Lateral Pectoral Nerve Identification through Ultrasound-Guided Methylene Blue Injection during Selective Peripheral Neurectomy for Shoulder Spasticity: Proposal for a New Procedure
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Selection and Indications
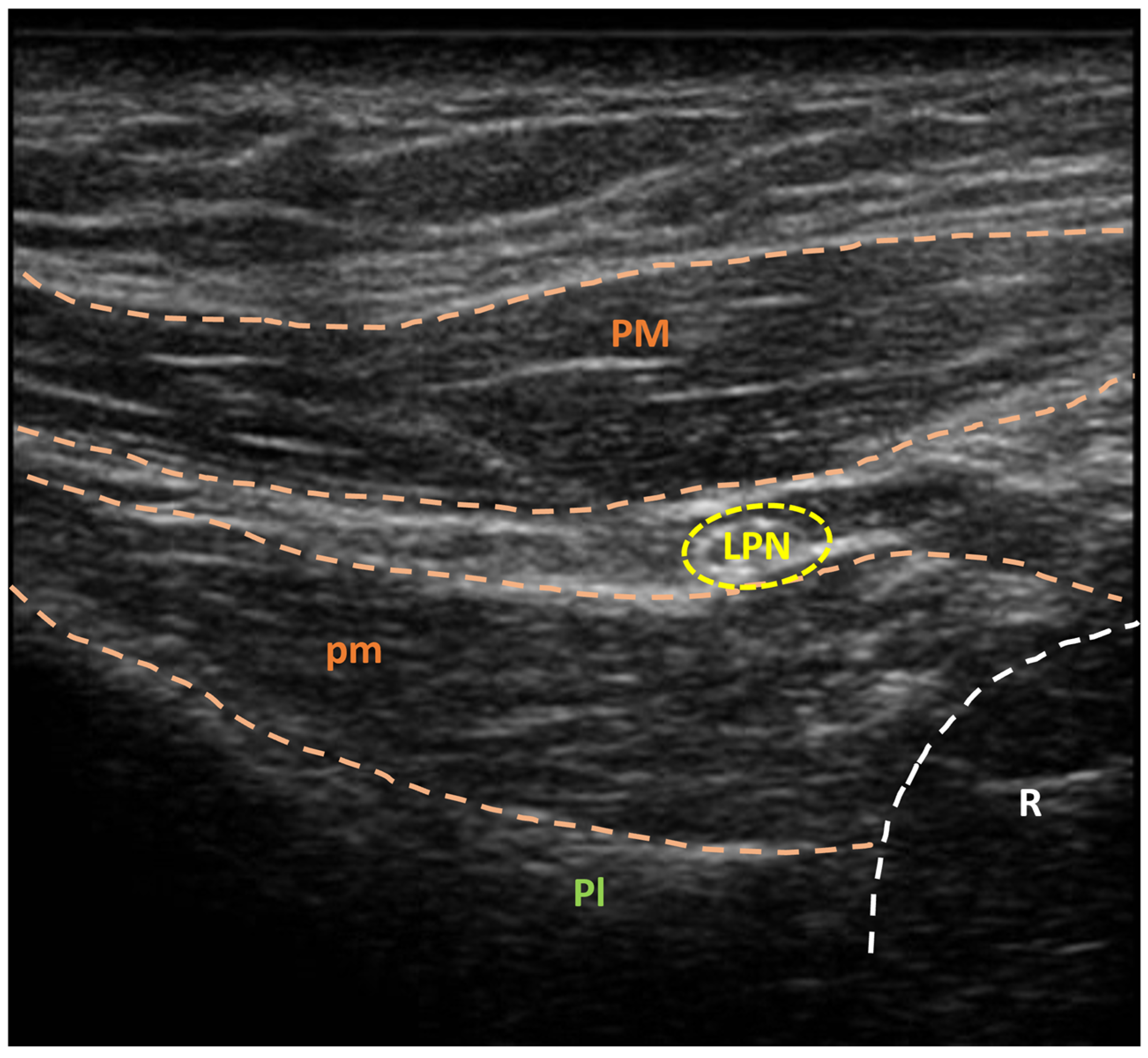
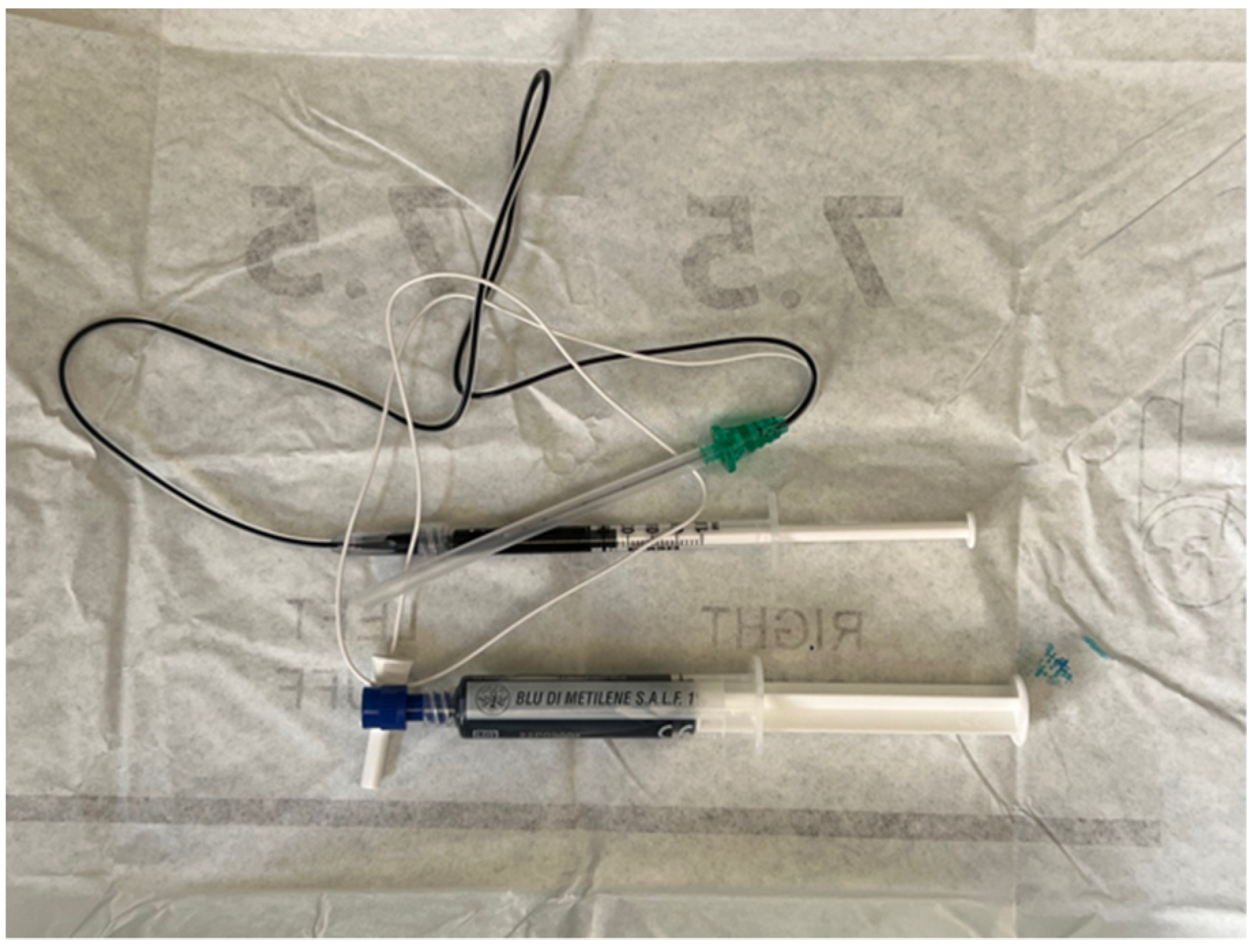
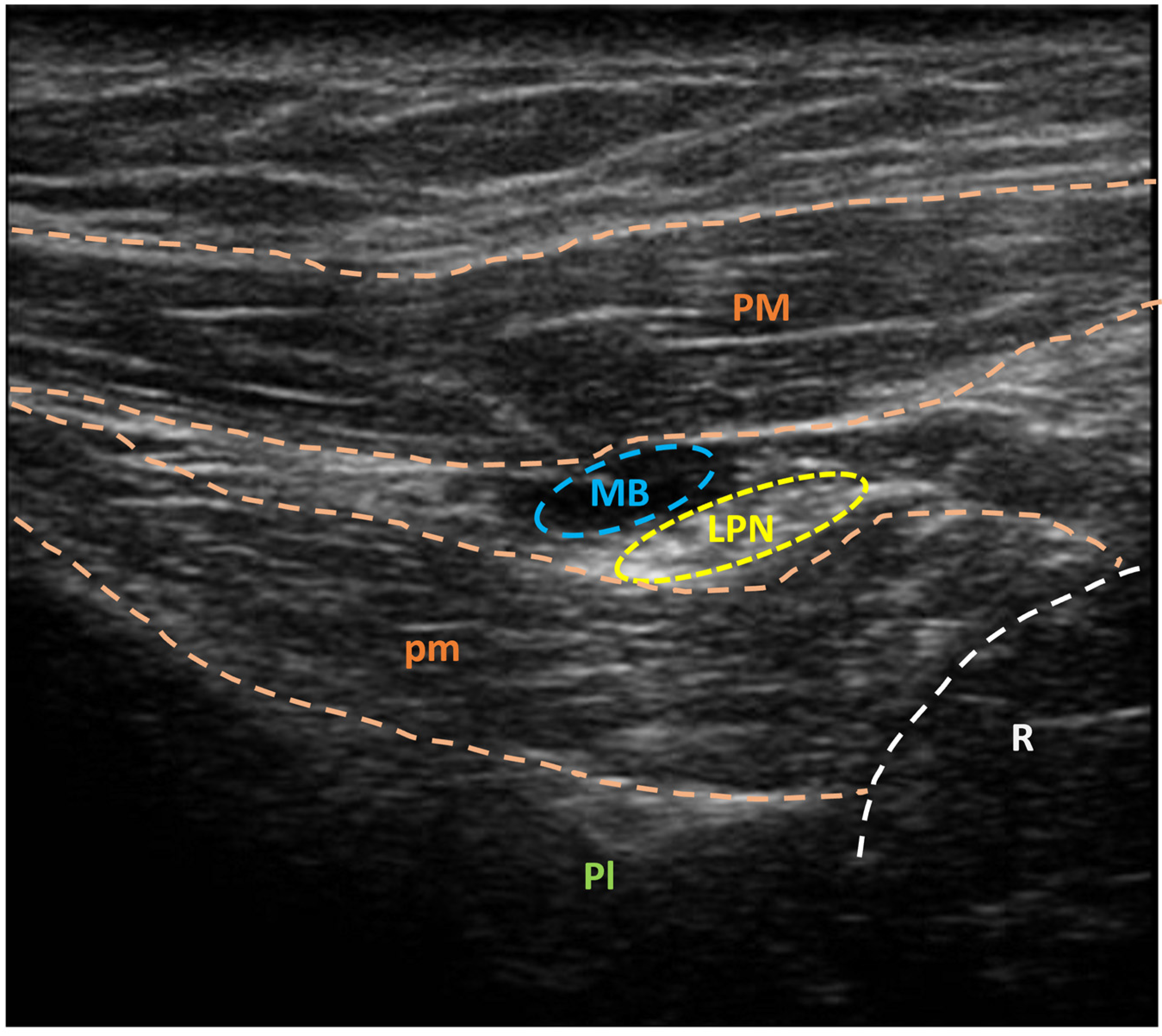
3.2. US-Guided Nerve Localization and Marking
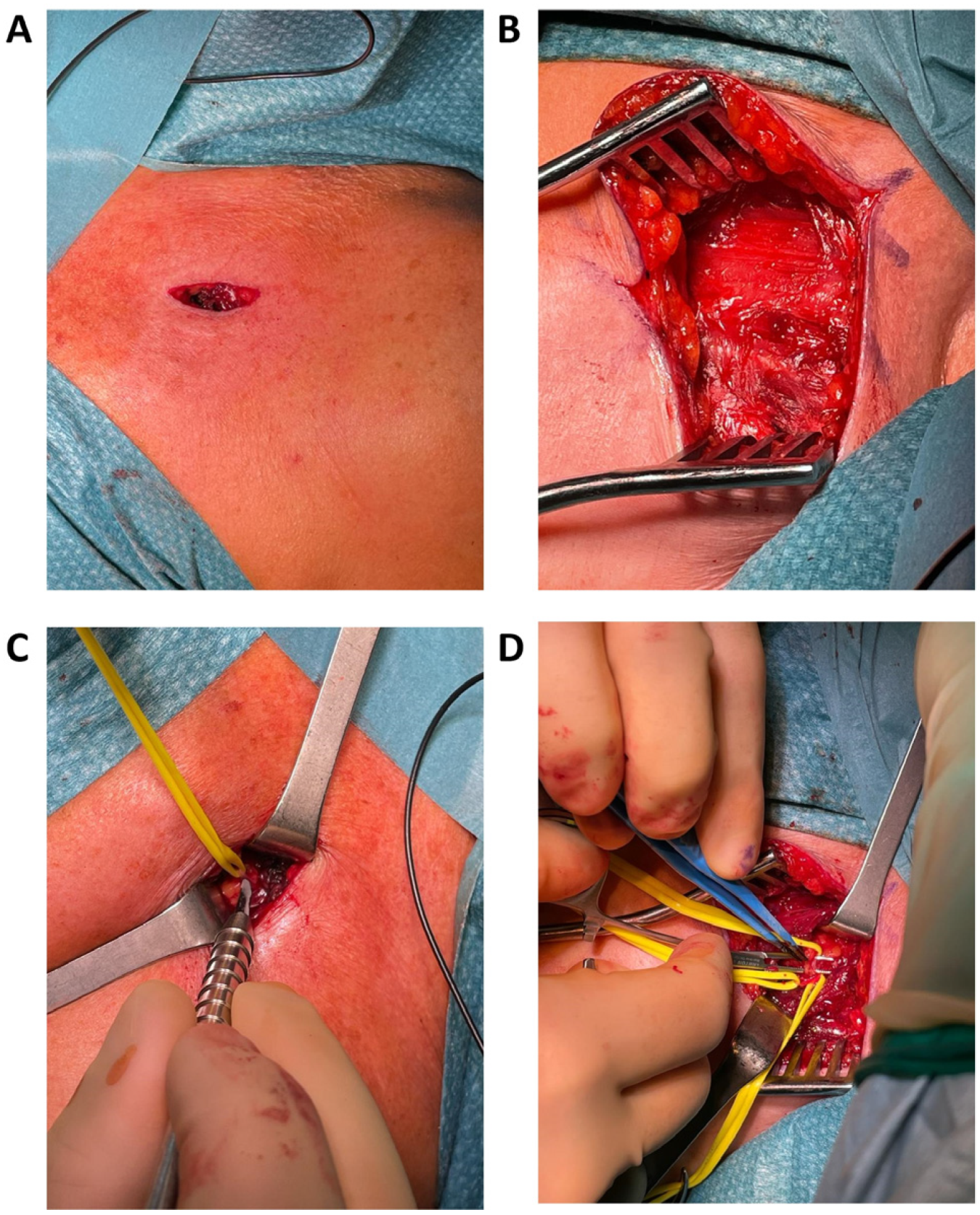
3.3. Setup
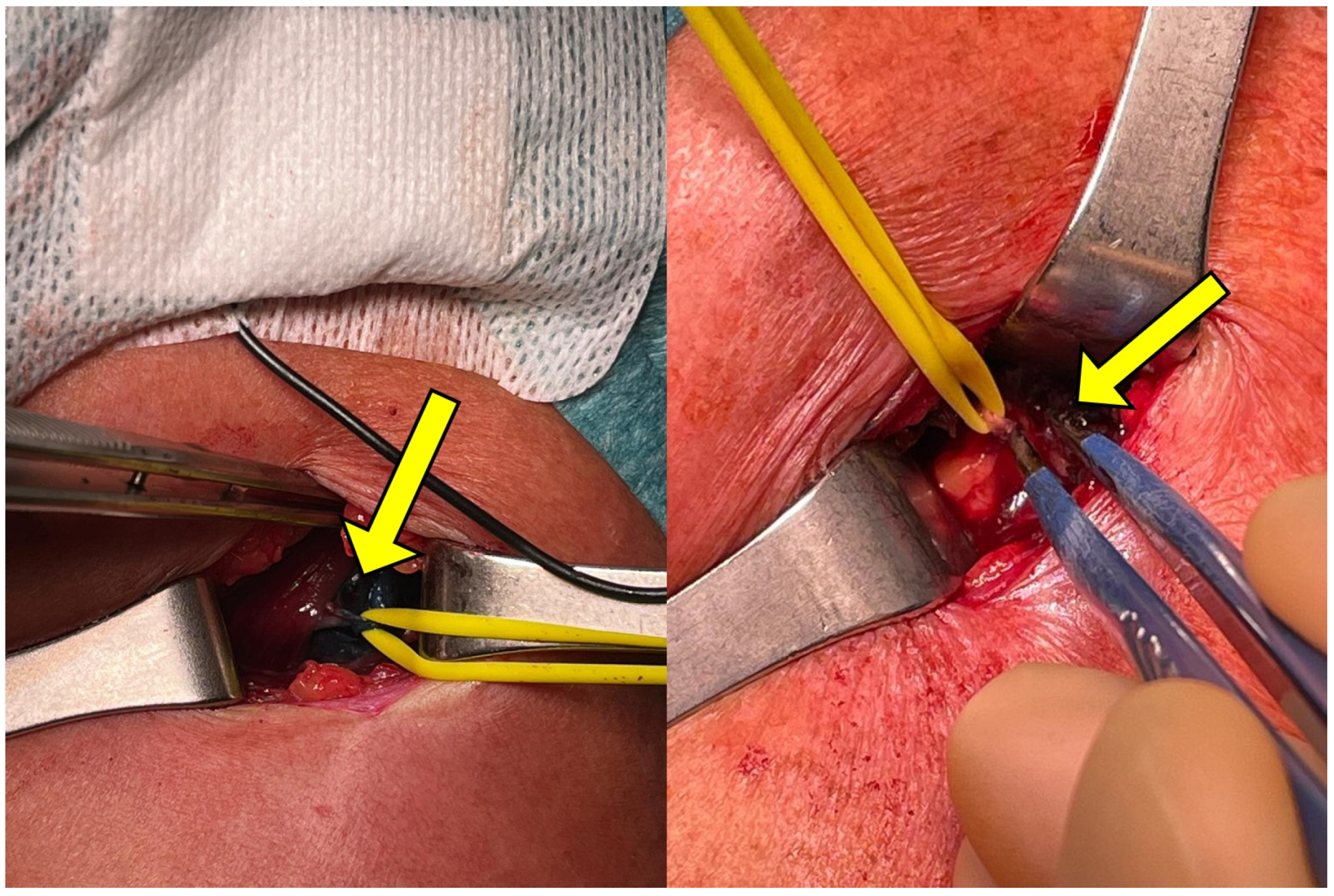
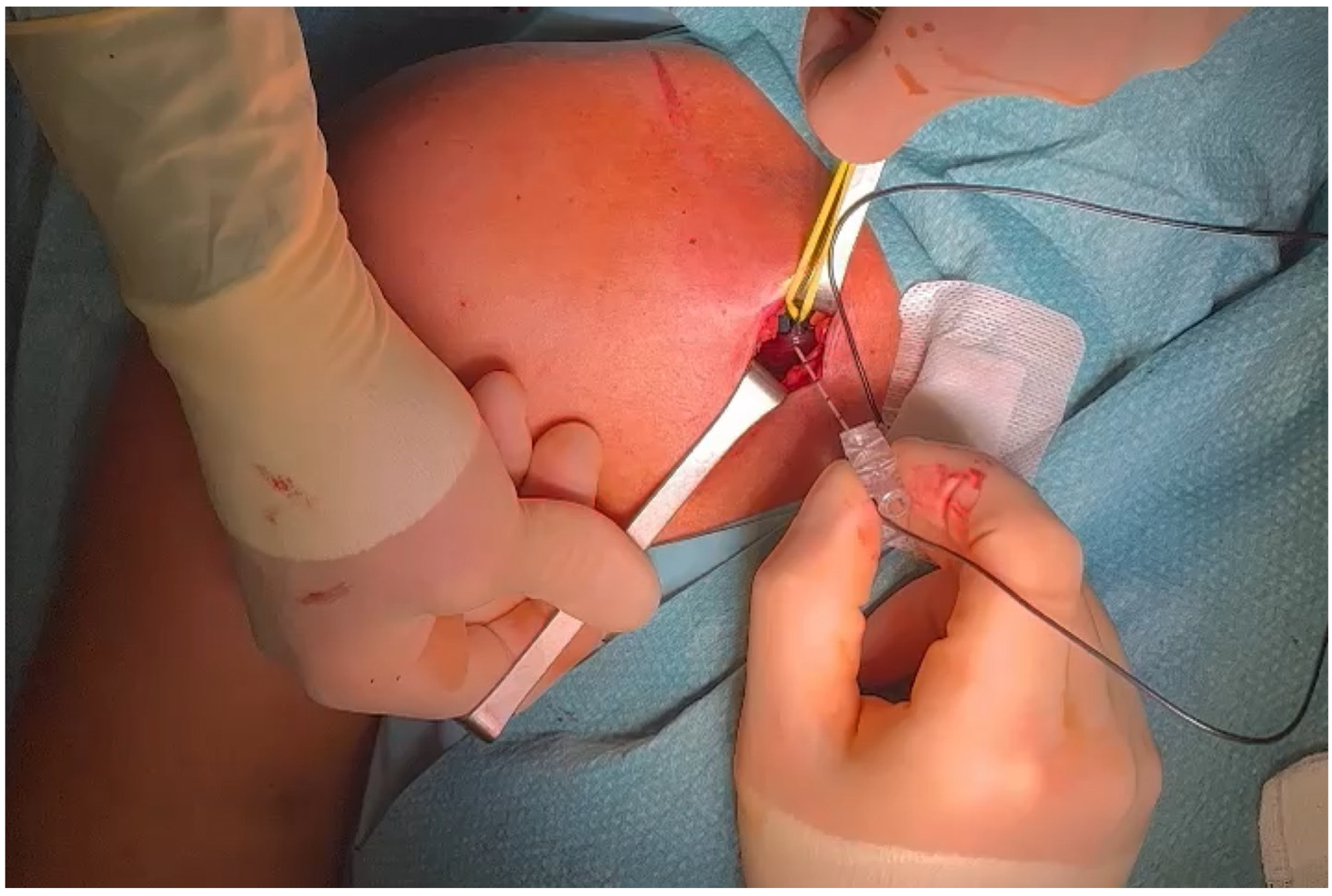
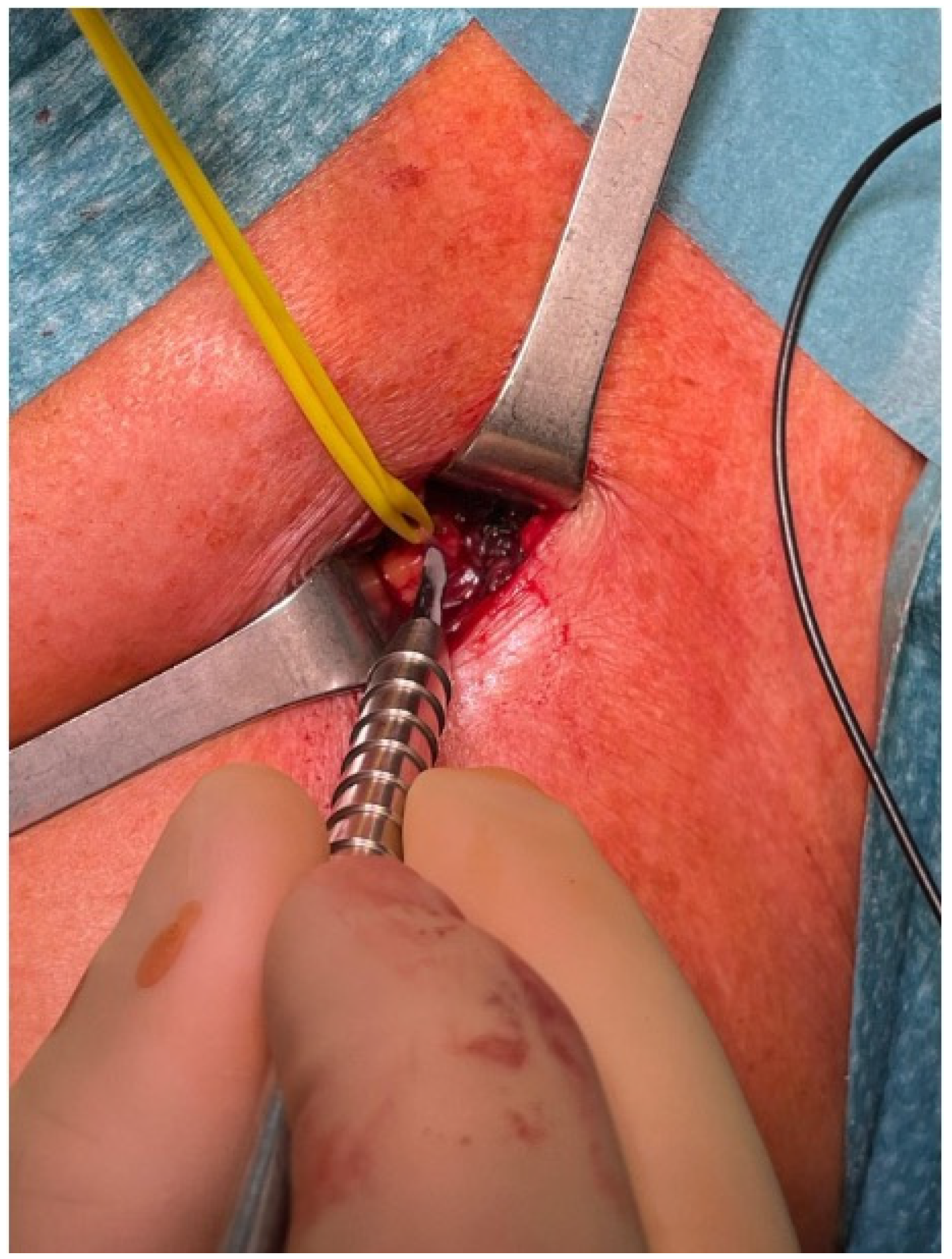
3.4. Exposure
3.5. Selective Peripheral Neurectomy
3.6. Closure and Postoperative Care
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sheean, G. The Pathophysiology of Spasticity. Eur. J. Neurol. 2002, 9 (Suppl. S1), 3–9; dicussion 53–61. [Google Scholar] [CrossRef] [PubMed]
- Kheder, A.; Nair, K.P.S. Spasticity: Pathophysiology, Evaluation and Management. Pract. Neurol. 2012, 12, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Sommerfeld, D.K.; Gripenstedt, U.; Welmer, A.-K. Spasticity after Stroke: An Overview of Prevalence, Test Instruments, and Treatments. Am. J. Phys. Med. Rehabil. 2012, 91, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Lundström, E.; Terént, A.; Borg, J. Prevalence of Disabling Spasticity 1 Year after First-Ever Stroke. Eur. J. Neurol. 2008, 15, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Wissel, J.; Manack, A.; Brainin, M. Toward an Epidemiology of Poststroke Spasticity. Neurology 2013, 80, S13–S19. [Google Scholar] [CrossRef] [PubMed]
