Developing a Model for Quantifying QTc-Prolongation Risk to Enhance Medication Safety Assessment: A Retrospective Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Settings
2.2. Patient Population
2.3. Design of the Models
3. Results
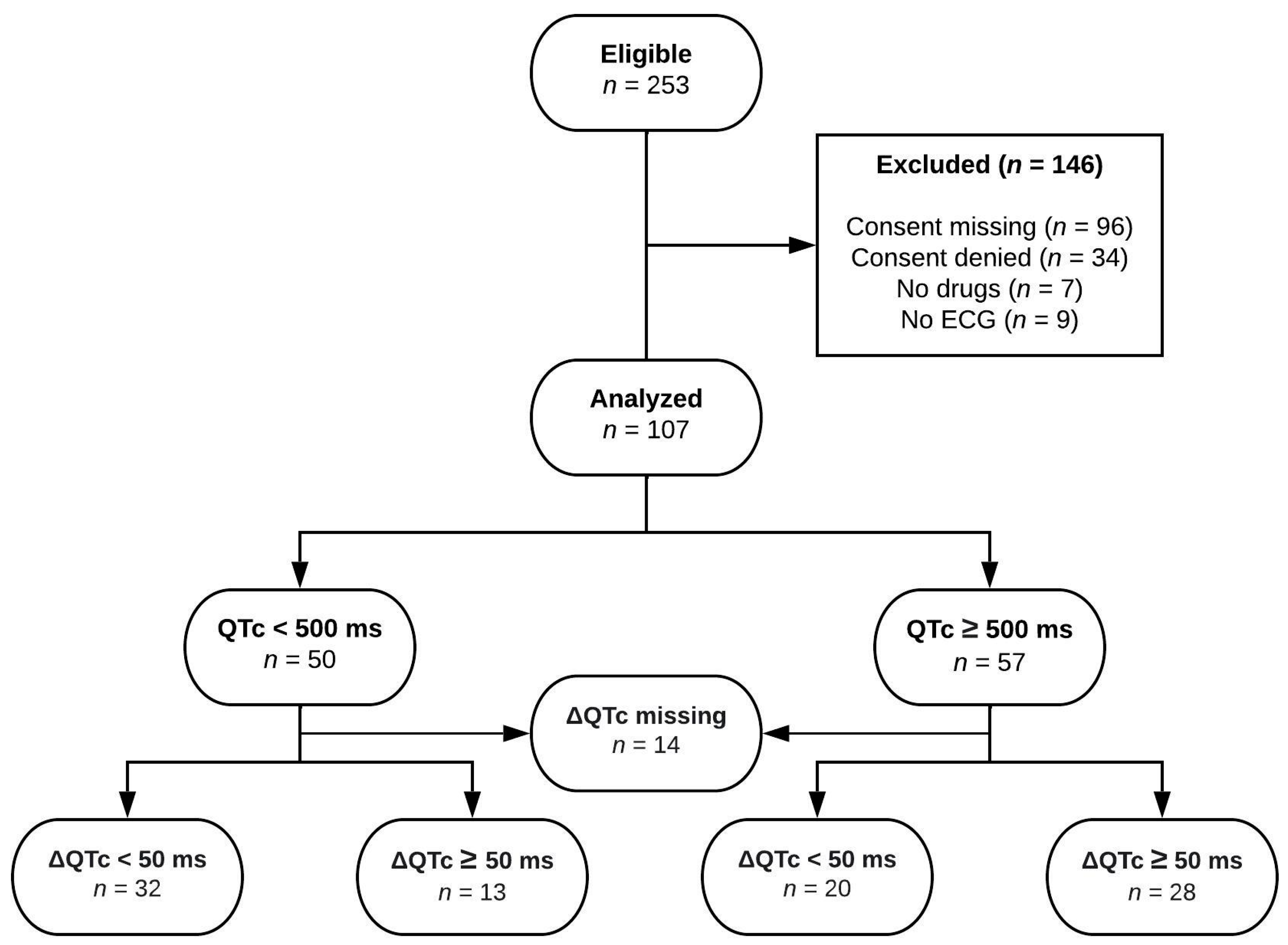
3.1. Patients
3.2. Final Models
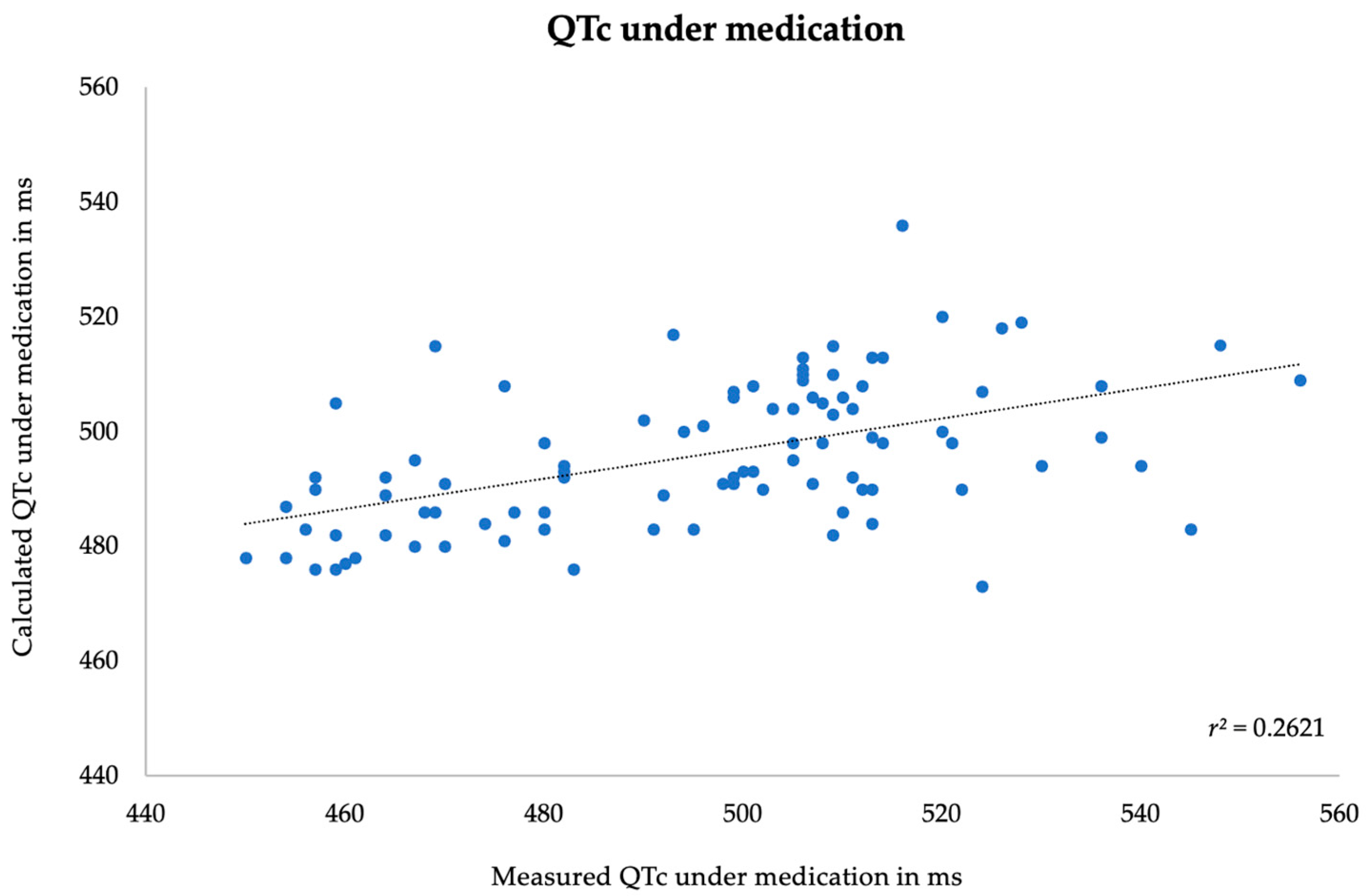
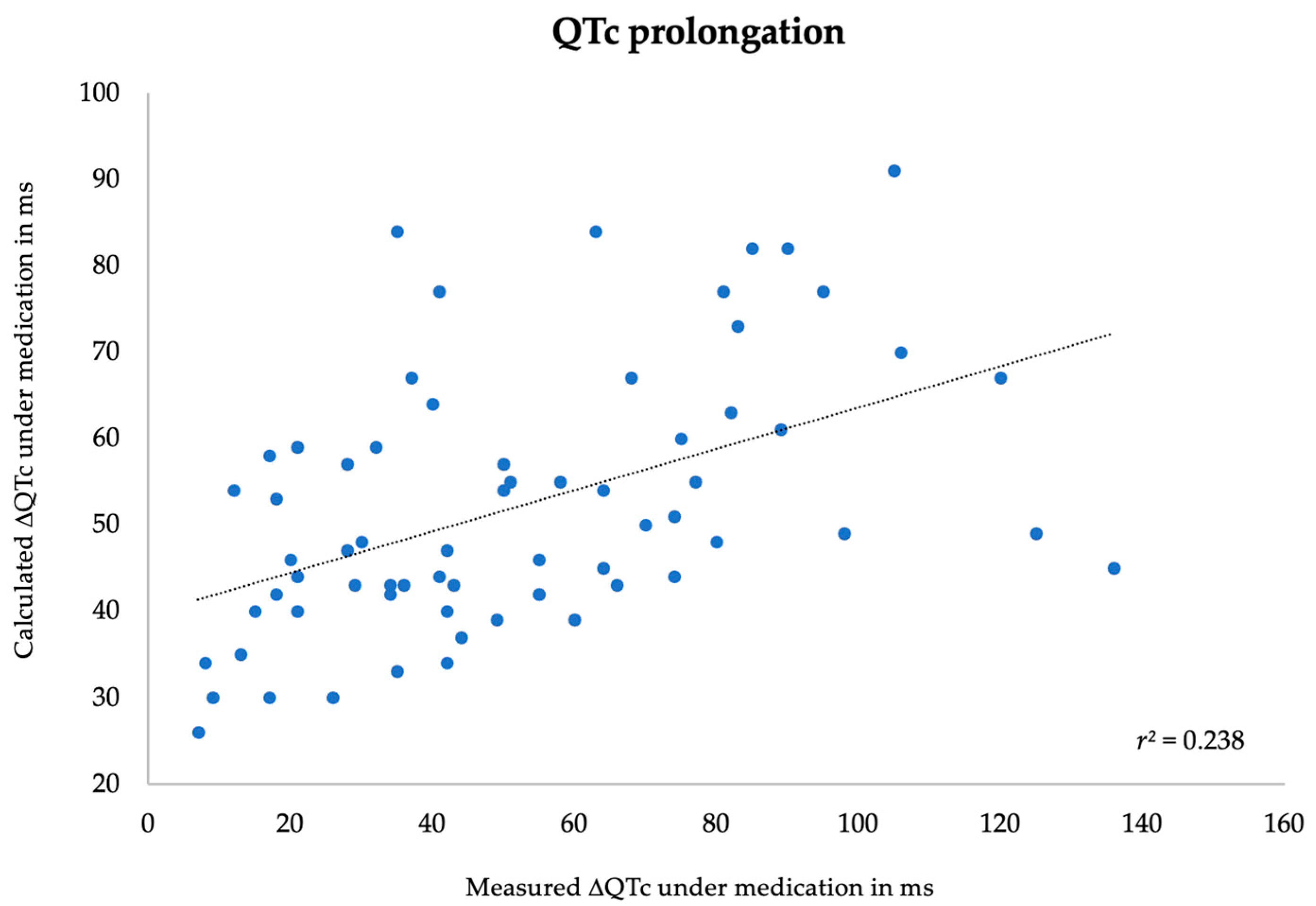
3.3. Correlation between Measured and Predicted Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rautaharju, P.M.; Surawicz, B.; Gettes, L.S. AHA/ACCF/HRS Recommendations for the Standardization and Interpretation of the Electrocardiogram. Part IV: The ST Segment, T and U Waves, and the QT Interval A Scientific Statement From the American Heart Association Electrocardiography and Arrhythmias Co. J. Am. Coll. Cardiol. 2009, 53, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, J.E.; Jaynes, H.A.; Kingery, J.R.; Mourad, N.A.; Trujillo, T.N.; Overholser, B.R.; Kovacs, R.J. Development and Validation of a Risk Score to Predict QT Interval Prolongation in Hospitalized Patients. Circ. Cardiovasc. Qual. Outcomes 2013, 6, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Heemskerk, C.P.M.; Pereboom, M.; van Stralen, K.; Berger, F.A.; van den Bemt, P.M.L.A.; Kuijper, A.F.M.; van der Hoeven, R.T.; Mantel-Teeuwisse, A.K.; Becker, M.L. Risk factors for QTc interval prolongation. Eur. J. Clin. Pharmacol. 2018, 74, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, K.; Lakshmanan, S.; Maan, A.; Kumar, N.; Dominic, P. Impact of Drug Induced Long QT Syndrome: A Systematic Review. J. Clin. Med. Res. 2018, 10, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatib, S.M.; Lapointe, N.M.A.; Kramer, J.M.; Califf, R.M. What Clinicians Should Know About the QT Interval. JAMA 2003, 289, 2120–2127. