Risk Factors for Recurrence of Primary Sclerosing Cholangitis after Liver Transplantation: Single-Center Data
Abstract
1. Introduction
2. Materials and Methods
3. Results
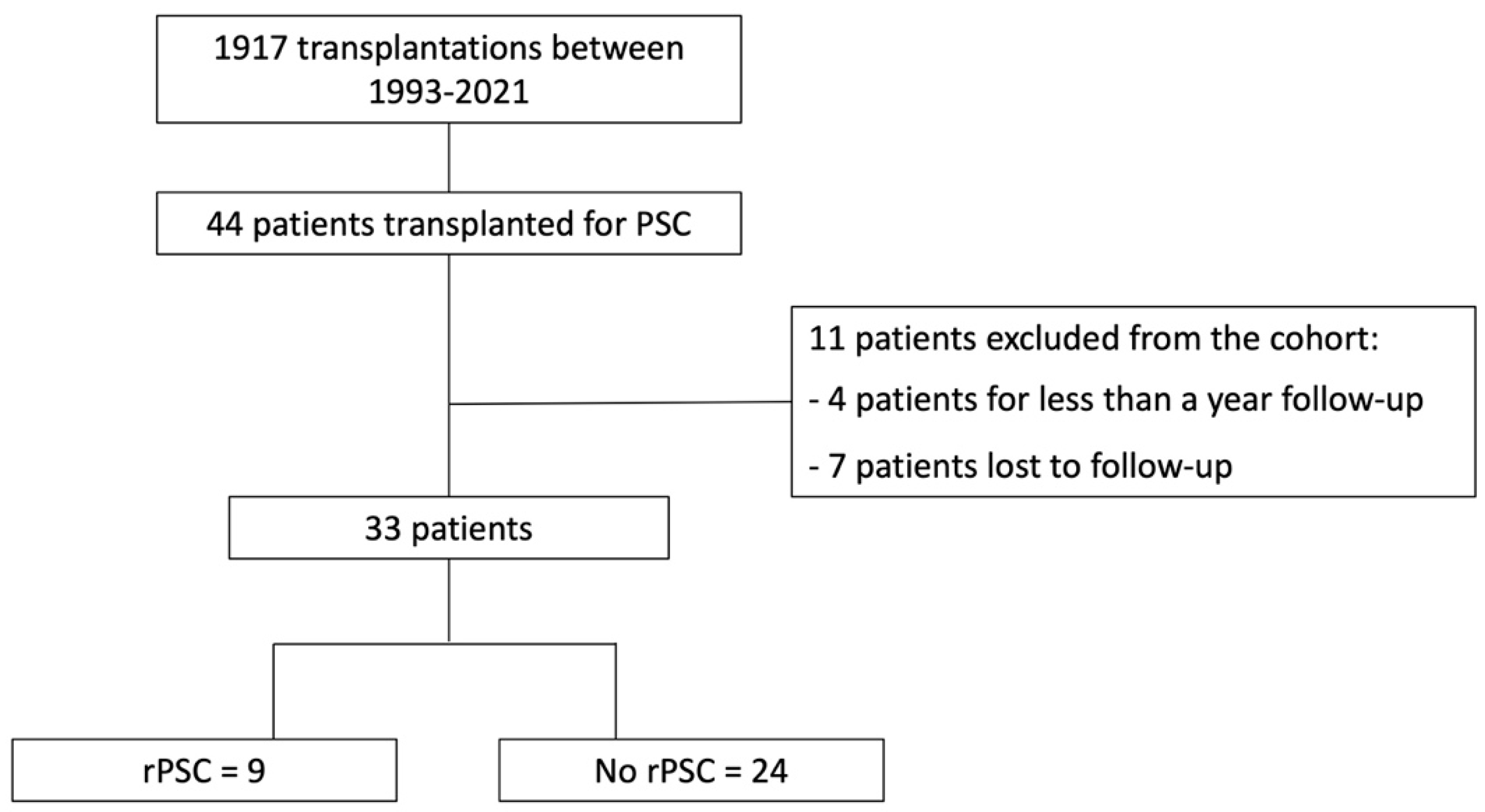
3.1. Patient Population
3.2. Transplant Features
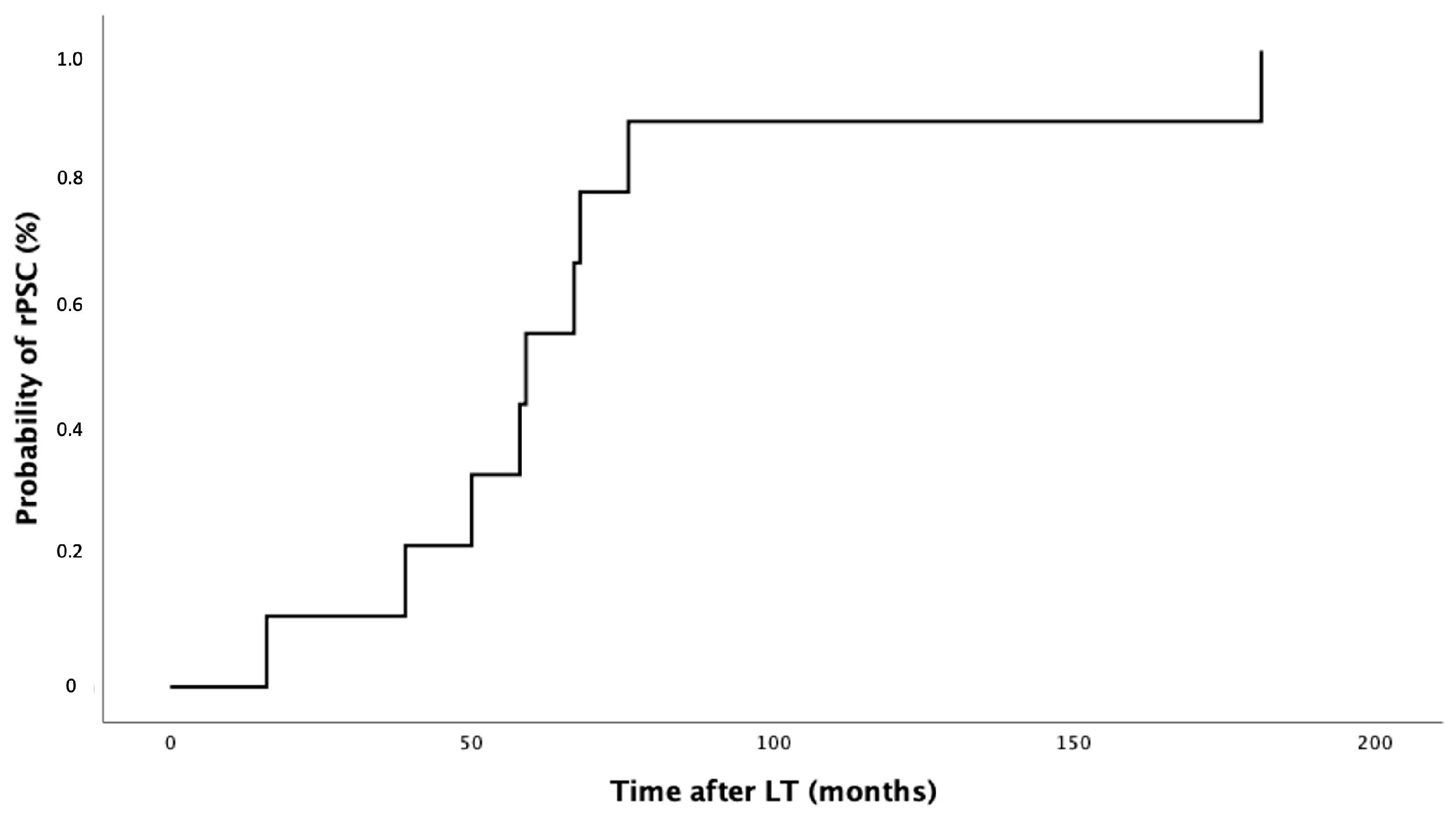
3.3. PSC Recurrence after LT
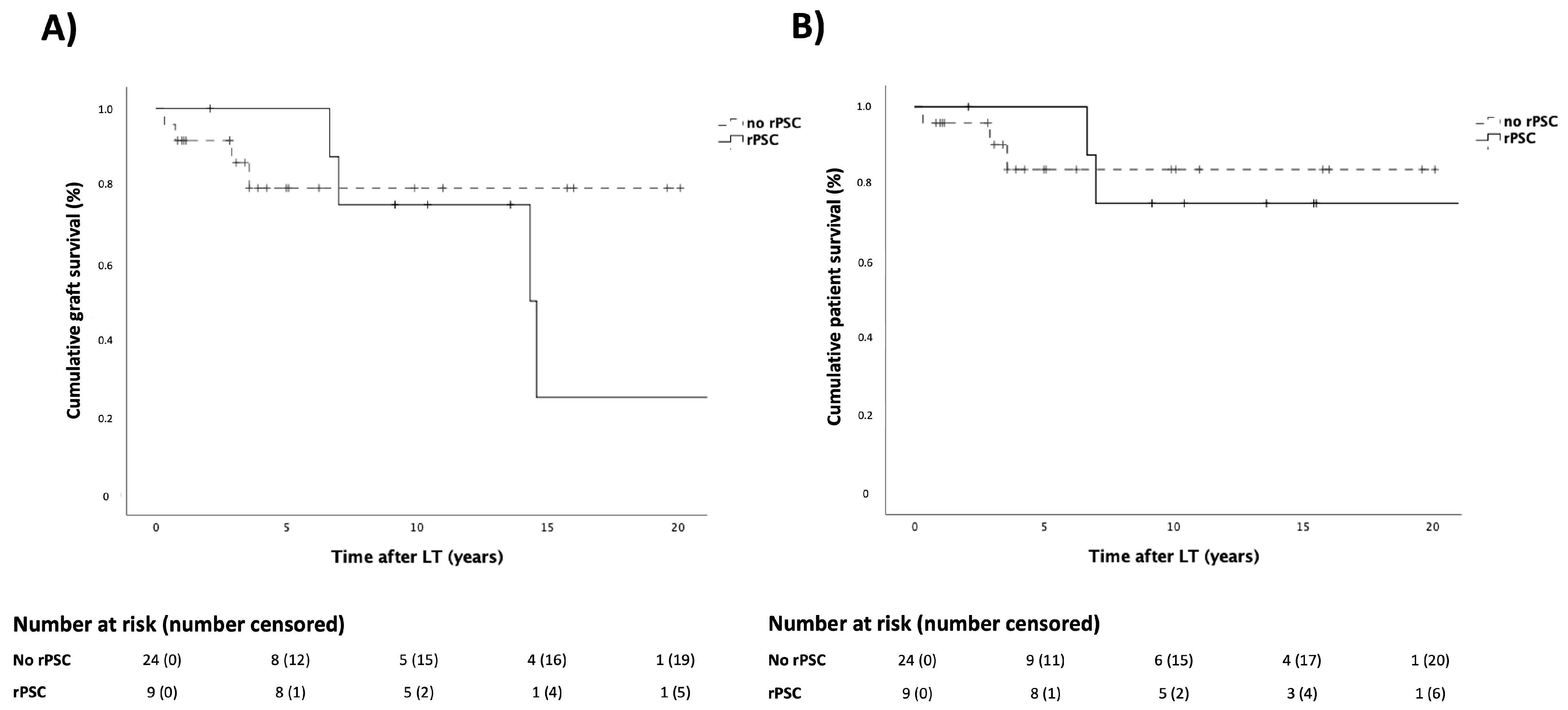
3.4. Patient and Graft Survival
3.5. Recipient, Donor and Transplant Features Risks of rPSC
3.6. IBD Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Tabibian, J.H.; Ali, A.H.; Lindor, K.D. Primary Sclerosing Cholangitis, Part 1: Epidemiology, Etiopathogenesis, Clinical Features, and Treatment. Gastroenterol. Hepatol. 2018, 14, 293–304. [Google Scholar]
- Hirschfield, G.M.; Karlsen, T.H.; Lindor, K.D.; Adams, D.H. Primary Sclerosing Cholangitis. Lancet 2013, 382, 1587–1599. [Google Scholar] [CrossRef]
- Weismüller, T.J.; Trivedi, P.J.; Bergquist, A.; Imam, M.; Lenzen, H.; Ponsioen, C.Y.; Holm, K.; Gotthardt, D.; Färkkilä, M.A.; Marschall, H.-U.; et al. Patient Age, Sex, and Inflammatory Bowel Disease Phenotype Associate With Course of Primary Sclerosing Cholangitis. Gastroenterology 2017, 152, 1975–1984.e8. [Google Scholar] [CrossRef] [PubMed]
- de Vries, A.B. Distinctive Inflammatory Bowel Disease Phenotype in Primary Sclerosing Cholangitis. World J. Gastroenterol. 2015, 21, 1956. [Google Scholar] [CrossRef] [PubMed]
- Tischendorf, J.J.W.; Hecker, H.; Krüger, M.; Manns, M.P.; Meier, P.N. Characterization, Outcome, and Prognosis in 273 Patients with Primary Sclerosing Cholangitis: A Single Center Study. Am. J. Gastroenterol. 2007, 102, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Eaton, J.E.; Talwalkar, J.A.; Lazaridis, K.N.; Gores, G.J.; Lindor, K.D. Pathogenesis of Primary Sclerosing Cholangitis and Advances in Diagnosis and Management. Gastroenterology 2013, 145, 521–536. [Google Scholar] [CrossRef] [PubMed]
- Bambha, K.; Kim, W.R.; Talwalkar, J.; Torgerson, H.; Benson, J.T.; Therneau, T.M.; Loftus, E.V.; Yawn, B.P.; Dickson, E.R.; Melton, L.J. Incidence, Clinical Spectrum, and Outcomes of Primary Sclerosing Cholangitis in a United States Community. Gastroenterology 2003, 125, 1364–1369. [Google Scholar] [CrossRef]
