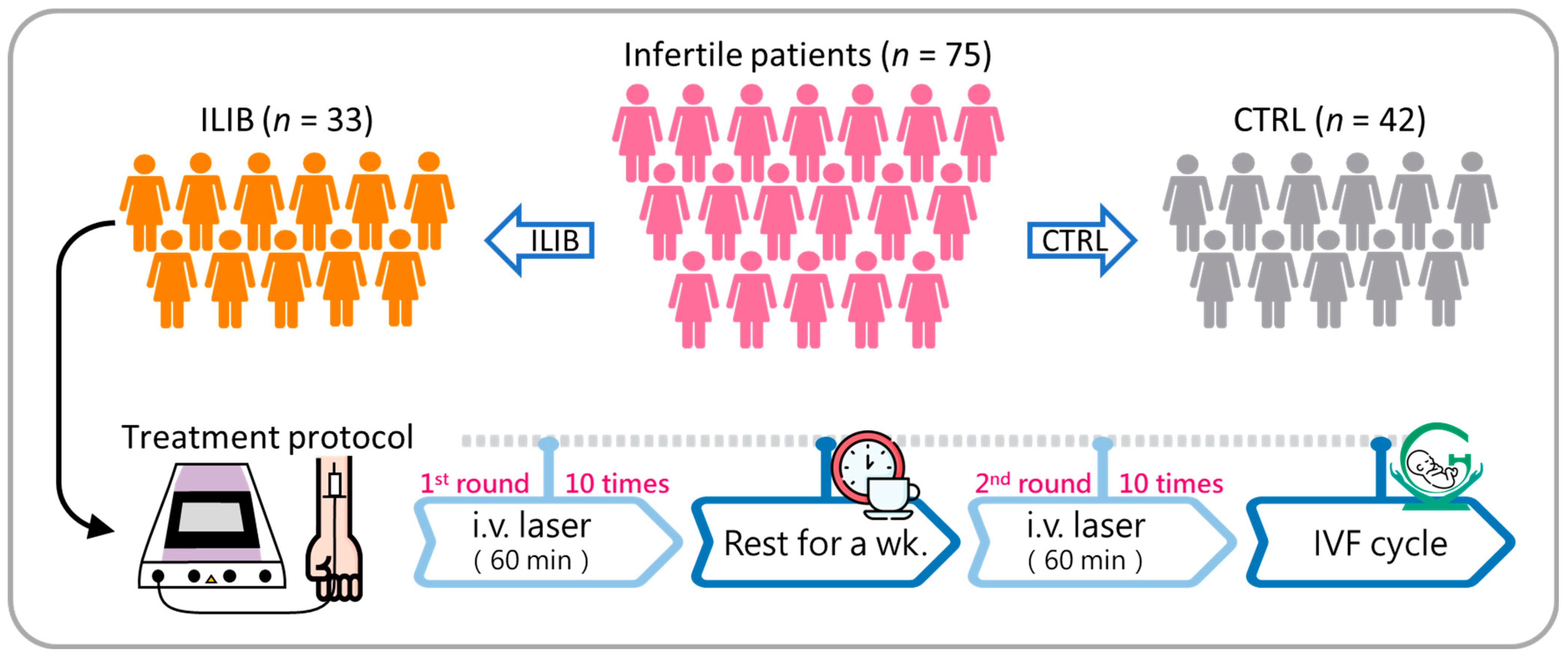
2.1. Patients and Design
2.2. Treatment Protocol
2.3. Collection of Patients’ Cumulus Cells
2.4. Ribonucleic Acid (RNA) Extraction and Real-Time Polymerase Chain Reaction (PCR)
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of Infertile Patients
3.2. ILIB Treatment Improved Oxeiptosis of Aging Ovarian Cells
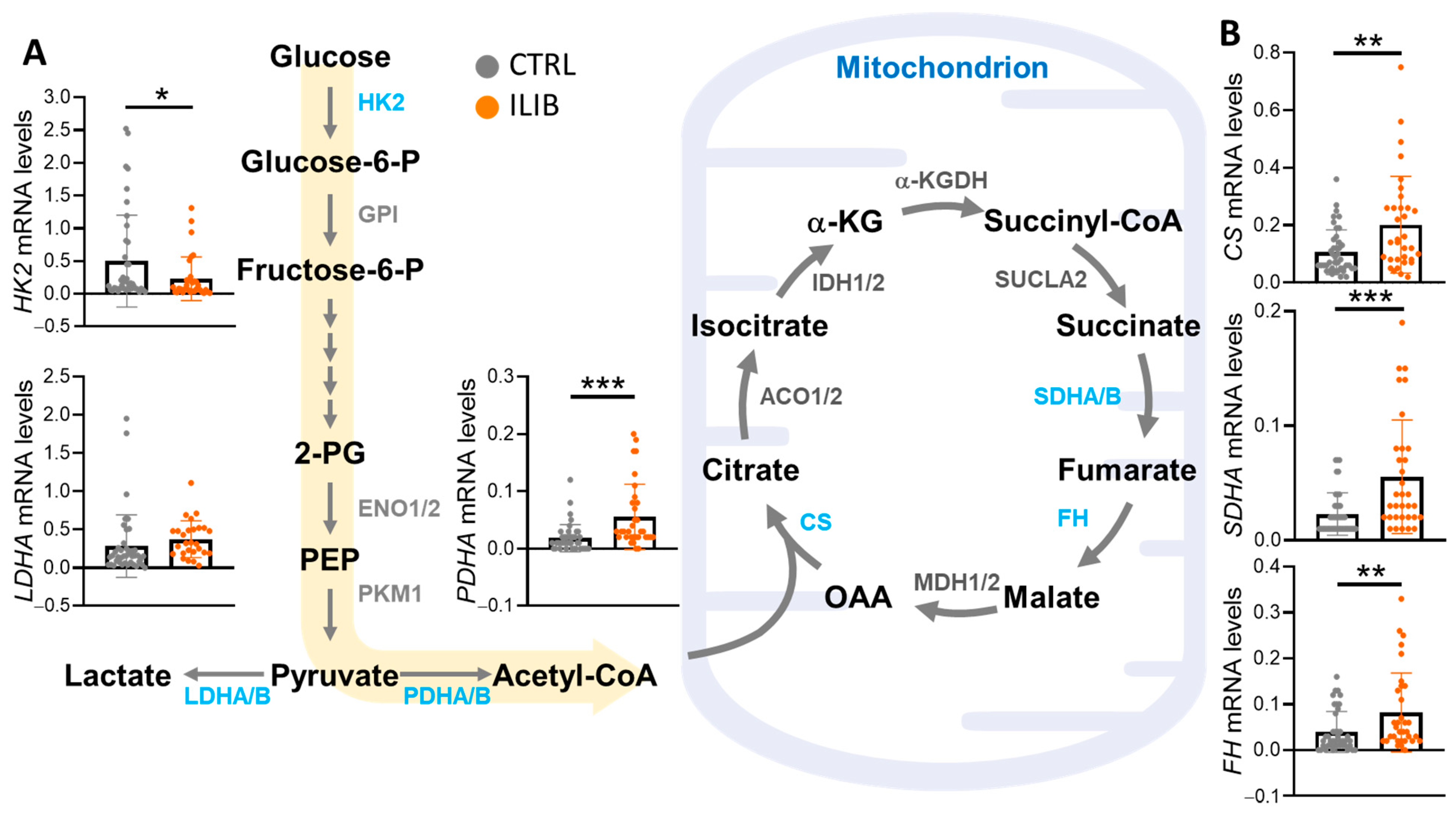
3.3. ILIB Altered the Energy Metabolism Reprogramming of Human Cumulus Cells
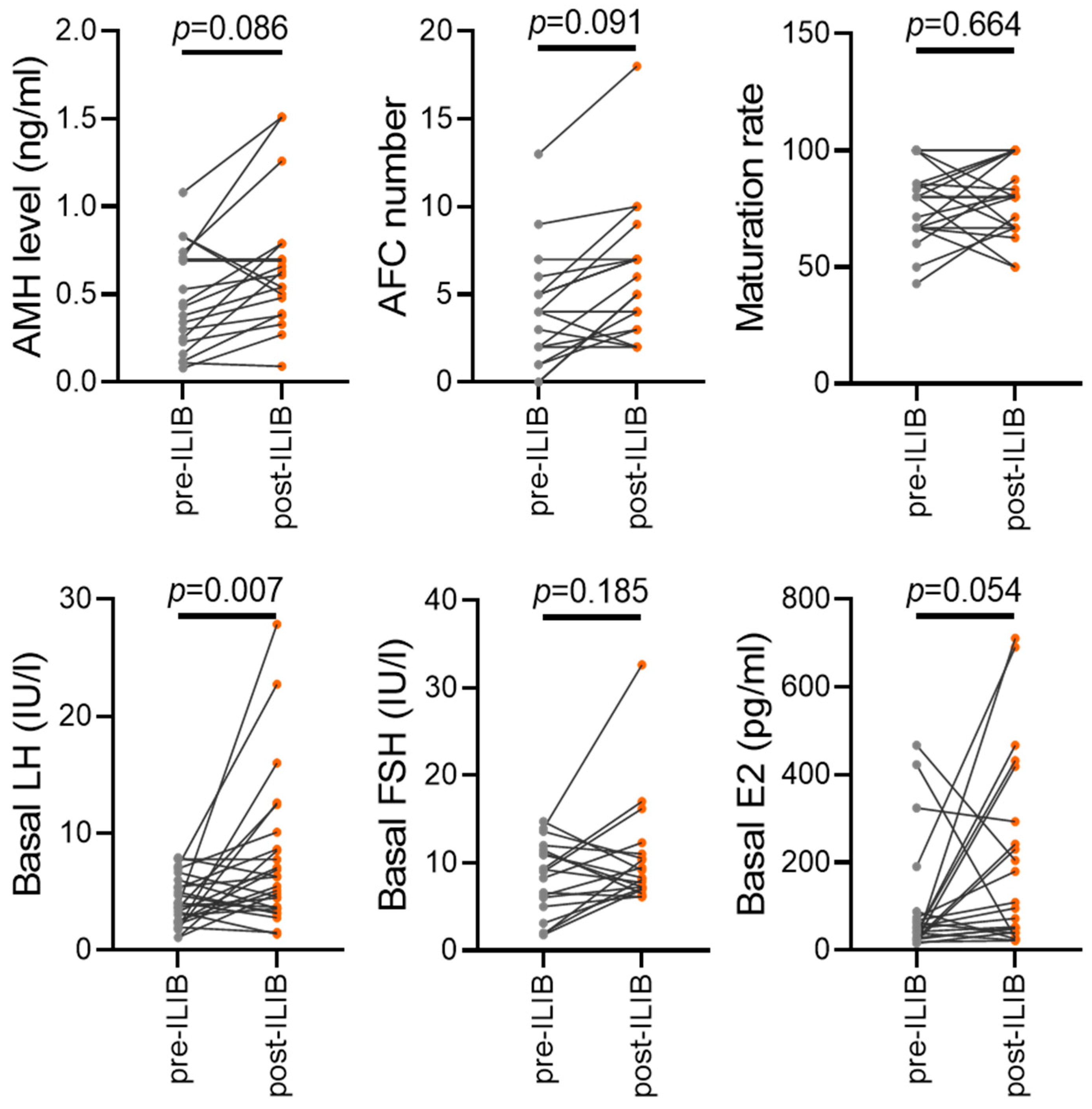
3.4. Demographic and Clinical Characteristics of Patients before and after ILIB Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society for Reproductive Endocrinology and Infertility. Optimizing natural fertility: A committee opinion. Fertil. Steril. 2017, 107, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Broekmans, F.J.; Soules, M.R.; Fauser, B.C. Ovarian aging: Mechanisms and clinical consequences. Endocr. Rev. 2009, 30, 465–493. [Google Scholar] [CrossRef] [PubMed]
- Gruhn, J.R.; Zielinska, A.P.; Shukla, V.; Blanshard, R.; Capalbo, A.; Cimadomo, D.; Nikiforov, D.; Chan, A.C.; Newnham, L.J.; Vogel, I.; et al. Chromosome errors in human eggs shape natural fertility over reproductive life span. Science 2019, 365, 1466–1469. [Google Scholar] [CrossRef] [PubMed]
- Charalambous, C.; Webster, A.; Schuh, M. Aneuploidy in mammalian oocytes and the impact of maternal ageing. Nat. Rev. Mol. Cell Biol. 2023, 24, 27–44. [Google Scholar] [CrossRef]
