Exploring Costimulatory Blockade-Based Immunologic Strategies in Transplantation: Are They a Promising Immunomodulatory Approach for Organ and Vascularized Composite Allotransplantation?
Abstract
1. Introduction
2. Materials and Methods
3. Immunologic Strategies in VCA
3.1. T Cell Activation
3.2. Targeting Costimulatory Pathways in Transplant Patients
3.3. The Immunoglobulin Superfamily
3.3.1. The B7 Family
3.3.2. The ICOS-B7h Pathway
3.3.3. The Programmed Death-1 (PD-1)/PD-1 Ligands
3.4. TNF Family Members
3.4.1. CD40/CD40L(CD154) Pathway
3.4.2. The OX40 and OX40L Pathway
3.4.3. GITR/GITRL Pathway
3.4.4. 4-1BB/4-1BBL Pathway
3.5. TIM Family
3.5.1. TIM-1 Signaling Pathways
3.5.2. TIM 3 Signaling Pathways
3.6. Adhesion Molecule Blockade
3.6.1. LFA-1 Pathway
3.6.2. LFA-3 Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, S.Y.; Merchant, J. Joseph Murray (1919–2012): First transplant surgeon. Singap. Med. J. 2019, 60, 162–163. [Google Scholar] [CrossRef] [PubMed]
- Bezinover, D.; Saner, F. Organ transplantation in the modern era. BMC Anesthesiol. 2019, 19, 32. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Siso, J.R.; Bueno, E.M.; Sisk, G.C.; Marty, F.M.; Pomahac, B.; Tullius, S.G. Vascularized composite tissue allotransplantation-state of the art. Clin. Transplant. 2013, 27, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Ton, C.; Salehi, S.; Abasi, S.; Aggas, J.R.; Liu, R.; Brandacher, G.; Guiseppi-Elie, A.; Grayson, W.L. Methods of ex vivo analysis of tissue status in vascularized composite allografts. J. Transl. Med. 2023, 21, 609. [Google Scholar] [CrossRef]
- Shores, J.T.; Malek, V.; Lee, W.P.A.; Brandacher, G. Outcomes after hand and upper extremity transplantation. J. Mater. Sci. Mater. Med. 2017, 28, 72. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S. Vascularised composite allotransplants: Transplant of upper extremities and face. Indian J. Plast. Surgery 2015, 48, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Sisk, G.; Carty, M.; Sampson, C.; Blazar, P.; Dyer, G.; Earp, B.; Pribaz, J.; Pomahac, B.; Talbot, S.G. Functional Outcomes after Bilateral Hand Transplantation: A 3.5-Year Comprehensive Follow-Up. Plast. Reconstr. Surg. 2016, 137, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Jonczyk, M.M.; Tratnig-Frankl, P.; Cetrulo, C.L., Jr. Genitourinary vascularized composite allotransplantation: A review of penile transplantation. Curr. Opin. Organ. Transplant. 2019, 24, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, S.; Petruzzo, P.; Morelon, E.; Hautz, T.; Kanitakis, J.; Weissenbacher, A.; Messner, F.; Bernardon, L.; Seulin, C.; Berchtold, V.; et al. 20-Year Follow-up of Two Cases of Bilateral Hand Transplantation. N. Engl. J. Med. 2020, 383, 1791–1792. [Google Scholar] [CrossRef]
- Kantar, R.S.; Ceradini, D.J.; Gelb, B.E.; Levine, J.P.; Staffenberg, D.A.; Saadeh, P.B.; Flores, R.L.; Sweeney, N.G.; Bernstein, G.L.; Rodriguez, E.D. Facial Transplantation for an Irreparable Central and Lower Face Injury: A Modernized Approach to a Classic Challenge. Plast. Reconstr. Surg. 2019, 144, 264e–283e. [Google Scholar] [CrossRef]
- Lewis, H.C.; Cendales, L.C. Vascularized composite allotransplantation in the United States: A retrospective analysis of the Organ Procurement and Transplantation Network data after 5 years of the Final Rule. Am. J. Transplant. 2021, 21, 291–296. [Google Scholar] [CrossRef]
- Cherikh, W.S.; Cendales, L.C.; Wholley, C.L.; Wainright, J.; Gorantla, V.S.; Klassen, D.K.; McDiarmid, S.V.; Scott Levin, L. Vascularized composite allotransplantation in the United States: A descriptive analysis of the Organ Procurement and Transplantation Network Data. Am. J. Transplant. 2019, 19, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Eun, S.C. Therapeutic application of T regulatory cells in composite tissue allotransplantation. J. Transl. Med. 2017, 15, 218. [Google Scholar] [CrossRef] [PubMed]
- Siemionow, M.Z.; Kulahci, Y.; Bozkurt, M. Composite tissue allotransplantation. Plast. Reconstr. Surg. 2009, 124 (Suppl. S6), e327–e339. [Google Scholar] [CrossRef] [PubMed]
- Iske, J.; Nian, Y.; Maenosono, R.; Maurer, M.; Sauer, I.M.; Tullius, S.G. Composite tissue allotransplantation: Opportunities and challenges. Cell Mol. Immunol. 2019, 16, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Johnson, A.C.; Colakoglu, S.; Huang, C.A.; Mathes, D.W. Clinical and preclinical tolerance protocols for vascularized composite allograft transplantation. Arch. Plast. Surg. 2021, 48, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Issa, F. Vascularized composite allograft-specific characteristics of immune responses. Transpl. Int. 2016, 29, 672–681. [Google Scholar] [CrossRef]
- Eun, S.C. Composite Tissue Allotransplantation Immunology. Arch. Plast. Surg. 2013, 40, 141–153. [Google Scholar] [CrossRef]
- Kueckelhaus, M.; Fischer, S.; Seyda, M.; Bueno, E.M.; Aycart, M.A.; Alhefzi, M.; ElKhal, A.; Pomahac, B.; Tullius, S.G. Vascularized composite allotransplantation: Current standards and novel approaches to prevent acute rejection and chronic allograft deterioration. Transpl. Int. 2016, 29, 655–662. [Google Scholar] [CrossRef]