- Bergquist, A.; Ekbom, A.; Olsson, R.; Kornfeldt, D.; Lööf, L.; Danielsson, Å.; Hultcrantz, R.; Lindgren, S.; Prytz, H.; Sandberg-Gertzén, H.; et al. Hepatic and Extrahepatic Malignancies in Primary Sclerosing Cholangitis. J. Hepatol. 2002, 36, 321–327. [Google Scholar] [CrossRef]
- Hohenester, S.; Maillette de Buy Wenniger, L.; Paulusma, C.C.; van Vliet, S.J.; Jefferson, D.M.; Oude Elferink, R.P.; Beuers, U. A Biliary HCO3− Umbrella Constitutes a Protective Mechanism against Bile Acid-Induced Injury in Human Cholangiocytes. Hepatology 2012, 55, 173–183. [Google Scholar] [CrossRef]
- Rossen, N.G.; Fuentes, S.; Boonstra, K.; D’Haens, G.R.; Heilig, H.G.; Zoetendal, E.G.; de Vos, W.M.; Ponsioen, C.Y. The Mucosa-Associated Microbiota of PSC Patients Is Characterized by Low Diversity and Low Abundance of Uncultured Clostridiales II. J. Crohn’s Colitis 2015, 9, 342–348. [Google Scholar] [CrossRef]
- Grant, A. MAdCAM-1 Expressed in Chronic Inflammatory Liver Disease Supports Mucosal Lymphocyte Adhesion to Hepatic Endothelium (MAdCAM-1 in Chronic Inflammatory Liver Disease). Hepatology 2001, 33, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.B.; Gish, R.G.; Harper, A.; Davis, G.L.; Vierling, J.; Lieblein, L.; Klintmalm, G.; Blazek, J.; Hunter, R.; Punch, J. Model for End-Stage Liver Disease (MELD) Exception Guidelines: Results and Recommendations from the MELD Exception Study Group and Conference (MESSAGE) for the Approval of Patients Who Need Liver Transplantation with Diseases Not Considered by the Standard MELD Formula. Liver Transpl. 2006, 12, S128–S136. [Google Scholar] [CrossRef] [PubMed]
- Klose, J.; Klose, M.A.; Metz, C.; Lehner, F.; Manns, M.P.; Klempnauer, J.; Hoppe, N.; Schrem, H.; Kaltenborn, A. Outcome Stagnation of Liver Transplantation for Primary Sclerosing Cholangitis in the Model for End-Stage Liver Disease Era. Langenbecks Arch. Surg. 2014, 399, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Graziadei, I.W.; Wiesner, R.H.; Batts, K.P.; Marotta, P.J.; LaRusso, N.F.; Porayko, M.K.; Hay, J.E.; Gores, G.J.; Charlton, M.R.; Ludwig, J.; et al. Recurrence of Primary Sclerosing Cholangitis Following Liver Transplantation. Hepatology 1999, 29, 1050–1056. [Google Scholar] [CrossRef]
