Decrease in Mortality after the Implementation of a Hospital Model to Improve Performance in Sepsis Care: Princess Sepsis Code
Abstract
1. Introduction
“For hospitals and health systems, we recommend using a performance improvement program for sepsis, including sepsis screening for patients with acute and high-risk illnesses and standard operating procedures for treatment. Strong Recommendation.”
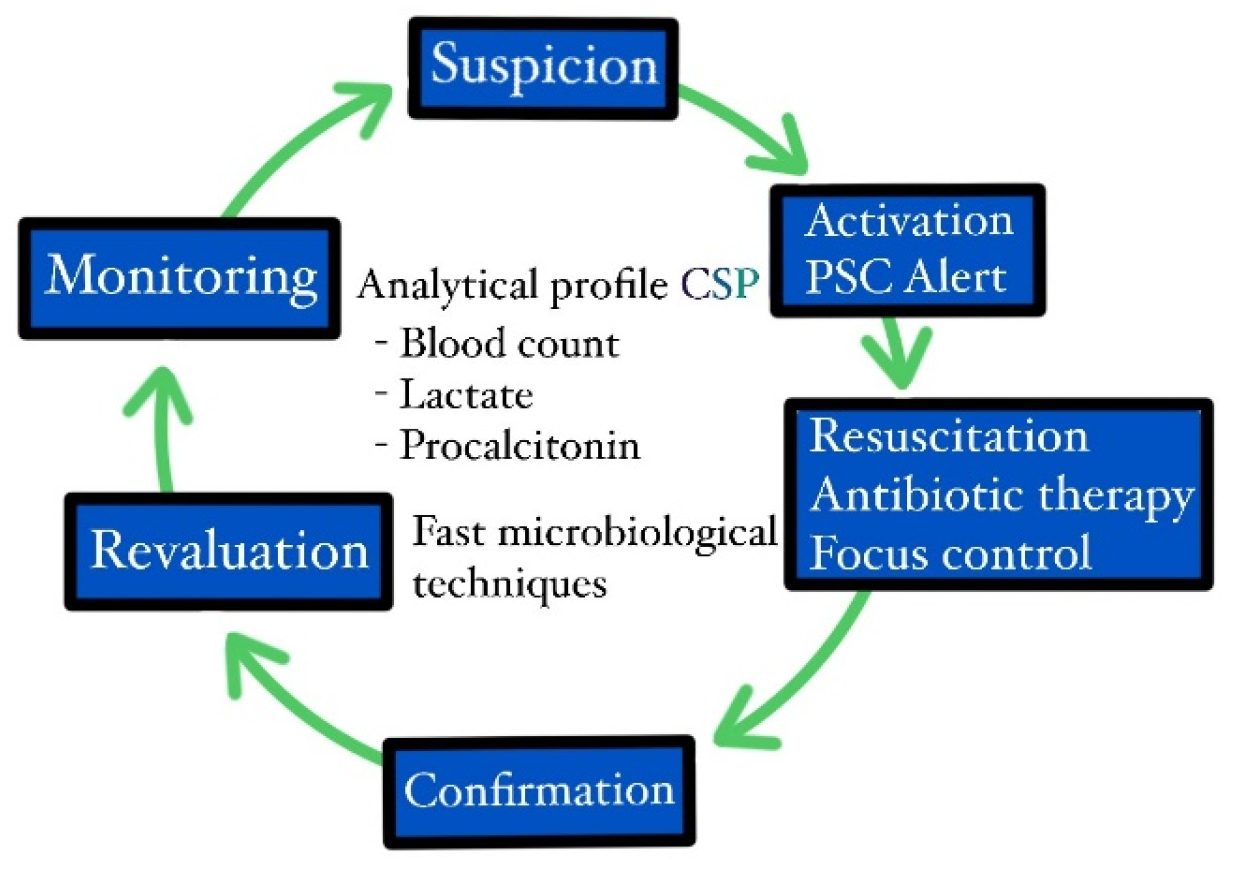
2. Materials and Methods
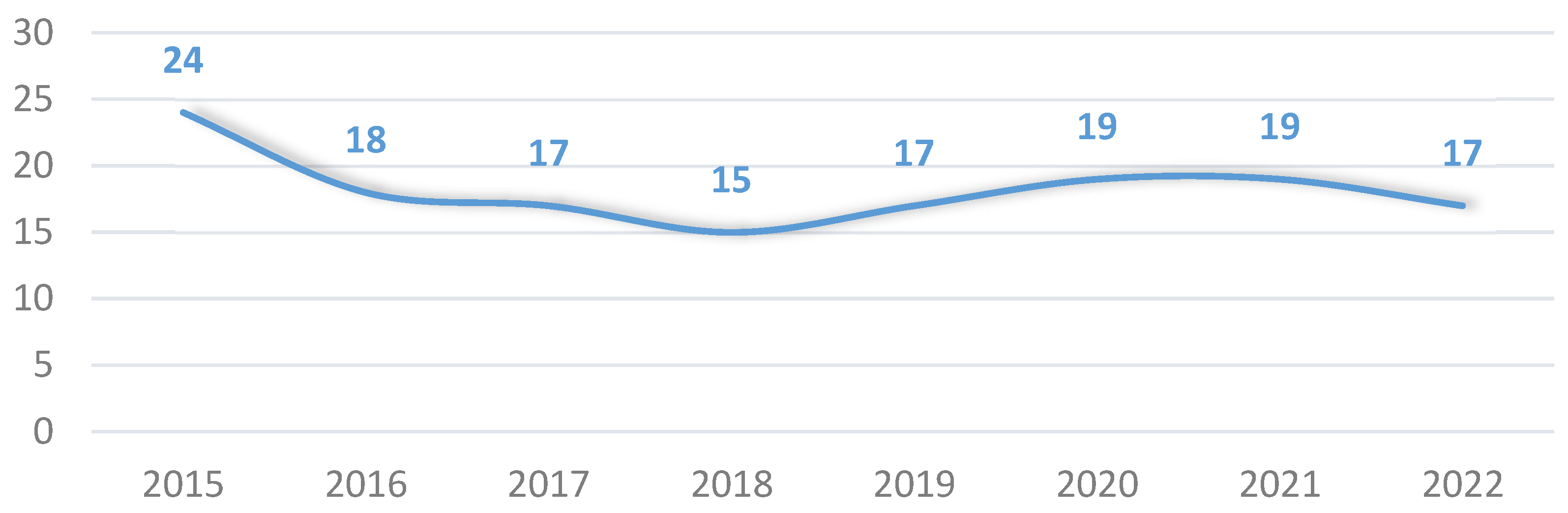
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Álvaro-Meca, A.; Jiménez-Sousa, M.A.; Micheloud, D.; Sánchez-Lopez, A.; Heredia-Rodríguez, M.; Tamayo, E.; Resino, S.; Group of Biomedical Research in Critical Care Medicine (BioCritic). Epidemiological trends of sepsis in the twenty-first century (2000–2013): An analysis of incidence, mortality, and associated costs in Spain. Popul. Health Metr. 2018, 16, 4. [Google Scholar] [CrossRef]
- Fleischmann-Struzek, C.; Mellhammar, L.; Rose, N.; Cassini, A.; Rudd, K.E.; Schlattmann, P.; Allegranzi, B.; Reinhart, K. Incidence and mortality of hospital- and ICU-treated sepsis: Results from an updated and expanded systematic review and meta-analysis. Intensive Care Med. 2020, 46, 1552–1562. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; van der Poll, T.; Marshall, J.C. The End of ‘One Size Fits All’ Sepsis Therapies: Toward an Individualized Approach. Biomedicines 2022, 10, 2260. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.P. Foreword. The Future of Sepsis Performance Improvement. Crit. Care Med. 2015, 43, 1787–1789. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Matthews, R. Safe Patients, Smart Hospitals: How One Doctor’s Checklist Can Help Us Change Health Care from the Inside Out. J. Nucl. Med. 2011, 52, 162–163. [Google Scholar] [CrossRef][Green Version]
