Exploring the Potential Use of Virtual Reality with a Supraorbital Keyhole Craniotomy for Anterior Skull Base Meningiomas: Two Case Reports
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Presurgical Planning
2.3. Neurosurgical Intervention
2.4. Consent and Waiver
3. Results
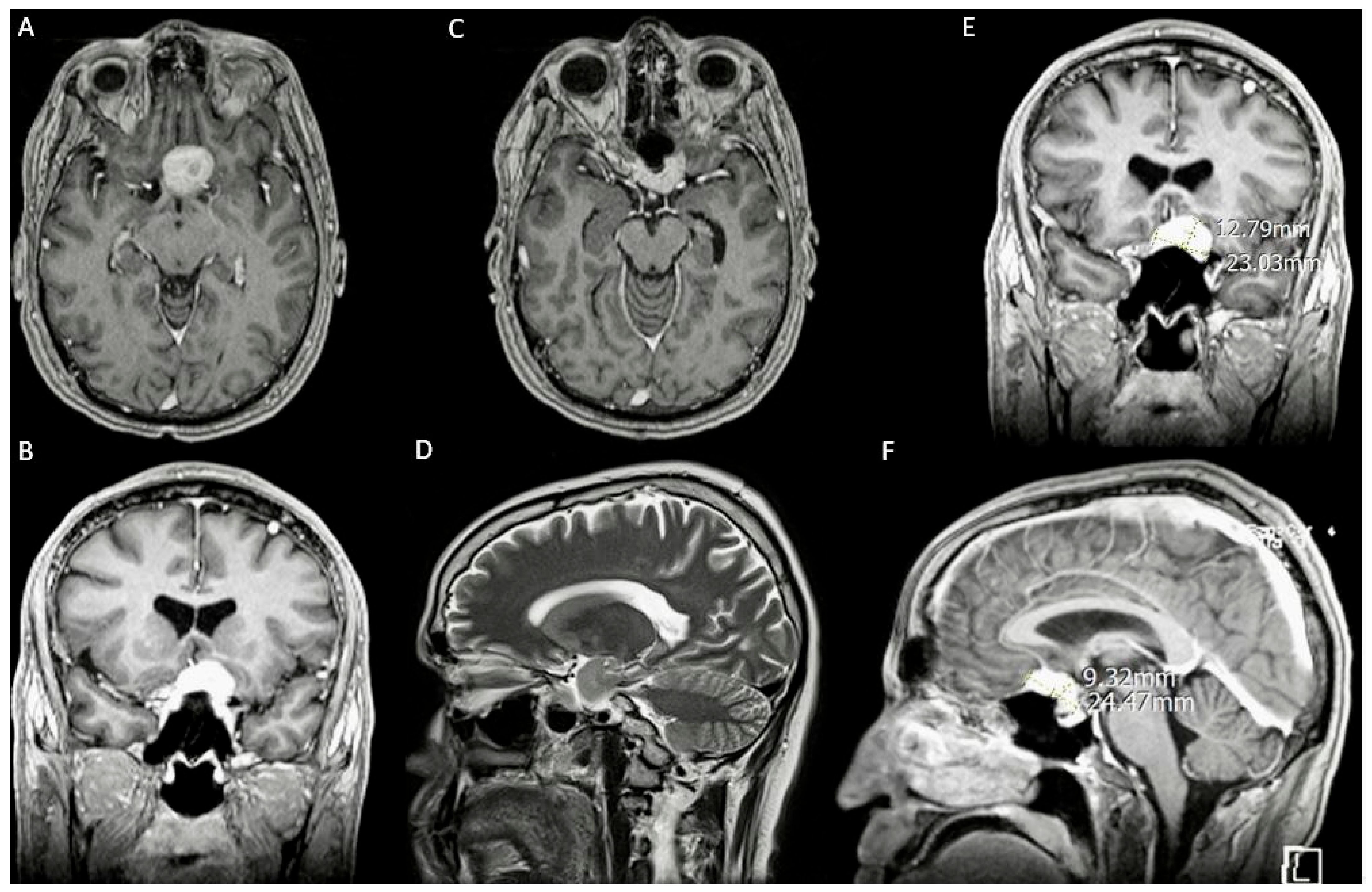
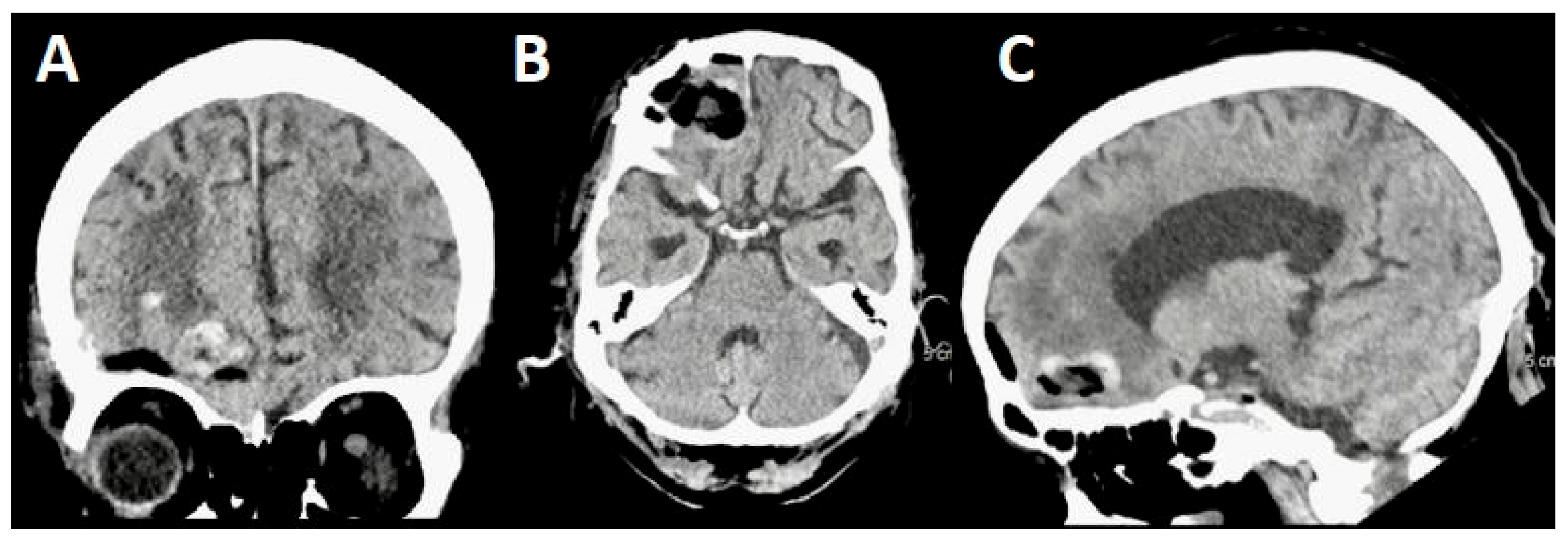
3.1. Case 1
3.1.1. Demography, Presentation, and Evaluation
3.1.2. Intervention
3.1.3. Postoperative Period
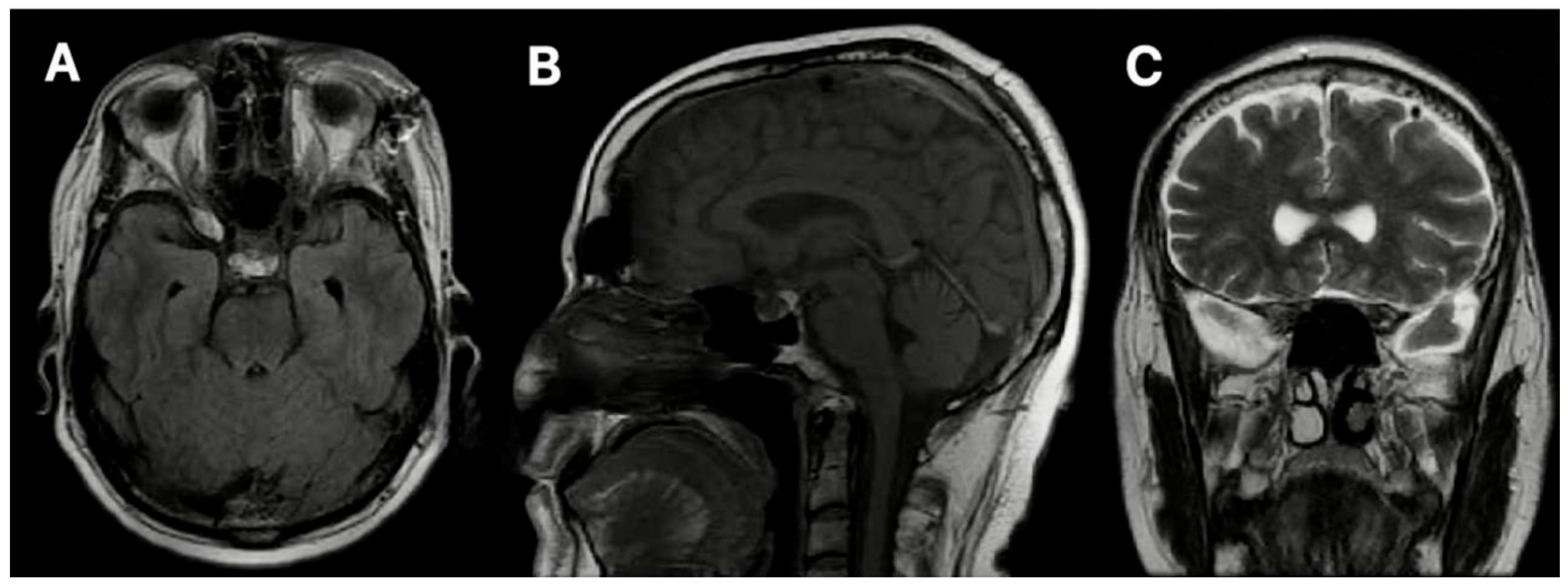
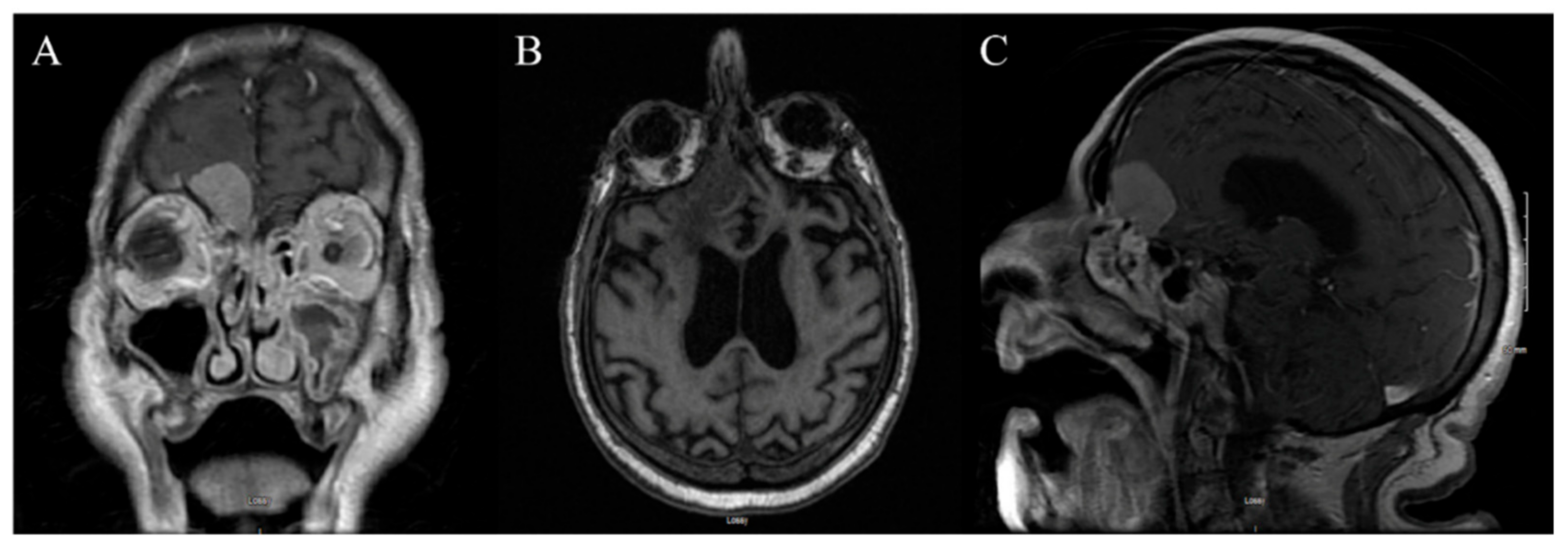
3.2. Case 2
3.2.1. Demography, Presentation, and Evaluation
3.2.2. Intervention
3.2.3. Postoperative Period
4. Discussion
4.1. Uses of VR and AR in Minimally Invasive Surgery
4.2. Supraorbital Keyhole Craniotomy
4.3. VR/AR-Assisted Supraorbital Keyhole Craniotomy
4.3.1. Patient Selection
4.3.2. Intraoperative Considerations
4.3.3. Surgical Learning Curve
4.3.4. Accessibility and Ethics
4.4. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro-Oncol. 2019, 21 (Suppl. S5), v1–v100. [Google Scholar] [CrossRef] [PubMed]
- Alruwaili, A.A.; De Jesus, O. StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560538/ (accessed on 4 September 2023).
- Buerki, R.A.; Horbinski, C.M.; Kruser, T.; Horowitz, P.M.; James, C.D.; Lukas, R.V. An overview of meningiomas. Future Oncol. 2018, 14, 2161–2177. [Google Scholar] [CrossRef] [PubMed]
- Vysotski, S.; Madura, C.; Swan, B.; Holdsworth, R.; Lin, Y.; Del Rio, A.M.; Wood, J.; Kundu, B.; Penwarden, A.; Voss, J.; et al. Preoperative FMRI associated with decreased mortality and morbidity in brain tumor patients. Interdiscip. Neurosurg. 