The Correlation between Sex Hormone-Binding Globulin and Clinical Characteristics According to Anti-Müllerian Hormone in Women with Regular Menstrual Cycles: A Prospective Study
Abstract
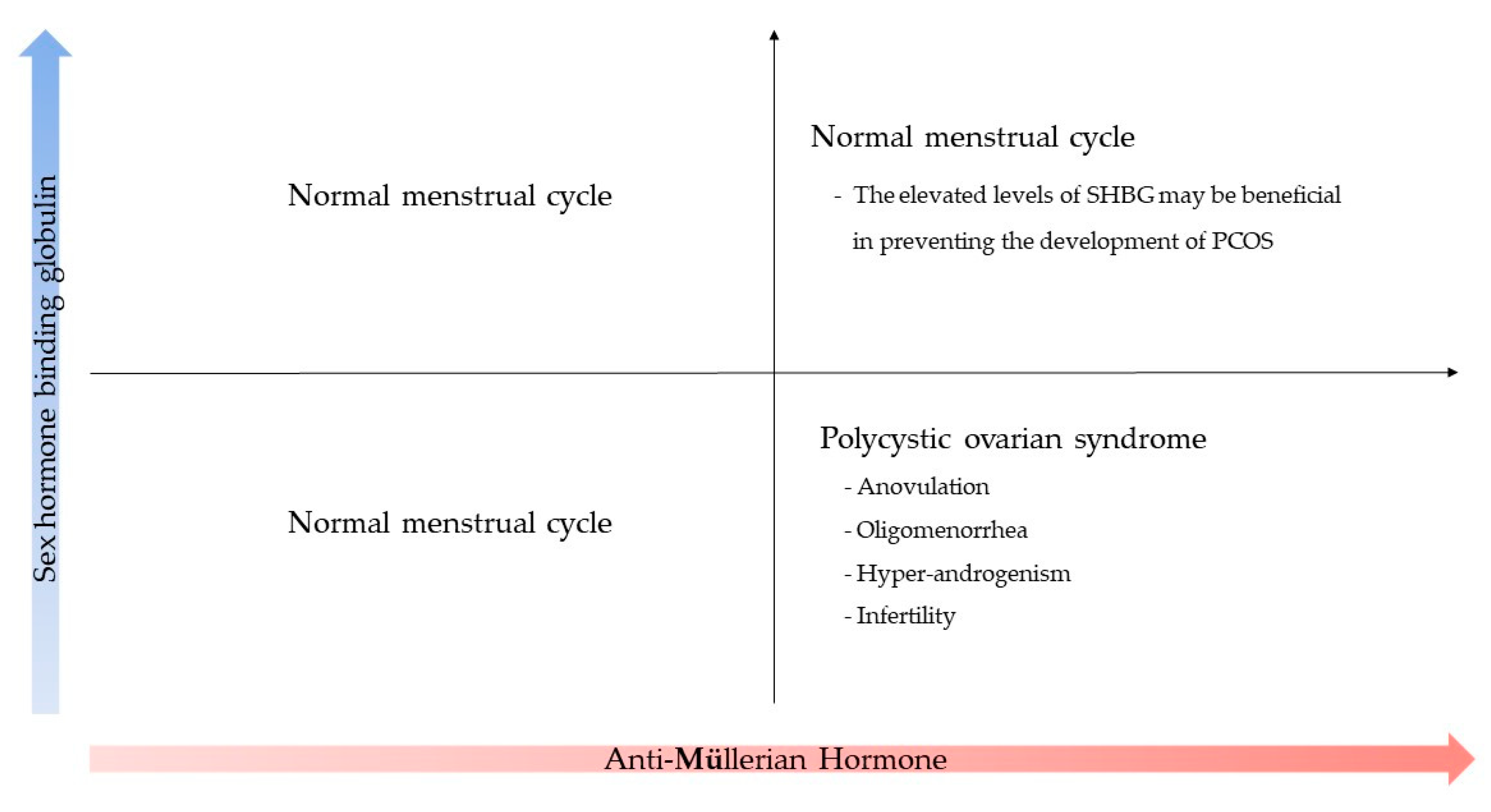
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Blood Test and Urine Pregnancy Test
2.3. Pelvic Ultrasonography
2.4. Determination of Ovulation
2.5. Determination of Luteal Phase Deficiency
2.6. Statistical Analysis
3. Results
3.1. Demographic Findings
3.2. Comparison of Blood Hormone Levels between Normal and High Groups for Anti-Müllerian Hormone
3.3. Relationship between Menstrual Cycle, Ovulation, and AMH
3.4. Relationship between Age and AMH
3.5. Body Mass Index and Anti-Müllerian Hormone Levels
3.6. Endometrial Thickness, Dominant Follicle Size, and Anti-Müllerian Hormone Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Josso, N.; Picard, J.-Y. Genetics of anti-Müllerian hormone and its signaling pathway. Best Pract. Res. Clin. Endocrinol. Metab. 2022, 36, 101634. [Google Scholar] [CrossRef] [PubMed]
- Weenen, C.; Laven, J.S.; Von Bergh, A.R.; Cranfield, M.; Groome, N.P.; Visser, J.A.; Kramer, P.; Fauser, B.C.; Themmen, A.P. Anti-Müllerian hormone expression pattern in the human ovary: Potential implications for initial and cyclic follicle recruitment. Mol. Hum. Reprod. 2004, 10, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Al Wattar, B.H.; Fisher, M.; Bevington, L.; Talaulikar, V.; Davies, M.; Conway, G.; Yasmin, E. Clinical practice guidelines on the diagnosis and management of polycystic ovary syndrome: A systematic review and quality assessment study. J. Clin. Endocrinol. Metab. 2021, 106, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Tal, R.; Seifer, D.B.; Khanimov, M.; Malter, H.E.; Grazi, R.V.; Leader, B. Characterization of women with elevated antimüllerian hormone levels (AMH): Correlation of AMH with polycystic ovarian syndrome phenotypes and assisted reproductive technology outcomes. Am. J. Obstet. Gynecol. 2014, 211, 59.e1–59.e8. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E. Polycystic ovarian syndrome: Pathophysiology, molecular aspects and clinical implications. Expert Rev. Mol. Med. 2008, 10, e3. [Google Scholar] [CrossRef] [PubMed]
- Legro, R.S.; Arslanian, S.A.; Ehrmann, D.A.; Hoeger, K.M.; Murad, M.H.; Pasquali, R.; Welt, C.K. Diagnosis and treatment of polycystic ovary syndrome: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2013, 98, 4565–4592. [Google Scholar] [CrossRef] [PubMed]
- Evliyaoglu, O.; Imöhl, M.; Weiskirchen, R.; van Helden, J. Age-specific reference values improve the diagnostic performance of AMH in polycystic ovary syndrome. Clin. Chem. Lab. Med. (CCLM) 2020, 58, 1291–1301. [Google Scholar] [CrossRef]