- Urban, P.P.; Wolf, T.; Uebele, M.; Marx, J.J.; Vogt, T.; Stoeter, P.; Bauermann, T.; Weibrich, C.; Vucurevic, G.D.; Schneider, A.; et al. Occurence and Clinical Predictors of Spasticity after Ischemic Stroke. Stroke 2010, 41, 2016–2020. [Google Scholar] [CrossRef]
- Katoozian, L.; Tahan, N.; Zoghi, M.; Bakhshayesh, B. The Onset and Frequency of Spasticity After First Ever Stroke. J. Natl. Med. Assoc. 2018, 110, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Hefter, H.; Jost, W.H.; Reissig, A.; Zakine, B.; Bakheit, A.M.; Wissel, J. Classification of Posture in Poststroke Upper Limb Spasticity: A Potential Decision Tool for Botulinum Toxin A Treatment? Int. J. Rehabil. Res. 2012, 35, 227–233. [Google Scholar] [CrossRef]
- Menoux, D.; Jousse, M.; Quintaine, V.; Tlili, L.; Yelnik, A.P. Decrease in Post-Stroke Spasticity and Shoulder Pain Prevalence over the Last 15 Years. Ann. Phys. Rehabil. Med. 2019, 62, 403–408. [Google Scholar] [CrossRef]
- Fheodoroff, K.; Rekand, T.; Medeiros, L.; Koßmehl, P.; Wissel, J.; Bensmail, D.; Scheschonka, A.; Flatau-Baqué, B.; Simon, O.; Dressler, D.; et al. Quality of Life in Subjects with Upper- and Lower-Limb Spasticity Treated with incobotulinumtoxinA. Health Qual. Life Outcomes 2020, 18, 51. [Google Scholar] [CrossRef]