2018, 13, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Morales-Valero, S.F.; Van Gompel, J.J.; Loumiotis, I.; Lanzino, G. Craniotomy for anterior cranial fossa meningiomas: Historical overview. Neurosurg. Focus 2014, 36, E14. [Google Scholar] [CrossRef]
- Reisch, R.; Perneczky, A.; Filippi, R. Surgical technique of the supraorbital key-hole craniotomy. Surg. Neurol. 2003, 59, 223–227. [Google Scholar] [CrossRef]
- Thakur, J.D.; Mallari, R.J.; Corlin, A.; Yawitz, S.; Eisenberg, A.; Rhee, J.; Sivakumar, W.; Krauss, H.; Martin, N.; Griffiths, C.; et al. Critical appraisal of minimally invasive keyhole surgery for intracranial meningioma in a large case series. PLoS ONE 2022, 17, e0264053. [Google Scholar] [CrossRef]
- Altay, T.; Couldwell, W.T. The Frontotemporal (Pterional) Approach: An historical perspective. Neurosurgery 2012, 71, 481–492. [Google Scholar] [CrossRef]
- Zhou, L.; Jing, X.; Wang, C.; Zhang, H.; Lei, P.; Song, P.; Li, Z.; Gao, L.; Lu, M.; Chen, Q.; et al. Clinical application of transcranial neuroendoscopy combined with supraorbital keyhole approach in minimally invasive surgery of the anterior skull base. Sci. Rep. 2024, 14, 14886. [Google Scholar] [CrossRef]
- Meola, A.; Cutolo, F.; Carbone, M.; Cagnazzo, F.; Ferrari, M.; Ferrari, V. Augmented reality in neurosurgery: A systematic review. Neurosurg. Rev. 2017, 40, 537–548. [Google Scholar] [CrossRef]
- Avery, M.B.; Mallari, R.J.; Barkhoudarian, G.; Kelly, D.F. Supraorbital and mini-pterional keyhole craniotomies for brain tumors: A clinical and anatomical comparison of indications and outcomes in 204 cases. J. Neurosurg. 2022, 136, 1314–1324. [Google Scholar] [CrossRef]