- Li, Y.; Fang, L.; Yan, Y.; Wang, Z.; Wu, Z.; Jia, Q.; Cheng, J.-C.; Sun, Y.-P. Association between human SHBG gene polymorphisms and risk of PCOS: A meta-analysis. Reprod. BioMed. Online 2021, 42, 227–236. [Google Scholar] [CrossRef]
- Teede, H.J.; Tay, C.T.; Laven, J.J.; Dokras, A.; Moran, L.J.; Piltonen, T.T.; Costello, M.F.; Boivin, J.; Redman, L.M.; Boyle, J.A. Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Eur. J. Endocrinol. 2023, 189, G43–G64. [Google Scholar] [CrossRef]
- Calzada, M.; López, N.; Noguera, J.A.; Mendiola, J.; Hernández, A.I.; Corbalán, S.; Sanchez, M.; Torres, A.M. AMH in combination with SHBG for the diagnosis of polycystic ovary syndrome. J. Obstet. Gynaecol. 2019, 39, 1130–1136. [Google Scholar] [CrossRef]
- Xita, N.; Tsatsoulis, A.; Chatzikyriakidou, A.; Georgiou, I. Association of the (TAAAA) n repeat polymorphism in the sex hormone-binding globulin (SHBG) gene with polycystic ovary syndrome and relation to SHBG serum levels. J. Clin. Endocrinol. Metab. 2003, 88, 5976–5980. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Young, J.R.; Jaffe, R.B. Strength-duration characteristics of estrogen effects on gonadotropin response to gonadotropin-releasing hormone in women. II. Effects of varying concentrations of estradiol. J. Clin. Endocrinol. Metab. 1976, 42, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Judd, S.; Terry, A.; Petrucco, M.; White, G. The source of pulsatile secretion of progesterone during the human follicular phase. J. Clin. Endocrinol. Metab. 1992, 74, 299–305. [Google Scholar] [PubMed]
- Pfister, A.; Crawford, N.M.; Steiner, A.Z. Association between diminished ovarian reserve and luteal phase deficiency. Fertil. Steril. 2019, 112, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Wiweko, B.; Maidarti, M.; Priangga, M.D.; Shafira, N.; Fernando, D.; Sumapraja, K.; Natadisastra, M.; Hestiantoro, A. Anti-mullerian hormone as a diagnostic and prognostic tool for PCOS patients. J. Assist. Reprod. Genet. 2014, 31, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Chua, S.; Danhof, N.; Mochtar, M.; Van Wely, M.; McLernon, D.; Custers, I.; Lee, E.; Dreyer, K.; Cahill, D.; Gillett, W. Age-related natural fertility outcomes in women over 35 years: A systematic review and individual participant data meta-analysis. Hum. Reprod. 2020, 35, 1808–1820. [Google Scholar] [CrossRef]
- Ramezani Tehrani, F.; Rahmati, M.; Mahboobifard, F.; Firouzi, F.; Hashemi, N.; Azizi, F. Age-specific cut-off levels of anti-Müllerian hormone can be used as diagnostic markers for polycystic ovary syndrome. Reprod. Biol. Endocrinol. 2021, 19, 76. [Google Scholar] [CrossRef] [PubMed]