- Lellouch, A.G.; Ng, Z.Y.; Rosales, I.A.; Schol, I.M.; Leonard, D.A.; Gama, A.R.; Colvin, R.B.; Lantieri, L.A.; Randolph, M.A.; Cetrulo, C.L., Jr. Toward Development of the Delayed Tolerance Induction Protocol for Vascularized Composite Allografts in Nonhuman Primates. Plast. Reconstr. Surg. 2020, 145, 757e–768e. [Google Scholar] [CrossRef]
- Ezekian, B.; Schroder, P.M.; Freischlag, K.; Yoon, J.; Kwun, J.; Knechtle, S.J. Contemporary Strategies and Barriers to Transplantation Tolerance. Transplantation 2018, 102, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Orlando, G.; Hematti, P.; Stratta, R.J.; Burke, G.W., 3rd; Di Cocco, P.; Pisani, F.; Soker, S.; Wood, K. Clinical operational tolerance after renal transplantation: Current status and future challenges. Ann. Surg. 2010, 252, 915–928, Erratum in Ann Surg. 2011, 253, 1236. [Google Scholar] [CrossRef] [PubMed]
- Sachs, D.H. Transplant tolerance: Bench to bedside—26th annual Samuel Jason Mixter Lecture. Arch. Surg. 2011, 146, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Moorman, C.D.; Sohn, S.J.; Phee, H. Emerging Therapeutics for Immune Tolerance: Tolerogenic Vaccines, T cell Therapy, and IL-2 Therapy. Front. Immunol. 2021, 12, 657768. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Layhadi, J.A.; Keane, S.T.; Cartwright, N.J.K.; Durham, S.R.; Shamji, M.H. Immunological mechanisms of tolerance: Central, peripheral and the role of T and B cells. Asia Pac. Allergy 2023, 13, 175–186. [Google Scholar] [CrossRef]
- Ding, M.; He, Y.; Zhang, S.; Guo, W. Recent Advances in Costimulatory Blockade to Induce Immune Tolerance in Liver Transplantation. Front. Immunol. 2021, 12, 537079. [Google Scholar] [CrossRef] [PubMed]
- Priyadharshini, B.; Greiner, D.L.; Brehm, M.A. T-cell activation and transplantation tolerance. Transplant. Rev. 2012, 26, 212–222. [Google Scholar] [CrossRef]
- Li, Q.; Lan, P. Activation of immune signals during organ transplantation. Signal Transduct. Target. Ther. 2023, 8, 110. [Google Scholar] [CrossRef]
- Ingulli, E. Mechanism of cellular rejection in transplantation. Pediatr. Nephrol. 2010, 25, 61–74. [Google Scholar] [CrossRef]
- Siemionow, M.; Klimczak, A. Basics of immune responses in transplantation in preparation for application of composite tissue allografts in plastic and reconstructive surgery: Part I. Plast. Reconstr. Surg. 2008, 121, 4e–12e. [Google Scholar] [CrossRef]
- Gutcher, I.; Becher, B. APC-derived cytokines and T cell polarization in autoimmune inflammation. J. Clin. Investig. 2007, 117, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Karahan, G.E.; Claas, F.H.J.; Heidt, S. Pre-existing Alloreactive T and B Cells and Their Possible Relevance for Pre-transplant Risk Estimation in Kidney Transplant Recipients. Front. Med. 2020, 7, 340. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Su, Y.; Jiao, A.; Wang, X.; Zhang, B. T cells in health and disease. Signal Transduct. Target. Ther. 2023, 8, 235. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.Y.; Grimmig, T.; Sayegh, M.H. Costimulation Blockade in Transplantation. Adv. Exp. Med. Biol. 2019, 1189, 267–312. [Google Scholar] [CrossRef] [PubMed]
- Pilat, N.; Sayegh, M.H.; Wekerle, T. Costimulatory pathways in transplantation. Semin. Immunol. 2011, 23, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, M.R.; Sayegh, M.H. T-cell costimulatory pathways in allograft rejection and tolerance. Transplantation 2005, 80, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Kauke, M.; Safi, A.F.; Panayi, A.C.; Palmer, W.J.; Haug, V.; Kollar, B.; Nelms, L.; Tchiloemba, B.; Pomahac, B. A systematic review of immunomodulatory strategies used in skin-containing preclinical vascularized composite allotransplant models. J. Plast. Reconstr. Aesthet. Surg. 2022, 75, 586–604. [Google Scholar] [CrossRef]
- Ville, S.; Poirier, N.; Branchereau, J.; Charpy, V.; Pengam, S.; Nerriere-Daguin, V.; Le Bas-Bernardet, S.; Coulon, F.; Mary, C.; Chenouard, A.; et al. Anti-CD28 Antibody and Belatacept Exert Differential Effects on Mechanisms of Renal Allograft Rejection. J. Am. Soc. Nephrol. 2016, 27, 3577–3588. [Google Scholar] [CrossRef]
- Vanhove, B.; Poirier, N.; Soulillou, J.P.; Blancho, G. Selective Costimulation Blockade with Antagonist Anti-CD28 Therapeutics in Transplantation. Transplantation 2019, 103, 1783–1789. [Google Scholar] [CrossRef]
- Leibler, C.; Thiolat, A.; Elsner, R.A.; El Karoui, K.; Samson, C.; Grimbert, P. Costimulatory blockade molecules and B-cell-mediated immune response: Current knowledge and perspectives. Kidney Int. 2019, 95, 774–786. [Google Scholar] [CrossRef]
- Kitchens, W.H.; Larsen, C.P.; Badell, I.R. Costimulatory Blockade and Solid Organ Transplantation: The Past, Present, and Future. Kidney Int. Rep. 2023, 8, 2529–2545. [Google Scholar] [CrossRef] [PubMed]