- Robinow, Z.M.; Peterson, C.; Waldau, B.; Shahlaie, K. Supraorbital Keyhole Craniotomy via Eyebrow Incision: A Systematic Review and Meta-Analysis. World Neurosurg. 2021, 158, e509–e542. [Google Scholar] [CrossRef] [PubMed]
- Ormond, D.R.; Hadjipanayis, C.G. The Supraorbital Keyhole Craniotomy through an Eyebrow Incision: Its Origins and Evolution. Minim. Invasive Surg. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Khanapure, K.; Joshi, K.C.; Jagannatha, A.T.; Perikal, P.J.; Quryshi, S.A.; Srikantha, U.; Verma, R.G.; Hegde, A.S. Supraorbital craniotomy for large anterior skull base meningiomas: A technical note. Asian J. Neurosurg. 2019, 14, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-C.; Tzaan, W.-C. Microsurgical supraorbital keyhole approach to the anterior cranial base. J. Clin. Neurosci. 2010, 17, 1510–1514. [Google Scholar] [CrossRef]
- Sutherland, J.; Belec, J.; Sheikh, A.; Chepelev, L.; Althobaity, W.; Chow, B.J.W.; Mitsouras, D.; Christensen, A.; Rybicki, F.J.; La Russa, D.J. Applying Modern Virtual and Augmented Reality Technologies to Medical Images and Models. J. Digit. Imaging 2019, 32, 38–53. [Google Scholar] [CrossRef]
- Liu, T.; Tai, Y.; Zhao, C.; Wei, L.; Zhang, J.; Pan, J.; Shi, J. Augmented reality in neurosurgical navigation: A survey. Int. J. Med. Robot. Comput. Assist. Surg. 2020, 16, 1–20. [Google Scholar] [CrossRef]
- Agha, R.A.; Sohrabi, C.; Mathew, G.; Franchi, T.; Kerwan, A.; O’neill, N. The PROCESS 2020 Guideline: Updating Consensus Preferred Reporting Of CasE Series in Surgery (PROCESS) Guidelines. Int. J. Surg. 2020, 84, 231–235. [Google Scholar] [CrossRef]