- Doan, Q.V.; Brashear, A.; Gillard, P.J.; Varon, S.F.; Vandenburgh, A.M.; Turkel, C.C.; Elovic, E.P. Relationship between Disability and Health-Related Quality of Life and Caregiver Burden in Patients with Upper Limb Poststroke Spasticity. PM&R. 2012, 4, 4–10. [Google Scholar] [CrossRef]
- Chang, E.; Ghosh, N.; Yanni, D.; Lee, S.; Alexandru, D.; Mozaffar, T. A Review of Spasticity Treatments: Pharmacological and Interventional Approaches. Crit. Rev. Phys. Rehabil. Med. 2013, 25, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Sturbois-Nachef, N.; Keenan, M.A.; Winston, P. Surgical Approaches to Upper Limb Spasticity in Adult Patients: A Literature Review. Front. Rehabil. Sci. 2021, 2, 709969. [Google Scholar] [CrossRef] [PubMed]
- Gras, M.; Leclercq, C. Spasticity and Hyperselective Neurectomy in the Upper Limb. Hand Surg. Rehabil. 2017, 36, 391–401. [Google Scholar] [CrossRef]
- Stoffel, A. The treatment of spastic contractures. JBJS 1913, s2–s10, 611. [Google Scholar]
- Brunelli, G.; Brunelli, F. Partial Selective Denervation in Spastic Palsies (Hyponeurotization). Microsurgery 1983, 4, 221–224. [Google Scholar] [CrossRef]
- Sindou, M.; Mertens, P. Selective Neurotomy of the Tibial Nerve for Treatment of the Spastic Foot. Neurosurgery 1988, 23, 738–744. [Google Scholar] [CrossRef]
- Mahan, M.A.; Eli, I.; Hamrick, F.; Abou-Al-Shaar, H.; Shingleton, R.; Tucker Balun, K.; Edgley, S.R. Highly Selective Partial Neurectomies for Spasticity: A Single-Center Experience. Neurosurgery 2021, 89, 827–835. [Google Scholar] [CrossRef]