- Van der Zwan, M.; Hesselink, D.A.; van den Hoogen, M.W.F.; Baan, C.C. Costimulation Blockade in Kidney Transplant Recipients. Drugs 2020, 80, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Parsons, R.F.; Larsen, C.P.; Pearson, T.C.; Badell, I.R. Belatacept and CD28 Costimulation Blockade: Preventing and Reducing Alloantibodies over the Long Term. Curr. Transplant. Rep. 2019, 6, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Ford, M.L.; Larsen, C.P. Translating costimulation blockade to the clinic: Lessons learned from three pathways. Immunol. Rev. 2009, 229, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Maltzman, J.S.; Turka, L.A. T-cell costimulatory blockade in organ transplantation. Cold Spring Harb. Perspect. Med. 2013, 3, a015537. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.Y.; McGrath, M.; Najafian, N. The emerging role of the TIM molecules in transplantation. Am. J. Transplant. 2011, 11, 2012–2019. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, C.; Su, Y.; Ke, N. Function and characteristics of TIM-4 in immune regulation and disease (Review). Int. J. Mol. Med. 2023, 51, 10. [Google Scholar] [CrossRef] [PubMed]
- Cherukuri, A.; Mohib, K.; Rothstein, D.M. Regulatory B cells: TIM-1, transplant tolerance, and rejection. Immunol. Rev. 2021, 299, 31–44. [Google Scholar] [CrossRef]
- Tang, R.; Rangachari, M.; Kuchroo, V.K. Tim-3: A co-receptor with diverse roles in T cell exhaustion and tolerance. Semin. Immunol. 2019, 42, 101302. [Google Scholar] [CrossRef]
- Esensten, J.H.; Helou, Y.A.; Chopra, G.; Weiss, A.; Bluestone, J.A. CD28 Costimulation: From Mechanism to Therapy. Immunity 2016, 44, 973–988. [Google Scholar] [CrossRef]
- Bour-Jordan, H.; Esensten, J.H.; Martinez-Llordella, M.; Penaranda, C.; Stumpf, M.; Bluestone, J.A. Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/B7 family. Immunol. Rev. 2011, 241, 180–205. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.R.; Choi, J.M. Current Understanding of Cytotoxic T Lymphocyte Antigen-4 (CTLA-4) Signaling in T-Cell Biology and Disease Therapy. Mol. Cells. 2022, 45, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Buchbinder, E.; Hodi, F.S. Cytotoxic T lymphocyte antigen-4 and immune checkpoint blockade. J. Clin. Investig. 2015, 125, 3377–3383. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Badell, I.R.; Ford, M.L. Selective CD28 blockade attenuates CTLA-4-dependent CD8+ memory T cell effector function and prolongs graft survival. JCI Insight. 2018, 3, e96378. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.; Jeffery, L.E.; Sansom, D.M. Understanding the CD28/CTLA-4 (CD152) pathway and its implications for costimulatory blockade. Am. J. Transplant. 2014, 14, 1985–1991. [Google Scholar] [CrossRef] [PubMed]
- Rowshanravan, B.; Halliday, N.; Sansom, D.M. CTLA-4: A moving target in immunotherapy. Blood 2018, 131, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Crepeau, R.L.; Ford, M.L. Challenges and opportunities in targeting the CD28/CTLA-4 pathway in transplantation and autoimmunity. Expert Opin. Biol. Ther. 2017, 17, 1001–1012. [Google Scholar] [CrossRef]
- Wei, S.C.; Meijers, W.C.; Axelrod, M.L.; Anang, N.A.S.; Screever, E.M.; Wescott, E.C.; Johnson, D.B.; Whitley, E.; Lehmann, L.; Courand, P.Y.; et al. A Genetic Mouse Model Recapitulates Immune Checkpoint Inhibitor-Associated Myocarditis and Supports a Mechanism-Based Therapeutic Intervention. Cancer Discov. 2021, 11, 614–625. [Google Scholar] [CrossRef]
- Kong, Y.C.; Flynn, J.C. Opportunistic Autoimmune Disorders Potentiated by Immune-Checkpoint Inhibitors Anti-CTLA-4 and Anti-PD-1. Front. Immunol. 2014, 5, 206. [Google Scholar] [CrossRef]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef]
- Sobhani, N.; Tardiel-Cyril, D.R.; Davtyan, A.; Generali, D.; Roudi, R.; Li, Y. CTLA-4 in Regulatory T Cells for Cancer Immunotherapy. Cancers 2021, 13, 1440. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.M.; Ma, Y.; Yin, Z.; Xia, Y.; Du, J.; Huang, J.Y.; Huang, J.J.; Zou, L.; Ye, Z.; Huang, Z. Current understanding of CTLA-4: From mechanism to autoimmune diseases. Front. Immunol. 2023, 14, 1198365. [Google Scholar] [CrossRef] [PubMed]
- Watkins, B.; Qayed, M.; McCracken, C.; Bratrude, B.; Betz, K.; Suessmuth, Y.; Yu, A.; Sinclair, S.; Furlan, S.; Bosinger, S.; et al. Phase II Trial of Costimulation Blockade with Abatacept for Prevention of Acute GVHD. J. Clin. Oncol. 2021, 39, 1865–1877. [Google Scholar] [CrossRef] [PubMed]
- Chandraker, A.; Russell, M.E.; Glysing-Jensen, T.; Willett, T.A.; Sayegh, M.H. T-cell costimulatory blockade in experimental chronic cardiac allograft rejection: Effects of cyclosporine and donor antigen. Transplantation 1997, 63, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Badell, I.R.; Karadkhele, G.M.; Vasanth, P.; Farris AB 3rd Robertson, J.M.; Larsen, C.P. Abatacept as rescue immunosuppression after calcineurin inhibitor treatment failure in renal transplantation. Am. J. Transplant. 2019, 19, 2342–2349. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.P.; Pearson, T.C.; Adams, A.B.; Tso, P.; Shirasugi, N.; Strobert, E.; Anderson, D.; Cowan, S.; Price, K.; Naemura, J.; et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am. J. Transplant. 2005, 5, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, S.; Shahid, K. Comparative Safety and Efficacy of Immunosuppressive Regimens Post-kidney Transplant: A Systematic Review. Cureus 2023, 15, e43903. [Google Scholar] [CrossRef]
- Melvin, G.; Sandhiya, S.; Subraja, K. Belatacept: A worthy alternative to cyclosporine? J. Pharmacol. Pharmacother. 2012, 3, 90–92. [Google Scholar] [CrossRef]
- Vincenti, F.; Rostaing, L.; Grinyo, J.; Rice, K.; Steinberg, S.; Gaite, L.; Moal, M.C.; Mondragon-Ramirez, G.A.; Kothari, J.; Polinsky, M.S.; et al. Belatacept and Long-Term Outcomes in Kidney Transplantation. N. Engl. J. Med. 2016, 374, 333–343, Erratum in N. Engl. J. Med. 2016, 374, 698. [Google Scholar] [CrossRef]