- Durrani, S.; Onyedimma, C.; Jarrah, R.; Bhatti, A.; Nathani, K.R.; Bhandarkar, A.R.; Mualem, W.; Ghaith, A.K.; Zamanian, C.; Michalopoulos, G.D.; et al. The Virtual Vision of Neurosurgery: How Augmented Reality and Virtual Reality are Transforming the Neurosurgical Operating Room. World Neurosurg. 2022, 168, 190–201. [Google Scholar] [CrossRef]
- Jean, W.C.; Britz, G.W.; DiMeco, F.; Elmi-Terander, A.; McIntyre, C. Introduction. Virtual and augmented reality in neurosurgery: A timeline. Neurosurg. Focus 2021, 51, E1. [Google Scholar] [CrossRef]
- Ferroli, P.; Tringali, G.; Acerbi, F.; Schiariti, M.; Broggi, M.; Aquino, D.; Broggi, G. Advanced 3-Dimensional Planning in Neurosurgery. Neurosurgery 2013, 72 (Suppl. S1), A54–A62. [Google Scholar] [CrossRef]
- Bernardo, A. Virtual Reality and Simulation in Neurosurgical Training. World Neurosurg. 2017, 106, 1015–1029. [Google Scholar] [CrossRef] [PubMed]
- Park, J. Supraorbital Keyhole Approach for Intracranial Aneurysms: Transitioning from Concerns to Confidence. J. Korean Neurosurg. Soc. 2020, 63, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Zumofen, D.W.; Rychen, J.; Roethlisberger, M.; Taub, E.; Kalbermatten, D.; Nossek, E.; Potts, M.; Guzman, R.; Riina, H.A.; Mariani, L. A Review of the Literature on the Transciliary Supraorbital Keyhole Approach. World Neurosurg. 2017, 98, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.Z.; Muskens, I.S.; Mekary, R.A.; Najafabadi, A.H.Z.; Helmy, A.E.; Reisch, R.; Broekman, M.L.D.; Marcus, H.J. The endoscope-assisted supraorbital “keyhole” approach for anterior skull base meningiomas: An updated meta-analysis. Acta Neurochir. 2021, 163, 661–676. [Google Scholar] [CrossRef]