- The ProCESS Investigators. A Randomized Trial of Protocol-Based Care for Early Septic Shock. N. Engl. J. Med. 2014, 370, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Mouncey, P.R.; Osborn, T.M.; Power, G.S.; Harrison, D.A.; Sadique, M.Z.; Grieve, R.D.; Jahan, R.; Harvey, S.E.; Bell, D.; Bion, J.F.; et al. Trial of Early, Goal-Directed Resuscitation for Septic Shock. N. Engl. J. Med. 2015, 372, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- ARISE Investigators; ANZICS Clinical Trials Group; Peake, S.L.; Delaney, A.; Bailey, M.; Bellomo, R.; Cameron, P.A.; Cooper, D.J.; Higgins, A.M.; Holdgate, A.; et al. Goal-directed resuscitation for patients with early septic shock. N. Engl. J. Med. 2014, 371, 1496–1506. [Google Scholar]
- Osborn, T.M. Severe Sepsis and Septic Shock Trials (ProCESS, ARISE, ProMISe): What is Optimal Resuscitation? Crit. Care Clin. 2017, 33, 323–344. [Google Scholar] [CrossRef]
- Angus, D.C.; Barnato, A.E.; Bell, D.; Bellomo, R.; Chong, C.-R.; Coats, T.J.; Davies, A.; Delaney, A.; Harrison, D.A.; Holdgate, A.; et al. A systematic review and meta-analysis of early goal-directed therapy for septic shock: The ARISE, ProCESS and ProMISe Investigators. Intensive Care Med. 2015, 41, 1549–1560. [Google Scholar] [CrossRef]
- Ramasco Rueda, F.; González de Castro, R. Manual de Infecciones Perioperatorias, 2nd ed.Editorial Médica Ergon: Madrid, Spain, 5 July 2023; pp. 233–267. Available online: https://ergon.es/producto/manual-infecciones-perioperatorias-2ed/ (accessed on 5 July 2023).