- Lindström, L.; Jørgensen, K.K.; Boberg, K.M.; Castedal, M.; Rasmussen, A.; Rostved, A.A.; Isoniemi, H.; Bottai, M.; Bergquist, A. Risk Factors and Prognosis for Recurrent Primary Sclerosing Cholangitis after Liver Transplantation: A Nordic Multicentre Study. Scand. J. Gastroenterol. 2018, 53, 297–304. [Google Scholar] [CrossRef]
- Visseren, T.; Erler, N.S.; Polak, W.G.; Adam, R.; Karam, V.; Vondran, F.W.R.; Ericzon, B.; Thorburn, D.; IJzermans, J.N.M.; Paul, A.; et al. Recurrence of Primary Sclerosing Cholangitis after Liver Transplantation—Analysing the European Liver Transplant Registry and Beyond. Transpl. Int. 2021, 34, 1455–1467. [Google Scholar] [CrossRef]
- Steenstraten, I.C.; Sebib Korkmaz, K.; Trivedi, P.J.; Inderson, A.; van Hoek, B.; Rodriguez Girondo, M.D.M.; Maljaars, P.W.J. Systematic Review with Meta-Analysis: Risk Factors for Recurrent Primary Sclerosing Cholangitis after Liver Transplantation. Aliment. Pharmacol. Ther. 2019, 49, 636–643. [Google Scholar] [CrossRef]
- Visseren, T.; Erler, N.S.; Heimbach, J.K.; Eaton, J.E.; Selzner, N.; Gulamhusein, A.; van der Heide, F.; Porte, R.J.; van Hoek, B.; Alwayn, I.P.J.; et al. Inflammatory Conditions Play a Role in Recurrence of PSC after Liver Transplantation—An International Multicentre Study. JHEP Rep. 2022, 4, 100599. [Google Scholar] [CrossRef]
- Cholongitas, E.; Shusang, V.; Papatheodoridis, G.V.; Marelli, L.; Manousou, P.; Rolando, N.; Patch, D.; Rolles, K.; Davidson, B.; Burroughs, A.K. Risk Factors for Recurrence of Primary Sclerosing Cholangitis after Liver Transplantation. Liver Transpl. 2008, 14, 138–143. [Google Scholar] [CrossRef]
- Chazouilleres, O.; Beuers, U.; Bergquist, A.; Karlsen, T.H.; Levy, C.; Samyn, M.; Schramm, C.; Trauner, M. EASL Clinical Practice Guidelines on Sclerosing Cholangitis. J. Hepatol. 2022, 77, 761–806. [Google Scholar] [CrossRef]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial Diagnosis, Monitoring of Known IBD, Detection of Complications. J. Crohn’s Colitis 2019, 13, 144–164K. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Gores, G.J.; Haddock, M.G.; Alberts, S.R.; Pedersen, R.; Kremers, W.; Nyberg, S.L.; Ishitani, M.B.; Rosen, C.B. Predictors of Disease Recurrence Following Neoadjuvant Chemoradiotherapy and Liver Transplantation for Unresectable Perihilar Cholangiocarcinoma. Transplantation 2006, 82, 1703–1707. