Assessment of Psychometric Characteristics of Parkinson’s Disease Sleep Scale 2 and Analysis of a Cut-Off Score for Detecting Insomnia in Italian Patients with Parkinson’s Disease: A Validation Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participant
2.2. Outcome Measure
2.3. PDSS-2
2.4. GSDS
2.5. Reliability
2.6. Construct Validity
2.7. Cut-Off Score of PDSS-2
2.8. Score Analysis
2.9. Statistical Analysis
3. Result
3.1. Participants
3.2. Reliability
3.3. Construct Validity
3.4. Score Analysis
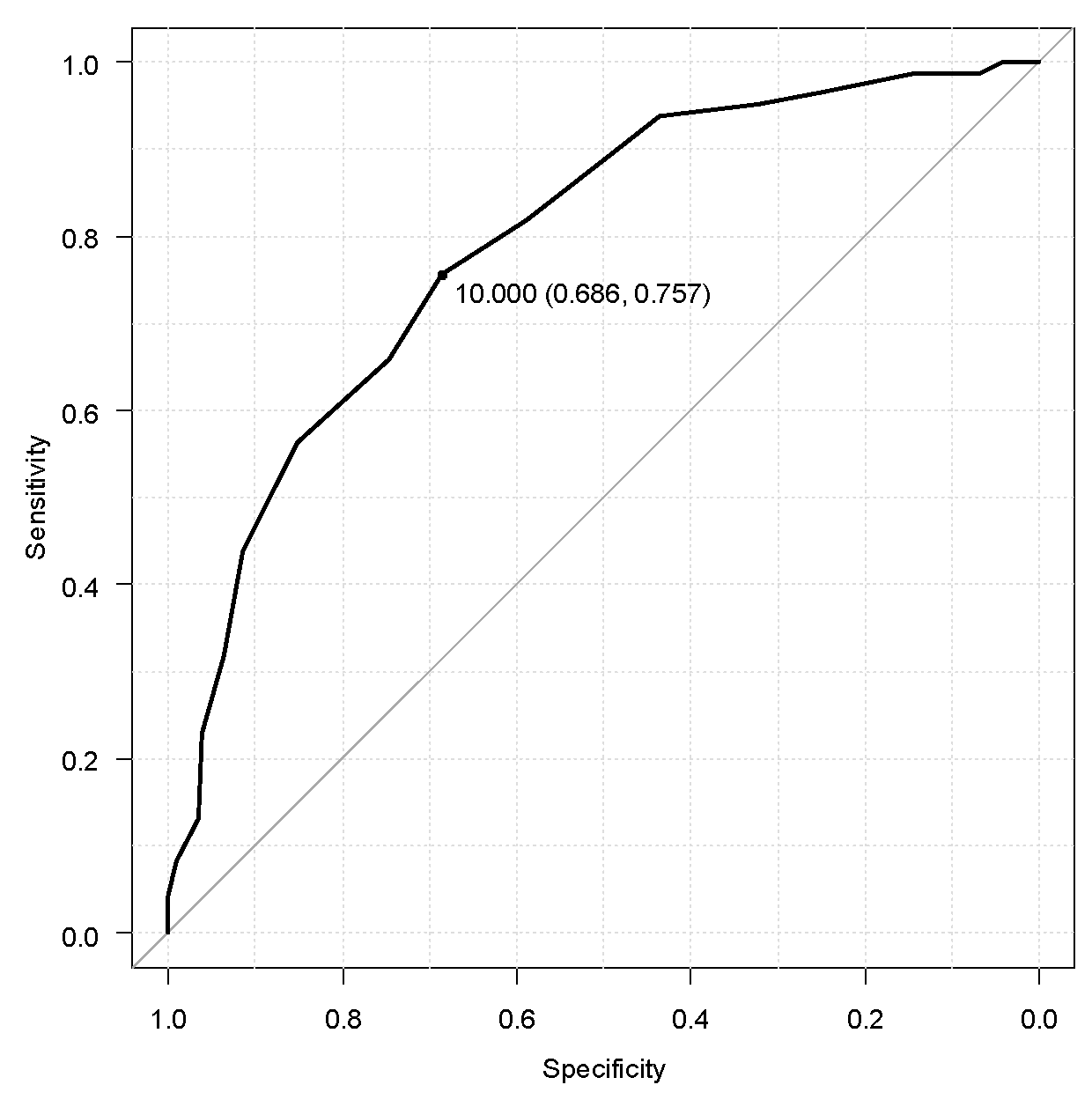
3.5. Cut-Off Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hobson, P.; Meara, J. Mortality and quality of death certification in a cohort of patients with Parkinson’s disease and matched controls in North Wales, UK at 18 years: A community-based cohort study. BMJ Open 2018, 8, e018969. [Google Scholar] [CrossRef] [PubMed]
- De Lau, L.M.; Breteler, M.M. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006, 5, 525–535. [Google Scholar] [CrossRef]
- Barone, P.; Antonini, A.; Colosimo, C.; Marconi, R.; Morgante, L.; Avarello, T.P.; Bottacchi, E.; Cannas, A.; Ceravolo, G.; Ceravolo, R.; et al. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2009, 24, 1641–1649. [Google Scholar] [CrossRef]
- Fernandes, M.; Pierantozzi, M.; Stefani, A.; Cattaneo, C.; Bonizzoni, E.A.; Cerroni, R.; Mercuri, N.B.; Liguori, C. Frequency of non-motor symptoms in Parkinson’s patients with motor fluctuations. Front. Neurol. 2021, 12, 678373. [Google Scholar] [CrossRef] [PubMed]
- Stefani, A.; Högl, B. Sleep in Parkinson’s disease. In Neuropsychopharmacology; Springer Nature: Berlin/Heidelberg, Germany, 2020; Volume 45, pp. 121–128. [Google Scholar] [CrossRef]
- Liguori, C.; De Franco, V.; Cerroni, R.; Spanetta, M.; Mercuri, N.B.; Stefani, A.; Pierantozzi, M.; Di Pucchio, A. Sleep problems affect quality of life in Parkinson’s disease along disease progression. Sleep Med. 2021, 81, 307–311. [Google Scholar] [CrossRef]