- Ramasco, F.; Figuerola, A.; Mendez, R.; Serrano, D.R.; Von Wernitz, A.; Hernández-Aceituno, A.; Sáez, C.; Cardeñoso, L.; Martin, E.; García-Vázquez, N.; et al. Initial clinical outcomes and prognostic variables in the implementation of a Code Sepsis in a high complexity University Hospital. Rev. Esp. Quimioter. 2019, 32, 238–245. [Google Scholar]
- Méndez, R.; Figuerola, A.; Chicot, M.; Barrios, A.; Pascual, N.; Ramasco, F.; Rodríguez, D.; García, Í.; von Wernitz, A.; Zurita, N.; et al. Código Sepsis: Esquivando la mortalidad en un hospital terciario. Rev. Esp. Quimioter. 2022, 35, 43–49. [Google Scholar] [CrossRef]
- Hernández, A.B.; de Vega-Ríos, E.; Ballesteros, J.S.; Braña, D.U.; Domingo, L.C.; Tejerina, A.F.; von Wernitz Teleki, A.; Jiménez, D.J.; de los Santos Gil, I.; Béjar, C.S. Impact of the implementation of a Sepsis Code Program in medical patient management: A cohort study in an Internal Medicine ward. Rev. Esp. Quimioter. 2022, 35, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Herrejón, E.P.; del Castillo, J.G.; Rueda, F.R.; González, F.J.C.; Artola, B.S.; von Teleki, A.W.; Vidal, F.G.; Iglesias, P.R.; Redondo, G.B.; Serrano, D.A.R.; et al. Documento de consenso para la implantación y desarrollo del Código Sepsis en la Comunidad de Madrid. Rev. Esp. Quimioter. 2019, 32, 400–409. [Google Scholar]
- Nunnally, M.E.; Ferrer, R.; Martin, G.S.; Martin-Loeches, I.; Machado, F.R.; De Backer, D.; Coopersmith, C.M.; Deutschman, C.S.; Antonelli, M.; Hellman, J.; et al. The Surviving Sepsis Campaign: Research priorities for the administration, epidemiology, scoring and identification of sepsis. Intensive Care Med. Exp. 2021, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Bouza, C.; López-Cuadrado, T.; Saz-Parkinson, Z.; Amate-Blanco, J.M. Epidemiology and recent trends of severe sepsis in Spain: A nationwide population-based analysis (2006–2011). BMC Infect. Dis. 2014, 14, 3863. [Google Scholar] [CrossRef]
- Schinkel, M.; Virk, H.S.; Nanayakkara, P.W.B.; van der Poll, T.; Wiersinga, W.J. What Sepsis Researchers Can Learn from COVID-19. Am. J. Respir. Crit. Care Med. 2021, 203, 125–127. [Google Scholar] [CrossRef]
- European Society of Intensive Care Medicine (ESICM); Global Sepsis Alliance (GSA); Society of Critical Care Medicine (SCCM). Reducing the global burden of sepsis: A positive legacy for the COVID-19 pandemic? Intensive Care Med. 2021, 47, 733–736. [Google Scholar] [CrossRef]
- Rosen, M.A.; DiazGranados, D.; Dietz, A.S.; Benishek, L.E.; Thompson, D.; Pronovost, P.J.; Weaver, S.J. Teamwork in healthcare: Key discoveries enabling safer, high-quality care. Am. Psychol. 2018, 73, 433–450. [Google Scholar] [CrossRef]
- Damiani, E.; Donati, A.; Serafini, G.; Rinaldi, L.; Adrario, E.; Pelaia, P.; Busani, S.; Girardis, M. Effect of Performance Improvement Programs on Compliance with Sepsis Bundles and Mortality: A Systematic Review and Meta-Analysis of Observational Studies. PLoS ONE 2015, 10, e0125827. [Google Scholar] [CrossRef] [PubMed]
- Alnababteh, M.H.; Huang, S.S.; Ryan, A.; McGowan, K.M.; Yohannes, S. A Multimodal Sepsis Quality-Improvement Initiative Including 24/7 Screening and a Dedicated Sepsis Response Team-Reduced Readmissions and Mortality. Crit. Care Explor. 2020, 2, e0251. [Google Scholar] [CrossRef]
- De Souza, D.R.X.; de Araújo, I.D.T.; Nobre, T.T.X.; da Silva Gama, Z.A.; Grabois, V.; de Araújo Nunes, V.M. Improving the quality of care for patients with sepsis in the context of an emergency service. Enf. Glob. 2022, 21, 1–49. [Google Scholar] [CrossRef]