- Emamhadi, M.; Alijani, B.; Haghani Dogahe, M.; Emamhadi, A. Hyper-Selective Neurectomy for Knee Flexion Spasticity: Anatomic Bases and Surgical Technique. Surg. Radiol. Anat. 2023, 45, 201–205. [Google Scholar] [CrossRef]
- Bollens, B.; Gustin, T.; Stoquart, G.; Detrembleur, C.; Lejeune, T.; Deltombe, T. A Randomized Controlled Trial of Selective Neurotomy versus Botulinum Toxin for Spastic Equinovarus Foot after Stroke. Neurorehabil Neural Repair. 2013, 27, 695–703. [Google Scholar] [CrossRef]
- Bollens, B.; Deltombe, T.; Detrembleur, C.; Gustin, T.; Stoquart, G.; Lejeune, T.M. Effects of Selective Tibial Nerve Neurotomy as a Treatment for Adults Presenting with Spastic Equinovarus Foot: A Systematic Review. J. Rehabil. Med. 2011, 43, 277–282. [Google Scholar] [CrossRef][Green Version]
- Salga, M.; Gatin, L.; Deltombe, T.; Gustin, T.; Carda, S.; Marque, P.; Winston, P.; Reebye, R.; Wein, T.; Esquenazi, A.; et al. International Recommendations to Manage Poststroke Equinovarus Foot Deformity Validated by a Panel of Experts Using Delphi. Arch. Phys. Med. Rehabil. 2022, 104, 372–379. [Google Scholar] [CrossRef]
- Lin, W.; Li, T.; Qi, W.; Shen, Y.; Xu, W. Hyperselective Neurectomy of Thoracodorsal Nerve for Treatment of the Shoulder Spasticity: Anatomical Study and Preliminary Clinical Results. Acta Neurochir. 2023, 165, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, C.; Perruisseau-Carrier, A.; Gras, M.; Panciera, P.; Fulchignoni, C.; Fulchignoni, M. Hyperselective Neurectomy for the Treatment of Upper Limb Spasticity in Adults and Children: A Prospective Study. J. Hand Surg. 2021, 46, 708–716. [Google Scholar] [CrossRef]