- Lombardi, Y.; François, H. Belatacept in Kidney Transplantation: What Are the True Benefits? A Systematic Review. Front. Med. 2022, 9, 942665. [Google Scholar] [CrossRef]
- Archdeacon, P.; Dixon, C.; Belen, O.; Albrecht, R.; Meyer, J. Summary of the US FDA approval of belatacept. Am. J. Transplant. 2012, 12, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, D.; Jethwani, P. Preventing Rejection of the Kidney Transplant. J. Clin. Med. 2023, 12, 5938. [Google Scholar] [CrossRef] [PubMed]
- Masson, P.; Henderson, L.; Chapman, J.R.; Craig, J.C.; Webster, A.C. Belatacept for kidney transplant recipients. Cochrane Database Syst. Rev. 2014, 2014, CD010699. [Google Scholar] [CrossRef] [PubMed]
- Kollar, B.; Pomahac, B.; Riella, L.V. Novel immunological and clinical insights in vascularized composite allotransplantation. Curr. Opin. Organ Transplant. 2019, 24, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Mazzola Poli de Figueiredo, S.; Johnson, J.C.; Lyapichev, K.A.; Stevenson, H.L.; Lea, A.; Hussain, S.; Kulkarni, R.D.; Fair, J.H.; Mujtaba, M.; Kueht, M.L. Fatal Case of EBV-negative Posttransplant Lymphoproliferative Disorder with Hemophagocytic Lymphohistiocytosis in an Adult Kidney Transplant Recipient. Transplant. Direct 2022, 8, e1368. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.T.; Powell, J.T.; Patel, M.; Tsapepas, D. Risk of posttransplant lymphoproliferative disorder associated with use of belatacept. Am. J. Health Syst. Pharm. 2013, 70, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Cendales, L.; Bray, R.; Gebel, H.; Brewster, L.; Elbein, R.; Farthing, D.; Song, M.; Parker, D.; Stillman, A.; Pearson, T.; et al. Tacrolimus to Belatacept Conversion Following Hand Transplantation: A Case Report. Am. J. Transplant. 2015, 15, 2250–2255. [Google Scholar] [CrossRef] [PubMed]
- Cendales, L.C.; Ruch, D.S.; Cardones, A.R.; Potter, G.; Dooley, J.; Dore, D.; Orr, J.; Ruskin, G.; Song, M.; Chen, D.F.; et al. De novo belatacept in clinical vascularized composite allotransplantation. Am. J. Transplant. 2018, 18, 1804–1809. [Google Scholar] [CrossRef]
- Giannis, D.; Moris, D.; Cendales, L.C. Costimulation Blockade in Vascularized Composite Allotransplantation. Front. Immunol. 2020, 11, 544186. [Google Scholar] [CrossRef]
- Grahammer, J.; Weissenbacher, A.; Zelger, B.G.; Zelger, B.; Boesmueller, C.; Ninkovic, M.; Mühlbacher, A.; Peschel, I.; Brandacher, G.; Öfner, D.; et al. Benefits and limitations of belatacept in 4 hand-transplanted patients. Am. J. Transplant. 2017, 17, 3228–3235. [Google Scholar] [CrossRef]
- Krezdorn, N.; Murakami, N.; Pomahac, B.; Riella, L.V. Immunological Characteristics of a Patient with Belatacept-Resistant Acute Rejection After Face Transplantation. Am. J. Transplant. 2016, 16, 3305–3307. [Google Scholar] [CrossRef] [PubMed]
- Krezdorn, N.; Tasigiorgos, S.; Wo, L.; Lopdrup, R.; Turk, M.; Kiwanuka, H.; Ahmed, S.; Petruzzo, P.; Bueno, E.; Pomahac, B.; et al. Kidney Dysfunction After Vascularized Composite Allotransplantation. Transplant. Direct 2018, 4, e362. [Google Scholar] [CrossRef] [PubMed]
- Atia, A.; Moris, D.; McRae, M.; Song, M.; Stempora, L.; Leopardi, F.; Williams, K.; Kwun, J.; Parker, W.; Cardones, A.R.; et al. Th17 cell inhibition in a costimulation blockade-based regimen for vascularized composite allotransplantation using a nonhuman primate model. Transpl. Int. 2020, 33, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Salama, A.D.; Sho, M.; Izawa, A.; Sandner, S.E.; Ito, T.; Akiba, H.; Yagita, H.; Sharpe, A.H.; Freeman, G.J.; et al. The role of the ICOS-B7h T cell costimulatory pathway in transplantation immunity. J. Clin. Investig. 2003, 112, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, K.; Anagnostou, T.; Boussiotis, V.A. The PD1:PD-L1/2 Pathway from Discovery to Clinical Implementation. Front. Immunol. 2016, 7, 550. [Google Scholar] [CrossRef] [PubMed]
- Riley, J.L. PD-1 signaling in primary T cells. Immunol. Rev. 2009, 229, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Handelsman, S.; Overbey, J.; Chen, K.; Lee, J.; Haj, D.; Li, Y. PD-L1’s Role in Preventing Alloreactive T Cell Responses Following Hematopoietic and Organ Transplant. Cells 2023, 12, 1609. [Google Scholar] [CrossRef] [PubMed]
- Beenen, A.C.; Sauerer, T.; Schaft, N.; Dörrie, J. Beyond Cancer: Regulation and Function of PD-L1 in Health and Immune-Related Diseases. Int. J. Mol. Sci. 2022, 23, 8599. [Google Scholar] [CrossRef]
- Laman, J.D.; Claassen, E.; Noelle, R.J. Functions of CD40 and Its Ligand, gp39 (CD40L). Crit. Rev. Immunol. 2017, 37, 371–420. [Google Scholar] [CrossRef]
- Kirk, A.D.; Burkly, L.C.; Batty, D.S.; Baumgartner, R.E.; Berning, J.D.; Buchanan, K.; Fechner, J.H., Jr.; Germond, R.L.; Kampen, R.L.; Patterson, N.B.; et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat. Med. 1999, 5, 686–693. [Google Scholar] [CrossRef]
- Perrin, S.; Magill, M. The Inhibition of CD40/CD154 Costimulatory Signaling in the Prevention of Renal Transplant Rejection in Nonhuman Primates: A Systematic Review and Meta Analysis. Front. Immunol. 2022, 13, 861471. [Google Scholar] [CrossRef]