- Meczekalski, B.; Czyzyk, A.; Kunicki, M.; Podfigurna-Stopa, A.; Plociennik, L.; Jakiel, G.; Maciejewska-Jeske, M.; Lukaszuk, K. Fertility in women of late reproductive age: The role of serum anti-Müllerian hormone (AMH) levels in its assessment. J. Endocrinol. Investig. 2016, 39, 1259–1265. [Google Scholar] [CrossRef]
- Kim, J.Y.; Tfayli, H.; Michaliszyn, S.F.; Lee, S.; Nasr, A.; Arslanian, S. Anti-Müllerian hormone in obese adolescent girls with polycystic ovary syndrome. J. Adolesc. Health 2017, 60, 333–339. [Google Scholar] [CrossRef]
- Rudnicka, E.; Kunicki, M.; Calik-Ksepka, A.; Suchta, K.; Duszewska, A.; Smolarczyk, K.; Smolarczyk, R. Anti-müllerian hormone in pathogenesis, diagnostic and treatment of PCOS. Int. J. Mol. Sci. 2021, 22, 12507. [Google Scholar] [CrossRef]
- Jamil, A.S.; Alalaf, S.K.; Al-Tawil, N.G.; Al-Shawaf, T. Comparison of clinical and hormonal characteristics among four phenotypes of polycystic ovary syndrome based on the Rotterdam criteria. Arch. Gynecol. Obstet. 2016, 293, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Pellatt, L.; Rice, S.; Mason, H.D. Anti-Müllerian hormone and polycystic ovary syndrome: A mountain too high? Reproduction 2010, 139, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Seifer, D.B.; Tal, R. Anti-Mullerian Hormone: Role in Ovarian Function and Clinical Significance; Nova Science: Hauppauge, NY, USA, 2016. [Google Scholar]
- Lin, Y.-H.; Chiu, W.-C.; Wu, C.-H.; Tzeng, C.-R.; Hsu, C.-S.; Hsu, M.-I. Antimüllerian hormone and polycystic ovary syndrome. Fertil. Steril. 2011, 96, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.-Y.; Lu, L.-k.-y.; Li, M.; Zhang, Q.-L.; Ying, C.-M. Threshold value of anti-Mullerian hormone for the diagnosis of polycystic ovary syndrome in Chinese women. PLoS ONE 2018, 13, e0203129. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, N.; Shab-Bidar, S.; Tehrani, F.R.; Mirmiran, P.; Azizi, F. Is ovarian reserve associated with body mass index and obesity in reproductive aged women? A meta-analysis. Menopause 2018, 25, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Rosenfield, R.L.; Ehrmann, D.A. The pathogenesis of polycystic ovary syndrome (PCOS): The hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef] [PubMed]
- Kiconco, S.; Teede, H.J.; Azziz, R.; Norman, R.J.; Joham, A.E. The need to reassess the diagnosis of polycystic ovary syndrome (PCOS): A review of diagnostic recommendations from the international evidence-based guideline for the assessment and management of PCOS. In Seminars in Reproductive Medicine; Thieme Medical Publishers, Inc.: New York, NY, USA, 2021; Volume 39, pp. 71–77. [Google Scholar]
- Zhu, R.; Lee, B.H.; Huang, Z.; Indran, I.R.; Li, J.; Shen, L.; Kramer, M.S.; Yong, E.L. Antimüllerian hormone, antral follicle count and ovarian volume predict menstrual cycle length in healthy women. Clin. Endocrinol. 2016, 84, 870–877. [Google Scholar] [CrossRef]