- Li, C.J.; Lin, L.T.; Tsai, H.W.; Chern, C.U.; Wen, Z.H.; Wang, P.H.; Tsui, K.H. The Molecular Regulation in the Pathophysiology in Ovarian Aging. Aging Dis. 2021, 12, 934–949. [Google Scholar] [CrossRef] [PubMed]
- Secomandi, L.; Borghesan, M.; Velarde, M.; Demaria, M. The role of cellular senescence in female reproductive aging and the potential for senotherapeutic interventions. Hum. Reprod. Update 2022, 28, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, L.; Xiang, W. Mechanisms of ovarian aging in women: A review. J. Ovarian Res. 2023, 16, 67. [Google Scholar] [CrossRef]
- Park, S.U.; Walsh, L.; Berkowitz, K.M. Mechanisms of ovarian aging. Reproduction 2021, 162, R19–R33. [Google Scholar] [CrossRef] [PubMed]
- May-Panloup, P.; Boucret, L.; Chao de la Barca, J.M.; Desquiret-Dumas, V.; Ferré-L’Hotellier, V.; Morinière, C.; Descamps, P.; Procaccio, V.; Reynier, P. Ovarian ageing: The role of mitochondria in oocytes and follicles. Hum. Reprod. Update 2016, 22, 725–743. [Google Scholar] [CrossRef]
- Van Blerkom, J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion 2011, 11, 797–813. [Google Scholar] [CrossRef]
- Tesarik, J.; Mendoza-Tesarik, R. Mitochondria in Human Fertility and Infertility. Int. J. Mol. Sci. 2023, 24, 8950. [Google Scholar] [CrossRef]
- Alberico, H.C.; Woods, D.C. Role of Granulosa Cells in the Aging Ovarian Landscape: A Focus on Mitochondrial and Metabolic Function. Front. Physiol. 2021, 12, 800739. [Google Scholar] [CrossRef]
- Chiang, J.L.; Shukla, P.; Pagidas, K.; Ahmed, N.S.; Karri, S.; Gunn, D.D.; Hurd, W.W.; Singh, K.K. Mitochondria in Ovarian Aging and Reproductive Longevity. Ageing Res. Rev. 2020, 63, 101168. [Google Scholar] [CrossRef] [PubMed]