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, R.; Gray, A.; Nabeebaccus, A.; Whyte, M.B. Incidence and outcomes of long QTc in acute medical admissions. Int. J. Clin. Pract. 2018, 72, e13250. [Google Scholar] [CrossRef]
- Khatib, R.; Sabir, F.R.N.; Omari, C.; Pepper, C.; Tayebjee, M.H. Managing drug-induced QT prolongation in clinical practice. Postgrad. Med. J. 2021, 97, 452–458. [Google Scholar] [CrossRef]
- Bindraban, A.N.; Rolvink, J.; Berger, F.A.; van den Bemt, P.M.L.A.; Kuijper, A.F.M.; van der Hoeven, R.T.M.; Mantel-Teeuwisse, A.K.; Becker, M.L. Development of a risk model for predicting QTc interval prolongation in patients using QTc-prolonging drugs. Int. J. Clin. Pharm. 2018, 40, 1372–1379. [Google Scholar] [CrossRef]
- Berger, F.A.; van der Sijs, H.; van Gelder, T.; van den Bemt, P.M.L.A. The use of a clinical decision support tool to assess the risk of QT drug–drug interactions in community pharmacies. Ther. Adv. Drug. Saf. 2021, 12, 2042098621996098. [Google Scholar] [CrossRef]
- Van Der Sijs, H.; Aarts, J.; Vulto, A.; Berg, M. Overriding of drug safety alerts in computerized physician order entry. J. Am. Med. Inform. Assoc. 2006, 13, 138–147. [Google Scholar] [CrossRef]
- Sigman, M. Introduction: Personalized medicine: What is it and what are the challenges? Fertil Steril. 2018, 109, 944–945. [Google Scholar] [CrossRef] [PubMed]
- Goetz, L.H.; Schork, N.J. Personalized medicine: Motivation, challenges, and progress. Fertil. Steril. 2018, 109, 952–963. [Google Scholar] [CrossRef]
- Anonymous. QT interval and drug therapy. Br. Med. J. 2016, 2732, 1–5. [Google Scholar]