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, M.; Di Maira, T.; Baumann, U.; Mirza, D.F.; Heneghan, M.A.; Klempnauer, J.L.; Bennet, W.; Ericzon, B.-G.; Line, P.-D.; Lodge, P.A.; et al. Characteristics, Trends, and Outcomes of Liver Transplantation for Primary Sclerosing Cholangitis in Female Versus Male Patients: An Analysis From the European Liver Transplant Registry. Transplantation 2021, 105, 2255–2262. [Google Scholar] [CrossRef] [PubMed]
- Graziadei, I.W.; Wiesner, R.H.; Marotta, P.J.; Porayko, M.K.; Hay, J.E.; Charlton, M.R.; Poterucha, J.J.; Rosen, C.B.; Gores, G.J.; LaRusso, N.F.; et al. Long-Term Results of Patients Undergoing Liver Transplantation for Primary Sclerosing Cholangitis. Hepatology 1999, 30, 1121–1127. [Google Scholar] [CrossRef]
- Gow, P.J.; Chapman, R.W. Liver Transplantation for Primary Sclerosing Cholangitis: Transplantation for PSC. Liver 2000, 20, 97–103. [Google Scholar] [CrossRef]
- Wiesner, R.H. Liver Transplantation for Primary Sclerosing Cholangitis: Timing, Outcome, Impact of Inflammatory Bowel Disease and Recurrence of Disease. Best Pract. Res. Clin. Gastroenterol. 2001, 15, 667–680. [Google Scholar] [CrossRef]
- Hildebrand, T.; Pannicke, N.; Dechene, A.; Gotthardt, D.N.; Kirchner, G.; Reiter, F.P.; Sterneck, M.; Herzer, K.; Lenzen, H.; Rupp, C.; et al. Biliary Strictures and Recurrence after Liver Transplantation for Primary Sclerosing Cholangitis: A Retrospective Multicenter Analysis: Biliary Strictures and PSC Recurrence. Liver Transpl. 2016, 22, 42–52. [Google Scholar] [CrossRef]
- Henson, J.B.; Patel, Y.A.; King, L.Y.; Zheng, J.; Chow, S.; Muir, A.J. Outcomes of Liver Retransplantation in Patients with Primary Sclerosing Cholangitis. Liver Transpl. 2017, 23, 769–780. [Google Scholar] [CrossRef]
- Fosby, B. Recurrence and Rejection in Liver Transplantation for Primary Sclerosing Cholangitis. World J. Gastroenterol. 2012, 18, 1. [Google Scholar] [CrossRef]