- Yong, L.Y.; Wong, C.H.L.; Gaston, M.; Lam, W.L. The Role of Selective Peripheral Neurectomy in the Treatment of Upper Limb Spasticity. J. Hand Surg. 2018, 23, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Heredia-Gutierrez, A.; Carbarin-Carbarin, M.E.; Torres-Garcia, S.; Gonzalez-Carranza, V.; de Leon, F.C.-P. The Utility of Selective Partial Neurectomy of the Musculocutaneous Nerve in Children with Bilateral Spastic Elbow. Childs Nerv. Syst. 2023, 39, 3509–3514. [Google Scholar] [CrossRef] [PubMed]
- Sitthinamsuwan, B.; Chanvanitkulchai, K.; Phonwijit, L.; Nunta-Aree, S.; Kumthornthip, W.; Ploypetch, T. Surgical Outcomes of Microsurgical Selective Peripheral Neurotomy for Intractable Limb Spasticity. Stereotact. Funct. Neurosurg. 2013, 91, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Rea, P. Chapter 2—Upper Limb Nerve Supply. In Essential Clinically Applied Anatomy of the Peripheral Nervous System in the Limbs; Rea, P., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 41–100. ISBN 978-0-12-803062-2. [Google Scholar]
- Simpson, D.M.; Hallett, M.; Ashman, E.J.; Comella, C.L.; Green, M.W.; Gronseth, G.S.; Armstrong, M.J.; Gloss, D.; Potrebic, S.; Jankovic, J.; et al. Practice Guideline Update Summary: Botulinum Neurotoxin for the Treatment of Blepharospasm, Cervical Dystonia, Adult Spasticity, and Headache: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2016, 86, 1818–1826. [Google Scholar] [CrossRef]