- Jallo, G.I.; Bognár, L. Eyebrow Surgery: The Supraciliary Craniotomy: Technical Note. Neurosurgery 2006, 59 (Suppl. S1), ONS-E157–ONS-E158. [Google Scholar] [CrossRef]
- Raza, S.M.; Garzon-Muvdi, T.; Boaehene, K.; Olivi, A.; Gallia, G.; Lim, M.; Subramanian, P.; Quinones-Hinojosa, A. The Supraorbital Craniotomy for Access to the Skull Base and Intraaxial Lesions: A Technique in Evolution. min-Minim. Invasive Neurosurg. 2010, 53, 1–8. [Google Scholar] [CrossRef]
- Telera, S.; Carapella, C.M.; Caroli, F.; Crispo, F.; Cristalli, G.; Raus, L.; Sperduti, I.; Pompili, A. Supraorbital keyhole approach for removal of midline anterior cranial fossa meningiomas: A series of 20 consecutive cases. Neurosurg. Rev. 2012, 35, 67–83. [Google Scholar] [CrossRef]
- Ansari, S.F.; Eisenberg, A.; Rodriguez, A.; Barkhoudarian, G.; Kelly, D.F. The Supraorbital Eyebrow Craniotomy for Intra- and Extra-Axial Brain Tumors: A Single-Center Series and Technique Modification. Oper. Neurosurg. 2020, 19, 667–677. [Google Scholar] [CrossRef]
- Lupret, V.; Sajko, T.; Bero, V.; Kudeliæ, N.; Lupret, V., Jr. Advantages and disadvantages of the supraorbitalkeyhole approach to intracranial aneurysms. Acta Clin. Croat. 2006, 45, 91–94. [Google Scholar]
- Zhang, M.Z.; Wang, L.; Zhang, W.; Qi, W.; Wang, R.; Han, X.D.; Zhao, J.Z. The supraorbital keyhole approach with eyebrow incisions for treating lesions in the anterior fossa and sellar region. Chin. Med. J. 2004, 117, 323–326. [Google Scholar]