- Gordon, F.D.; Goldberg, D.S.; Goodrich, N.P.; Lok, A.S.F.; Verna, E.C.; Selzner, N.; Stravitz, R.T.; Merion, R.M. Recurrent Primary Sclerosing Cholangitis in the Adult-to-Adult Living Donor Liver Transplantation Cohort Study: Comparison of Risk Factors between Living and Deceased Donor Recipients. Liver Transpl. 2016, 22, 1214–1222. [Google Scholar] [CrossRef]
- Alabraba, E.; Nightingale, P.; Gunson, B.; Hubscher, S.; Olliff, S.; Mirza, D.; Neuberger, J. A Re-Evaluation of the Risk Factors for the Recurrence of Primary Sclerosing Cholangitis in Liver Allografts: Recurrence of Primary Sclerosing Cholangitis. Liver Transpl. 2009, 15, 330–340. [Google Scholar] [CrossRef]
- Lai, Q.; Giovanardi, F.; Melandro, F.; Laureiro, Z.L.; Merli, M.; Lattanzi, B.; Hassan, R.; Rossi, M.; Mennini, G. Donor-to-Recipient Gender Match in Liver Transplantation: A Systematic Review and Meta-Analysis. World J. Gastroenterol. 2018, 24, 2203–2210. [Google Scholar] [CrossRef]
- Stahl, J.E.; Kreke, J.E.; Malek, F.A.A.; Schaefer, A.J.; Vacanti, J. Consequences of Cold-Ischemia Time on Primary Nonfunction and Patient and Graft Survival in Liver Transplantation: A Meta-Analysis. PLoS ONE 2008, 3, e2468. [Google Scholar] [CrossRef]
- Kang, K.J. Mechanism of Hepatic Ischemia/Reperfusion Injury and Protection against Reperfusion Injury. Transplant. Proc. 2002, 34, 2659–2661. [Google Scholar] [CrossRef]
- Ben-Ari, Z. Intrahepatic Cholestasis after Liver Transplantation. Liver Transpl. 2003, 9, 1005–1018. [Google Scholar] [CrossRef]
- Tian, F.; Cheng, L.; Li, D.; Liu, Z.; Zeng, Y.; Xu, J.; Li, X.; Wang, S. Downregulation of Mucins in Graft Bile Ducts After Liver Transplantation in Rats. Transplantation 2011, 92, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Briceño, J.; Marchal, T.; Padillo, J.; Solórzano, G.; Pera, C. Influence of Marginal Donors on Liver Preservation Injury. Transplantation 2002, 74, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Heidenhain, C.; Pratschke, J.; Puhl, G.; Neumann, U.; Pascher, A.; Veltzke-Schlieker, W.; Neuhaus, P. Incidence of and Risk Factors for Ischemic-Type Biliary Lesions Following Orthotopic Liver Transplantation. Transpl. Int. 2010, 23, 14–22. [Google Scholar] [CrossRef]