- Denton, M.D.; Reul, R.M.; Dharnidharka, V.R.; Fang, J.C.; Ganz, P.; Briscoe, D.M. Central role for CD40/CD40 ligand (CD154) interactions in transplant rejection. Pediatr. Transplant. 1998, 2, 6–15. [Google Scholar] [PubMed]
- Reul, R.M.; Fang, J.C.; Denton, M.D.; Geehan, C.; Long, C.; Mitchell, R.N.; Ganz, P.; Briscoe, D.M. CD40 and CD40 ligand (CD154) are coexpressed on microvessels in vivo in human cardiac allograft rejection. Transplantation 1997, 64, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Subbotin, V.; Chen, C.; Aitouche, A.; Valdivia, L.A.; Sayegh, M.H.; Linsley, P.S.; Fung, J.J.; Starzl, T.E.; Rao, A.S. Prevention of chronic rejection in mouse aortic allografts by combined treatment with CTLA4-Ig and anti-CD40 ligand monoclonal antibody. Transplantation 1997, 64, 1838–1843. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ford, M.L. CD11b is a novel alternate receptor for CD154 during alloimmunity. Am. J. Transplant. 2020, 20, 2216–2225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Pierson, R.N., 3rd; Azimzadeh, A.M. Update on CD40 and CD154 blockade in transplant models. Immunotherapy 2015, 7, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Pinelli, D.F.; Ford, M.L. Novel insights into anti-CD40/CD154 immunotherapy in transplant tolerance. Immunotherapy 2015, 7, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Harland, R.C.; Klintmalm, G.; Jensik, S.; Yang, H.; Bromberg, J.; Holman, J.; Kumar, M.S.A.; Santos, V.; Larson, T.J.; Wang, X. Efficacy and safety of bleselumab in kidney transplant recipients: A phase 2, randomized, open-label, noninferiority study. Am. J. Transplant. 2020, 20, 159–171. [Google Scholar] [CrossRef]
- Ford, M.L.; Adams, A.B.; Pearson, T.C. Targeting co-stimulatory pathways: Transplantation and autoimmunity. Nat. Rev. Nephrol. 2014, 10, 14–24. [Google Scholar] [CrossRef]
- Fu, Y.; Lin, Q.; Zhang, Z.; Zhang, L. Therapeutic strategies for the costimulatory molecule OX40 in T-cell-mediated immunity. Acta Pharm. Sin. B 2020, 10, 414–433. [Google Scholar] [CrossRef]
- Louis, K.; Macedo, C.; Metes, D. Targeting T Follicular Helper Cells to Control Humoral Allogeneic Immunity. Transplantation 2021, 105, e168–e180. [Google Scholar] [CrossRef] [PubMed]
- Ward-Kavanagh, L.K.; Lin, W.W.; Šedý, J.R.; Ware, C.F. The TNF Receptor Superfamily in Co-stimulating and Co-inhibitory Responses. Immunity 2016, 44, 1005–1019. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Zeng, D.; Liu, G.; Zhu, Y.; Wang, J.; Wu, H.; Jiang, J.; Huang, J. Crucial role of OX40/OX40L signaling in a murine model of asthma. Mol. Med. Rep. 2018, 17, 4213–4220. [Google Scholar] [CrossRef] [PubMed]
- Vu, M.D.; Clarkson, M.R.; Yagita, H.; Turka, L.A.; Sayegh, M.H.; Li, X.C. Critical, but conditional, role of OX40 in memory T cell-mediated rejection. J. Immunol. 2006, 176, 1394–1401. [Google Scholar] [CrossRef] [PubMed]
- Curti, B.D.; Kovacsovics-Bankowski, M.; Morris, N.; Walker, E.; Chisholm, L.; Floyd, K.; Walker, J.; Gonzalez, I.; Meeuwsen, T.; Fox, B.A.; et al. OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res. 2013, 73, 7189–7198. [Google Scholar] [CrossRef]
- Sonawane, S.B.; Kim, J.I.; Lee, M.K.; Lee, S.H.; Duff, P.E.; Moore, D.J.; Lian, M.M.; Deng, S.; Choi, Y.; Yeh, H.; et al. GITR Blockade Facilitates Treg Mediated Allograft Survival. Transplantation 2009, 88, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Manzanet, R.; DeKruyff, R.; Kuchroo, V.K.; Umetsu, D.T. The costimulatory role of TIM molecules. Immunol. Rev. 2009, 229, 259–270. [Google Scholar] [CrossRef]
- Mizui, M.; Shikina, T.; Arase, H.; Suzuki, K.; Yasui, T.; Rennert, P.D.; Kumanogoh, A.; Kikutani, H. Bimodal regulation of T cell-mediated immune responses by TIM-4. Int. Immunol. 2008, 20, 695–708. [Google Scholar] [CrossRef]
- Zhu, C.; Anderson, A.C.; Schubart, A.; Xiong, H.; Imitola, J.; Khoury, S.J.; Zheng, X.X.; Strom, T.B.; Kuchroo, V.K. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005, 6, 1245–1252. [Google Scholar] [CrossRef]
- Walling, B.L.; Kim, M. LFA-1 in T Cell Migration and Differentiation. Front. Immunol. 2018, 9, 952. [Google Scholar] [CrossRef]
- Schneeberger, S.; Hautz, T.; Sucher, R.; Lee, W.P.A.; Pratschke, J.; Brandacher, G. Hand Transplantation. Trends Transpl. 2012, 6, 4–16. [Google Scholar] [CrossRef]
- Posselt, A.M.; Bellin, M.D.; Tavakol, M.; Szot, G.L.; Frassetto, L.A.; Masharani, U.; Kerlan, R.K.; Fong, L.; Vincenti, F.G.; Hering, B.J.; et al. Islet transplantation in type 1 diabetics using an immunosuppressive protocol based on the anti-LFA-1 antibody efalizumab. Am. J. Transplant. 2010, 10, 1870–1880. [Google Scholar] [CrossRef] [PubMed]
- Rostaing, L.; Charpentier, B.; Glyda, M.; Rigotti, P.; Hettich, F.; Franks, B.; Houbiers, J.G.; First, R.; Holman, J.M. Alefacept combined with tacrolimus, mycophenolate mofetil and steroids in de novo kidney transplantation: A randomized controlled trial. Am. J. Transplant. 2013, 13, 1724–1733. [Google Scholar] [CrossRef] [PubMed]
- Rostaing, L.; Kamar, N. Alefacept Treatment in the Setting of Transplantation. Trends Transpl. 2014, 8, 35–40. [Google Scholar]