- Zhu, K.; van Hilten, J.J.; Marinus, J. The course of insomnia in Parkinson’s disease. Park. Relat. Disord. 2016, 33, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Shafazand, S.; Wallace, D.M.; Arheart, K.L.; Vargas, S.; Luca, C.C.; Moore, H.; Katzen, H.; Levin, B.; Singer, C. Insomnia, sleep quality, and quality of life in mild to moderate Parkinson’s disease. Ann. Am. Thorac. Soc. 2017, 14, 412–419. [Google Scholar] [CrossRef]
- Gros, P.; Videnovic, A. Overview of Sleep and Circadian Rhythm Disorders in Parkinson Disease. In Clinics in Geriatric Medicine; W.B. Saunders: Philadelphia, PA, USA, 2020; Volume 36, pp. 119–130. [Google Scholar] [CrossRef]
- Shen, Y.; Huang, J.Y.; Li, J.; Liu, C.F. Excessive Daytime Sleepiness in Parkinson’s Disease: Clinical Implications and Management. Chin. Med. J. 2018, 131, 974–981. [Google Scholar] [CrossRef]
- Mery, V.P.; Gros, P.; Lafontaine, A.-L.; Robinson, A.; Benedetti, A.; Kimoff, R.J.; Kaminska, M. Reduced cognitive function in patients with Parkinson disease and obstructive sleep apnea. Neurology 2017, 88, 1120–1128. [Google Scholar] [CrossRef]
- Mantovani, S.; Smith, S.S.; Gordon, R.; O’Sullivan, J.D. An overview of sleep and circadian dysfunction in Parkinson’s disease. J. Sleep Res. 2018, 27, e12673. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, J.-R.; Shen, Y.; Liu, C.-F. Clinical Significance of REM Sleep Behavior Disorders and Other Non-motor Symptoms of Parkinsonism. Neurosci. Bull. 2017, 33, 576–584. [Google Scholar] [CrossRef]
- Högl, B.; Arnulf, I.; Comella, C.; Ferreira, J.; Iranzo, A.; Tilley, B.; Trenkwalder, C.; Poewe, W.; Rascol, O.; Sampaio, C.; et al. Scales to assess sleep impairment in Parkinson’s disease: Critique and recommendations. In Movement Disorders; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2010; Volume 25, pp. 2704–2716. [Google Scholar] [CrossRef]
- Singh, R.; Rai, N.K.; Chouhan, S.; Pakhare, A. Translation of Parkinson’s Disease Sleep Scale-2 (PDSS-2) in Hindi (H-PDSS-2) and its Validation for Assessment of Sleep Disturbances among Indian Parkinson’s Disease Patients. Neurol. India 2023, 71, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Joghataei, M.T.; Fereshtehnejad, S.-M.; Mehdizadeh, M.; Goudarzi, S.; Habibi, S.A.H.; Meimandi, M.; Dehmiyani, A.; Taghizadeh, G. Validity and Reliability of the Persian Version of Parkinson’s Disease Sleep Scale-2. Park. Dis. 2021, 2021, 2015123. [Google Scholar] [CrossRef]