- Sutton, M.E.; Bense, R.D.; Lisman, T.; van der Jagt, E.J.; van den Berg, A.P.; Porte, R.J. Duct-to-Duct Reconstruction in Liver Transplantation for Primary Sclerosing Cholangitis Is Associated with Fewer Biliary Complications in Comparison with Hepaticojejunostomy: Biliary Reconstruction in Liver Transplantation for PSC. Liver Transpl. 2014, 20, 457–463. [Google Scholar] [CrossRef]
- Wells, M.M.; Croome, K.P.; Boyce, E.; Chandok, N. Roux-En-Y Choledochojejunostomy Versus Duct-to-Duct Biliary Anastomosis in Liver Transplantation for Primary Sclerosing Cholangitis: A Meta-Analysis. Transplant. Proc. 2013, 45, 2263–2271. [Google Scholar] [CrossRef] [PubMed]
- Pandanaboyana, S.; Bell, R.; Bartlett, A.J.; McCall, J.; Hidalgo, E. Meta-Analysis of Duct-to-Duct versus Roux-En-Y Biliary Reconstruction Following Liver Transplantation for Primary Sclerosing Cholangitis. Transpl. Int. 2015, 28, 485–491. [Google Scholar] [CrossRef]
- Welsh, F.K.S.; Wigmore, S.J. ROUX-EN-Y Choledochojejunostomy Is the Method of Choice for Biliary Reconstruction in Liver Transplantation for Primary Sclerosing Cholangitis. Transplantation 2004, 77, 602–604. [Google Scholar] [CrossRef]
- Buis, C.I.; Verdonk, R.C.; Van Der Jagt, E.J.; Van Der Hilst, C.S.; Slooff, M.J.H.; Haagsma, E.B.; Porte, R.J. Nonanastomotic Biliary Strictures after Liver Transplantation, Part 1: Radiological Features and Risk Factors for Early vs. Late Presentation. Liver Transpl. 2007, 13, 708–718. [Google Scholar] [CrossRef]
- Guichelaar, M.M.J.; Benson, J.T.; Malinchoc, M.; Krom, R.A.F.; Wiesner, R.H.; Charlton, M.R. Risk Factors for and Clinical Course of Non-Anastomotic Biliary Strictures After Liver Transplantation. Am. J. Transplant. 2003, 3, 885–890. [Google Scholar] [CrossRef]
- Chuang, J.-H.; Chen, W.-J.; Lee, S.-Y.; Chang, N.-K. Prompt Colonization of the Hepaticojejunostomy and Translocation of Bacteria to Liver after Bile Duct Reconstruction. J. Pediatr. Surg. 1998, 33, 1215–1218. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Loftus, E.V.; Talwalkar, J.A. Inflammatory Bowel Disease after Liver Transplantation for Primary Sclerosing Cholangitis. Am. J. Gastroenterol. 2013, 108, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Peverelle, M.; Paleri, S.; Hughes, J.; De Cruz, P.; Gow, P.J. Activity of Inflammatory Bowel Disease After Liver Transplantation for Primary Sclerosing Cholangitis Predicts Poorer Clinical Outcomes. Inflamm. Bowel Dis. 2020, 26, 1901–1908. [Google Scholar] [CrossRef] [PubMed]