- Rhee, C.; Dantes, R.; Epstein, L.; Murphy, D.J.; Seymour, C.W.; Iwashyna, T.J.; Kadri, S.S.; Angus, D.C.; Danner, R.L.; Fiore, A.E.; et al. Incidence and Trends of Sepsis in US Hospitals Using Clinical vs. Claims Data, 2009–2014. JAMA 2017, 318, 1241–1249. [Google Scholar] [CrossRef]
- Levy, M.M.; Rhodes, A.; Phillips, G.S.; Townsend, S.R.; Schorr, C.A.; Beale, R.; Osborn, T.; Lemeshow, S.; Chiche, J.-D.; Artigas, A.; et al. Surviving Sepsis Campaign: Association between performance metrics and outcomes in a 7.5-year study. Intensive Care Med. 2014, 40, 1623–1633. [Google Scholar] [CrossRef]
- Cardoso, T.; Carneiro, A.H.; Ribeiro, O.; Teixeira-Pinto, A.; Costa-Pereira, A. Reducing mortality in severe sepsis with the implementation of a core 6-h bundle: Results from the Portuguese community-acquired sepsis study (SACiUCI study). Crit. Care 2010, 14, R83. [Google Scholar] [CrossRef] [PubMed]
- Girardis, M.; Rinaldi, L.; Donno, L.; Marietta, M.; Codeluppi, M.; Marchegiano, P.; Venturelli, C.; The ‘Sopravvivere alla Seps’ Group of the Modena-University Hospital. Effects on management and outcome of severe sepsis and septic shock patients admitted to the intensive care unit after implementation of a sepsis program: A pilot study. Crit. Care 2009, 13, R143. [Google Scholar] [CrossRef] [PubMed]
- Schinkel, M.; Nanayakkara, P.W.B.; Wiersinga, W.J. Sepsis Performance Improvement Programs: From Evidence toward Clinical Implementation. Crit. Care 2022, 26, 77. [Google Scholar] [CrossRef]
- Ospina-Tascón, G.A.; Hernandez, G.; Alvarez, I.; Calderón-Tapia, L.E.; Manzano-Nunez, R.; Sánchez-Ortiz, A.I.; Quiñones, E.; Ruiz-Yucuma, J.E.; Aldana, J.L.; Teboul, J.-L.; et al. Effects of very early start of norepinephrine in patients with septic shock: A propensity score-based analysis. Crit. Care 2020, 24, 52. [Google Scholar] [CrossRef]
- Monnet, X.; Lai, C.; Ospina-Tascon, G.; De Backer, D. Evidence for a personalized early start of norepinephrine in septic shock. Crit. Care 2023, 27, 322. [Google Scholar] [CrossRef]
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 2006, 34, 1589–1596. [Google Scholar] [CrossRef]
- Dougnac, L.A.; Mercado, F.M.; Cornejo, R.R.; Cariaga, V.M.; Hernández, P.G.; Andresen, H.M.; Bugedo, G.; Castillo, L. Prevalencia de sepsis grave en las Unidades de Cuidado Intensivo: Primer estudio nacional multicéntrico. Rev. Méd. Chile 2007, 135, 620–630. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pieroni, M.; Olier, I.; Ortega-Martorell, S.; Johnston, B.W.; Welters, I.D. In-Hospital Mortality of Sepsis Differs Depending on the Origin of Infection: An Investigation of Predisposing Factors. Front. Med. 2022, 9, 915224. [Google Scholar] [CrossRef]
- Stortz, J.A.; Cox, M.C.; Hawkins, R.B.; Ghita, G.L.; Brumback, B.A.; Mohr, A.M.; Moldawer, L.L.; Efron, P.A.; Brakenridge, S.C.; Moore, F.A. Phenotypic heterogeneity by site of infection in surgical sepsis: A prospective longitudinal study. Crit. Care 2020, 24, 203. [Google Scholar] [CrossRef] [PubMed]
- Leligdowicz, A.; Dodek, P.M.; Norena, M.; Wong, H.; Kumar, A.; Kumar, A. Association between Source of Infection and Hospital Mortality in Patients Who Have Septic Shock. Am. J. Respir. Crit. Care Med. 2014, 189, 1204–1213. [Google Scholar] [CrossRef]