- Ho, D.S.W.; Zecchin, R.P.; Richards, D.A.B.; Uther, J.B.; Ross, D.L. Double-blind trial of lignocaine versus sotalol for acute termination of spontaneous sustained ventricular tachycardia. Lancet 1994, 344, 18–23. [Google Scholar] [CrossRef]
- Frick, S.; Müller, D.; Kullak-Ublick, G.A.; Jetter, A. Do clinical and laboratory parameters predict thiopurine metabolism and clinical outcome in patients with inflammatory bowel diseases? Eur. J. Clin. Pharmacol. 2019, 75, 335–342. [Google Scholar] [CrossRef]
- Woosley, R.L.; Black, K.; Heise, C.W.; Romero, K. CredibleMeds.org: What does it offer? Trends Cardiovasc. Med. 2018, 28, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Vandael, E.; Vandenberk, B.; Vandenberghe, J.; Willems, R.; Foulon, V. Risk factors for QTc-prolongation: Systematic review of the evidence. Int. J. Clin. Pharm. 2017, 39, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F., III; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Johnson, J.N.; Ackerman, M.J. QTc: How long is too long? Br. J. Sports Med. 2009, 43, 657–662. [Google Scholar] [CrossRef]
- Laerd Statistics. One-Way ANOVA in SPSS Statistics. Available online: https://statistics.laerd.com/spss-tutorials/one-way-anova-using-spss-statistics.php (accessed on 9 May 2022).
- Portet, S. A primer on model selection using the Akaike Information Criterion. Infect. Dis. Model. 2020, 5, 111–128. [Google Scholar] [CrossRef]
- Rabe-Hesketh, S.; Skrondal, A. Generalized Linear Mixed Models. In International Encyclopedia of Education, 3rd ed.; Peterson, P., Baker, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 171–177. [Google Scholar]
- Chaudhry, G.M.; Haffajee, C.I. Antiarrhythmic agents and proarrhythmia. Crit. Care Med. 2000, 28 (Suppl. S10), N158–N164. [Google Scholar] [CrossRef] [PubMed]