- Quraishi, M.N.; Sergeant, M.; Kay, G.; Iqbal, T.; Chan, J.; Constantinidou, C.; Trivedi, P.; Ferguson, J.; Adams, D.H.; Pallen, M.; et al. The Gut-Adherent Microbiota of PSC–IBD Is Distinct to That of IBD. Gut 2017, 66, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Kummen, M.; Holm, K.; Anmarkrud, J.A.; Nygård, S.; Vesterhus, M.; Høivik, M.L.; Trøseid, M.; Marschall, H.-U.; Schrumpf, E.; Moum, B.; et al. The Gut Microbial Profile in Patients with Primary Sclerosing Cholangitis Is Distinct from Patients with Ulcerative Colitis without Biliary Disease and Healthy Controls. Gut 2017, 66, 611. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, K.K.; Lindström, L.; Cvancarova, M.; Karlsen, T.H.; Castedal, M.; Friman, S.; Schrumpf, E.; Foss, A.; Isoniemi, H.; Nordin, A.; et al. Immunosuppression After Liver Transplantation for Primary Sclerosing Cholangitis Influences Activity of Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2013, 11, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Åberg, F.; Sallinen, V.; Tuominen, S.; Adam, R.; Karam, V.; Mirza, D.; Heneghan, M.A.; Line, P.-D.; Bennet, W.; Ericzon, B.-G.; et al. Cyclosporine vs. Tacrolimus after Liver Transplantation for Primary Sclerosing Cholangitis—A Propensity Score-Matched Intention-to-Treat Analysis. J. Hepatol. 2024, 80, 99–108. [Google Scholar] [CrossRef] [PubMed]
| Patients, n Median (IQR), n (%) | Cohort (n = 33) | rPSC (n = 9) | No rPSC (n = 24) | p-Value |
|---|---|---|---|---|
| Recipient features | ||||
| Recipient gender (male) | 16 (48) | 5 (56) | 12 (50) | 1.000 |
| Recipient age (years) | 44 (31.3–56.7) | 37.0 (27–47.5) | 45.0 (31.0–52.8) | 0.290 |
| MELD score at LT | 17 (10.5–26.0) ¶ | 19.5 (10.5–26.0) | 17 (9.0–27.0) | 0.866 |
| Variant syndromes | 8 (24) | 3 (33) | 5 (21) | 0.651 |
| Cirrhotic evolution | 25 (76) | 7 (78) | 18 (75) | 1.000 |
| Recurrent cholangitis before LT | 16 (48) | 4 (44) | 12 (50) | 1.000 |
| Autoimmune overlap | 6 (18) | 2 (22) | 4 (17) | 1.000 |
| Donor features | ||||
| Donor gender (male) | 17 (52) § | 2 (22) | 15 (65) | 0.049 |
| Donor age (years) | 52.0 (26.5–68.6) § | 55.0 (21.0–67.5) | 52.0 (34.0–69.0) | 0.773 |