- Fitterer, J.W.; Picelli, A.; Winston, P. A Novel Approach to New-Onset Hemiplegic Shoulder Pain With Decreased Range of Motion Using Targeted Diagnostic Nerve Blocks: The ViVe Algorithm. Front. Neurol. 2021, 12, 668370. [Google Scholar] [CrossRef]
- Porzionato, A.; Macchi, V.; Stecco, C.; Loukas, M.; Tubbs, R.S.; De Caro, R. Surgical Anatomy of the Pectoral Nerves and the Pectoral Musculature. Clin. Anat. 2012, 25, 559–575. [Google Scholar] [CrossRef]
- Creze, M.; Peltier, J.; Havet, E.; Potier, A.; Lefranc, M.; Foulon, P.; Le Gars, D. Anatomy and Surgical Landmarks for the Ansa Pectoralis: Application to Pectoralis Major Nerve Selective Neurotomy. Surg. Radiol. Anat. 2012, 34, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Macchi, V.; Tiengo, C.; Porzionato, A.; Parenti, A.; Stecco, C.; Mazzoleni, F.; De Caro, R. Medial and Lateral Pectoral Nerves: Course and Branches. Clin. Anat. 2007, 20, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Maarrawi, J.; Mertens, P.; Luaute, J.; Vial, C.; Chardonnet, N.; Cosson, M.; Sindou, M. Long-Term Functional Results of Selective Peripheral Neurotomy for the Treatment of Spastic Upper Limb: Prospective Study in 31 Patients. J. Neurosurg. 2006, 104, 215–225. [Google Scholar] [CrossRef]
- Puligopu, A.K.; Purohit, A.K. Outcome of Selective Motor Fasciculotomy in the Treatment of Upper Limb Spasticity. J. Pediatr. Neurosci. 2011, 6, S118–S125. [Google Scholar] [CrossRef] [PubMed]