- Haugaa, K.H.; Bos, J.M.; Tarrell, R.F.; Morlan, B.W.; Caraballo, P.J.; Ackerman, M.J. Institution-wide QT alert system identifies patients with a high risk of mortality. Mayo Clin. Proc. 2013, 88, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ramos, L.G. Drug-Induced QT Prolongation And Torsades de Pointes. Pharmacovigil. Forum. 2017, 42, 473–477. [Google Scholar]
- Riad, F.S.; Davis, A.M.; Moranville, M.P.; Beshai, J.F. Drug-Induced QTc Prolongation. Am. J. Cardiol. 2017, 119, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Nachimuthu, S.; Assar, M.D.; Schussler, J.M. Drug-induced QT interval prolongation: Mechanisms and clinical management. Ther. Adv. Drug Saf. 2012, 3, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, I.; Fujita, H.; Ohe, K. Early detection of QT interval prolongation from the ECG database system. Stud. Health Technol. Inform. 2013, 192, 1043. [Google Scholar]
- Shah, R.R. Drug-induced QT interval prolongation: Does ethnicity of the thorough QT study population matter? Br. J. Clin. Pharmacol. 2013, 75, 347–358. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, P.; He, N.; Zhai, S. Drug-induced torsades de pointes: Disproportionality analysis of the United States Food and Drug Administration adverse event reporting system. Front. Cardiovasc. Med. 2022, 9, 966331. [Google Scholar] [CrossRef]
- Díez-Escuté, N.; Arbelo, E.; Martínez-Barrios, E.; Cerralbo, P.; Cesar, S.; Cruzalegui, J.; Chipa, F.; Fiol, V.; Zschaeck, I.; Hernández, C.; et al. Sex differences in long QT syndrome. Front. Cardiovasc. Med. 2023, 10, 1164028. [Google Scholar] [CrossRef]