- Weaver, T.A.; Charafeddine, A.H.; Agarwal, A.; Turner, A.P.; Russell, M.; Leopardi, F.V.; Kampen, R.L.; Stempora, L.; Song, M.; Larsen, C.P.; et al. Alefacept promotes co-stimulation blockade based allograft survival in nonhuman primates. Nat. Med. 2009, 15, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Black, C.K.; Termanini, K.M.; Aguirre, O.; Hawksworth, J.S.; Sosin, M. Solid organ transplantation in the 21st century. Ann. Transl. Med. 2018, 6, 409. [Google Scholar] [CrossRef] [PubMed]
- Grinyó, J.M. Why is organ transplantation clinically important? Cold Spring Harb. Perspect. Med. 2013, 3, a014985. [Google Scholar] [CrossRef]
- Kaballo, M.A.; Canney, M.; O’Kelly, P.; Williams, Y.; O’Seaghdha, C.M.; Conlon, P.J. A comparative analysis of survival of patients on dialysis and after kidney transplantation. Clin. Kidney J. 2018, 11, 389–393. [Google Scholar] [CrossRef]
- Pullen, L.C. VCA Joins UNOS: A Five-Year Update. Am. J. Transplant. 2019, 19, 615–616. [Google Scholar] [CrossRef]
- Milek, D.; Reed, L.T.; Echternacht, S.R.; Shanmugarajah, K.; Cetrulo CLJr Lellouch, A.G.; Langstein, H.N.; Leckenby, J.I. A Systematic Review of the Reported Complications Related to Facial and Upper Extremity Vascularized Composite Allotransplantation. J. Surg. Res. 2023, 281, 164–175. [Google Scholar] [CrossRef]
- Uluer, M.C.; Brazio, P.S.; Woodall, J.D.; Nam, A.J.; Bartlett, S.T.; Barth, R.N. Vascularized Composite Allotransplantation: Medical Complications. Curr. Transplant. Rep. 2016, 3, 395–403. [Google Scholar] [CrossRef] [PubMed]
- McDermott, J.K. Contemporary Heart Transplantation. Organ and Tissue Transplantation. In Complications of Immunosuppression; Bogar, L., Stempien-Otero, A., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Parlakpinar, H.; Gunata, M. Transplantation and immunosuppression: A review of novel transplant-related immunosuppressant drugs. Immunopharmacol. Immunotoxicol. 2021, 43, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, C.L.; Kant, S. Pathophysiology of Rejection in Kidney Transplantation. J. Clin. Med. 2023, 12, 4130. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.; Alvey, N.; Bowman, L.; Schulte, J.; Segovia, M.C.; McDermott, J.; Te, H.S.; Kapila, N.; Levine, D.J.; Gottlieb, R.L.; et al. Consensus recommendations for use of maintenance immunosuppression in solid organ transplantation: Endorsed by the American College of Clinical Pharmacy, American Society of Transplantation, and the International Society for Heart and Lung Transplantation. Pharmacotherapy 2022, 42, 599–633. [Google Scholar] [CrossRef] [PubMed]
- Szumilas, K.; Wilk, A.; Wiśniewski, P.; Gimpel, A.; Dziedziejko, V.; Kipp, M.; Pawlik, A. Current Status Regarding Immunosuppressive Treatment in Patients after Renal Transplantation. Int. J. Mol. Sci. 2023, 24, 10301. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, A.C. Immunosuppressive Medications. Clin. J. Am. Soc. Nephrol. 2016, 11, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowski, D.; Wiseman, A. Long-Term Immunosuppression Management: Opportunities and Uncertainties. Clin. J. Am. Soc. Nephrol. 2021, 16, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Small, B.; Au, J.; Brink, H.; Shah, I.; Strah, H. Induction and maintenance immunosuppression in lung transplantation. Indian. J. Thorac. Cardiovasc. Surg. 2022, 38 (Suppl. S2), 300–317. [Google Scholar] [CrossRef]
- Hussain, Y.; Khan, H. Immunosuppressive Drugs. Encycl. Infect. Immun. 2022, 4, 726–740. [Google Scholar] [CrossRef]
- Huelsboemer, L.; Boroumand, S.; Kochen, A.; Dony, A.; Moscarelli, J.; Hauc, S.C.; Stögner, V.A.; Formica, R.N.; Pomahac, B.; Kauke-Navarro, M. Immunosuppressive strategies in face and hand transplantation: A comprehensive systematic review of current therapy regimens and outcomes. Front. Transplant. 2024, 3, 1366243. [Google Scholar] [CrossRef]
- Claeys, E.; Vermeire, K. Immunosuppressive drugs in organ transplantation to prevent allograft rejection: Mode of action and side effects. J. Immunol. Sci. 2019, 3, 14–21. [Google Scholar] [CrossRef]
- Acharya, S.; Lama, S.; Kanigicherla, D.A. Anti-thymocyte globulin for treatment of T-cell-mediated allograft rejection. World J. Transplant. 2023, 13, 299–308. [Google Scholar] [CrossRef]
- Hart, A.; Lentine, K.L.; Smith, J.M.; Miller, J.M.; Skeans, M.A.; Prentice, M.; Robinson, A.; Foutz, J.; Booker, S.E.; Israni, A.K.; et al. OPTN/SRTR 2019 Annual Data Report: Kidney. Am. J. Transplant. 2021, 21 (Suppl. S2), 21–137. [Google Scholar] [CrossRef] [PubMed]
- Lentine, K.L.; Smith, J.M.; Miller, J.M.; Bradbrook, K.; Larkin, L.; Weiss, S.; Handarova, D.K.; Temple, K.; Israni, A.K.; Snyder, J.J. OPTN/SRTR 2021 Annual Data Report: Kidney. Am. J. Transplant. 2023, 23 (Suppl. S1), S21–S120. [Google Scholar] [CrossRef] [PubMed]
- Kandaswamy, R.; Stock, P.G.; Miller, J.M.; White, J.; Booker, S.E.; Israni, A.K.; Snyder, J.J. OPTN/SRTR 2021 Annual Data Report: Pancreas. Am. J. Transplant. 2023, 23 (Suppl. S1), S121–S177. [Google Scholar] [CrossRef] [PubMed]
- Kwong, A.J.; Ebel, N.H.; Kim, W.R.; Lake, J.R.; Smith, J.M.; Schladt, D.P.; Schnellinger, E.M.; Handarova, D.; Weiss, S.; Cafarella, M.; et al. OPTN/SRTR 2021 Annual Data Report: Liver. Am. J. Transplant. 2023, 23 (Suppl. S1), S178–S263. [Google Scholar] [CrossRef] [PubMed]
- Horslen, S.P.; Wood, N.L.; Cafarella, M.; Schnellinger, E.M. OPTN/SRTR 2021 Annual Data Report: Intestine. Am. J. Transplant. 2023, 23 (Suppl. S1), S264–S299. [Google Scholar] [CrossRef] [PubMed]