- Esteves, S.C.; Yarali, H.; Vuong, L.N.; Carvalho, J.F.; Ozbek, I.Y.; Polat, M.; Le, H.L.; Pham, T.D.; Ho, T.M.; Humaidan, P.; et al. Cumulative delivery rate per aspiration IVF/ICSI cycle in POSEIDON patients: A real-world evidence study of 9073 patients. Hum. Reprod. 2021, 36, 2157–2169. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, A.; Mawal, N. The risk of poor ovarian response during repeat IVF. Reprod. Biomed. Online 2021, 42, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Irani, M.; Reichman, D.; Robles, A.; Melnick, A.; Davis, O.; Zaninovic, N.; Xu, K.; Rosenwaks, Z. Morphologic grading of euploid blastocysts influences implantation and ongoing pregnancy rates. Fertil. Steril. 2017, 107, 664–670. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; Romero, A.; Manucha, W.; Tan, D.X.; Zuccari, D.; Chuffa, L.G.A. Aging-Related Ovarian Failure and Infertility: Melatonin to the Rescue. Antioxidants 2023, 12, 695. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Zhao, Q.; Li, Y.; Zheng, Z.; Kong, X.; Shu, C.; Liu, Y.; Shi, Y. The role of oxidative stress in ovarian aging: A review. J. Ovarian Res. 2022, 15, 100. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, S.Y.; Kim, E.Y.; Kim, K.H.; Koong, M.K.; Lee, K.A. The Antioxidant Auraptene Improves Aged Oocyte Quality and Embryo Development in Mice. Antioxidants 2022, 12, 87. [Google Scholar] [CrossRef]
- Fu, J.C.; Wang, N.K.; Cheng, Y.Y.; Chang, S.T. The Adjuvant Therapy of Intravenous Laser Irradiation of Blood (ILIB) on Pain and Sleep Disturbance of Musculoskeletal Disorders. J. Pers. Med. 2022, 12, 1333. [Google Scholar] [CrossRef]