- Eroglu, T.E.; Blom, M.T.; Souverein, P.C.; De Boer, A.; Tan, H.L. Multiple categories of non-cardiac QT-prolonging drugs are associated with increased risk of out-of-hospital cardiac arrest: Real-world data from a population-based study. Europace 2022, 24, 630–638. [Google Scholar] [CrossRef]
- Deng, C.; Niu, J.; Xuan, L.; Zhu, W.; Dai, H.; Zhao, Z.; Li, M.; Lu, J.; Xu, Y.; Chen, Y.; et al. Association of QTc interval with risk of cardiovascular diseases and related vascular traits: A prospective and longitudinal analysis. Glob. Heart 2020, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Aburisheh, K.; AlKheraiji, M.F.; Alwalan, S.I.; Isnani, A.C.; Rafiullah, M.; Mujammami, M.; Alfadda, A.A. Prevalence of QT prolongation and its risk factors in patients with type 2 diabetes. BMC Endocr Disord. 2023, 23, 50. [Google Scholar] [CrossRef] [PubMed]
- TeBay, C.; Hill, A.P.; Windley, M.J. Metabolic and electrolyte abnormalities as risk factors in drug-induced long QT syndrome. Biophys. Rev. 2022, 14, 353–367. [Google Scholar] [CrossRef]
- Wiśniowska, B.; Tylutki, Z.; Wyszogrodzka, G.; Polak, S. Drug-drug interactions and QT prolongation as a commonly assessed cardiac effect—Comprehensive overview of clinical trials. BMC Pharmacol Toxicol. 2016, 17, 12. [Google Scholar] [CrossRef]
- Keller, G.A.; Alvarez, P.A.; Ponte, M.L.; Belloso, W.H.; Bagnes, C.; Sparanochia, C.; Gonzalez, C.D.; Cecilia Villa Etchegoyen, M.; Diez, R.A.; Di Girolamo, G. Drug-Induced QTc Interval Prolongation: A Multicenter Study to Detect Drugs and Clinical Factors Involved in Every Day Practice. Curr. Drug Saf. 2015, 11, 86–98. [Google Scholar] [CrossRef] [PubMed]
| Parameters | N | ||
|---|---|---|---|
| Sex | male | 68 | |
| female | 39 | ||
| Setting | ICU | yes | 41 |
| no | 66 | ||
| Postoperative | yes | 22 | |
| no | 85 | ||
| Underlying medical conditions | Ischemic heart disease | yes | 26 |