| Gender mismatch | 13 (41) § | 4 (44) | 9 (39) | 1.000 |
| Ischemia time (h) | ||||
| CIT | 8.0 (6.3–9.0) § | 9.1 (8.3–9.5) | 7.3 (6.0–8.8) | 0.026 |
| Split graft | 4 (12) | 1 (11) | 3 (13) | 1.000 |
| Type of anastomosis | ||||
| 14 (42) | 7 (78) | 7 (29) | 0.019 |
| 19 (58) | 2 (22) | 17 (71) | |
| Acute rejection | 5 (15) | 1 (11) | 4 (17) | 1.000 |
| Steroid maintenance therapy | 10 (30) | 2 (22) | 8 (33) | 0.669 |
| Patients, n Median (IQR), n (%) | Cohort (n = 33) | rPSC (n = 9) | No rPSC (n = 24) | p-Value |
|---|---|---|---|---|
| Diagnosis of IBD | 24 (73) | 9 (100) | 15 (63) | 0.039 |
| 21 (88) | |||
| 3 (12) | |||
| Type of IBD | ||||
| 16 (67) | |||
| 8 (33) | |||
| 5-aminosalicylic acid treatment before LT | 16 (67) | 5 (56) | 11 (46) | 0.708 |
| AZA treatment before LT | 5 (21) | 1 (11) | 4 (17) | 1.000 |
| Biologic treatment before LT | 4 (17) | 1 (11) | 3 (13) | 1.000 |
| History of IBD reactivation after LT | 12 (50) | 7 (78) | 5 (21) | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catanzaro, E.; Gringeri, E.; Cazzagon, N.; Floreani, A.; Cillo, U.; Burra, P.; Gambato, M. Risk Factors for Recurrence of Primary Sclerosing Cholangitis after Liver Transplantation: Single-Center Data. J. Pers. Med. 2024, 14, 222. https://doi.org/10.3390/jpm14030222
Catanzaro E, Gringeri E, Cazzagon N, Floreani A, Cillo U, Burra P, Gambato M. Risk Factors for Recurrence of Primary Sclerosing Cholangitis after Liver Transplantation: Single-Center Data. Journal of Personalized Medicine. 2024; 14(3):222. https://doi.org/10.3390/jpm14030222
Chicago/Turabian StyleCatanzaro, Elisa, Enrico Gringeri, Nora Cazzagon, Annarosa Floreani, Umberto Cillo, Patrizia Burra, and Martina Gambato. 2024. "Risk Factors for Recurrence of Primary Sclerosing Cholangitis after Liver Transplantation: Single-Center Data" Journal of Personalized Medicine 14, no. 3: 222. https://doi.org/10.3390/jpm14030222
APA StyleCatanzaro, E., Gringeri, E., Cazzagon, N., Floreani, A., Cillo, U., Burra, P., & Gambato, M. (2024). Risk Factors for Recurrence of Primary Sclerosing Cholangitis after Liver Transplantation: Single-Center Data. Journal of Personalized Medicine, 14(3), 222. https://doi.org/10.3390/jpm14030222