- Yang, H.-J.; Kim, H.-J.; Koh, S.-B.; Kim, J.-S.; Ahn, T.-B.; Cheon, S.-M.; Cho, J.W.; Kim, Y.-J.; Ma, H.-I.; Park, M.Y.; et al. Subtypes of Sleep Disturbance in Parkinson’s Disease Based on the Cross-Culturally Validated Korean Version of Parkinson’s Disease Sleep Scale-2. J. Clin. Neurol. 2020, 16, 66–74. [Google Scholar] [CrossRef]
- Martinez-Martin, P.; Wetmore, J.B.; Rodríguez-Blázquez, C.; Arakaki, T.; Bernal, O.; Campos-Arillo, V.; Cerda, C.; Estrada-Bellmann, I.; Garretto, N.; Ginsburg, L.; et al. The Parkinson’s Disease Sleep Scale–2 (PDSS-2): Validation of the Spanish Version and Its Relationship With a Roommate-Based Version. Mov. Disord. Clin. Pract. 2019, 6, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Arnaldi, D.; Cordano, C.; De Carli, F.; Accardo, J.; Ferrara, M.; Picco, A.; Tamburini, T.; Brugnolo, A.; Abbruzzese, G.; Nobili, F. Parkinson’s Disease Sleep Scale 2: Application in an Italian population. Neurol. Sci. 2015, 37, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Trenkwalder, C.; Kohnen, R.; Högl, B.; Metta, V.; Sixel-Döring, F.; Frauscher, B.; Hülsmann, J.; Martinez-Martin, P.; Chaudhuri, K.R. Parkinson’s disease sleep scale—Validation of the revised version PDSS-2. Mov. Disord. 2011, 26, 644–652. [Google Scholar] [CrossRef]
- Muntean, M.-L.; Benes, H.; Sixel-Döring, F.; Chaudhuri, K.R.; Suzuki, K.; Hirata, K.; Zimmermann, J.; Trenkwalder, C. Clinically relevant cut-off values for the Parkinson’s Disease Sleep Scale-2 (PDSS-2): A validation study. Sleep Med. 2016, 24, 87–92. [Google Scholar] [CrossRef]
- Suzuki, K.; Miyamoto, T.; Miyamoto, M.; Suzuki, S.; Numao, A.; Watanabe, Y.; Tatsumoto, M.; Sakuta, H.; Watanabe, Y.; Fujita, H.; et al. Evaluation of cutoff scores for the Parkinson’s disease sleep scale-2. Acta Neurol. Scand. 2015, 131, 426–430. [Google Scholar] [CrossRef]
- Galeoto, G.; Scialpi, A.; Grassi, M.L.; Berardi, A.; Valente, D.; Tofani, M.; Paoloni, M. General Sleep Disturbance Scale: Translation, cultural adaptation, and psychometric properties of the Italian version. Cranio J. Craniomandib. Pract. 2021, 39, 326–334. [Google Scholar] [CrossRef]