- Mewes, C.; Runzheimer, J.; Böhnke, C.; Büttner, B.; Nemeth, M.; Hinz, J.; Quintel, M.; Mansur, A. Differences in Mortality and Sepsis-Associated Organ Dysfunction between Surgical and Non-Surgical Sepsis Patients. Biomedicines 2023, 11, 2233. [Google Scholar] [CrossRef]
- Schorr, C.; Odden, A.; Evans, L.; Escobar, G.J.; Gandhi, S.; Townsend, S.; Levy, M. Implementation of a multicenter performance improvement program for early detection and treatment of severe sepsis in general medical–surgical wards. J. Hosp. Med. 2016, 11, S32–S39. [Google Scholar] [CrossRef]
- Shankar-Hari, M.; Harrison, D.A.; Ferrando-Vivas, P.; Rubenfeld, G.D.; Rowan, K. Risk Factors at Index Hospitalization Associated with Longer-term Mortality in Adult Sepsis Survivors. JAMA Netw. Open 2019, 2, e194900. [Google Scholar] [CrossRef] [PubMed]
- Skei, N.V.; Nilsen, T.I.L.; Mohus, R.M.; Prescott, H.C.; Lydersen, S.; Solligård, E.; Damås, J.; Gustad, L. Trends in mortality after a sepsis hospitalization: A nationwide prospective registry study from 2008 to 2021. Infection 2023, 51, 1773–1786. [Google Scholar] [CrossRef]
- Roh, J.; Jo, E.-J.; Eom, J.S.; Mok, J.; Kim, M.H.; Kim, K.U.; Park, H.-K.; Lee, M.K.; Yeom, S.; Lee, K. Factors predicting long-term survival of patients with sepsis on arrival at the emergency department. Medicine 2019, 98, e16871. [Google Scholar] [CrossRef] [PubMed]
- Méndez Hernández, R.; Ramasco Rueda, F. Biomarkers as Prognostic Predictors and Therapeutic Guide in Critically ill Patients: Clinical Evidence. J. Pers. Med. 2023, 13, 333. [Google Scholar] [CrossRef]
- Asfar, P.; Meziani, F.; Hamel, J.-F.; Grelon, F.; Megarbane, B.; Anguel, N.; Mira, J.-P.; Dequin, P.-F.; Gergaud, S.; Weiss, N.; et al. High versus Low Blood-Pressure Target in Patients with Septic Shock. N. Engl. J. Med. 2014, 370, 1583–1593. [Google Scholar] [CrossRef] [PubMed]
- De Backer, D.; Cecconi, M.; Chew, M.S.; Hajjar, L.; Monnet, X.; Ospina-Tascón, G.A.; Ostermann, M.; Pinsky, M.R.; Vincent, J.-L. A plea for personalization of the hemodynamic management of septic shock. Crit. Care 2022, 26, 372. [Google Scholar] [CrossRef]
- Sterling, S.A.; Miller, W.R.; Pryor, J.; Puskarich, M.A.; Jones, A.E. The Impact of Timing of Antibiotics on Outcomes in Severe Sepsis and Septic Shock: A Systematic Review and Meta-Analysis. Crit. Care Med. 2015, 43, 1907–1915. [Google Scholar] [CrossRef]
- Pea, F.; Viale, P. The antimicrobial therapy puzzle: Could pharmacokinetic-pharmacodynamic relationships be helpful in addressing the issue of appropriate pneumonia treatment in critically ill patients? Clin. Infect. Dis. 2006, 42, 1764–1771. [Google Scholar]
- Zonneveld, L.E.; van Wijk, R.J.; Olgers, T.J.; Bouma, H.R.; ter Maaten, J.C. Prognostic value of serial score measurements of the national early warning score, the quick sequential organ failure assessment and the systemic inflammatory response syndrome to predict clinical outcome in early sepsis. Eur. J. Emerg. Med. 2022, 29, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Brink, A.; Alsma, J.; Verdonschot, R.J.C.G.; Rood, P.P.M.; Zietse, R.; Lingsma, H.F.; Schuit, S.C.E. Predicting mortality in patients with suspected sepsis at the Emergency Department; A retrospective cohort study comparing qSOFA, SIRS and National Early Warning Score. PLoS ONE 2019, 14, e0211133. [Google Scholar] [CrossRef]
- Spoto, S.; Nobile, E.; Carnà, E.P.R.; Fogolari, M.; Caputo, D.; De Florio, L.; Valeriani, E.; Benvenuto, D.; Costantino, S.; Ciccozzi, M.; et al. Best diagnostic accuracy of sepsis combining SIRS criteria or qSOFA score with Procalcitonin and Mid-Regional pro-Adrenomedullin outside ICU. Sci. Rep. 2020, 10, 16605. [Google Scholar] [CrossRef]