- Fouad, W. Management of Spastic Hand by Selective Peripheral Neurotomies. Alex. J. Med. 2011, 47, 201–208. [Google Scholar] [CrossRef][Green Version]
- Mikalef, P.; Power, D. The Role of Neurectomy in the Management of Spasticity of the Upper Limb. EFORT Open Rev. 2017, 2, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Gavilán, J.; Gavilán, C.; Tomás, M.D. Methylene Blue Infusion for Intraoperative Identification of the Parathyroid Glands. Laryngoscope 1986, 96, 1389–1390. [Google Scholar] [CrossRef]
- Aoun, H.D.; Littrup, P.J.; Heath, K.E.; Adam, B.; Prus, M.; Beydoun, R.; Baciewcz, F. Methylene Blue/Collagen Mixture for CT-Guided Presurgical Lung Nodule Marking: High Efficacy and Safety. J. Vasc. Interv. Radiol. 2020, 31, 1682.e1–1682.e7. [Google Scholar] [CrossRef]
- Vegunta, R.K.; Rawlings, A.L.; Jeziorczak, P.M. Methylene Blue: A Simple Marker for Intraoperative Detection of Gastroduodenal Perforations during Laparoscopic Pyloromyotomy. Surg. Innov. 2010, 17, 11–13. [Google Scholar] [CrossRef]
- Bužga, M.; Machytka, E.; Dvořáčková, E.; Švagera, Z.; Stejskal, D.; Máca, J.; Král, J. Methylene Blue: A Controversial Diagnostic Acid and Medication? Toxicol. Res. 2022, 11, 711–717. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zerbinati, P.; Bemporad, J.; Massimiani, A.; Bianchini, E.; Mazzoli, D.; Glorioso, D.; della Vecchia, G.; De Luca, A.; De Blasiis, P. Lateral Pectoral Nerve Identification through Ultrasound-Guided Methylene Blue Injection during Selective Peripheral Neurectomy for Shoulder Spasticity: Proposal for a New Procedure. J. Pers. Med. 2024, 14, 116. https://doi.org/10.3390/jpm14010116