- Hammond, G.L.; Wu, T.-S.; Simard, M. Evolving utility of sex hormone-binding globulin measurements in clinical medicine. Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 183–189. [Google Scholar] [CrossRef]
- Alinezhad, A.; Jafari, F. The relationship between components of metabolic syndrome and plasma level of sex hormone-binding globulin. Eur. J. Transl. Myol. 2019, 29, 8196. [Google Scholar] [CrossRef]
- Basualto-Alarcón, C.; Llanos, P.; García-Rivas, G.; Troncoso, M.F.; Lagos, D.; Barrientos, G.; Estrada, M. Classic and novel sex hormone binding globulin effects on the cardiovascular system in men. Int. J. Endocrinol. 2021, 2021, 5527973. [Google Scholar] [CrossRef]
- Qu, X.; Donnelly, R. Sex hormone-binding globulin (SHBG) as an early biomarker and therapeutic target in polycystic ovary syndrome. Int. J. Mol. Sci. 2020, 21, 8191. [Google Scholar] [CrossRef]
- Lin, Y.-M.; Velmurugan, B.K.; Yeh, Y.-L.; Tu, C.C.; Ho, T.-J.; Lai, T.Y.; Tsai, C.-H.; Tsai, F.J.; Tsai, C.-H.; Huang, C.-Y. Activation of estrogen receptors with E2 downregulates peroxisome proliferator-activated receptor γ in hepatocellular carcinoma. Oncol. Rep. 2013, 30, 3027–3031. [Google Scholar] [CrossRef] [PubMed]
- Meenakumari, K.; Agarwal, S.; Krishna, A.; Pandey, L. Effects of metformin treatment on luteal phase progesterone concentration in polycystic ovary syndrome. Braz. J. Med. Biol. Res. 2004, 37, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, K.A. Luteal phase defect: Etiology, diagnosis, and management. Endocrinol. Metab. Clin. N. Am. 1992, 21, 85–104. [Google Scholar] [CrossRef]
- Pugeat, M.; Nader, N.; Hogeveen, K.; Raverot, G.; Déchaud, H.; Grenot, C. Sex hormone-binding globulin gene expression in the liver: Drugs and the metabolic syndrome. Mol. Cell. Endocrinol. 2010, 316, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Morgante, G.; Cappelli, V.; Troìa, L.; De Leo, V. Evaluation of different antiandrogenic progestins on clinical and biochemical variables in polycystic ovary syndrome. Eur. J. Contracept. Reprod. Health Care 2020, 25, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Assens, M.; Dyre, L.; Henriksen, L.S.; Brocks, V.; Sundberg, K.; Jensen, L.N.; Pedersen, A.T.; Main, K.M. Menstrual pattern, reproductive hormones, and transabdominal 3D ultrasound in 317 adolescent girls. J. Clin. Endocrinol. Metab. 2020, 105, e3257–e3266. [Google Scholar] [CrossRef]
- Januszewski, M.; Issat, T.; Jakimiuk, A.A.; Santor-Zaczynska, M.; Jakimiuk, A.J. Metabolic and hormonal effects of a combined Myo-inositol and d-chiro-inositol therapy on patients with polycystic ovary syndrome (PCOS). Ginekol. Pol. 2019, 90, 7–10. [Google Scholar] [CrossRef]

| Variables | Number (n = 68) | Percentage | Mean | SD |
|---|---|---|---|---|