- Panuccio, F.; Galeoto, G.; Marquez, M.A.; Grassi, M.L.; Scialpi, A.; Tofani, M.; Berardi, A. General Sleep Disturbance Scale (GSDS-IT) in people with spinal cord injury: A psychometric study. Spinal Cord 2020, 58, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Galeoto, G.; Scialpi, A.; Grassi, M.L.; Berardi, A.; Valente, D.; Tofani, M.; Paoloni, M. The Italian version of the General Sleep Disturbance Scale (GSDS-IT): Psychometric properties in a sample with hip and knee replacement. Cranio 2023, 41, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Mignolli, E.; Scialpi, A.; Valente, D.; Berardi, A.; Galeoto, G.; Tofani, M. Sleep Disturbance Scale for Children: Italian Validation in Autism Spectrum Disorder Population. Int. J. Environ. Res. Public Health 2022, 19, 10163. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.T.; McCrae, C.S.; Cheung, J.; Martin, J.L.; Harrod, C.G.; Heald, J.L.; Carden, K.A. Use of Actigraphy for the Evaluation of Sleep Disorders and Circadian Rhythm Sleep-Wake Disorders: An American Academy of Sleep Medicine Systematic Review, Meta-Analysis, and GRADE Assessment. J. Clin. Sleep Med. 2018, 14, 1209–1230. [Google Scholar] [CrossRef]
- Terwee, C.B.; Bot, S.D.M.; de Boer, M.R.; van der Windt, D.A.W.M.; Knol, D.L.; Dekker, J.; Bouter, L.M.; de Vet, H.C.W. Quality criteria were proposed for measurement properties of health status questionnaires. J. Clin. Epidemiol. 2007, 60, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Andresen, E.M. Criteria for assessing the tools of disability outcomes research. Arch. Phys. Med. Rehabil. 2000, 81 (Suppl. S2), S15–S20. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983, 148, 839–843. [Google Scholar] [CrossRef]
- Gonçalves, L.; Subtil, A.; Oliveira, M.R.; De, P.; Bermudez, Z. Roc Curve Estimation: An Overview. REVSTAT-Stat. J. 2014, 12, 1–20. [Google Scholar]
- Kurtis, M.M.; Balestrino, R.; Rodriguez-Blazquez, C.; Forjaz, M.J.; Martinez-Martin, P. A Review of Scales to Evaluate Sleep Disturbances in Movement Disorders. Front. Neurol. 2018, 9, 369. [Google Scholar] [CrossRef]