Zerbinati P, Bemporad J, Massimiani A, Bianchini E, Mazzoli D, Glorioso D, della Vecchia G, De Luca A, De Blasiis P. Lateral Pectoral Nerve Identification through Ultrasound-Guided Methylene Blue Injection during Selective Peripheral Neurectomy for Shoulder Spasticity: Proposal for a New Procedure. Journal of Personalized Medicine. 2024; 14(1):116. https://doi.org/10.3390/jpm14010116
Chicago/Turabian StyleZerbinati, Paolo, Jonathan Bemporad, Andrea Massimiani, Edoardo Bianchini, Davide Mazzoli, Davide Glorioso, Giuseppe della Vecchia, Antonio De Luca, and Paolo De Blasiis. 2024. "Lateral Pectoral Nerve Identification through Ultrasound-Guided Methylene Blue Injection during Selective Peripheral Neurectomy for Shoulder Spasticity: Proposal for a New Procedure" Journal of Personalized Medicine 14, no. 1: 116. https://doi.org/10.3390/jpm14010116
APA StyleZerbinati, P., Bemporad, J., Massimiani, A., Bianchini, E., Mazzoli, D., Glorioso, D., della Vecchia, G., De Luca, A., & De Blasiis, P. (2024). Lateral Pectoral Nerve Identification through Ultrasound-Guided Methylene Blue Injection during Selective Peripheral Neurectomy for Shoulder Spasticity: Proposal for a New Procedure. Journal of Personalized Medicine, 14(1), 116. https://doi.org/10.3390/jpm14010116