- Schapochnik, A.; Alonso, P.T.; de Souza, V.; Rodrigues, V.; Quintela, K.; Cruz, M.D.P.; Ferreira, C.M.; Cecatto, R.B.; Rodrigues, M.; Hamblin, M.R.; et al. Intravascular laser irradiation of blood (ILIB) used to treat lung diseases: A short critical review. Lasers Med. Sci. 2023, 38, 93. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.F.; Tsai, Y.A.; Wu, S.B.; Wei, Y.H.; Tsai, P.Y.; Chuang, T.Y. Effects of intravascular laser irradiation of blood in mitochondria dysfunction and oxidative stress in adults with chronic spinal cord injury. Photomed. Laser Surg. 2012, 30, 579–586. [Google Scholar] [CrossRef]
- Li, C.J.; Lin, L.T.; Tsai, H.W.; Wen, Z.H.; Tsui, K.H. Phosphoglycerate mutase family member 5 maintains oocyte quality via mitochondrial dynamic rearrangement during aging. Aging Cell 2022, 21, e13546. [Google Scholar] [CrossRef]
- Tsui, K.H.; Li, C.J. Mitoquinone shifts energy metabolism to reduce ROS-induced oxeiptosis in female granulosa cells and mouse oocytes. Aging 2023, 15, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Su, W.P.; Li, C.J.; Lin, L.T.; Lin, P.H.; Wen, Z.H.; Sheu, J.J.; Tsui, K.H. Boosting mitochondrial function and metabolism in aging female germ cells with dual ROCK/ROS inhibition. Biomed. Pharmacother. 2023, 163, 114888. [Google Scholar] [CrossRef]
- Yang, W.H.; Lin, S.P.; Chang, S.T. Case report: Rapid improvement of crossed cerebellar diaschisis after intravascular laser irradiation of blood in a case of stroke. Medicine 2017, 96, e5646. [Google Scholar] [CrossRef] [PubMed]