- Colvin, M.; Smith, J.M.; Ahn, Y.; Skeans, M.A.; Messick, E.; Bradbrook, K.; Gauntt, K.; Israni, A.K.; Snyder, J.J.; Kasiske, B.L. OPTN/SRTR 2020 Annual Data Report: Heart. Am. J. Transplant. 2022, 22 (Suppl. S2), 350–437. [Google Scholar] [CrossRef]
- Valapour, M.; Lehr, C.J.; Schladt, D.P.; Smith, J.M.; Goff, R.; Mupfudze, T.G.; Swanner, K.; Gauntt, K.; Snyder, J.J. OPTN/SRTR 2021 Annual Data Report: Lung. Am. J. Transplant. 2023, 23 (Suppl. S1), S379–S442. [Google Scholar] [CrossRef]
- Fischer, S.; Lian, C.G.; Kueckelhaus, M.; Strom, T.B.; Edelman, E.R.; Clark, R.A.; Murphy, G.F.; Chandraker, A.K.; Riella, L.V.; Tullius, S.G.; et al. Acute rejection in vascularized composite allotransplantation. Curr. Opin. Organ Transplant. 2014, 19, 531–544. [Google Scholar] [CrossRef]
- Lellouch, A.G.; Andrews, A.R.; Saviane, G.; Ng, Z.Y.; Schol, I.M.; Goutard, M.; Gama, A.R.; Rosales, I.A.; Colvin, R.B.; Lantieri, L.A.; et al. Tolerance of a Vascularized Composite Allograft Achieved in MHC Class-I-mismatch Swine via Mixed Chimerism. Front. Immunol. 2022, 13, 829406. [Google Scholar] [CrossRef] [PubMed]
- Panackel, C.; Mathew, J.F.; Fawas, N.M.; Jacob, M. Immunosuppressive Drugs in Liver Transplant: An Insight. J. Clin. Exp. Hepatol. 2022, 12, 1557–1571. [Google Scholar] [CrossRef] [PubMed]
- Safi, A.F.; Kauke, M.; Nelms, L.; Palmer, W.J.; Tchiloemba, B.; Kollar, B.; Haug, V.; Pomahač, B. Local immunosuppression in vascularized composite allotransplantation (VCA): A systematic review. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, J.M.; Bechstein, W.O.; Kuypers, D.R.; Burra, P.; Citterio, F.; De Geest, S.; Duvoux, C.; Jardine, A.G.; Kamar, N.; Krämer, B.K.; et al. Practical Recommendations for Long-term Management of Modifiable Risks in Kidney and Liver Transplant Recipients: A Guidance Report and Clinical Checklist by the Consensus on Managing Modifiable Risk in Transplantation (COMMIT) Group. Transplantation 2017, 101 (Suppl. S2), S1–S56. [Google Scholar] [CrossRef] [PubMed]
- Salvadori, M.; Tsalouchos, A. Biomarkers in renal transplantation: An updated review. World J. Transplant. 2017, 7, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R. Donor-Specific Antibodies in Kidney Transplant Recipients. Clin. J. Am. Soc. Nephrol. 2018, 13, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Moris, D.; Cendales, L.C. Sensitization and Desensitization in Vascularized Composite Allotransplantation. Front. Immunol. 2021, 12, 682180. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, C.L.; Kanitakis, J.; Weissenbacher, A.; Brandacher, G.; Mehra, M.R.; Amer, H.; Zelger, B.G.; Zelger, B.; Pomahac, B.; McDiarmid, S.; et al. Defining chronic rejection in vascularized composite allotransplantation—The American Society of Reconstructive Transplantation and International Society of Vascularized Composite Allotransplantation chronic rejection working group: 2018 American Society of Reconstructive Transplantation meeting report and white paper Research goals in defining chronic rejection in vascularized composite allotransplantation. SAGE Open Med. 2020, 8, 2050312120940421. [Google Scholar] [CrossRef]
- Park, S.H.; Eun, S.C.; Kwon, S.T. Hand Transplantation: Current Status and Immunologic Obstacles. Exp. Clin. Transplant. 2019, 17, 97–104. [Google Scholar] [CrossRef]
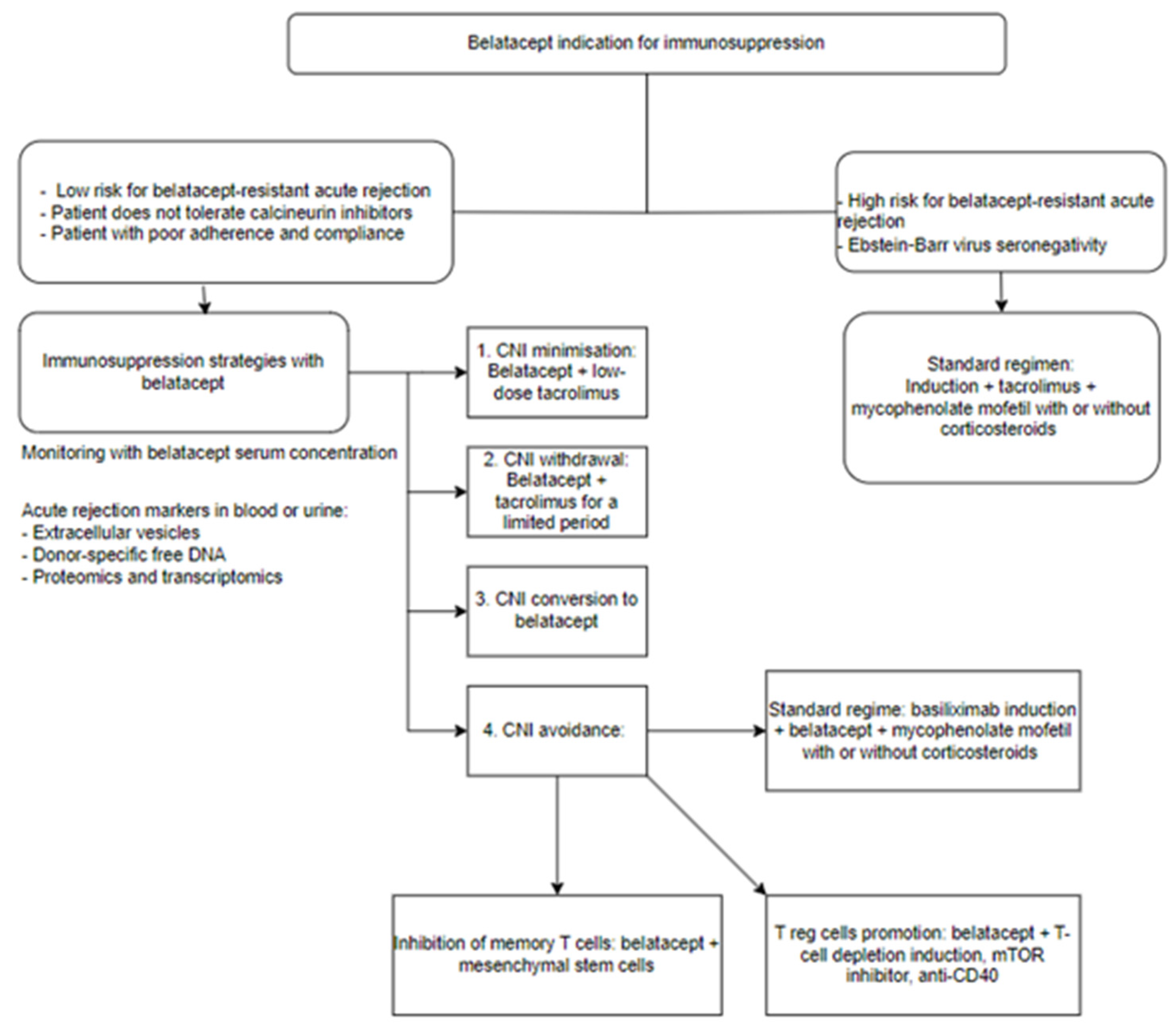
- Budde, K.; Prashar, R.; Haller, H.; Rial, M.C.; Kamar, N.; Agarwal, A.; de Fijter, J.W.; Rostaing, L.; Berger, S.P.; Djamali, A.; et al. Conversion from Calcineurin Inhibitor- to Belatacept-Based Maintenance Immunosuppression in Renal Transplant Recipients: A Randomized Phase 3b Trial. J. Am. Soc. Nephrol. 2021, 32, 3252–3264. [Google Scholar] [CrossRef]
- Chavarot, N.; Divard, G.; Scemla, A.; Amrouche, L.; Aubert, O.; Leruez-Ville, M.; Timsit, M.O.; Tinel, C.; Zuber, J.; Legendre, C.; et al. Increased incidence and unusual presentations of CMV disease in kidney transplant recipients after conversion to belatacept. Am. J. Transplant. 2021, 21, 2448–2458. [Google Scholar] [CrossRef] [PubMed]
- Grinyó, J.M.; Del Carmen Rial, M.; Alberu, J.; Steinberg, S.M.; Manfro, R.C.; Nainan, G.; Vincenti, F.; Jones-Burton, C.; Kamar, N. Safety and Efficacy Outcomes 3 Years after Switching to Belatacept from a Calcineurin Inhibitor in Kidney Transplant Recipients: Results from a Phase 2 Randomized Trial. Am. J. Kidney Dis. 2017, 69, 587–594. [Google Scholar] [CrossRef]
- Bray, R.A.; Gebel, H.M.; Townsend, R.; Roberts, M.E.; Polinsky, M.; Yang, L.; Meier-Kriesche, H.U.; Larsen, C.P. De novo donor-specific antibodies in belatacept-treated vs cyclosporine-treated kidney-transplant recipients: Post hoc analyses of the randomized phase III BENEFIT and BENEFIT-EXT studies. Am. J. Transplant. 2018, 18, 1783–1789. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, C.E.; Anglicheau, D.; Snanoudj, R.; Scemla, A.; Martinez, F.; Timsit, M.O.; Legendre, C.; Sberro-Soussan, R. Conversion from Calcineurin Inhibitors to Belatacept in HLA-sensitized Kidney Transplant Recipients with Low-level Donor-specific Antibodies. Transplantation 2019, 103, 2150–2156. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.M.; Samy, K.P.; Farris, A.B.; Leopardi, F.V.; Song, M.; Stempora, L.; Strobert, E.A.; Jenkins, J.A.; Kirk, A.D.; Cendales, L.C. Studies Introducing Costimulation Blockade for Vascularized Composite Allografts in Nonhuman Primates. Am. J. Transplant. 2015, 15, 2240–2249. [Google Scholar] [CrossRef] [PubMed]