- Kataoka, H.; Saeki, K.; Kurumatani, N.; Sugie, K.; Obayashi, K. Objective sleep measures between patients with Parkinson’s disease and community-based older adults. Sleep Med. 2019, 68, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Luo, Y.-J.; Gu, H.-Y.; Wang, Y.-M.; Liu, M.-H.; Li, K.; Li, J.; Zhuang, S.; Shen, Y.; Jin, H.; et al. Sex and onset-age-related features of excessive daytime sleepiness and night-time sleep in patients with Parkinson’s disease. BMC Neurol. 2021, 21, 165. [Google Scholar] [CrossRef] [PubMed]
- Marinus, J.; Zhu, K.; Marras, C.; Aarsland, D.; van Hilten, J.J. Risk factors for non-motor symptoms in Parkinson’s disease. Lancet Neurol. 2018, 17, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Palmeri, R.; Lo Buono, V.; Bonanno, L.; Sorbera, C.; Cimino, V.; Bramanti, P.; Di Lorenzo, G.; Marino, S. Potential predictors of quality of life in Parkinson’s Disease: Sleep and mood disorders. J. Clin. Neurosci. 2019, 70, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Maggi, G.; Trojano, L.; Barone, P.; Santangelo, G. Sleep Disorders and Cognitive Dysfunctions in Parkinson’s Disease: A Meta-Analytic Study. Neuropsychol. Rev. 2021, 31, 643–682. [Google Scholar] [CrossRef]
- He, G.; Liu, C.-F.; Ye, Q.; Liu, Z.; Jin, M.; Shang, H.; Chen, L.; Tuo, H.; Jiang, H.; Cai, J.; et al. Prevalence and profile of nocturnal disturbances in Chinese patients with advanced-stage Parkinson’s disease: A cross-sectional epidemiology study. BMC Neurol. 2021, 21, 194. [Google Scholar] [CrossRef]
- Chahine, L.M.; Amara, A.W.; Videnovic, A. A systematic review of the literature on disorders of sleep and wakefulness in Parkinson’s disease from 2005 to 2015. Sleep Med. Rev. 2016, 35, 33–50. [Google Scholar] [CrossRef]

| Sample N = 350 | ||
|---|---|---|
| Age | N° | % |
| 18–30 | 1 | 0.3 |
| 31–40 | 3 | 0.9 |
| 41–50 | 30 | 8.6 |
| 51–60 | 73 | 20.9 |
| 61–70 | 121 | 34.6 |
| 71–80 | 99 | 28.3 |
| 81+ | 23 | 6.6 |
| Sex | N° | % |
| F | 169 | 48.3 |
| M | 181 | 51.7 |
| Insomnia Disorder | N° | % |
| No | 215 | 61.4 |
| Yes | 135 | 38.6 |
| Mean | Standard Deviation | Alpha Deleted | Cronbach’s Alpha | |
|---|---|---|---|---|
| item 1 | 1.87 | 1.29 | 0.815 | |
| item 2 | 1.47 | 1.37 | 0.821 | |