- Lemée, J.-M.; Corniola, M.V.; Da Broi, M.; Schaller, K.; Meling, T.R. Early Postoperative Complications in Meningioma: Predictive Factors and Impact on Outcome. World Neurosurg. 2019, 128, e851–e858. [Google Scholar] [CrossRef] [PubMed]
- Ottenhausen, M.; Rumalla, K.; Alalade, A.F.; Nair, P.; La Corte, E.; Younus, I.; Forbes, J.A.; Ben Nsir, A.; Banu, M.A.; Tsiouris, A.J.; et al. Decision-making algorithm for minimally invasive approaches to anterior skull base meningiomas. Neurosurg. Focus 2018, 44, E7. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Castilla, L.; Russin, J.J.; Spetzler, R.F. Surgical management of skull base tumors. Rep. Pract. Oncol. Radiother. 2016, 21, 325–335. [Google Scholar] [CrossRef]
- Shahid, A.H.; Butler, D.; Dyess, G.; Bassett, M.; Harris, L.; Hummel, U.; Chason, D.; Thakur, J.D. Supraorbital keyhole approaches in the first 3 years of practice: Outcomes and lessons learned. Patient series. J. Neurosurg. Case Lessons 2024, 7, CASE23744. [Google Scholar] [CrossRef] [PubMed]
- Valerio, J.E.; Ramirez-Velandia, F.; Fernandez-Gomez, M.P.; Rea, N.S.; Alvarez-Pinzon, A.M. Bridging the Global Technology Gap in Neurosurgery: Disparities in Access to Advanced Tools for Brain Tumor Resection. Neurosurg. Pract. 2024, 5, e00090. [Google Scholar] [CrossRef]
- Pinzón, A.A.; Stanford, L. Overcoming Barriers: Bioethics for equity in rare diseases and neurooncology in Latin America and the Caribbean. Rev. Med. 2024, 31, 7–10. [Google Scholar] [CrossRef]
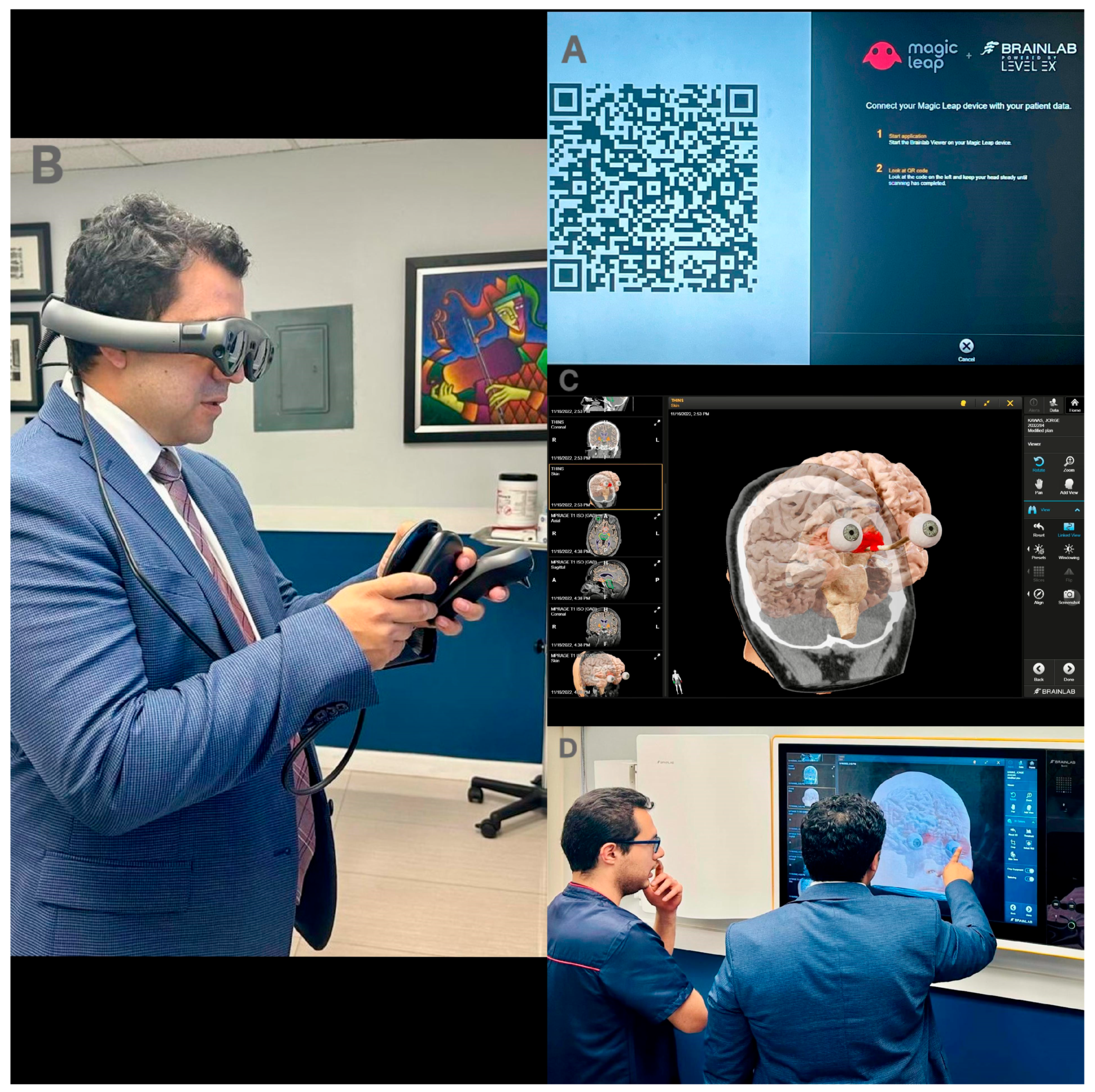


| Step | Procedure |
|---|---|
| The patient is positioned supinely. |
| General anesthesia is administered. | |
| The head is elevated approximately 15 degrees and retro-flexed 25 to 30 degrees. | |