- Tome, R.F.F.; Silva, D.F.B.; Dos Santos, C.A.O.; de Vasconcelos Neves, G.; Rolim, A.K.A.; de Castro Gomes, D.Q. ILIB (intravascular laser irradiation of blood) as an adjuvant therapy in the treatment of patients with chronic systemic diseases-an integrative literature review. Lasers Med. Sci. 2020, 35, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, A.; Mirmiranpor, H.; Khandani, S.; Sobhani, S.O.; Shafaee, Y. Intravenous Laser Wavelength Irradiation Effect on Interleukins: IL-1α, IL-1β, IL6 in Diabetic Rats. Laser Ther. 2019, 28, 267–273. [Google Scholar]
- Momenzadeh, S.; Abbasi, M.; Ebadifar, A.; Aryani, M.; Bayrami, J.; Nematollahi, F. The intravenous laser blood irradiation in chronic pain and fibromyalgia. J. Lasers Med. Sci. 2015, 6, 6–9. [Google Scholar]

| Parameters | CTRL (n = 42) | ILIB (n = 33) |
|---|---|---|
| Age (years) | 38.9 ± 3.2 | 39.8 ± 3.4 |
| BMI (kg/m2) | 23.3 ± 4.5 | 23.1 ± 4.3 |
| Duration of infertility (years) | 4.2 ± 3.3 | 4.9 ± 3.2 |
| Previous IVF failure (n) | 1.4 ± 2.3 | 1.8 ± 1.2 |
| Types of infertility n (%) | ||
| Primary infertility | 20/42 (47.6%) | 15/33 (45.5%) |
| Secondary infertility | 23/43 (53.4%) | 18/33 (54.5%) |
| Basal FSH (IU/L) | 9.8 ± 7.5 | 10.9 ± 6.7 |
| Basal E2 (pg/mL) | 112.8 ± 148.8 | 266.5 ± 394.6 |
| Basal LH (IU/L) | 8.4 ± 13.6 | 7.6 ± 6.0 |
| Parameters | CTRL (n = 42) | ILIB (n = 33) |
|---|---|---|
| Stimulation duration (days) | 10.6 ± 2.3 | 10.3 ± 2.8 |
| No. of oocytes retrieved (n) | 5.6 ± 4.2 | 4.7 ± 3.9 |