| item 3 | 2.27 | 1.34 | 0.811 | |
| item 4 | 1.42 | 1.39 | 0.845 | |
| item 5 | 1.21 | 1.37 | 0.806 | |
| item 6 | 1.36 | 1.29 | 0.815 | |
| item 7 | 0.55 | 1 | 0.819 | |
| item 8 | 2.61 | 1.29 | 0.829 | |
| item 9 | 1.83 | 1.51 | 0.815 | |
| item 10 | 1.32 | 1.36 | 0.806 | |
| item 11 | 1.35 | 1.25 | 0.812 | |
| item 12 | 1.58 | 1.37 | 0.810 | |
| item 13 | 0.97 | 1.27 | 0.828 | |
| item 14 | 2.19 | 1.22 | 0.814 | |
| item 15 | 0.70 | 1.03 | 0.820 | |
| Total score | 0.828 |
| Mean | Standard Deviation | Alpha Deleted | Cronbach’s Alpha | |
|---|---|---|---|---|
| Item 1 | 2.2 | 2.38 | 0.823 | |
| Item 2 | 4.86 | 2.41 | 0.823 | |
| Item 3 | 3.92 | 2.57 | 0.821 | |
| Item 4 | 2.72 | 2.26 | 0.867 | |
| Item 5 | 3.48 | 2.55 | 0.812 | |
| Item 6 | 4.03 | 2.33 | 0.813 | |
| Item 7 | 2.56 | 2.46 | 0.816 | |
| Item 8 | 2.64 | 2.3 | 0.817 | |
| Item 9 | 4.19 | 2.28 | 0.806 | |
| Item 10 | 4.12 | 2.32 | 0.808 | |
| Item 11 | 4.11 | 2.33 | 0.806 | |
| Item 12 | 4.15 | 2.3 | 0.813 | |
| Item 13 | 3.78 | 2.57 | 0.819 | |
| Item 14 | 2.83 | 2.62 | 0.841 | |
| Item 15 | 1.83 | 2.14 | 0.829 | |
| Item 16 | 0.23 | 0.93 | 0.834 | |
| Item 17 | 0.22 | 1.11 | 0.835 | |
| Item 18 | 0.95 | 2.12 | 0.833 | |
| Item 19 | 0.33 | 1.34 | 0.834 | |
| Item 20 | 2.06 | 3.06 | 0.835 | |
| Item 21 | 0.47 | 1.46 | 0.831 | |
| Total score | 0.832 |
| PDSS-2 | ||||||
|---|---|---|---|---|---|---|
| GSDS | Subscale 1 | Subscale 2 | Subscale 3 | Subscale 4 | Subscale 5 | Total |
| Subscale 1 | 0.292 ** | 0.447 ** | 0.214 ** | 0.394 ** | 0.595 ** | 0.500 ** |
| Subscale 2 | 0.375 ** | 0.568 ** | 0.293 ** | 0.637 ** | 0.332 ** | 0.596 ** |
| Subscale 3 | 0.005 | −0.047 | −0.041 | 0.157 ** | 0.163 ** | 0.058 |
| Subscale 4 | 0.268 ** | 0.515 ** | 0.238 ** | 0.346 ** | 0.286 ** | 0.432 ** |
| Subscale 5 | 0.315 ** | 0.538 ** | 0.387 ** | 0.413 ** | 0.300 ** | 0.516 ** |
| Subscale 6 | 0.151 ** | 0.290 ** | 0.194 ** | 0.218 ** | 0.216 ** | 0.275 ** |
| Total | 0.372 ** | 0.600 ** | 0.402 ** | 0.548 ** | 0.402 ** | 0.615 ** |
| Age Band | ||||||
|---|---|---|---|---|---|---|
| 31–40 | 41–50 | 51–60 | 61–70 | 71–80 | 81+ | |