| A Mayfield head holder is used to stabilize the head. | |
| A precise incision is made without shaving, preserving cosmetic appearance and providing a controlled entry point (measuring around 2.5 to 3 cm in length). |
| Subcutaneous dissection: performed using monopolar electrocautery. |
| Subperiosteal dissection: remove the galea from the squamous portion of the frontal bone | |
| A 2.5 cm × 2 cm “D-shaped” craniotomy is executed, facing the linear part downwards, along the anterior cranial fossa floor. |
| The internal part of the orbital portion of the frontal bone is smoothed, optimizing the surgical field for tumor access. |
| The dura is carefully opened in an oblique or cross fashion, exposing the underlying brain and meningioma. |
| Precise identification of the tumor borders and boundaries is performed to lay the groundwork for a successful resection. |
| Rhoton forceps, cotton balls, and cotonoids are used, providing a clear line of sight to the tumor. |
| To release cerebrospinal fluid (CSF) and alleviate surrounding pressure. |
| Crucial for deeply seated lesions and widening the field. | |
| Meticulous dissection is aided by neuronavigation for precision and safety. |
| Nearby neurovascular structures are gently retracted using microsurgery principles. | |
| Ensures meticulous control of bleeding sources, minimizing the risk of a postoperative hemorrhage. |
| The surgical site is meticulously closed in layers to promote wound healing and reduce the risk of complications. |
| Aspect | Advantages | Disadvantages or Possible Complications |
|---|---|---|
| Patient positioning and preparation |
|
|