| No. of metaphase II oocytes (n) | 4.3 ± 4.2 | 3.8 ± 3.1 |
| Maturation rate (%) | 80.7 ± 3.6 | 84.7 ± 3.8 |
| No. of fertilized oocytes (n) | 3.6 ± 3.5 | 3.0 ± 2.7 |
| Fertilization rate (%) | 84.3 ± 3.7 | 78.8 ± 79.6 |
| No. of Day 3 embryos (n) | 3.3 ± 2.8 | 2.9 ± 2.7 |
| No. of top-quality D3 embryos (n) | 3.3 ± 2.8 | 2.9 ± 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.-T.; Li, C.-J.; Chern, C.-U.; Lin, P.-H.; Lin, P.-W.; Chen, Y.-C.; Tsai, H.-W.; Tsui, K.-H. Intravascular Laser Blood Irradiation (ILIB) Enhances Antioxidant Activity and Energy Metabolism in Aging Ovaries. J. Pers. Med. 2024, 14, 551. https://doi.org/10.3390/jpm14060551
Lin L-T, Li C-J, Chern C-U, Lin P-H, Lin P-W, Chen Y-C, Tsai H-W, Tsui K-H. Intravascular Laser Blood Irradiation (ILIB) Enhances Antioxidant Activity and Energy Metabolism in Aging Ovaries. Journal of Personalized Medicine. 2024; 14(6):551. https://doi.org/10.3390/jpm14060551
Chicago/Turabian StyleLin, Li-Te, Chia-Jung Li, Chyi-Uei Chern, Pei-Hsuan Lin, Po-Wen Lin, Yu-Chen Chen, Hsiao-Wen Tsai, and Kuan-Hao Tsui. 2024. "Intravascular Laser Blood Irradiation (ILIB) Enhances Antioxidant Activity and Energy Metabolism in Aging Ovaries" Journal of Personalized Medicine 14, no. 6: 551. https://doi.org/10.3390/jpm14060551
APA StyleLin, L.-T., Li, C.-J., Chern, C.-U., Lin, P.-H., Lin, P.-W., Chen, Y.-C., Tsai, H.-W., & Tsui, K.-H. (2024). Intravascular Laser Blood Irradiation (ILIB) Enhances Antioxidant Activity and Energy Metabolism in Aging Ovaries. Journal of Personalized Medicine, 14(6), 551. https://doi.org/10.3390/jpm14060551