| no | 81 | ||
| Acute myocardial infarction | yes | 6 | |
| no | 101 | ||
| Chronic heart failure | yes | 24 | |
| no | 83 | ||
| Diabetes mellitus | no | 90 | |
| yes | 17 | ||
| Sepsis | yes | 13 | |
| no | 94 | ||
| Liver failure | yes | 9 | |
| no | 98 | ||
| Arrhythmia | yes | 44 | |
| no | 63 | ||
| Structural heart disease | yes | 9 | |
| no | 98 | ||
| Hypertension | yes | 73 | |
| no | 34 | ||
| Medications | Loop diuretics | yes | 65 |
| no | 42 | ||
| Antiarrhythmics | yes | 34 | |
| no | 73 | ||
| Antihypertensives | yes | 50 | |
| no | 57 | ||
| Other drugs | yes | 106 | |
| no | 1 | ||
| Valid (N) | Mean | SD (σ) | Median | IQR | Minimum | Maximum | |
|---|---|---|---|---|---|---|---|
| Age (y) | 107 (100%) | 64.2 | 15.4 | 17.0 | 97.0 | ||
| LVEF (%) | 50 (47%) | 45.0 | 17.4 | 10.0 | 70.0 | ||
| SOFA-Score (N) | 30 (28%) | 9.4 | 5.0 | 1.0 | 20.0 | ||
| Potassium (mmol/L) | 107 (100%) | 4.1 | 0.6 | 2.3 | 5.7 | ||
| Calcium (mmol/L) | 74 (70%) | 2.4 | 0.2 | 1.2 | 2.8 | ||
| Magnesium (mmol/L) | 80 (75%) | 0.9 | 0.2 | 0.4 | 1.5 | ||
| eGFR (mL/min/1.73m2) | 107 (100%) | 67.5 | 31.4 | 4.0 | 140.0 | ||
| TSH (mU/L) | 70 (65%) | 3.6 | 3.5 | 0.0 | 20.1 | ||
| Initial QTc (ms) | 93 (87%) | 445.2 | 25.4 | 388.0 | 509.0 | ||
| QTc under medication (ms) | 107 (100%) | 497.7 | 26.4 | 450.0 | 570.0 | ||
| QTc prolongation (ms) | 93 (87%) | 50.7 | 29.9 | 7.0 | 136.0 | ||
| KR drugs (N) | 107 (100%) | 2.0 | 2.0 | 0.0 | 5.0 | ||
| PR drugs (N) | 107 (100%) | 1.0 | 1.0 | 0.0 | 3.0 | ||
| KR + PR (N) | 107 (100%) | 2.0 | 2.0 | 1.0 | 7.0 | ||
| MELD-Score (N) | 10 (9%) | 33.0 | 10.0 | 10.0 | 40.0 | ||
| HbA1c (%) | 20 (19%) | 6.2 | 1.5 | 4.9 | 11.4 |
| QTc under Medication | Coefficient | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|