| Mean ± (SD) | 25.67 (15.04) | 18.27 (10.16) | 22.93 (11.56) | 22.59 (10.37) | 24.11 (10.47) | 21.22 (7.75) |
| Median | 18.00 | 16.50 | 22.00 | 22.00 | 23.00 | 20.00 |
| Variance | 226,333 | 103,306 | 133,648 | 107,711 | 109,712 | 60,178 |
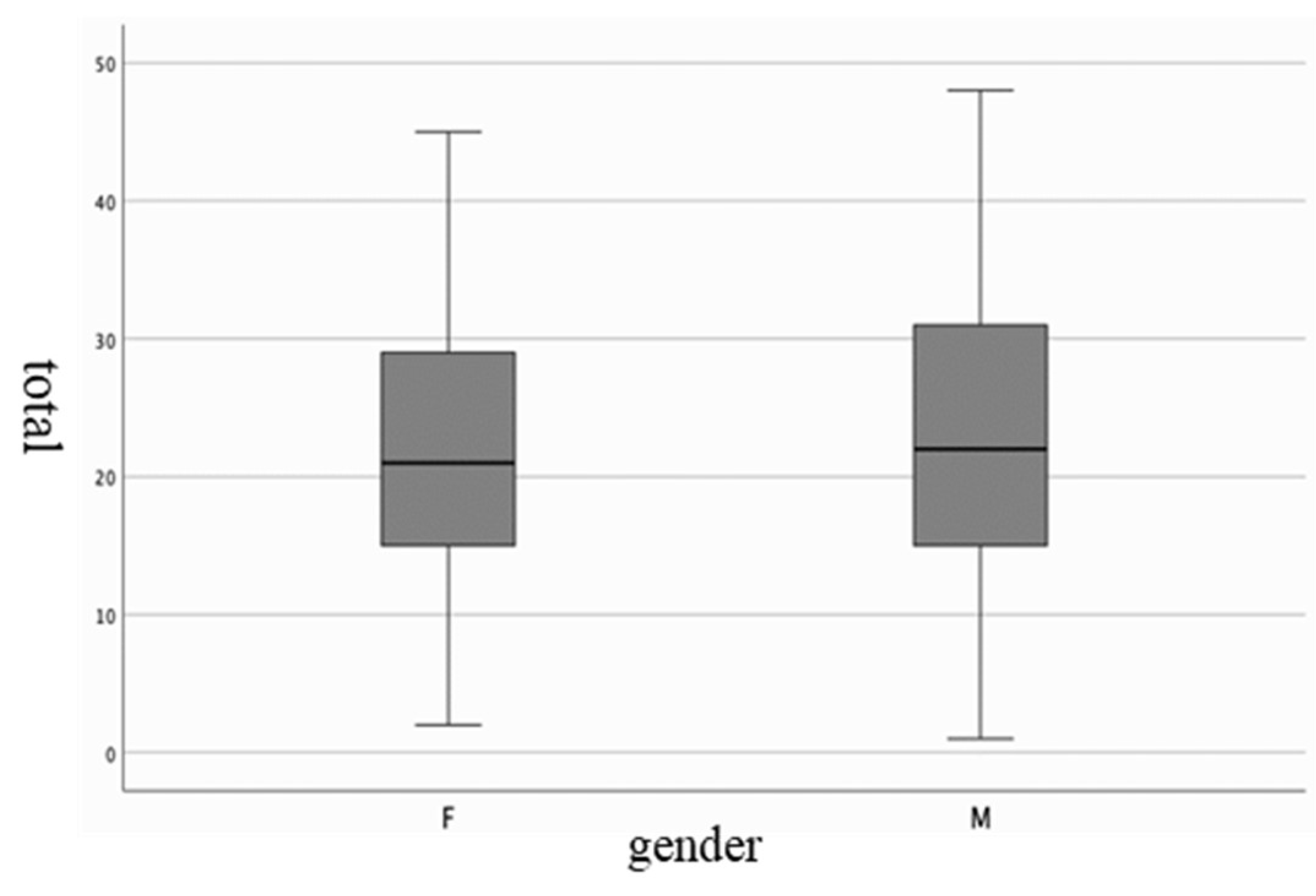
| F | M | |
|---|---|---|
| Mean ± (SD) | 22.54 (10.48) | 22.82 (10.69) |
| Median | 21.00 | 22.00 |
| Variance | 109,821 | 114,283 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liguori, C.; Frontani, F.; Francescangeli, G.; Pierantozzi, M.; Cerroni, R.; Schirinzi, T.; Stefani, A.; Mercuri, N.B.; Galeoto, G. Assessment of Psychometric Characteristics of Parkinson’s Disease Sleep Scale 2 and Analysis of a Cut-Off Score for Detecting Insomnia in Italian Patients with Parkinson’s Disease: A Validation Study. J. Pers. Med. 2024, 14, 298. https://doi.org/10.3390/jpm14030298
Liguori C, Frontani F, Francescangeli G, Pierantozzi M, Cerroni R, Schirinzi T, Stefani A, Mercuri NB, Galeoto G. Assessment of Psychometric Characteristics of Parkinson’s Disease Sleep Scale 2 and Analysis of a Cut-Off Score for Detecting Insomnia in Italian Patients with Parkinson’s Disease: A Validation Study. Journal of Personalized Medicine. 2024; 14(3):298. https://doi.org/10.3390/jpm14030298
Chicago/Turabian StyleLiguori, Claudio, Francesco Frontani, Giulia Francescangeli, Mariangela Pierantozzi, Rocco Cerroni, Tommaso Schirinzi, Alessandro Stefani, Nicola Biagio Mercuri, and Giovanni Galeoto. 2024. "Assessment of Psychometric Characteristics of Parkinson’s Disease Sleep Scale 2 and Analysis of a Cut-Off Score for Detecting Insomnia in Italian Patients with Parkinson’s Disease: A Validation Study" Journal of Personalized Medicine 14, no. 3: 298. https://doi.org/10.3390/jpm14030298
APA StyleLiguori, C., Frontani, F., Francescangeli, G., Pierantozzi, M., Cerroni, R., Schirinzi, T., Stefani, A., Mercuri, N. B., & Galeoto, G. (2024). Assessment of Psychometric Characteristics of Parkinson’s Disease Sleep Scale 2 and Analysis of a Cut-Off Score for Detecting Insomnia in Italian Patients with Parkinson’s Disease: A Validation Study. Journal of Personalized Medicine, 14(3), 298. https://doi.org/10.3390/jpm14030298