| Skin incision, dural and arachnoid layer opening, and plane closure |
|
|
| Surgical approach |
|
|
| Postoperative care |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valerio, J.; Fernandez Gomez, M.P.; Ayala Arcipreste, A.; Santiago Rea, N.; Mantilla, P.; Olarinde, I.O.; Alvarez-Pinzon, A.M. Exploring the Potential Use of Virtual Reality with a Supraorbital Keyhole Craniotomy for Anterior Skull Base Meningiomas: Two Case Reports. J. Pers. Med. 2024, 14, 1074. https://doi.org/10.3390/jpm14111074
Valerio J, Fernandez Gomez MP, Ayala Arcipreste A, Santiago Rea N, Mantilla P, Olarinde IO, Alvarez-Pinzon AM. Exploring the Potential Use of Virtual Reality with a Supraorbital Keyhole Craniotomy for Anterior Skull Base Meningiomas: Two Case Reports. Journal of Personalized Medicine. 2024; 14(11):1074. https://doi.org/10.3390/jpm14111074
Chicago/Turabian StyleValerio, Jose, Maria P. Fernandez Gomez, Arturo Ayala Arcipreste, Noe Santiago Rea, Penelope Mantilla, Immanuel O. Olarinde, and Andres M. Alvarez-Pinzon. 2024. "Exploring the Potential Use of Virtual Reality with a Supraorbital Keyhole Craniotomy for Anterior Skull Base Meningiomas: Two Case Reports" Journal of Personalized Medicine 14, no. 11: 1074. https://doi.org/10.3390/jpm14111074
APA StyleValerio, J., Fernandez Gomez, M. P., Ayala Arcipreste, A., Santiago Rea, N., Mantilla, P., Olarinde, I. O., & Alvarez-Pinzon, A. M. (2024). Exploring the Potential Use of Virtual Reality with a Supraorbital Keyhole Craniotomy for Anterior Skull Base Meningiomas: Two Case Reports. Journal of Personalized Medicine, 14(11), 1074. https://doi.org/10.3390/jpm14111074