| Diabetes mellitus | 10.652 | −0.94 | 22.245 | 0.07 |
| Age | −0.348 | −0.608 | −0.089 | 0.01 |
| Ischemic cardiomyopathy | 14.251 | 2.126 | 26.375 | 0.02 |
| Loop diuretics | 6.671 | −2.328 | 15.669 | 0.14 |
| Initial QTc | 0.228 | 0.038 | 0.418 | 0.02 |
| Arrhythmia | 9.48 | −0.321 | 19.282 | 0.06 |
| QTc prolongation | Coefficient | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|
| Arrhythmia | 20.668 | 8.892 | 32.443 | <0.001 |
| Antihypertensives | 11.596 | 0.238 | 22.953 | 0.045 |
| Age | −0.307 | −0.636 | 0.022 | 0.067 |
| Potassium | −5.591 | −7.806 | 6.623 | 0.365 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovannoni, L.; Kullak-Ublick, G.A.; Jetter, A. Developing a Model for Quantifying QTc-Prolongation Risk to Enhance Medication Safety Assessment: A Retrospective Analysis. J. Pers. Med. 2024, 14, 172. https://doi.org/10.3390/jpm14020172
Giovannoni L, Kullak-Ublick GA, Jetter A. Developing a Model for Quantifying QTc-Prolongation Risk to Enhance Medication Safety Assessment: A Retrospective Analysis. Journal of Personalized Medicine. 2024; 14(2):172. https://doi.org/10.3390/jpm14020172
Chicago/Turabian StyleGiovannoni, Luis, Gerd A. Kullak-Ublick, and Alexander Jetter. 2024. "Developing a Model for Quantifying QTc-Prolongation Risk to Enhance Medication Safety Assessment: A Retrospective Analysis" Journal of Personalized Medicine 14, no. 2: 172. https://doi.org/10.3390/jpm14020172
APA StyleGiovannoni, L., Kullak-Ublick, G. A., & Jetter, A. (2024). Developing a Model for Quantifying QTc-Prolongation Risk to Enhance Medication Safety Assessment: A Retrospective Analysis. Journal of Personalized Medicine, 14(2), 172. https://doi.org/10.3390/jpm14020172