| Age (years) | ||||
| 20–29 | 21 | 30.8 | ||
| 30–34 | 23 | 33.8 | ||
| 35–40 | 19 | 27.9 | ||
| 41–45 | 5 | 7.3 | ||
| BMI (kg/m2) | 21.97 | 3.24 | ||
| <18.5 | 5 | 7 | ||
| 18.5–22.9 | 40 | 58.8 | ||
| 23–25 | 11 | 16 | ||
| >25 | 12 | 17 | ||
| Menstrual cycle length (days) | 29.01 | 2.29 | ||
| ≤28 | 34 | 50 | ||
| >28 | 34 | 50 | ||
| AMH (ng/mL) | 4.1 | 2.61 | ||
| TSH (uIU/mL) | 1.88 | 1.02 | ||
| Prolactin (ng/mL) | 13.13 | 6.33 | ||
| Testosterone (ng/mL) | 0.32 | 0.17 | ||
| SHBG (nmol/L) | 74.68 | 36.62 | ||
| FAI | 1.7 | 1.16 | ||
| Variables | Number AMH (<4.45) (n = 39) | High AMH (≥45) (n = 39) | p Value |
|---|---|---|---|
| AMH (ng/mL) | 2.28 ± 1.28 | 6.55 ± 1.81 | <0.001 |
| Age (years) | 33.2 ± 5.14 | 32.24 ± 4.63 | 0.428 |
| BMI (kg/m2) | 22.19 ± 3.51 | 21.65 ± 2.85 | 0.501 |
| TSH (uIU/mL) | 1.81 ± 1.03 | 1.98 ± 1.01 | 0.499 |
| Prolactin (ng/mL) | 13.31 ± 7.55 | 12.89 ± 4.28 | 0.786 |
| Testosterone (ng/mL) | 0.311 ± 0.141 | 0.349 ± 0.205 | 0.389 |
| SHBG (nmol/L) | 65.46 ± 25.78 | 87.08 ± 45.05 | 0.025 |
| FAI | 1.79 ± 1.46 | 1.58 ± 1.26 | 0.23 |
| Variables | Normal AMH (n = 39) | High AMH (n = 29) | p Value |
|---|---|---|---|
| Menstrual cycle (days) | 28.5 ± 2.06 | 29.76 ± 2.44 | 0.025 |
| Ovulation rate (%) | 1 ± 0.00 | 1 ± 0.00 | |
| Expected ovulation day (MCD) | 15.69 ± 3.20 | 16.33 ± 3.69 | 0.69 |
| The length of luteal phase (days) | 12.59 ± 2.71 | 13.3 ± 2.40 | 0.324 |
| Variables | Age (<35) (n = 44) | Age (≥35) (n = 24) | p-Value |
| Age (years) | 29.75 ± 2.72 | 38.37 ± 2.53 | <0.001 |
| BMI (kg/m2) | 21.76 ± 2.99 | 22.34 ± 3.69 | 0.489 |
| AMH (ng/mL) | 4.57 ± 2.65 | 3.24 ± 2.36 | 0.044 |
| SHBG (nmol/L) | 77.34 ± 41.64 | 69.80 ± 25.02 | 0.421 |
| Below 35 years old (n = 44) | |||
| Variables | Normal AMH (n = 25) | High AMH (n = 19) | p-Value |
| Age (years) | 29.92 ± 2.73 | 29.43 ± 2.92 | 0.595 |
| AMH (ng/mL) | 2.64 ± 1.08 | 7.06 ± 1.95 | <0.001 |
| SHBG (nmol/L) | 61.33 ± 27.26 | 97.03 ± 50.81 | 0.006 |
| Menstrual cycle (days) | 28.62 ± 2.08 | 30.23 ± 2.80 | 0.045 |
| Length of luteal phase (days) | 12.67 ± 3.02 | 12.96 ± 2.97 | 0.784 |
| Variables | Normal AMH (<4.45) (n = 14) | High AMH (≥4.45) (n = 10) | p-Value |
| Age (years) | 39.07 ± 2.36 | 37.40 ± 2.54 | 0.056 |
| AMH (ng/mL) | 1.64 ± 1.41 | 5.48 ± 1.36 | <0.001 |
| SHBG (nmol/L) | 72.83 ± 21.87 | 65.55 ± 29.57 | 0.494 |
| Menstrual cycle (days) | 28.28 ± 2.09 | 28.80 ± 1.68 | 0.528 |
| Length of luteal phase (days) | 12.46 ± 2.18 | 13.87 ± 0.86 | 0.51 |
| Variables | Normal AMH (<3.72) (n = 12) | High AMH (≥3.72) (n = 12) | p-Value |
| Age (years) | 39.33 ± 2.22 | 37.42 ± 2.54 | 0.062 |
| AMH (ng/mL) | 1.24 ± 1.06 | 5.24 ± 1.36 | 0 |
| SHBG (nmol/L) | 72.13 ± 67.47 | 67.47 ± 27.95 | 0.659 |
| Menstrual cycle (days) | 27.67 ± 1.23 | 29.33 ± 2.14 | 0.032 |