- Bischop, L.A.; Wilson, D.P.K.; Wise, R.D.; Savarimuthu, S.M.; Anesi, G.L. Prognostic value of the Quick Sepsis-related Organ Failure Assessment (qSOFA) score among critically ill medical and surgical patients with suspected infection in a resourcelimited setting. Afr. J. Thorac. Crit. Care Med. 2021, 24, 145–150. [Google Scholar] [CrossRef]
- Deng, H.-F.; Sun, M.-W.; Wang, Y.; Zeng, J.; Yuan, T.; Li, T.; Li, D.-H.; Chen, W.; Zhou, P.; Wang, Q.; et al. Evaluating machine learning models for sepsis prediction: A systematic review of methodologies. iScience 2021, 25, 103651. [Google Scholar] [CrossRef] [PubMed]
- Seymour, C.W.; Kennedy, J.N.; Wang, S.; Chang, C.-C.H.; Elliott, C.F.; Xu, Z.; Berry, S.; Clermont, G.; Cooper, G.; Gomez, H.; et al. Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. JAMA 2019, 321, 2003–2017. [Google Scholar] [CrossRef]
- Sinapidis, D.; Kosmas, V.; Vittoros, V.; Koutelidakis, I.M.; Pantazi, A.; Stefos, A.; Katsaros, K.E.; Akinosoglou, K.; Bristianou, M.; Toutouzas, K.; et al. Progression into sepsis: An individualized process varying by the interaction of comorbidities with the underlying infection. BMC Infect. Dis. 2018, 18, 242. [Google Scholar] [CrossRef] [PubMed]
- Bolte, T.B.B.; Swanson, M.B.B.; Kaldjian, A.M.B.; Mohr, N.M.; McDanel, J.; Ahmed, A.M. Hospitals That Report Severe Sepsis and Septic Shock Bundle Compliance Have More Structured Sepsis Performance Improvement. J. Patient Saf. 2022, 18, e1231–e1236. [Google Scholar] [CrossRef] [PubMed]



| 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | ||
|---|---|---|---|---|---|---|---|---|---|
| Age in years (mean, SD) | 73 ± 15 | 71 ± 15 | 72 ± 15 | 73 ± 15 | 75 ± 14 | 76 ± 14 | 76 ± 14 | 75 ± 15 | p < 0.05 |
| Gender: male (%) | 59.9 | 56.5 | 59.6 | 58.2 | 61.5 | 60.6 | 59.4 | 61.0 | n.s. |
| Activation in the emergency department (%) | 79.7 | 77.7 | 85.7 | 76.5 | 70.4 | 80.1 | 85.6 | 70.8 | p < 0.05 |
| RH lpm (average, SD) | 103 ± 25 | 98 ± 25 | 104 ± 23 | 102 ± 24 | 101 ± 23 | 98 ± 22 | 101 ± 59 | 97 ± 23 | p < 0.05 |
| RR rpm (average, SD) | - | - | 27 ± 8.7 | 27 ± 5.9 | 27 ± 12 | 28 ± 6.3 | 27 ± 6.9 | 26 ± 7.8 | p < 0.05 |
| SAP mmHg (mean, SD) | 108 ± 28 | 106 ± 27 | 102 ± 25 | 99 ± 25 | 98 ± 24 | 101 ± 27 | 101 ± 27 | 101 ± 26 | p < 0.05 |
| SpO2 (average, SD) | - | - | 93 ± 5.6 | 93 ± 5.7 | 93 ± 5.4 | 93 ± 5.3 | 93 ± 4.7 | 93 ± 5.2 | n.s. |
| Temperature °C (average, SD) | - | - | 37.4 ± 1.2 | 37.4 ± 1.2 | 38.4 ± 19 | 37.4 ± 2.9 | 37.3 ± 1.3 | 37.2 ± 1.2 | n.s. |
| Low level of consciousness (%) | - | - | 35.2 | 32.6 | 42.1 | 46.7 | 41.0 | 24.6 | p < 0.05 |
| Lactate mmol/L (mean, SD) | 3.4 ± 3.1 | 2.8 ± 2.1 | 3.3 ± 2.6 | 3.2 ± 2.2 | 3.1 ± 2.2 | 3.3 ± 2.3 | 3.2 ± 2.5 | 3.1 ± 2.52 | n.s. |
| Procalcit. mg/dL (mean, SD) | 15 ± 27 | 9 ± 19 | 15 ± 26 | 11 ± 20 | 11 ± 22 | 13 ± 24 | 14 ± 26 | 14 ± 25 | n.s. |
| Creatinine mg/dL (mean, SD) | 1.7 ± 1.1 | 1.7 ± 1.8 | 1.2 ± 1.1 | 1.3 ± 1.2 | 1.3 ± 1.0 | 1.1 ± 0.6 | 1.8 ± 1.2 | 1.7 ± 1.3 | p < 0.05 |
| Amines, first 6 h (%) | 33.5 | 28.6 | 29.2 | 25.3 | 28.3 | 26.1 | 23.7 | 31.7 | n.s. |
| Antibiotics, first 6 h (%) | 76.3 | 99.5 | 100 | 99.7 | 99.7 | 100 | 100 | 99.2 | p < 0.05 |
| Blood cultures collected (%) | 97.8 | 94.4 | 90.8 | 93.9 | 88.6 | 91.6 | 94.3 | 89.4 | p < 0.05 |