- Mager, L.; Gardeen, S.; Carr, D.R.; Shahwan, K.T. Cemiplimab for the Treatment of Advanced Cutaneous Squamous Cell Carcinoma: Appropriate Patient Selection and Perspectives. Clin. Cosmet. Investig. Dermatol. 2023, 16, 2135–2142. [Google Scholar] [CrossRef] [PubMed]
- Hanna, G.J.; Dharanesswaran, H.; Giobbie-Hurder, A.; Harran, J.J.; Liao, Z.; Pai, L.; Tchekmedyian, V.; Ruiz, E.S.; Waldman, A.H.; Schmults, C.D.; et al. Cemiplimab for Kidney Transplant Recipients with Advanced Cutaneous Squamous Cell Carcinoma. J. Clin. Oncol. 2024, 42, JCO2301498. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, F.; Klintmalm, G.; Yang, H.; Ram Peddi, V.; Blahunka, P.; Conkle, A.; Santos, V.; Holman, J. A randomized, phase 1b study of the pharmacokinetics, pharmacodynamics, safety, and tolerability of bleselumab, a fully human, anti-CD40 monoclonal antibody, in kidney transplantation. Am. J. Transplant. 2020, 20, 172–180. [Google Scholar] [CrossRef]
- Shahbaz, S.K.; Pourrezagholi, F.; Barabadi, M.; Foroughi, F.; Hosseinzadeh, M.; Ahmadpoor, P.; Nafar, M.; Yekaninejad, M.S.; Amirzargar, A. High expression of TIM-3 and KIM-1 in blood and urine of renal allograft rejection patients. Transplant. Immunol. 2017, 43–44, 11–20. [Google Scholar] [CrossRef]
- Anazawa, T.; Okajima, H.; Masui, T.; Uemoto, S. Current state and future evolution of pancreatic islet transplantation. Ann. Gastroenterol. Surg. 2018, 3, 34–42. [Google Scholar] [CrossRef]
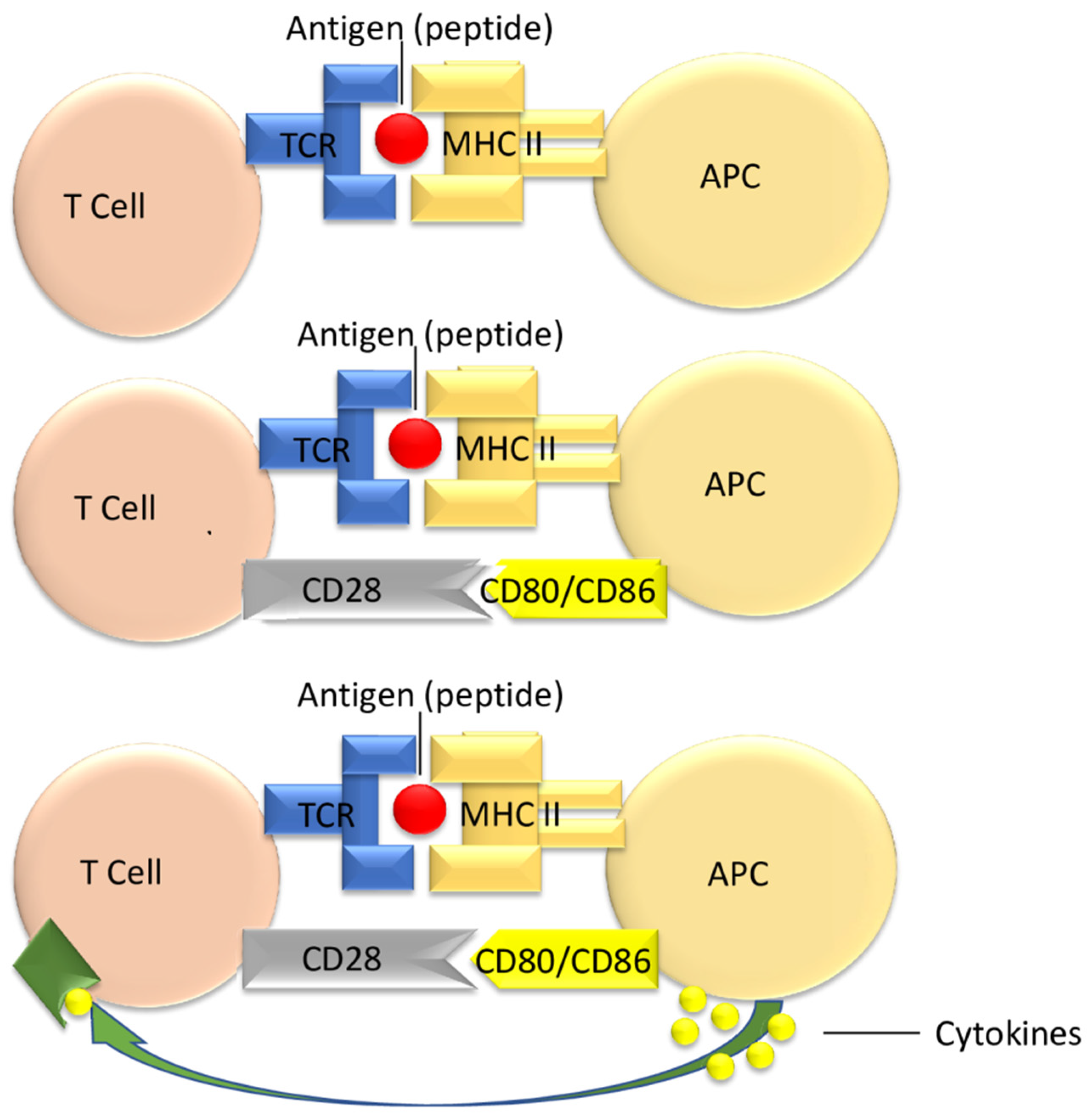

| Group | Costimulatory Molecules on T Cell | Correspondent Ligands on the Antigen-Presenting Cell (APC) |
Functional Attributes: Positive/Stimulatory Negative/Inhibitory | |
|---|---|---|---|---|
| 1 | Immunoglobulin superfamily | CD 28 CTLA-4(CD152) ICOS PD1 | CD 86 CD80 ICOS-L PDL-1/2 | Stimulatory Inhibitory Stimulatory Inhibitory |
| 2 | TNF superfamily | CD40-L(CD154) CD27 CD30 OX40(CD 134) GITR 4-1 BB(CD137) | CD40 CD70 CD30-L OX40-L GITRL 4-1 BBL | Stimulatory |
| 3 | TIM family (T-cell immunoglobulin and mucin domain molecules) | TIM-1 TIM-3 | TIM-4 Galectin-9 | Stimulatory Inhibitory |
| 4 | Cell adhesion molecules | LFA-1 CD2 | ICAM-1(CD54) LFA-3 | Stimulatory |
| Phase of Immunosuppressive Therapy | Agents Used and Proportion of Cases Using the Therapeutic Agent | |
|---|---|---|
| Face Transplant | Upper Limb Transplant | |
| Induction Therapy | Thymoglobulin (88.9%). Methylprednisolone (73.3%) MMF (60.0%), Tacrolimus (53.3%), Prednisone (22.2%) Basiliximab (6.7%), Donor hematopoietic stem cell transplant (6.7%), Rituximab (4.4%), Extracorporeal photochemotherapy (2.2%), Anti-IL-2 mAb (2.2%), Alemtuzumab (2.2%). | Thymoglobulin (53.8%). Methylprednisolone (50.5%), MMF/MPA (35.2%), Tacrolimus (33.0%), Basiliximab (31.9%), Alemtuzumab (28.6%), Prednisone/Prednisolone/Steroids (26.4%). Mesenchymal stem cell transplant (12.1%), Cyclophosphamide (12.1%), Bone marrow cell infusion (3.3%) |
| Maintenance Therapy | Tacrolimus (97.8%) MMF (95.6%), Prednisolone/prednisone/steroids (73.3%) Methylprednisolone (24.4%) Extra-corporeal photopheresis (15.6%) Sirolimus (11.1%), Belatacept (2.2%), Everolimus (2.2%), Cyclosporine A (2.2%), Azathioprine (2.2%) | Tacrolimus (100%). Prednisone/Prednisolone/Steroids (94.5%) MMF/MPA (90.1%) Sirolimus (27.5%), Everolimus (9.9%), Belatacept (6.6%), Donor bone marrow infusion (5.5%). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grosu-Bularda, A.; Hodea, F.-V.; Zamfirescu, D.; Stoian, A.; Teodoreanu, R.N.; Lascăr, I.; Hariga, C.S. Exploring Costimulatory Blockade-Based Immunologic Strategies in Transplantation: Are They a Promising Immunomodulatory Approach for Organ and Vascularized Composite Allotransplantation? J. Pers. Med. 2024, 14, 322. https://doi.org/10.3390/jpm14030322
Grosu-Bularda A, Hodea F-V, Zamfirescu D, Stoian A, Teodoreanu RN, Lascăr I, Hariga CS. Exploring Costimulatory Blockade-Based Immunologic Strategies in Transplantation: Are They a Promising Immunomodulatory Approach for Organ and Vascularized Composite Allotransplantation? Journal of Personalized Medicine. 2024; 14(3):322. https://doi.org/10.3390/jpm14030322
Chicago/Turabian StyleGrosu-Bularda, Andreea, Florin-Vlad Hodea, Dragos Zamfirescu, Alexandru Stoian, Răzvan Nicolae Teodoreanu, Ioan Lascăr, and Cristian Sorin Hariga. 2024. "Exploring Costimulatory Blockade-Based Immunologic Strategies in Transplantation: Are They a Promising Immunomodulatory Approach for Organ and Vascularized Composite Allotransplantation?" Journal of Personalized Medicine 14, no. 3: 322. https://doi.org/10.3390/jpm14030322
APA StyleGrosu-Bularda, A., Hodea, F.-V., Zamfirescu, D., Stoian, A., Teodoreanu, R. N., Lascăr, I., & Hariga, C. S. (2024). Exploring Costimulatory Blockade-Based Immunologic Strategies in Transplantation: Are They a Promising Immunomodulatory Approach for Organ and Vascularized Composite Allotransplantation? Journal of Personalized Medicine, 14(3), 322. https://doi.org/10.3390/jpm14030322