| Variables | Normal BMI (<23) (n = 45) | High BMI (≥23) (n = 23) | p-Value |
| BMI (kg/m2) | 19.98 ± 1.23 | 25.76 ± 2.38 | <0.001 |
| Age (years) | 28.75 ± 2.10 | 29.54 ± 2.66 | 0.191 |
| AMH (ng/mL) | 2.81 ± 1.98 | 6.53 ± 1.90 | <0.001 |
| SHBG (nmol/L) | 73.19 ± 37.50 | 74.74 ± 33.80 | 0.869 |
| Menstrual cycle (days) | 28.75 ± 2.10 | 29.54 ± 2.66 | 0.191 |
| BMI < 23 (n = 45) | |||
| Variables | Normal AMH (<4.45) (n = 39) | High AMH (≥4.45) (n = 6) | p-Value |
| BMI (kg/m2) | 19.19 ± 0.83 | 22.08 ± 0.37 | <0.001 |
| AMH (ng/mL) | 2.58 ± 1.10 | 6.95 ± 1.52 | <0.001 |
| Age (years) | 30.11 ± 2.86 | 29.00 ± 2.91 | 0.432 |
| SHBG (nmol/L) | 62.48 ± 27.35 | 133.46 ± 60.67 | <0.001 |
| Menstrual cycle (days) | 28.55 ± 2.07 | 30.70 ± 1.30 | 0.035 |
| BMI (≥23) (n = 23) | |||
| Variables | Normal AMH (<4.45) (n = 15) | High AMH (≥4.45) (n = 8) | p-Value |
| BMI (kg/m2) | 26.22 ± 2.43 | 25.05 ± 2.26 | 0.264 |
| AMH (ng/mL) | 2.32 ± 1.25 | 6.86 ± 1.68 | <0.001 |
| Age (years) | 33.07 ± 5.86 | 33.77 ± 5.69 | 0.8 |
| SHBG (nmol/L) | 44.83 ± 17.70 | 78.53 ± 38.33 | 0.009 |
| Menstrual cycle (days) | 28.85 ± 2.38 | 30.70 ± 1.38 | 0.041 |
| Variables | Normal AMH (n = 36) | High AMH (n = 26) | p-Value |
|---|---|---|---|
| Endometrial thickness (mm) | 12.49 ± 2.79 | 12.12 ± 2.02 | 0.567 |
| Largest dominant follicle diameter (mm) | 19.85 ± 4.64 | 18.01 ± 4.10 | 0.112 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keum, J.; Kim, Y.J.; Choi, S.K.; Lee, W.M.; Bae, J. The Correlation between Sex Hormone-Binding Globulin and Clinical Characteristics According to Anti-Müllerian Hormone in Women with Regular Menstrual Cycles: A Prospective Study. J. Pers. Med. 2024, 14, 274. https://doi.org/10.3390/jpm14030274
Keum J, Kim YJ, Choi SK, Lee WM, Bae J. The Correlation between Sex Hormone-Binding Globulin and Clinical Characteristics According to Anti-Müllerian Hormone in Women with Regular Menstrual Cycles: A Prospective Study. Journal of Personalized Medicine. 2024; 14(3):274. https://doi.org/10.3390/jpm14030274
Chicago/Turabian StyleKeum, Jihyun, Yong Jin Kim, Sae Kyung Choi, Won Moo Lee, and Jaeman Bae. 2024. "The Correlation between Sex Hormone-Binding Globulin and Clinical Characteristics According to Anti-Müllerian Hormone in Women with Regular Menstrual Cycles: A Prospective Study" Journal of Personalized Medicine 14, no. 3: 274. https://doi.org/10.3390/jpm14030274
APA StyleKeum, J., Kim, Y. J., Choi, S. K., Lee, W. M., & Bae, J. (2024). The Correlation between Sex Hormone-Binding Globulin and Clinical Characteristics According to Anti-Müllerian Hormone in Women with Regular Menstrual Cycles: A Prospective Study. Journal of Personalized Medicine, 14(3), 274. https://doi.org/10.3390/jpm14030274