| Positive blood cultures (%) | 13.4 | 11.9 | 36.9 | 34.7 | 36.9 | 38.5 | 43.2 | 37.8 | p < 0.05 |
| ICU admission (%) | 32.3 | 37.5 | 30.0 | 33.0 | 30.3 | 29.2 | 23.1 | 38.0 | p < 0.05 |
| SOFA (median, SD) | 7.3 ± 3.6 | 5.2 ± 2.9 | 3.6 ± 1.8 | 3.9 ± 2.2 | 4.1 ± 2.2 | 4.4 ± 2.4 | 4.6 ± 2.7 | 1.4 ± 1.2 | p < 0.05 |
| Bivariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| OR (CI 95%) | Significance | OR (CI 95%) | Significance | |
| Age > 70 | 2.32 (1.86–2.89) | p < 0.05 | 2.40 (1.45–4.00) | p < 0.05 |
| Sex (% male) | 1.21 (1.02–1.42) | p < 0.05 | 0.73 (0.51–1.06) | n.s. |
| RH (mean bpm) | 102 ± 26 vs. 100 ± 32 | n.s. | ||
| RR > 22 rpm | 1.72 (1.18–2.51) | p < 0.05 | 2.03 (1.14–3.61) | p < 0.05 |
| SAP < 100 mmHg | 1.26 (1.05–1.51) | p < 0.05 | 1.52 (1.00–2.31) | n.s. |
| SpO2 < 90% | 1.52 (1.25–1.87) | p < 0.05 | 1.23 (0.84–1.81) | n.s. |
| Low level of consciousness | 2.66 (2.16–3.28) | p < 0.05 | 2.83 (1.95–4.12) | p < 0.05 |
| Serum lactate > 2 mmol/L | 2.16 (1.74–2.67) | p < 0.05 | 2.46 (1.58–3.81) | p < 0.05 |
| Procalcitonin > 2 mg/dL | 1.09 (0.91–1.30) | n.s. | ||
| Creatinine > 1.6 | 1.65 (1.39–1.95) | p < 0.05 | 1.55 (1.06–2.26) | p < 0.05 |
| Amines, first 6 h | 1.65 (1.40–1.94) | p < 0.05 | 0.67 (0.43–1.02) | n.s. |
| Antibiotic therapy, first 6 h | 0.60 (0.43–0.82) | p < 0.05 | 0.91 (0.12–6.89) | n.s. |
| Blood cultures collected | 0.74 (0.56–0.97) | p < 0.05 | 0.69 (0.36–1.35) | n.s. |
| Positive blood cultures | 0.95 (0.80–1.13) | n.s. | ||
| Origin of sepsis | p < 0.05 | 0.87 (0.78–0.99) | p < 0.05 | |
| Activation in the emergency department | 0.76 (0.64–0.91) | p < 0.05 | 0.82 (0.47–1.43) | n.s. |
| Admission to ICU | 1.08 (0.91–1.29) | n.s. | ||
| Days in ICU (average) | 2.8 ± 6.6 vs. 3.3 ± 11 | n.s. | ||
| Abdominal Sepsis | Bivariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (CI 95%) | Significance | OR (CI 95%) | Significance | |
| Age > 70 | 2.58 (1.75–3.81) | p < 0.05 | 2.43 (1.46–4.05) | p < 0.05 |
| Sex (% male) | 1.05 (0.79–1.39) | n.s. | ||
| RH (mean bpm) | 102 ± 26 vs. 100 ± 32 | n.s. | ||
| RR > 22 rpm | 1.33 (0.71–2.49) | n.s. | ||
| SAP < 100 mmHg | 1.39 (1.01–1.93) | p < 0.05 | 1.69 (0.81–3.54) | n.s. |
| SpO2 < 90% | 1.40 (0.92–2.15) | n.s. | ||
| Low level of consciousness | 2.46 (1.66–3.64) | p < 0.05 | 2.61 (1.47–4.64) | p < 0.05 |
| Serum lactate > 2 mmol/L | 2.39 (1.58–3.63) | p < 0.05 | 3.77 (1.60–8.86) | p < 0.05 |
| Procalcitonin > 2 mg/dL | 1.17 (0.85–1.62) | n.s. | ||
| Creatinine > 1.6 | 1.90 (1.43–2.53) | p < 0.05 | 2.08 (1.15–3.76) | p < 0.05 |
| Amines, first 6 h | 2.22 (1.67–2.96) | p < 0.05 | 0.14 (0.06–0.34) | p < 0.05 |
| Antibiotic therapy, first 6 h | 0.39 (0.26–0.58) | p < 0.05 | 3.70 (0.19–71.9) | n.s. |
| Blood cultures collected | 0.79 (0.53–1.17) | n.s. | ||
| Positive blood cultures | 1.05 (0.78–1.41) | n.s. | ||
| Activation in the emergency department | 0.71 (0.54–0.94) | p < 0.05 | 0.55 (0.28–1.05) | n.s. |
| Admission to ICU | 1.33 (1.00–1.77) | p < 0.05 | 0.35 (0.14–0.86) | p < 0.05 |
| Urinary Sepsis | Bivariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (CI 95%) | Significance | OR (CI 95%) | Significance | |
| Age > 70 | 2.52 (1.37–4.65) | p < 0.05 | 2.52 (0.53–11.9) | n.s. |
| Sex (% male) | 1.04 (0.69–1.57) | n.s. | ||
| RH (mean bpm) | 102 ± 24 vs. 100 ± 46 | n.s. | ||
| RR > 22 rpm | 2.99 (1.78–5.03) | p < 0.05 | 3.20 (0.86–11.9) | n.s. |
| SAP < 100 mmHg | 1.10 (0.71–1.69) | n.s. | ||
| SpO2 < 90% | 1.84 (1.13–2.99) | p < 0.05 | 1.27 (0.55–2.95) | n.s. |
| Low level of consciousness | 4.30 (2.49–7.40) | p < 0.05 | 4.32 (1.78–10.5) | p < 0.05 |
| Serum lactate > 2 mmol/L | 2.47 (1.40–4.37) | p < 0.05 | 4.03 (1.32–12.3) | p < 0.05 |
| Procalcitonin > 2 mg/dL | 1.35 (0.87–2.09) | n.s. | ||
| Creatinine > 1.6 | 1.61 (1.07–2.43) | p < 0.05 | 1.58 (0.74–3.35) | n.s. |
| Amines, first 6 h | 1.01 (0.61–1.69) | n.s. | ||
| Antibiotic therapy, first 6 h | 0.86 (0.29–2.53) | n.s. | ||
| Blood cultures collected | 0.41 (0.20–0.83) | p < 0.05 | 0.34 (0.06–1.80) | n.s. |
| Positive blood cultures | 0.97 (0.65–1.45) | n.s. | ||
| Activation in the emergency department | 0.82 (0.46–1.49) | n.s. | ||
| Admission to ICU | 0.61 (0.33–1.11) | n.s. | ||
| Respiratory Sepsis | Bivariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (CI 95%) | Significance | OR (CI 95%) | Significance | |
| Age > 70 | 2.57 (1.71–3.85) | p < 0.05 | 3.15 (1.62–6.11) | p < 0.05 |
| Sex (% male) | 1.16 (0.87–1.55) | n.s. | ||
| RH (mean bpm) | 103 ± 25 vs. 103 ± 24 | n.s. | ||
| RR > 22 rpm | 1.14 (0.58–2.26) | n.s. | ||
| SAP < 100 mmHg | 1.27 (0.93–1.73) | n.s. | ||
| SpO2 < 90% | 1.51 (1.09–2.11) | p < 0.05 | 1.48 (0.89–2.47) | n.s. |
| Low level of consciousness | 2.49 (1.78–3.50) | p < 0.05 | 2.66 (1.61–4.42) | p < 0.05 |
| Serum lactate > 2 mmol/L | 1.68 (1.22–2.32) | p < 0.05 | 1.67 (0.99–2.88) | n.s. |
| Procalcitonin > 2 mg/dL | 1.00 (0.71–1.40) | n.s. | ||
| Creatinine > 1.6 | 1.58 (1.17–2.12) | p < 0.05 | 1.23 (0.71–2.21) | n.s. |
| Amines, first 6 h | 1.40 (1.03–1.91) | p < 0.05 | 0.48 (0.26–0.91) | p < 0.05 |
| Antibiotic therapy, first 6 h | 0.81 (0.38–1.73) | n.s. | ||
| Blood cultures collected | 0.84 (0.49–1.43) | n.s. | ||
| Positive blood cultures | 1.09 (0.79–1.50) | n.s. | ||
| Activation in the emergency department | 0.84 (0.58–1.22) | n.s. | ||
| Admission to ICU | 0.90 (0.63–1.28) | n.s. | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méndez, R.; Figuerola, A.; Ramasco, F.; Chicot, M.; Pascual, N.F.; García, Í.; von Wernitz, A.; Zurita, N.D.; Semiglia, A.; Pizarro, A.; et al. Decrease in Mortality after the Implementation of a Hospital Model to Improve Performance in Sepsis Care: Princess Sepsis Code. J. Pers. Med. 2024, 14, 149. https://doi.org/10.3390/jpm14020149
Méndez R, Figuerola A, Ramasco F, Chicot M, Pascual NF, García Í, von Wernitz A, Zurita ND, Semiglia A, Pizarro A, et al. Decrease in Mortality after the Implementation of a Hospital Model to Improve Performance in Sepsis Care: Princess Sepsis Code. Journal of Personalized Medicine. 2024; 14(2):149. https://doi.org/10.3390/jpm14020149
Chicago/Turabian StyleMéndez, Rosa, Angels Figuerola, Fernando Ramasco, Marta Chicot, Natalia F. Pascual, Íñigo García, Andrés von Wernitz, Nelly D. Zurita, Auxiliadora Semiglia, Alberto Pizarro, and et al. 2024. "Decrease in Mortality after the Implementation of a Hospital Model to Improve Performance in Sepsis Care: Princess Sepsis Code" Journal of Personalized Medicine 14, no. 2: 149. https://doi.org/10.3390/jpm14020149
APA StyleMéndez, R., Figuerola, A., Ramasco, F., Chicot, M., Pascual, N. F., García, Í., von Wernitz, A., Zurita, N. D., Semiglia, A., Pizarro, A., Saez, C., & Rodríguez, D. (2024). Decrease in Mortality after the Implementation of a Hospital Model to Improve Performance in Sepsis Care: Princess Sepsis Code. Journal of Personalized Medicine, 14(2), 149. https://doi.org/10.3390/jpm14020149







